Abstract
Purpose
To assess retinal vascular reactivity in healthy controls and subjects with diabetic retinopathy (DR).
Methods
A total of 22 healthy control eyes and 16 eyes with DR were enrolled. Images were acquired using a commercially available swept-source optical coherence tomography angiography (SS-OCTA) system. Three conditions were tested for each patient (hyperoxia, hypercapnia, and room-air) by employing a non-rebreathing apparatus that delivered appropriate gas mixtures (100% O2, 5% CO2, room air). Vessel skeleton density (VSD) and vessel diameter index (VDI) were compared between the conditions using mixed-model ANOVA adjusting for age and hypertension. Significant gas or interaction effects were followed by a Bonferroni adjusted pairwise post hoc analysis. Statistical significance was defined at P < 0.05.
Results
The mixed-model ANOVA of the VSD found a significant intraindividual gas effect (F[2, 70] = 20.3, P < 0.001) and intergroup effect (F[1, 35] = 6.9, P = 0.001), and interaction effects (F[2, 70] = 4.6, P = 0.03). The post hoc pairwise comparison found significant differences among all three gas conditions in the healthy controls. In the subjects with DR, there were significant differences in VSD between hyperoxic and room air, and between hyperoxic and hypercapnic conditions, but not between hypercapnic and room-air conditions. Similar results were found for VDI.
Conclusions
The retinal capillaries, assessed with SS-OCTA, in subjects with DR preferentially reacted to hyperoxia but not hypercapnia, while the healthy controls reacted to both. The difference in the vascular reactivity may be indicative of the underlying pathophysiology of DR.
Keywords: diabetic retinopathy, optical coherence tomography angiography, oxygen, carbon dioxide, capillary, reactivity
Vascular supply changes in response to metabolism of neural tissue. This vascular function, referred to as vascular reactivity, has been suggested as a potential indicator of vascular health.1,2 In the retina, vascular reactivity has been studied using flickering light stimulus to alter the oxygen demand of the neural retina.3 Retinal vascular reactivity (RVR) has also been studied using gas perturbation experiments that alter the partial pressures of CO2 and O2 (PCO2 and PO2) in blood.1,2,4–8 These studies have clearly demonstrated that under normal conditions retinal arteries and arterioles can constrict or dilate in response to changes in metabolic demand or supply.1,4 Furthermore, this vascular reactivity is impaired in subjects with prevalent retinal vascular diseases such as diabetic retinopathy (DR).5
Many studies have demonstrated that the earliest pathophysiologic changes in diseases such as DR occur at the capillary level before clinical changes are evident.9–11 A recent study using adaptive optics demonstrated that retinal capillaries in the inner retina of normal human subjects constrict and dilate in response to hyperoxia and hypercapnia respectively.4 The close proximity between the capillary bed and neural tissue makes the capillary bed a very desirable location for assessing RVR, especially since it has been implicated in the earliest stages of DR.9,10
Swept-source optical coherence tomography angiography (SS-OCTA) is a commercially available and FDA approved technology that allows the imaging of retinal capillaries based on the movement of red blood cells.12 In this study, we apply SS-OCTA imaging together with gas perturbation to assess the retinal capillary reactivity of healthy controls and subjects with DR. The findings provide insights into potential strategies that can enhance the use of OCTA quantifying metrics for detecting the earliest changes in DR in a clinically meaningful way.
Methods
This was a single-armed, prospective, and observational study of patients with DR and healthy controls. The study was approved by the USC Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.
Subjects
Subjects with DR and healthy controls were recruited at the USC Roski Eye Institute. Informed consents were obtained before enrollment. Clinical and demographic data were derived from clinical examination findings available from the medical record. The study excluded subjects with significant media opacity such as cataract, vitreous hemorrhage, or other obscuration of the fundus. Subjects with comorbid retinal pathologies such as diabetic macular edema, hypertensive retinopathy, age-related macular degeneration, and glaucoma or ocular hypertension were also excluded. When a subject reported a medical history of syncope, shortness of breath, lung disease, congestive heart failure, or recent hospitalization, the subject was excluded. Snellen acuities of the subjects studied were first converted to decimal, 50th percentile was computed and then converted back to the nearest Snellen notation.
Gas Delivery System
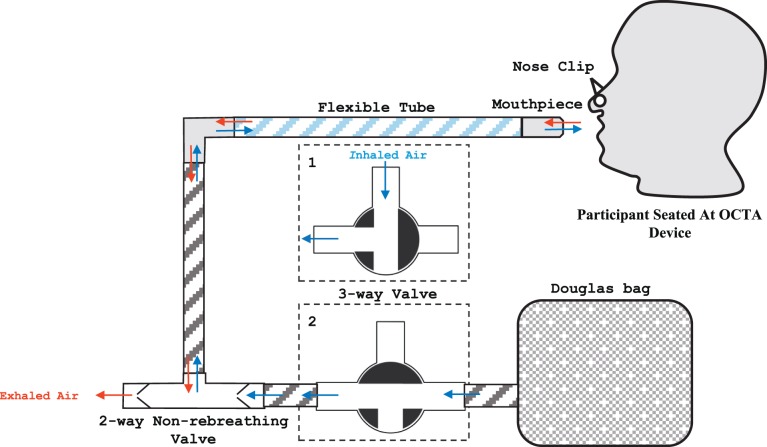
A nonrebreathing apparatus adapted from Lu et al.13 was used to deliver the gas perturbations so that simultaneous delivery of gas mixtures and image acquisition could be performed (Fig. 1). The apparatus consisted of a Douglas bag containing the desired gas mixture connected to a mouthpiece through a series of tubes and multiway valves. Three gas mixtures were used including 100% oxygen, a mix of 5% carbon dioxide, atmospheric oxygen and balanced nitrogen, and room air. Subjects were encouraged to breathe through their mouth with a nose clip in place. A fingertip pulse oximeter was used to monitor the subject's oxygen saturation. Patients with a baseline saturation below 95% were excluded from the study.
Figure 1.
Schematic of nonrebreathing apparatus and air flow used during acquisition of SS-OCTA images. Blue and red arrows indicate inhaled and exhaled air respectively. The three-way valve (outlined in short dashes) is tunable to two configurations, which determine the source of inhaled air. In configuration 1, room air is inhaled; in configuration 2, gas from the Douglas bag is inhaled. In both configurations, exhaled gas exits the circuit from the 2-way valve which prevents the same gas from being inhaled again, hence the term “nonrebreathing.”
SS-OCTA Imaging Protocol
The study was conducted using a 100 kHz SS-OCTA system (PLEX Elite 9000, software version 1.7; Carl Zeiss Meditec, Dublin, CA, USA) and the previously described optical microangiography (OMAGc) algorithm14 using both phase and amplitude data to determine decorrelation signal associated with red blood cell movement. The light source has a central wavelength of 1060 nm and a bandwidth of 100 nm, providing axial resolution of ∼6 μm and lateral resolution of ∼20 μm in retinal tissue. For each subject, one eye was imaged, based on the absence of co-morbid disease, absence of ocular media opacity and good fixation ability. When both eyes were suitable for imaging one eye was randomly selected and three 3 × 3-mm scans centered on the fovea were acquired 60 seconds after the start of gas breathing for each gas condition. The order of gas conditions is as follows: Room air condition to hypercapnic condition to hyperoxic condition. There was a 10-minute refractory period between the hypercapnic and the hyperoxic conditions. The order of hypercapnic and hyperoxic conditions was not altered due to persistent vasoconstriction after-effects when hyperoxia is induced before hypercapnia.6 Of the three 3 × 3-mm scans, the scan with best image quality and least motion artifacts under each gas condition was selected for analysis. Only images with centered foveal avascular zone (FAZ) and scan quality greater than seven were selected for analysis. Images with significant imaging artifacts from eye motion or vitreous floaters were excluded. If all three images from a subject under a given condition had imaging artifacts, the subject was not included in the analysis.
OCTA en face images of the superficial retinal layer (SRL), deep retinal layer (DRL), and full retinal layer (RET) were extracted and analyzed. The retinal segmentation schemes used for this study are commercially available and have been previously described.15,16 The SRL encompasses the superficial 60% of retina extending from the inner limiting membrane (ILM) to 110 μm above the RPE. The DRL spans the remaining 40% of the thickness between the ILM and RPE. RET used a single nonsegmented analysis from the ILM to the RPE.
Quantitative Vessel Morphometric Measures
The images were analyzed using OCTA metrics described previously16,17 and adapted for SS-OCTA. The analysis technique uses a thresholding method to suppress the noise from the noncapillary regions of the image, and uses filters to enhance regions with capillaries. Two capillary morphometric measures were assessed: vessel skeleton density (VSD) and vessel diameter index (VDI). In brief, VSD is a measure of the total linear length of vessels in an image. VSD is derived from a binarized OCTA image in which the vessels are reduced to a single pixel thickness. The absolute length of these vessels is then divided by the area of the image to derive VSD. VDI quantifies the vessel caliber in relation to the absolute length of vessels in the image. VDI is calculated by a ratio of the binarized vessel area (representing both length and width of vessels) to the skeletonized vessel length. VDI is therefore an index and not a direct measure of vessel caliber. This is important since vessel caliber is not detectable on OCTA for capillaries that lack flow. For example, an increase in vessel caliber of a capillary may be reflected by either the appearance of flow in a capillary segment that was not previously apparent or the dilation of a capillary segment that is already apparent on OCTA. VDI would capture both of these changes in a quantitative fashion. VSD and VDI have been described in previous publications.15,16
Statistical Analysis
Statistical software (Statistical Package for Social Science (SPSS) for Windows, version 24; SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. VSD and VDI were displayed as scatter plots for all three experimental conditions—hyperoxia, hypercapnia, and room air control. The statistical analysis and primary inferences for this study were only drawn from the full thickness retina to limit the number of statistical analyses to be made and to limit the extent of type 1 error. However, all layers demonstrated similar trends.
Two-way mixed model ANOVA was used for each of the vessel morphometric measures. The DR status—present or absent—was the between-group factor and the capillary morphometric findings of the three gas breathing conditions were the within-subject variables. All analyses were adjusted for the effects of age and hypertensive status as covariates. One healthy control whose full retinal layer VSD and VDI were outside the 99% z-score distribution range was excluded. In addition, Box test of equality of covariance matrices, Levene test of equality of error variances, and Mauchly test of sphericity were inspected to ensure the assumptions of the test statistic were met. We reported on the intraindividual gas effect, DR status (group) effect and gas/DR status interaction. When the gas effect or interaction effect was significant a Bonferroni adjusted post hoc analysis was done to assess pairwise comparisons—hyperoxia to room-air, hypercapnia to room-air, and hyperoxia to hypercapnia. Statistical significance was defined at P < 0.05. We also computed the mean and standard deviation of the percentage change of the pairwise comparisons.
The findings of the mixed model ANOVA were reported as F(a, b) = Y, P = n. Where a and b are the degrees of freedom of the factor and error terms respectively, Y is the F value and n is the calculated probability that the null hypothesis is true.
Results
Table 1 shows the number, age, and clinical information of subjects studied. An independent sample t-test found that healthy controls are significantly younger than DR subjects (P = 0.007). Similarly, a Pearson's χ2 analysis found that a greater proportion of subjects with diabetes were also hypertensive compared to controls—χ2 (1) = 6.51, P = 0.01. Therefore, age and hypertension were adjusted for in the mixed-model ANOVA.
Table 1.
Subject Demographics
|
Disease Classification |
||
|
Controls |
Diabetic Retinopathy |
|
| Number | 22 | 16 |
| Age (y), mean (SD) | 47 (19) | 60 (13) |
| Snellen visual acuity 50th percentile (range) | 20/20 (20/16, 20/25) | 20/25 (20/20, 20/40) |
| Severity of DR | NA | 10 mild NPDR, 2 moderate NPDR and 4 severe NPDR |
| Number with diagnosis of systemic hypertension | 5 | 9 |
The number, age, visual acuity, and ocular diagnosis of subjects studied is summarized.
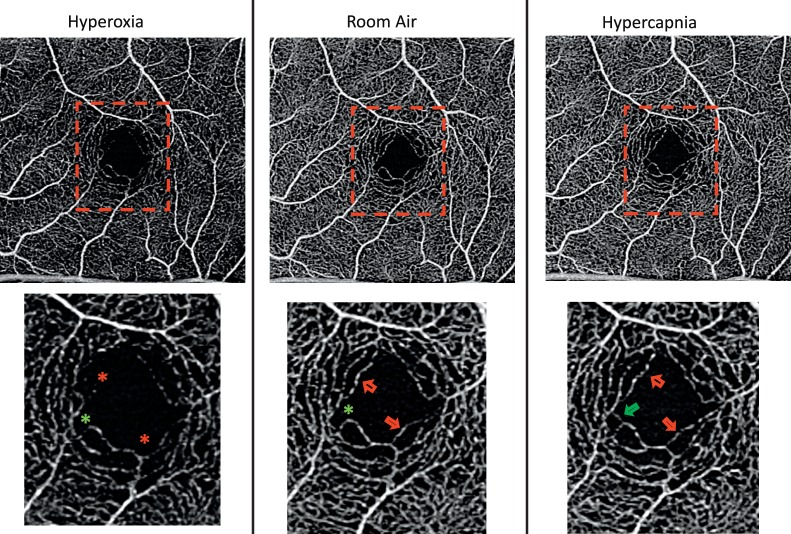
The SS-OCTA images in Figure 2 show changes in the parafoveal capillaries under the three experimental conditions. Capillary density qualitatively appeared greatest during the hypercapnic condition, followed by room-air and then hyperoxic condition. This qualitative finding was confirmed with the quantitative measures of capillary skeleton density, VSD (Fig. 3). In contrast, for patients with DR there was a significantly greater VSD during room air conditions than during hyperoxic conditions, but there was no significant change between room air and hypercapnic conditions.
Figure 2.
SS-OCTA images of a control subject acquired under three gas nonrebreathing conditions. The first row illustrates the 3 × 3-mm2 images of the full retinal layer. The regions enclosed by the red boxes are magnified in the second row. The red arrows point to retinal locations where flow was detected under hypercapnic and control conditions but not under hyperoxic conditions. The green arrow shows an example of a retinal location under hypercapnic condition where flow was detected but not under hyperoxic or control condition. A star identifies a location where flow was detected in the other gas conditions.
Figure 3.
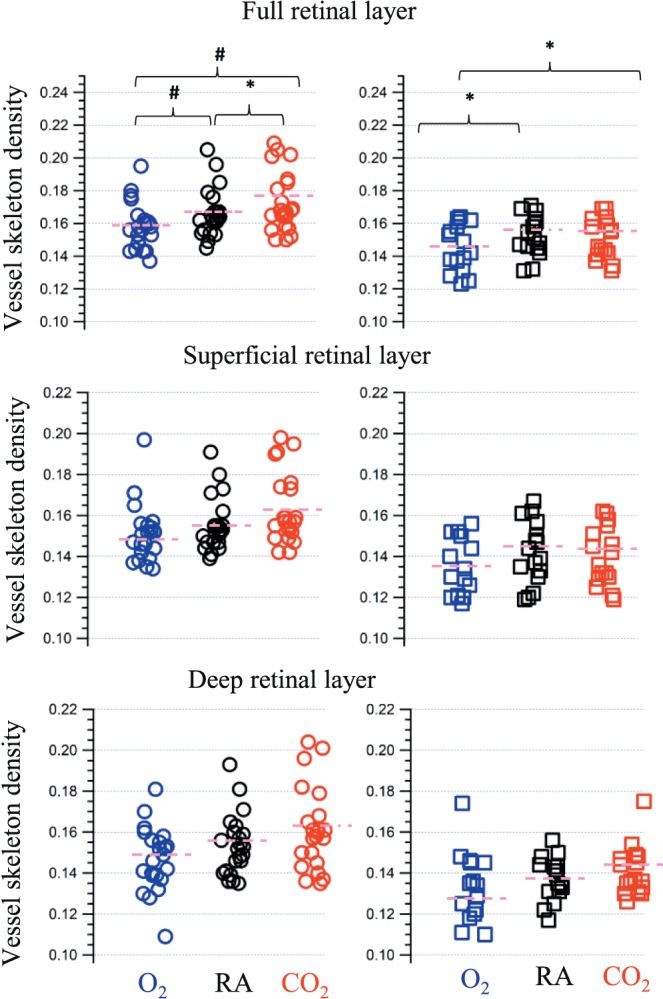
VSD under three conditions: hyperoxia shown in blue, room air shown in black, and hypercapnia shown in red. The circle and square markers represent controls and patients with DR respectively. The pink short dashes are the mean values of VSD measures. A star and a hash symbol are used to depict post hoc pairwise comparisons between conditions that were significant at P < 0.05 and P < 0.01, respectively.
The 2-way ANOVA investigating the VSD found a significant intraindividual gas condition effect (F[2, 70] = 20.3, P < 0.001) and a significant group effect (F[1, 35] = 6.9, P = 0.01). There was also a significant interaction between the gas condition and the DR status of the participants (F[2, 70] = 4.6, P = 0.03). The findings of post hoc pairwise comparisons are shown in Table 2. In brief, there was a significant change in the VSD across all three conditions in healthy controls. However, in subjects with DR there was a significant change between room air and hyperoxia, but not between room air and hypercapnia. The mean percentage changes are in Table 3.
Table 2.
Pairwise Post Hoc Analysis of the Mixed-Model ANOVA
|
Pairwise Comparison* |
Absolute Mean Difference (P
Value) |
|||
|
VSD |
VDI |
|||
| Controls |
Subjects With DR |
Controls |
Subjects With DR |
|
| O2–RA | 0.010 (P = 0.002) | 0.07 (P = 0.017) | 0.016 (1.00) | 0.052 (P = 0.82) |
| CO2–RA | 0.006 (P = 0.017) | 0.002 (P = 1.00) | 0.061 (P = 0.04) | 0.048 (P = 0.35) |
| CO2–O2 | 0.013 (P < 0.001) | 0.05 (P = 0.03) | 0.079 (P = 0.002) | 0.02 (P = 1.00) |
The table shows the absolute mean difference between any given pair of gas nonrebreathing conditions across the healthy control and DR subjects. Italicized-boldface cells are significant at an alpha level of P = 0.05.
O2, hyperoxic conditions; CO2, hypercapnic conditions; RA, room air conditions.
Table 3.
Percentage Change of the OCTA Morphometric Measures*
| Retinal Layer Segment |
Gas Condition |
Mean (SD) Percentage Change of Pairwise Comparisons |
|||
|
VSD |
VDI |
||||
| Controls |
Subjects With DR |
Controls |
Subjects With DR |
||
| Full retinal layer | O2–RA | −4.6 (5) | −5.0 (5) | 1.0 (5) | 2.10 (3) |
| CO2–RA | 3.9 (7) | −1.0 (3) | −2.2 (4) | 1.70 (3) | |
| CO2–O2 | 8.5 (8) | 4.0 (5) | −3.2 (4) | 0.0 (2) | |
| Superficial retinal layer | O2–RA | −2.2 (6) | −3.3 (4) | 0.1 (5) | 1.7 (4) |
| CO2–RA | 4.9 (7) | −0.8 (4) | −2.5 (4) | 1.5 (4) | |
| CO2–O2 | 7.1 (8) | 2.5 (3) | −2.7 (5) | 0.1 (2) | |
| Deep retinal layer | O2–RA | −4.4 (7) | −4.8 (11) | 0.4 (5) | 1.4 (5) |
| CO2–RA | 4.9 (8) | 3.2 (7) | −2.3 (6) | −0.2 (2) | |
| CO2–O2 | 9.3 (9) | 8.0 (13) | −2.9 (5) | −1.6 (5) | |
The percentage change was computed as (A-B)/C. Where A-B is label on the second column, and C is the measure of the metric during room air rebreathing.
The 2-way ANOVA investigating the VDI of the full retinal layer found a significant intraindividual gas condition effect (F[2, 70] = 7.3, P = 0.002), and a significant group effect of subjects with DR versus controls (F[1, 35] = 4.1, P = 0.04). There was also significant interaction between the gas condition and the DR status of the participants (F[2, 70] = 5.10, P = 0.03). The pairwise comparisons showed a significant difference between hypercapnia and hyperoxia, and hypercapnia and room air in the healthy controls, but not in the subjects with DR (Fig. 4).
Figure 4.
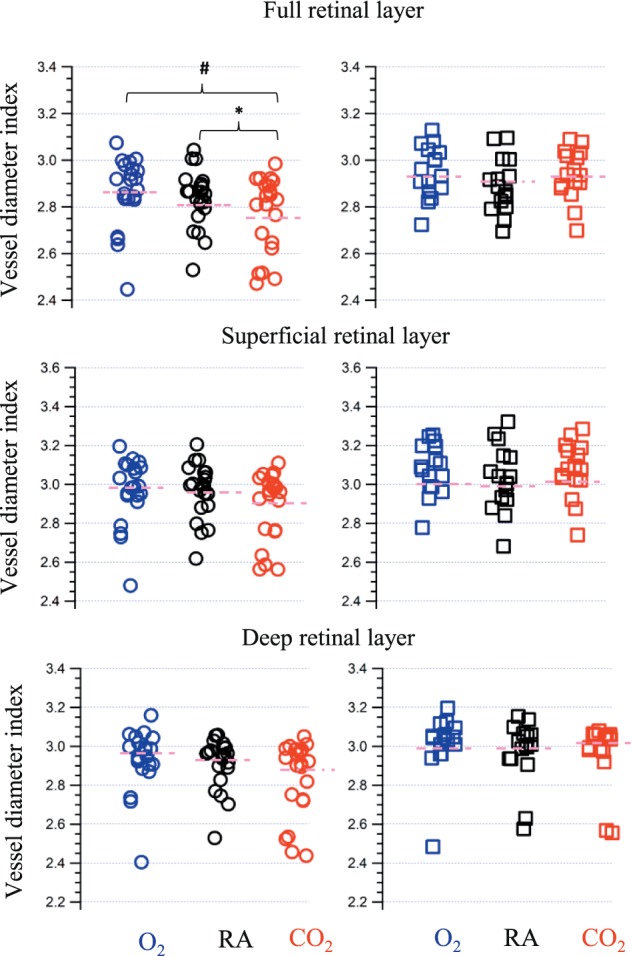
VDI under three conditions: hyperoxia shown in blue, room air shown in black, and hypercapnia shown in red. The circle and square markers represent controls and patients with DR, respectively. The pink short dashes are the mean values of VDI measures. A star and a hash symbol are used to depict post hoc pairwise comparisons between conditions that were significant at P < 0.05 and P < 0.01, respectively.
Discussion
We investigated RVR in controls and subjects with DR in vivo using SS-OCTA. Overall, we found that hyperoxia and hypercapnia significantly alter retinal capillary density (VSD) and caliber (VDI) in healthy subjects more so than in subjects with diabetic retinopathy. Specifically, capillary density was significantly reduced during hyperoxia as compared to room air, in both healthy controls and subjects with DR. However, during hypercapnia there was a significant increase in capillary density in controls but not subjects with DR. The impaired vascular reactivity to hypercapnia is consistent with the impaired vascular autoregulation in DR that is attributable to the loss of pericytes and endothelial cells, as well the thickening of the capillary basement membrane.18,19
Our index of vessel caliber, quantified as VDI, decreased significantly during hypercapnia in the healthy controls but not in subjects with DR. VDI is computed as the ratio of the vessel area (representing both length and width of vessels) to the skeletonized vessel length. The decreasing VDI in the healthy controls indicates an increase in the skeletonized vessel length compared to the binarized vessel width. This suggests that vasodilatory response to hypercapnia4,20 reflects on OCTA imaging as the appearance of flow in regions that were previously devoid of flow (i.e., dilation of previously constricted or closed capillary segments). The absence of this decreasing trend in the subjects with DR further supports the impairment in retinal vascular reactivity in diabetic subjects. Notably, our results were still statistically significant even if only subjects with mild nonproliferative diabetic retinopathy (NPDR) were included in the disease category. This suggests that changes in retinal vascular reactivity may be an early sign of retinopathy.
Hagag et al.21 recently described changes in the density of only the deep retinal capillaries of healthy human subjects as a result of hyperoxia.21 Our study confirms these findings and extends them in several ways. For example, we have found statistically significant changes in vessel density in the parafoveal retina with notable and similar trends in both the inner and outer retinal layers. Our findings in the more superficial layers are confirmed by adaptive optics-based studies and suggest that retinal capillaries throughout the retina respond to hyperoxia and hypercapnia. There are some significant methodological and technical differences between our study and Hagag et al.,21 which could explain why their observations were limited to the deep retinal plexus only. Notable among these is that Hagag et al.21 did not use a nonrebreathing apparatus that was amenable to simultaneous image acquisition and does not report a controlled scan time in relation to the breathing stimulus. Also, there are differences in the OCTA devices and amplitude decorrelation algorithms between the two studies. Nevertheless, the overall findings of both studies suggest that retinal capillary reactivity is a measurable and potentially useful phenomenon.
The findings of our study are not without limitations. One such limitation is our small sample size, however the findings from this study are novel and convincing. Larger studies with a broader spectrum of disease severity will be useful in assessing the magnitude of the changes we have observed, and exploring the effect of other potential factors such as HbA1c level. Another limitation of our study stems from the inherent limitation of any study using OCTA. The appearance or disappearance of capillaries, under the different gas breathing conditions reflects a change in the nature of the flow through the vessels and not the absolute presence or absence of vessels. This latter limitation does not however limit the use of methods and quantifying metrics investigated in this study as they pertain to the clinical utility of OCTA imaging for assessing diabetic retinopathy.
To conclude, we combined an FDA approved OCTA imaging device with a custom gas delivery apparatus that allows in vivo assessment of retinal vascular reactivity in a clinically feasible manner. We found significant changes in the vessel skeletal density in response to both hyperoxia and hypercapnia in the healthy controls, while subjects with DR showed a preferential response to hyperoxia but not hypercapnia. We also found that hypercapnic vasodilation most likely manifests as recruitment of previously closed capillary segments (without flow) on OCTA imaging. These findings provide a basis for the use of OCTA for assessing retinal vascular reactivity to enhance the understanding of the pathophysiology of DR, as well as identifying potential clinically feasible biomarkers for the early diagnosis of the disease.
Acknowledgments
Supported by NIH K08EY027006, Research Grants from Carl Zeiss Meditec Inc (Dublin, CA, USA) and Unrestricted Department Funding from Research to Prevent Blindness (New York, NY, USA). The authors alone are responsible for the content and writing of the paper.
Disclosure: B.S. Ashimatey, None; K.M. Green, None; Z. Chu, None; R.K. Wang, Carl Zeiss Meditec (C), Insight Phototonic Solutions (C), P; A.H. Kashani, Carl Zeiss Meditec (F, R)
References
- 1.Chapman N, Haimes G, Stanton AV, Thom SA, Hughes AD. Acute effects of oxygen and carbon dioxide on retinal vascular network geometry in hypertensive and normotensive subjects. Clin Sci (Lond) 2000;99:483–488. [PubMed] [Google Scholar]
- 2.Venkataraman ST, Hudson C, Rachmiel R, et al. Retinal arteriolar vascular reactivity in untreated and progressive primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2043–2050. doi: 10.1167/iovs.09-3630. [DOI] [PubMed] [Google Scholar]
- 3.Duan A, Bedggood PA, Bui BV, Metha AB. Evidence of flicker-induced functional hyperaemia in the smallest vessels of the human retinal blood supply. PLoS One. 2016;11:e0162621. doi: 10.1371/journal.pone.0162621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan A, Bedggood PA, Metha AB, Bui BV. Reactivity in the human retinal microvasculature measured during acute gas breathing provocations. Sci Rep. 2017;7:2113. doi: 10.1038/s41598-017-02344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore ED, Hudson C, Nrusimhadevara RK, et al. Retinal arteriolar diameter, blood velocity, and blood flow response to an isocapnic hyperoxic provocation in early sight-threatening diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:1744–1750. doi: 10.1167/iovs.06-1016. [DOI] [PubMed] [Google Scholar]
- 6.Tayyari F, Venkataraman ST, Gilmore ED, Wong T, Fisher J, Hudson C. The relationship between retinal vascular reactivity and arteriolar diameter in response to metabolic provocation. Invest Ophthalmol Vis Sci. 2009;50:4814–4821. doi: 10.1167/iovs.09-3373. [DOI] [PubMed] [Google Scholar]
- 7.Klefter ON, Lauritsen AO, Larsen M. Retinal hemodynamic oxygen reactivity assessed by perfusion velocity, blood oximetry and vessel diameter measurements. Acta Ophthalmol. 2015;93:232–241. doi: 10.1111/aos.12553. [DOI] [PubMed] [Google Scholar]
- 8.Palkovits S, Lasta M, Told R, et al. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci. 2014;55:4707–4713. doi: 10.1167/iovs.14-14593. [DOI] [PubMed] [Google Scholar]
- 9.Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 11.Bosch AJ, Harazny JM, Kistner I, Friedrich S, Wojtkiewicz J, Schmieder RE. Retinal capillary rarefaction in patients with untreated mild-moderate hypertension. BMC Cardiovasc Disord. 2017;17:300. doi: 10.1186/s12872-017-0732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Progress Retin Eye Res. 2017;60:66–100. doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp. 2014;94(52306) doi: 10.3791/52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int J Biomed Imaging. 2012;2012:1–11. doi: 10.1155/2012/509783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koulisis N, Kim AY, Chu Z, et al. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PLoS One. 2017;12:e0176404. doi: 10.1371/journal.pone.0176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT362–OCT370. doi: 10.1167/iovs.15-18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt. 2016;21:66008. doi: 10.1117/1.JBO.21.6.066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu X, Gens JS, Glazier JA, Burns SA, Gast TJ. Progression of diabetic capillary occlusion: a model. PLoS Comput Biol. 2016;12:e1004932. doi: 10.1371/journal.pcbi.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology. 2017;24:229–241. doi: 10.1016/j.pathophys.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataraman ST, Hudson C, Fisher JA, Rodrigues L, Mardimae A, Flanagan JG. Retinal arteriolar and capillary vascular reactivity in response to isoxic hypercapnia. Exp Eye Res. 2008;87:535–542. doi: 10.1016/j.exer.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Hagag AM, Pechauer AD, Liu L, et al. OCT Angiography changes in the 3 parafoveal retinal plexuses in response to hyperoxia. Ophthalmol Retina. 2018;2:329–336. doi: 10.1016/j.oret.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]