ABSTRACT
Individual differences in cognitive function are due to a combination of heritable and non-heritable factors. A large body of evidence from clinical, cognitive, and pharmacological neuroscience implicates dopaminergic gene variants as modulators of cognitive functions. Neuroepigenetic studies demonstrate environmental factors also influence complex phenotypes by affecting gene expression regulation. To evaluate the mechanism of environmental influence on cognitive abilities, we examined if epigenetic regulation of dopaminergic genes plays a role in cognition. Using a DNA methylation profiling microarray, we used a monozygotic (MZ) twin difference design to evaluate if co-twin differences in methylation of CpG sites near six dopaminergic genes predicted differences in response inhibition and memory performance. Studying MZ twins allows us to assess if environmentally driven differences in methylation affect differences in phenotype while controlling for the influence of genotype and shared family environment. Response inhibition was assessed with the flanker task and short-term and working memory were assessed with digit span recall. We found MZ co-twin differences in DRD4 gene methylation predicted differences in short-term memory. MZ differences in COMT, DBH, DAT1, DRD1, and DRD2 gene methylation predicted differences in response inhibition. Taken together, findings suggest methylation status of dopaminergic genes may influence cognitive functions in a dissociable manner. Our results highlight the importance of the epigenome and environment, over and above the influence of genotype, in supporting complex cognitive functions.
KEYWORDS: DNA methylation, cognition, memory, dopamine, epigenetics, childhood, twin, response inhibition
Introduction
Complex cognitive functions are centrally important for childhood development and adult health. They facilitate regulation of thoughts, emotions, and actions, which is required for effective interactions with our external world. This is clear when noting the overlap of cognitive dysfunction seen across neurodevelopmental, neurodegenerative, and psychiatric disorders that contribute to reduced quality of life and independence [1,2]. Cognitive functions involve dopaminergic neurocircuitry; dopamine is a catecholamine neuromodulator highly implicated in motivation, mood, and cognition [3]. The mesocortical and mesolimbic dopaminergic systems originate in the ventral tegmental area and project to the prefrontal cortex; anterior cingulate cortex; anterior temporal structures such as the amygdala, hippocampus, and entorhinal cortex; and the basal forebrain [4]. The executive attention network is a neural network involving the anterior cingulate cortex and prefrontal cortex while various memory processes also include brain regions such as the prefrontal cortex, hippocampus, and entorhinal cortex. Pharmacological manipulation of dopaminergic tone and receptors impacts cognitive capacities [5]. Genetic variation in dopaminergic genes is associated with cognitive performance in both healthy and clinical populations [6–8]. According to twin studies, cognitive ability is influenced by individual genetics with heritability estimates ranging from 40 −80%, with genetic influence increasing with age [9].
Figure 2.
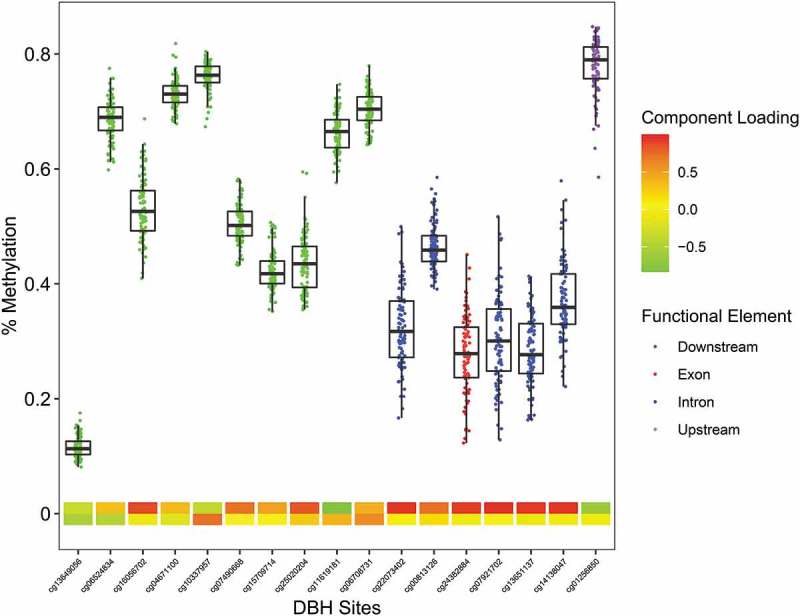
The Infinium MethylationEPIC β-values representing percent methylation were plotted against DBH CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point color. The two heat map rows represent the first and second component loadings from PCA analysis.
Figure 3.
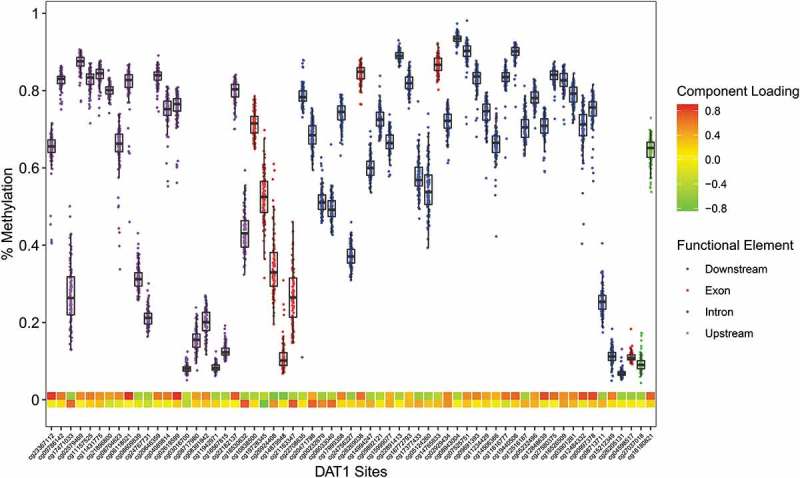
The Infinium MethylationEPIC β-values representing percent methylation were plotted against DAT1 CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point colors. The two heat map rows represent the first and second component loadings from PCA analysis.
Figure 4.
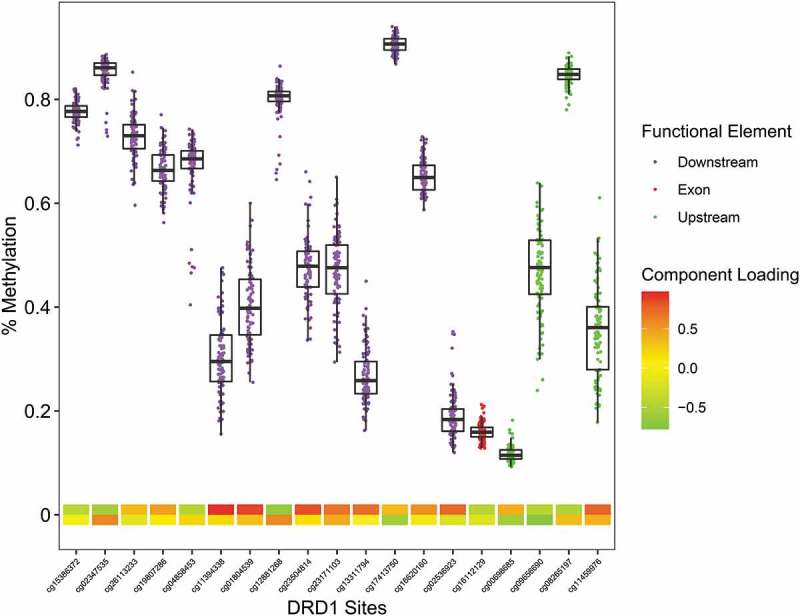
The Infinium MethylationEPIC β-values representing percent methylation were plotted against DRD1 CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point colors. The two heat map rows represent the first and second component loadings from PCA analysis.
Figure 5.
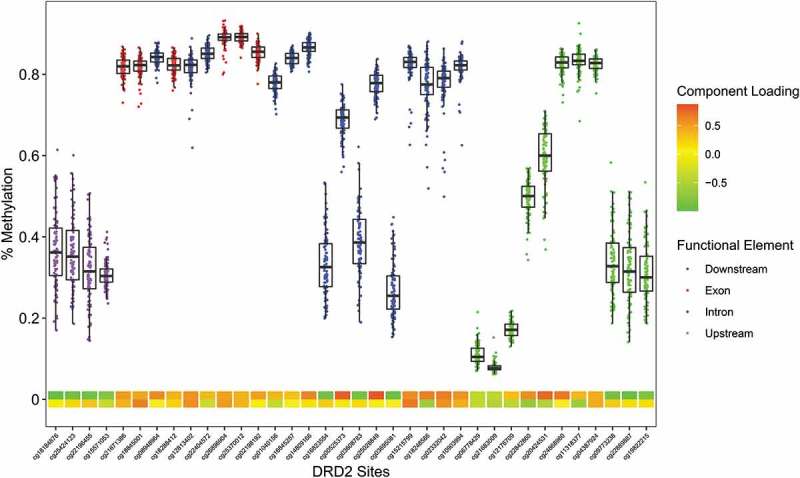
The Infinium MethylationEPIC β-values representing percent methylation were plotted against DRD2 CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point colors. The two heat map rows represent the first and second component loadings from PCA analysis.
Allelic variation can determine protein structure, function, and expression. Slight gene sequence alterations can have consequential effects on behavior. Variation in genes regulating dopamine conversion, degradation, and reuptake influence diverse cognitive abilities. For example, genetic polymorphisms in β-hydroxylase (DBH), which codes for an enzyme that catalyzes dopamine into norepinephrine, has been associated with individual differences in cognitive and memory tasks [10,11]. Polymorphisms of the catechol O-methyltransferase (COMT) gene, whose product metabolizes dopamine, are related to individual differences in cognitive function: the slower-acting Met/Met polymorphism, functionally increasing the amount of time dopamine is active in prefrontal cortex synapses, is associated with an advantage in behavioral and brain measures compared to the Val/Val genotype which metabolizes dopamine faster [12,13]. The dopamine transporter is responsible for clearing dopamine from the synaptic cleft and polymorphisms in this gene (DAT1) are associated with individual differences in cognitive tasks [14].
Dopamine’s effects are primarily mediated by G-coupled protein receptors and downstream cell signaling pathways [15]. Genetic variations in dopamine receptors have also been associated with cognitive functions in both healthy and clinical populations (DRD1 and DRD4) and in cognitive deficits after traumatic brain injury (DRD2) [6,16–19]. Generally, DRD1 and DRD2 activation is associated with increased cognitive abilities whereas DRD4 with decreased performance [20–23]. This body of literature demonstrates how individual genetic variation can partially account for individual differences in complex cognitive performance, however there are also non-heritable factors influencing cognitive abilities.
Genetic variability does not solely determine disease risk or complex behavior [24]. For example, neurodevelopmental, neurodegenerative, and psychiatric disorders all have genetic and environmental etiological factors (excluding rare monogenic disorders). While heritability estimates are considered moderate to high for the majority of these sporadic disorders (30–77%), there still remains an important environmental component [25–27]. Neuroepigenetic studies demonstrate environmental factors influence complex behavior and disease risk through the epigenome [28]. The epigenome is a complex regulatory code outside of the genetic nucleotide sequence. Through many molecular processes including electrostatic interactions, transcriptional machinery blockade, and RNA interference, the epigenetic code plays a role in determining what genes, and how much of a specific gene, is expressed [29]. Epigenetic processes can be dynamic and respond to social and environmental exposures. A seminal paper demonstrated early life stress can have long-lasting effects on DNA methylation, glucocorticoid receptor expression, stress physiology, and behavior in rats [30]. Since then, several studies replicated these findings in animal and human models, suggesting an important role for early life stress in adult physiology and behavior. Importantly, this implicates epigenetic processes as molecular mechanisms by which environmental factors can shape neurobiology and related complex behavior. Together, this suggests variability in cognitive function between individuals may be partially determined by experience dependent epigenetic regulation of cognitive genes.
Middle childhood is an important developmental time period to assess cognitive task performance. While there is substantial development of executive functions and memory processes between early- and middle-childhood largely considered under genetic control, research suggests environment affects cognitive development during this period as well [14,31]. Because cognition and the development of cognitive brain networks are malleable during this period, it may be an ideal period for interventions [32–35].
One of the most powerful methods of assessing environmental influences is the monozygotic (MZ) twin difference design. Trying to disentangle environmental influences on complex human behavior is difficult due to gene-environment interplay and the heritability of environmental assessments, such as socioeconomic status, stress, exercise, and nutrition. MZ twins share identical genomes except for somatic mutations that may confer differential disease risk between co-twins [36]. However, MZ twin difference designs are still the best method for assessing the influence of environment while controlling for genetic influence on complex phenotypes [37]. In this study, we extend the MZ difference approach to measure differences in DNA methylation as a product of the molecular effects of non-shared experiences. This approach is critically important since genotype as well as environment affect both methylation and gene expression [38], and much of the research on environmental effects on epigenetics lacks control for genetic influences.
The objective of this study was to examine the relation between dopaminergic gene methylation and cognitive task performance controlling for the confounding effects of genetic and early familial environmental factors using a childhood MZ twin difference design. We used the conflict flanker task to assess response inhibition and the Digit Span to assess short-term and working memory [39,40]. We hypothesized that greater co-twin differences in dopamine gene methylation would predict greater co-twin differences in cognitive performance.
Methods
Sample
Participants were members of Arizona Twin Project, a longitudinal study investigating the genetic and environmental influences on childhood health [41]. Participants were recruited from state birth records. We recruited a sub-sample of 48 MZ twin pairs (N = 96; 51% male; 50% Non-Hispanic White, 14.6% Hispanic/Latinx, 8.3% African American, 4.2% Asian American), mean age = 8.5 years, SD = .45. Seventy-one percent of primary caregivers reported currently being married. Total household income ranged from $6,400 – $300,000 USD (M = $97,057, SD = $64,893) and 21% of our families met Federal Medicaid Eligibility based on 2016 standards (Arizona Median Household Income = $53,510, US Census Bureau). Assessments were conducted when participants were ages 12 months, 30 months, 5 years, and 8 years. This study analyzed behavior and buccal cell samples collected during the 8-year home visit. All study procedures were approved by institutional review boards and are in accordance with the Helsinki Declaration of 1975.
Cognitive function and memory tasks
Digit span
Short-term and working memory were assessed with a standard digit span task. Participants were read an increasingly longer series of numbers (started with three and ended with 12) and asked to repeat it back either in the same order (forward) to assess short-term memory or the reverse order (backward) to assess working memory.
Flanker task
Participants were administered the flanker task from The Psychology Experiment Building Language (PEBL) [42,43], an open source software system, during home visits. Briefly, participants were presented with a white fixation cross for 500 ms, which was immediately followed by a horizontal array of five equally sized and spaced white arrows for 800 ms. Participants were instructed to attend to the middle arrow and ignore the four flanking arrows. Participants were instructed to press the left key for a left facing middle arrow and the right key for a right facing middle arrow. The flanking arrows either all pointed in the same direction as the target arrow (e.g., ‘< < < < <’; Congruent Trial), or they all pointed in the opposite direction (e.g., ‘< < > < <’; Incongruent Trial). Performance on the incongruent trials provides a measure of response inhibition [40].
Participants received a total of 160 trials (80 congruent and 80 incongruent ones) in a random order, requiring an equal number of left or right responses. Prior to the experiment subjects received 8 practice trials that were not analyzed. The task lasted approximately 10 min.
DNA methylation
Buccal cells were collected with Mawi iSWAB DNA collection tubes (Mawi DNA Technologies LLC, Hayward, CA) from 96 participants during the 8-year home visit. DNA was extracted with a Qiagen DNeasy Blood & Tissue isolation kit (Qiagen, Hilden, Germany). Sample yield and purity were assessed spectrophotometrically using a NanoDrop ND-1000 (ThermoScientific, Wilmington, DE) and using Qubit fluorometric methods. Approximately 500 ng of DNA was treated with sodium bisulfite using the EZ-96 DNA Methylation Kit (Zymo Research, Irvine, CA). DNA methylation was quantified using the Infinium MethylationEPIC BeadChip (IlluminaEPICarray) run on an Illumina iScanSystem (Illumina, San Diego, CA). Twin pairs were in adjacent positions. Raw IDAT files were exported for preprocessing in R with the minfi package [44]. We applied a filter to remove probes located on the sex chromosomes. Data was subjected to quality control analyses, which included quantile normalization, checking for sex mismatches, and excluding low-intensity samples (p < .01). Samples from 93 twins passed our quality control pipeline, including 45 complete twin pairs. Using the minfi package, data were normalized and annotated with Illumina CpG site probe names. Using the R package EpiDISH (Epigenetic Dissection of Intra-Sample Heterogeneity, 3.8) RPC method, we included the proportion of estimated epithelial cells as a covariate in our statistical models (m = 69%) [45,46]. Array was included as a covariate in all analyses to control for batch effects.
We interrogated dopamine genes involved in cognitive function and memory by identifying probes on the array that were annotated to pre-specified dopaminergic genes. Average beta values were calculated by dividing the methylated probe signal intensity by the sum of methylated and unmethylated probe signal intensities. Average beta values range from 0 (completely unmethylated) to 1 (fully methylated) and provide a quantitative readout of relative DNA methylation for each CpG site (Figure 1–6). The M-value was calculated as the log2 ratio of the intensities of methylated probe versus unmethylated probe. An M-value close to 0 indicates a similar intensity between the methylated and unmethylated probes, which means the CpG site is about half-methylated. Positive M-values indicate that more molecules are methylated than unmethylated, while negative M-values mean the opposite [47].
Figure 1.
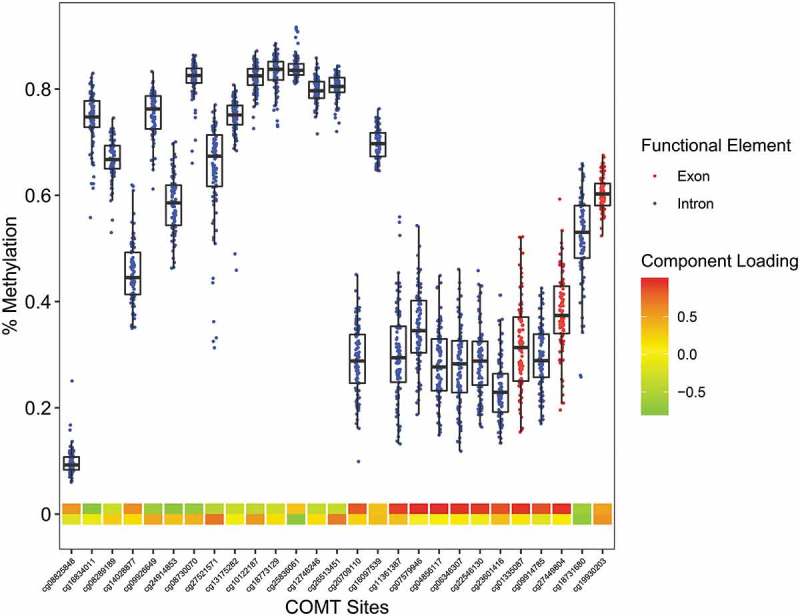
The Infinium MethylationEPIC β-values representing percent methylation were plotted against COMT CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point color. The two heat map rows represent the first and second component loadings from PCA analysis.
Figure 6.

The Infinium MethylationEPIC β-values representing percent methylation were plotted against DRD4 CpG sites. Data points represent individuals. CpG location in relation to the gene is indicated by the data point colors. The heat map row represents the component loadings from PCA analysis.
Statistical analysis
The genes of interest had 20–41 associated CpG probes. Instead of performing 20–41 tests of classical regression with methylation values, we used principal components analysis (PCA) to reduce data dimensionality. This procedure allowed us to use gene methylation summary statistics to characterize the data and reduce the number of statistical tests to avoid Type 1 errors. PCA is an established technique that has been used to reduce high-dimensionality of methylation data [37,48–50]. PCA projects data into new orthogonal directions corresponding to the directions of maximum variance (not amount). PCA appropriateness was assed with the Kaiser-Meyer-Olkin Measure of Sampling Adequacy and the Barlett’s Test of Sphericity. We extracted the first two components after removing all sites with loading values between -0.3 and 0.3 . Component scores were computed as the raw CpG M-values weighted by the factor loadings. Components with negative and positive loadings represent variance in methylation rather than methylation amount (Figure 1–6 and supplemental data). M-values were used for methylation analysis as has been recommended, especially for the homoscedasticity [47].
Short-term and working memory were assessed with standard correct count variables (Total Forward and Total Backward). Response inhibition was assessed with Linear Integration Speed Accuracy Scores [LISAS] for incongruent trials from the flanker task [48]. LISAS were computed with the following variables: RT (reaction time) and PE (proportion of error) are specific to the condition (congruent, incongruent). SRT and SPE are the standard deviations across both conditions. LISAS were computed with the following equation: RTcondition-specific + [(SRT-overall/SPE-overall)*PEcondition-specific. Multiplying PE by the ratio of the Standard Deviations gives both RT and PE an equal weight. Higher LISAS values indicate lower efficiency (higher RT and greater PE), these were reverse coded for analyses and ease of understanding [48].
MZ twin difference scores were computed from components and task performance for linear regression analysis controlling for sex, array, and cell count. Socioeconomic status was tested as an additional covariate in all analyses but failed to show significance and so was omitted from final models. We report the standardized beta value for all regression results with the p and corresponding FDR corrected p values. Differences in methylation were used as predictor variables and differences in cognitive performance were used as dependent variables. Mixed model regressions were conducted in SPSS 25. For all variables, participants > 3 standard deviations from the mean were considered outliers and dropped from the analysis (2 removed from Total Forward; 2 removed from Total Backward; 3 removed from LISAS incongruent).
Results
Preliminary analyses
We extracted the first and second components after removing all CpGs sites with loading values in between -0.3 and 0.3. For the fist component, all eigenvalues were > 6.5 and variance explained ranged from 31.34–70.15% (Table 1). For all analyses, Kaiser-Meyer-Olkin Measure of Sampling Adequacy > .80, indicating sampling was adequate. For all analyses, Barlett’s Test of Sphericity was significant at p < .001, indicating that it is highly unlikely for us to have obtained the observed correlation matrix by chance. Zero order correlations among all variables are reported (Table 2). All components and task performance had significant twin intra-class correlations, indicating that co-twins were similar on methylation and cognitive functions (Table 3).
Table 1.
Methylation probe reduction using PCA.
| Gene | CpG Probes | Component Probes | Eigenvalue | Variance Explained |
|---|---|---|---|---|
| COMT | 41 | 28 | 13.11 | 45.21% |
| DBH | 31 | 17 | 8.97 | 49.85% |
| DAT1 | 116 | 63 | 20.96 | 33.28% |
| DRD1 | 38 | 20 | 6.59 | 31.34% |
| DRD2 | 61 | 35 | 14.96 | 41.57% |
| DRD4 | 20 | 9 | 7.02 | 70.15% |
Table 2.
Zero-order correlations among individual twin variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | M | SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total Forward | - | 6.44 | 2.10 | |||||||||||||
| 2. Total Backward | 0.081 | - | 4.26 | 1.37 | ||||||||||||
| 3 LISAS Incongruent | −0.176 | −0.034 | - | 308.38 | 86.21 | |||||||||||
| 4. COMT C1 | −0.165 | −0.107 | −0.062 | - | ||||||||||||
| 5. COMT C2 | −0.059 | 0.027 | 0.185 | 0.076 | - | |||||||||||
| 6. DBH C1 | −0.175 | −0.086 | −0.105 | .961** | 0.040 | - | ||||||||||
| 7. DBH C2 | −0.005 | .257* | 0.095 | 0.091 | .617** | 0.019 | - | |||||||||
| 8. DAT1 C1 | −0.018 | .232* | 0.114 | −.491** | .315** | −.491** | .422** | - | ||||||||
| 9. DAT1 C2 | −0.128 | −0.059 | 0.103 | .717** | .604** | .667** | .477** | −0.045 | - | |||||||
| 10. DRD1 C1 | −0.166 | −0.089 | −0.132 | .916** | 0.059 | .918** | −0.001 | −.615** | .551** | - | ||||||
| 11. DRD1 C2 | −0.084 | 0.033 | 0.130 | .281** | .757** | .300** | .387** | −0.074 | .628** | .268* | - | |||||
| 12. DRD2 C1 | 0.150 | 0.119 | 0.066 | −.954** | 0.075 | −.959** | 0.044 | .596** | −.592** | −.937** | −.218* | - | ||||
| 13. DRD2 C2 | −0.188 | 0.081 | 0.102 | .386** | .839** | .399** | .522** | 0.063 | .711** | .421** | .812** | −.277** | - | |||
| 14. DRD4 C1 | 0.088 | 0.065 | −0.086 | 0.041 | .304** | 0.048 | .285** | 0.084 | .252* | 0.027 | 0.157 | 0.011 | .216* | - | ||
| 15. SES | 0.179 | 0.070 | 0.099 | −.306** | 0.109 | −.345** | 0.131 | −0.028 | −0.155 | −.273* | −0.136 | .317** | −0.070 | 0.177 | −0.25 | 0.70 |
** = Correlation is significant at the 0.01 level (2-tailed).
* = Correlation is significant at the 0.05 level (2-tailed).
C1 and C2: Component 1 and Component 2
Table 3.
Intra-twin correlations.
| Digit Span | r | p |
|---|---|---|
| Total Forward Score | 0.627 | < 0.001 |
| Total Backward Score | 0.426 | 0.003 |
| Flanker Task | ||
| LISAS (incongruent) | 0.294 | 0.047 |
| Gene Methylation C1 | ||
| COMT | 0.461 | 0.001 |
| DAT1 | 0.344 | 0.022 |
| DBH | 0.405 | 0.006 |
| DRD1 | 0.462 | 0.001 |
| DRD2 | 0.451 | 0.002 |
| DRD4 | 0.977 | < 0.001 |
| Gene Methylation C2 | ||
| COMT | 0.525 | < 0.001 |
| DAT1 | 0.449 | 0.002 |
| DBH | 0.553 | < 0.001 |
| DRD1 | 0.412 | 0.005 |
| DRD2 | 0.316 | 0.037 |
| DRD4 | NA | NA |
C1 and C2: First and second component
The descriptive statistics for task performance are presented in Table 3. Twin performance on Digit Span aligned well with normative Forward and Backward results in eight year old boys and girls (Table 3) [49]. Twin performance on the flanker task (Table 3) was also in normative ranges based on previous reports [50].
Associations between methylation and task performance
Using linear regression analysis controlling for sex, array, and cell count we found MZ differences in dopamine genes predicted differences in short-term memory and response inhibition (Table 4). Twin differences in DRD4 (Total Forward: b = 0.321, p = 0.019) predicted twin differences in short-term memory performance (Table 4). MZ differences in COMT (LISA Incongruent: b = 0.704, p = 0.023), DBH (LISA Incongruent: b = 0.302, p = 0.033), DAT1 (LISA Incongruent: b = 0.458, p = 0.037), DRD1 (LISA Incongruent: b = 0.473, p = 0.042), and DRD2 (LISA Incongruent: b = 0.334, p = 0.026) predicted differences in response inhibition (Table 4).
Table 4.
Standardized beta estimates for regression analyses.
| Short Term Memory |
Response Inhibition |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gene | β | SE | p | FDR p | β | SE | p | FDR p |
| COMT | ||||||||
| C1 | −0.002 | 0.270 | 0.993 | 0.993 | 0.704 | 0.353 | 0.023 | 0.049 |
| C2 | 0.143 | 0.181 | 0.409 | 0.409 | 0.209 | 0.236 | 0.258 | 0.962 |
| DBH | ||||||||
| C1 | 0.198 | 0.224 | 0.422 | 0.820 | 0.594 | 0.302 | 0.033 | 0.049 |
| C2 | 0.191 | 0.139 | 0.186 | 0.279 | −0.007 | 0.187 | 0.962 | 0.387 |
| DAT1 | ||||||||
| C1 | 0.086*^ | 0.270 | 0.547 | 0.820 | −0.038 | 0.372 | 0.811 | 0.811 |
| C2 | 0.322*^ | 0.178 | 0.096 | 0.279 | 0.458 | 0.245 | 0.037 | 0.111 |
| DRD1 | ||||||||
| C1 | −0.276# | 0.212 | 0.187 | 0.280 | 0.473 | 0.284 | 0.042 | 0.063 |
| C2 | 0.022# | 0.175 | 0.889 | 0.889 | 0.094 | 0.234 | 0.590 | 0.59 |
| DRD2 | ||||||||
| C1 | −0.064 | 0.252 | 0.802 | 0.802 | 0.639 | 0.334 | 0.026 | 0.063 |
| C2 | 0.215 | 0.177 | 0.210 | 0.420 | 0.305 | 0.235 | 0.102 | 0.204 |
| DRD4 | ||||||||
| C1 | 0.321*^# | 0.575 | 0.019 | 0.057 | 0.060 | 0.859 | 0.708 | 0.708 |
FDR: false discovery rate
C1 and C2: first and second component from PCA
* = Sex < .05, ^ = Array < .05, # = Cell Count < .05
Discussion
We used the MZ twin difference design to investigate dopaminergic gene methylation and cognitive performance in middle childhood [51]. We provide evidence for non-genetic contributions to cognitive function via epigenetic regulation of dopaminergic genes. As expected, we found intra-twin correlations of methylation and cognitive task performance were moderate to high. In line with our hypotheses, we report novel findings that differences in methylation of dopamine regulating genes were associated with twin difference in cognitive performance. Differences in methylation of COMT, DBH, and DAT1 were associated with differences in inhibitory control. Next, we found differences in dopamine receptor gene methylation were also associated with twin difference in inhibitory control and short-term memory. These results align well with the literature concerning the relationship between dopamine function and cognitive performance. Further, these findings add to the burgeoning field of neuroepigenetics demonstrating that experience-dependent methylomes contribute to complex behavior.
Our results suggest methylation of the dopamine regulating genes COMT, DBH, and DAT1 is broadly associated with response inhibition. Perhaps methylation of these genes influences overall dopaminergic tone, affecting individual performance in dopamine dependent complex tasks. This fits with the current understanding that dopaminergic cortical innervation contributes to complex cognitive functions [52]. DBH is an enzyme in the dopamine degradation pathway and earlier studies demonstrated its role in prefrontal dopamine inactivation and cognitive performance [53,54]. A functional polymorphism in DBH has been associated with cognitive performance in both healthy and clinical populations [7,8]. The dopamine transporter (DAT1) actively clears dopamine from the synaptic cleft and genetic polymorphisms have been associated with individual differences in cognitive abilities [14,55,56]. Variation in the COMT genotype influences dopaminergic conversion and is associated with response inhibition performance and neuronal activity during inhibition tasks [57,58]. Dopaminergic function is a key player in cognition and our results suggest methylation of genes involved in dopamine degradation and clearing may be a mechanism by which experiences shape individual variation in cognitive function.
We also found twin-differences in DRD4 methylation predict twin-differences in short-term memory performance. This finding is consistent with past studies demonstrating dopamine receptor gene polymorphisms are implicated in cognitive dysfunction [17,59–61]. It is of particular interest that this effect was restricted to short-term memory and not also seen with working memory. Indeed, DRD4 variation and modulation has been implicated in working memory performance in adults [62]. Perhaps this discrepancy is due to the difficulty of the task for this age group whom does not yet have full working memory capacity [63].
This study extends evidence for the contribution of dopaminergic neural function in response inhibition. Impaired inhibitory control can disrupt goal-directed behavior and is a hallmark of both attention deficit hyperactivity disorder (ADHD) and substance use disorders, syndromes that both involve dopamine dysfunction [64,65]. We demonstrate DNA methylation of dopamine degradation and receptor genes are related to inhibitory control. These findings support the literature demonstrating overall dopaminergic tone and dopamine receptor expression is related to response inhibition [66,67].
Research in the last two decades has established a role of epigenetic processes in typical and disordered cognitive behaviors. Most research to date establishes DNA and histone modifications as cognitive and memory regulators (for an overview see Miller and Sweatt, 2007; Rudenko and Tsai, 2014) [68,69]. However, much of these findings come from non-genetically informed studies in which an association between methylation and cognition could possibly be driven by genetic variation [70]. Our study adds to a growing and important body of literature utilizing the twin design to isolate environmentally driven peripheral DNA methylation signatures associated with cognitive functioning. A recent epigenome wide association study in middle aged MZ twins used pathway analyses to find methylation of neurobiology-related genes to be involved in cognitive functioning [71]. Genetic control of DNA methylation levels at specific loci has been described [38,72,73]. For example, genetic variation in the COMT gene and stress interact with and influence methylation levels, which are associated with prefrontal activity and cognitive performance [74]. Again, it is important that our design controlled for genetic influence on methylation status since DRD4 genotype is associated with methylation levels of the gene [75]. It will be important to use genetically informed designs to clearly parse out the unique influences and interactions of genetics versus epigenetics in shaping complex phenotypes. While epigenetically targeted pharmacological interventions may one day be able to rescue or improve cognitive function, our results highlight the possibility of using experience-based treatments to enhance the epigenome underlying cognition [76].
Even though the current participants were all healthy children, these results can shed light on understanding neuro-disorders resulting from dopaminergic dysfunction that include environmental etiology. A better understanding of epigenetic modulation of cognitive capacities could inform early interventions for many neuro-based disorders. Disorders involving dopamine dysfunction such as Parkinson’s, Schizophrenia, ADHD, and Major Depressive Disorder are not fully heritable and have an environmental component in their etiology. Much international research has focused attention on large-scale genome wide association studies searching for the genetic profile of these disorders. Here, we provide evidence suggesting gene methylome profiles early in development may also be a productive pursuit and lead to novel experience based early interventions for cognitive function. We used knowledge of genetic associations to inform hypothesis driven research into epigenetic modulation of cognitive functions. If dopaminergic gene methylation regulates overall dopaminergic tone and function and is malleable to experiences, perhaps interventions can be designed specifically for complex behaviors and disorder vulnerability. Moreover, since epigenetic differences between twin pairs becomes larger with age [77], these findings are of major importance for understanding the accumulation of environmental factors and how they relate to disease discordance, age of onset, and disease severity between twin pairs. Perhaps, the magnitude of our findings in a middle childhood MZ twin difference design may increase in an older sample, or between co-twins reared separately.
One of the limitations of our study is our sample tissue. Tissue specificity, often called the ‘tissue issue’, refers to the assumption that peripheral methylation measures at least partially mirror neuronal methylation. This is an inherent limitation and a barrier in understanding in vivo behavior and neuro-epigenetics with human research. Levels of methylation vary between tissue types and may relate differently to specific genes, cell types, traits, exposures, and genetic-by-exposure interactions. Nonetheless, much work has been done establishing high blood, buccal, and brain methylation correlations. From this work, buccal cells are the best surrogate tissue for brain methylation studies [78,79]. These results should be followed up with a larger human sample and animal studies using tissue from relevant brain regions and experimental designs. DNA methylation is only one player in an astonishingly complex epigenomic regulatory system; therefore, it is possible that other epigenetic mechanisms such as histone modifications and non-coding RNAs are stronger mechanisms linking dopamine genes and cognitive function. However, DNA methylation was the ideal measurement for this initial study since it is the most widely studied and has well validated methods of measurement.
The current study revealed that dopaminergic gene methylation is consistently related to childhood differences in cognitive abilities. Performance in short-term memory and inhibitory control tasks were influenced by the presence of methylation. Our study is the first to examine the relationship between dopaminergic gene methylation and cognitive performance using an MZ twin model. Because our sample is relatively young and reared together, the twins have had minimal non-shared experiences up to this point, yet we were still able to identify moderate effects in methylation differences and behavior. This finding has important implications for the degree to which environmentally driven methylation patterns are shaping complex behavior in development. Unraveling the experience based epigenetic enigma may open doors into a new frontier of understanding complex human behavior. Interrogating how experiences inform neurobiology allows the integration of both nature and nurture and moves us past their longstanding and false dichotomy. Indeed, elucidating how experiences alter the epigenome and downstream behavior and health likely will provide us with modifiable intervention targets. Future studies illuminating the interplay between genotype, DNA methylation, gene expression, and experiences have the potential to inform 21st century precision treatment plans for neurodevelopmental, neurodegenerative, and psychiatric disorders.
Funding Statement
This work was supported by the National Institute of Child Health and Human Development [HD079520];National Institute of Child Health and Human Development [HD086085];Science Foundation Arizona [Bisgrove].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Moreira HS, Costa AS, Castro SL, et al. Assessing executive dysfunction in neurodegenerative disorders: a critical review of brief neuropsychological tools. Front Aging Neurosci. 2017;9:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hosenbocus S, Chahal R.. A review of executive function deficits and pharmacological management in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2012;21:223. [PMC free article] [PubMed] [Google Scholar]
- [3].Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. [DOI] [PubMed] [Google Scholar]
- [4].Bannon MJ, Roth RH.. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- [5].Vijayraghavan S, Major AJ, Everling S. Neuromodulation of prefrontal cortex in non-human primates by dopaminergic receptors during rule-guided flexible behavior and cognitive control. Front Neural Circuits. 2017;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bombin I, Arango C, Mayoral M, et al. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147:873–879. [DOI] [PubMed] [Google Scholar]
- [7].Hui L, Zhang X, Yu YQ, et al. Association between DBH 19bp insertion/deletion polymorphism and cognition in first-episode schizophrenic patients. Schizophr Res. 2013;147:236–240. [DOI] [PubMed] [Google Scholar]
- [8].Togsverd M, Werge TM, Tankó L, et al. Cognitive performance in elderly women: significance of the 19bp insertion/deletion polymorphism in the 5′ flank of the dopamine beta-hydroxylase gene, educational level, body fat measures, serum triglyceride, alcohol consumption and age. Int J Geriatr Psychiatry. 2007;22:883–889. [DOI] [PubMed] [Google Scholar]
- [9].Haworth CMA, Wright MJ, Luciano M, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2010;15:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Voelker P, Rothbart MK, Posner MK. A polymorphism related to methylation influences attention during performance of speeded skills. AIMS Neurosci. 2016;3:40–55. [Google Scholar]
- [11].Parasuraman R, Greenwood PM, Kumar R, et al. Beyond heritability: neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychol Sci. 2005;16:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Diamond A, Briand L, Fossella J, et al. Genetic and neurochemicl modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. [DOI] [PubMed] [Google Scholar]
- [13].Bertolino A, Rubino V, Sambataro F, et al. Prefrontal-hippocampal coupling during memory processing is modulated by comt val158met genotype. Biol Psychiatry. 2006;60:1250–1258. [DOI] [PubMed] [Google Scholar]
- [14].Rueda MR, Rueda MR, Rothbart MK, et al. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci U S A. 2005;102:14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghanemi A. Targeting G protein coupled receptor-related pathways as emerging molecular therapies. Saudi Pharm J. 2015;23:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Szekeres G, Kéri S, Juhász A, et al. Role of dopamine d3 receptor (DRD3) and dopamine transporter (DAT) polymorphism in cognitive dysfunctions and therapeutic response to atypical antipsychotics in patients with schizophrenia. Am J Med Genet Neuropsychiatr Genet. 2004;124:B:1–5. [DOI] [PubMed] [Google Scholar]
- [17].Fan J, Fossella J, Sommer T, et al. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci USA. 2003;100:7406–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao L, Lin Y, Lao G, et al. Association study of dopamine receptor genes polymorphism with cognitive functions in bipolar i disorder patients. J Affect Disord. 2015;170:85–90. [DOI] [PubMed] [Google Scholar]
- [19].Yue JK, Winkler EA, Rick JW, et al. DRD2 C957T polymorphism is associated with improved 6-month verbal learning following traumatic brain injury. Neurogenetics. 2017;18:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Takahashi H, Yamada M, Suhara T. Functional significance of central D1 receptors in cognition: beyond working memory. J Cereb Blood Flow Metab. 2012;32:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Holstein M, Aarts E, van der Schaaf ME, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl). 2011;218:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakajima S, Gerretsen P, Takeuchi H, et al. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013;23:799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Furth KE, Mastwal S, Wang KH, et al. Dopamine, cognitive function, and gamma oscillations: role of D4 receptors. Front Cell Neurosci. 2013;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Asbury K, Plomin R. G is for genes: the impact of genetics on education and achievement. 2013;24. [Google Scholar]
- [25].Johnson W, McGue M, Gaist D, et al. Frequency and heritability of depression symptomatology in the second half of life: evidence from danish twins over 45. Psychol Med. 2002;32:1175–1185. [DOI] [PubMed] [Google Scholar]
- [26].Colvert E, Tick B, McEwen F, et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. [DOI] [PubMed] [Google Scholar]
- [28].Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barter JD, Foster TC. Aging in the brain: new roles of epigenetics in cognitive decline. Neuroscientist. 2018;24:516–525. [DOI] [PubMed] [Google Scholar]
- [30].Weaver ICG, Diorio J, Seckl JR, et al. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. [DOI] [PubMed] [Google Scholar]
- [31].Fan J, Wu Y, Fossella JA, et al. Assessing the heritability of attnetional networks. BioMed Cent Neurosci. 2001;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rueda MR, Fan J, McCandliss BD, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. [DOI] [PubMed] [Google Scholar]
- [33].Shaywitz BA, Shaywitz SE, Blachman B, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 2004;55:926–933. [DOI] [PubMed] [Google Scholar]
- [34].Temple E, Deutsch GK, Poldrack RA, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci. 2003;100:2860–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huber E, Donnelly PM, Rokem A, et al. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat Commun. 2018;9:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morimoto Y, Ono S, Imamura A, et al. Deep sequencing reveals variations in somatic cell mosaic mutations between monozygotic twins with discordant psychiatric disease. Hum Genome Var. 2017;4:17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Beaver KM, Vaughn MG, Delisi M. Nonshared environmental effects on adulthood psychopathic personality traits: results from a monozygotic twin difference scores analysis. Psychiatr Q. 2013;84:381–393. [DOI] [PubMed] [Google Scholar]
- [38].Bell JT, Pai AA, Pickrell JK, et al. Dna methylation patterns associate with genetic and gene expression variation in hapmap cell lines. Genome Biol. 2011;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- [40].Akshoomoff N, Newman E, Wk T, et al. The NIH Toolbox Cognition Battery: Results from a Large Normative Developmental Sample (PING). NIH Public Access. 2014;28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lemery-Chalfant K, Clifford S, McDonald K, et al. Arizona twin project: a focus on early resilience. Twin Res Hum Genet. 2013;16:404–411. [DOI] [PubMed] [Google Scholar]
- [42].Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and pebl test battery. J Neurosci Methods. 2014;222:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stins J, Polderman J, Boomsma D, et al. Conditional accuracy in response interference tasks: evidence from the eriksen flanker task and the spatial conflict task. Adv Cogn Psychol. 2007;3:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aryee MJ, Jaffe AE, Corrada-Bravo H, et al. Minfi: a flexible and comprehensive bioconductor package for the analysis of infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Teschendorff AE, Breeze CE, Zheng SC, et al. A comparison of reference-based algorithms for correcting cell-type heterogeneity in epigenome-wide association studies. BMC Bioinformatics. 2017;18:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Du P, Zhang X, Huang CC, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vandierendonck A. A comparison of methods to combine speed and accuracy measures of performance: a rejoinder on the binning procedure. Behav Res Methods. 2017;49:653–673. [DOI] [PubMed] [Google Scholar]
- [49].Gardner RA. Digits forward and digits backward as two separate tests: normative data on 1567 school children. J Clin Child Psychol. 1981;10:131–135. [Google Scholar]
- [50].Buss KA, Dennis TA, Brooker RJ, et al. An ERP study of conflict monitoring in 4–8-year old children: associations with temperament. Dev Cogn Neurosci. 2011;1:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pike A, Hetherington EM, Reiss D, et al. Using MZ differences in the search for nonshared environmental effects. J Child Psychol Psychiatry Allied Discip. 1996;36:695–704. [DOI] [PubMed] [Google Scholar]
- [52].Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. [DOI] [PubMed] [Google Scholar]
- [53].Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci. 1998;95:9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kieling C, Genro JP, Hutz MH, et al. The −1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147:485–490. [DOI] [PubMed] [Google Scholar]
- [55].Garcia-Garcia M, Barceló F, Clemente IC, et al. The role of the dopamine transporter DAT1 genotype on the neural correlates of cognitive flexibility. Eur J Neurosci. 2010;31:754–760. [DOI] [PubMed] [Google Scholar]
- [56].Cornish KM, Manly T, Savage R, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–698. [DOI] [PubMed] [Google Scholar]
- [57].Congdon E, Constable RT, Lesch KP, et al. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol Psychol. 2009;81:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stokes PRA, Rhodes RA, Grasby PM, et al. The effects of the COMT val108/158met polymorphism on bold activation during working memory, planning, and response inhibition: a role for the posterior cingulate cortex. Neuropsychopharmacology. 2011;36:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Müller J, Dreisbach G, Brocke B, et al. Dopamine and cognitive control: the influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Res. 2007;1131:155–162. [DOI] [PubMed] [Google Scholar]
- [60].Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014;123:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Barnes JJM, Dean AJ, Nandam LS, et al. The molecular genetics of executive function: role of monoamine system genes. Biol Psychiatry. 2011;69:e127–43. [DOI] [PubMed] [Google Scholar]
- [62].Herrmann MJ, Walter A, Schreppel T, et al. D4 receptor gene variation modulates activation of prefrontal cortex during working memory. Eur J Neurosci. 2007;26:2713–2718. [DOI] [PubMed] [Google Scholar]
- [63].Cowan N. Working memory maturation: can we get at the essence of cognitive growth? Perspect Psychol Sci. 2016;11:239–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li D, Sham PC, Owen MJ, et al. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15:2276–2284. [DOI] [PubMed] [Google Scholar]
- [65].Noble EP. Addiction and its reward process through polymorphisms of the D2dopamine receptor gene: a review. Eur Psychiatry. 2000;15:79–89. [DOI] [PubMed] [Google Scholar]
- [66].Robertson CL, Ishibashi K, Mandelkern MA, et al. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci. 2015;35:5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Grillner S, Hellgren J, Ménard A, et al. Mechanisms for selection of basic motor programs - roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. [DOI] [PubMed] [Google Scholar]
- [68].Miller CA, Jd S. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. [DOI] [PubMed] [Google Scholar]
- [69].Rudenko A, L-H T. Epigenetic regulation in memory and cognitive disorders. Neuroscience. 2013;264C:51–63. [DOI] [PubMed] [Google Scholar]
- [70].Yu NK, Baek SH, Kaang BK. DNA methylation-mediated control of learning and memory. Mol Brain. 2011;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Starnawska A, Tan Q, McGue M, et al. Epigenome-wide association study of cognitive functioning in middle-aged monozygotic twins. Front Aging Neurosci. 2017;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Boks MP, Derks EM, Weisenberger DJ, et al. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. PLoS One. 2009;4:21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang D, Cheng L, Badner JA, et al. genetic control of individual differences in gene-specific methylation in human brain. Am J Hum Genet. 2010;86:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ursini G, Bollati V, Fazio L, et al. Stress-related methylation of the catechol-o-methyltransferase val158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dadds MR, Schollar-Root O, Lenroot R, et al. Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur Child Adolesc Psychiatry. 2016;25:1081–1089. [DOI] [PubMed] [Google Scholar]
- [76].Kleefstra T, Schenck A, Kramer JM, et al. The genetics of cognitive epigenetics. Neuropharmacology. 2014;80:83–94. [DOI] [PubMed] [Google Scholar]
- [77].Fraga MF, Ballestar E, Paz MF, et al. From the cover: epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci. 2005;102:10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Smith AK, Kilaru V, Klengel T, et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet Part B Neuropsychiatr Genet. 2015;168:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lowe R, Gemma C, Beyan H, et al. Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics. 2013;8:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


