Abstract
Study Objectives:
Controversy exists as to whether elevated loop gain is a cause or consequence of obstructive sleep apnea (OSA). Upper airway surgery is commonly performed in Asian patients with OSA who have failed positive airway pressure therapy and who are thought to have anatomical predisposition to OSA. We hypothesized that high loop gain would decrease following surgical treatment of OSA due to reduced sleep apnea severity.
Methods:
Polysomnography was performed preoperatively and postoperatively to assess OSA severity in 30 Chinese participants who underwent upper airway surgery. Loop gain was calculated using a validated clinically-applicable method by fitting a feedback control model to airflow.
Results:
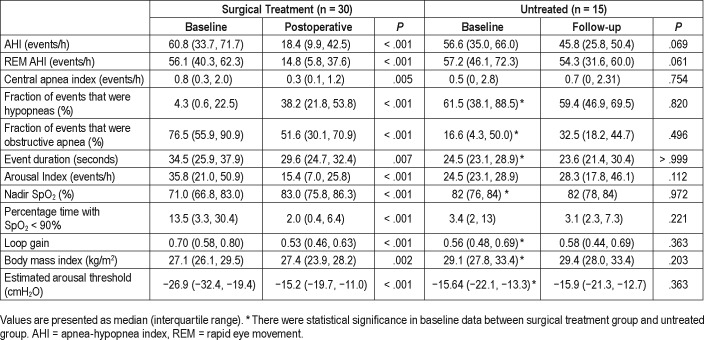
Patients were followed up for a median (interquartile range) of 130 (62, 224) days after surgery. Apnea-hypopnea index (AHI) changed from 60.8 (33.7, 71.7) to 18.4 (9.9, 42.5) events/h (P < .001). Preoperative and postoperative loop gain was 0.70 (0.58, 0.80) and 0.53 (0.46, 0.63) respectively (P < .001). There was a positive association between the decrease in loop gain and the improvement of AHI (P = .025).
Conclusions:
High loop gain was reduced by surgical treatment of OSA in our cohort. These data suggest that elevated loop gain may be acquired in OSA and may provide mechanistic insight into improvement in OSA with upper airway surgery.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Title: The Impact of Sleep Apnea Treatment on Physiology Traits in Chinese Patients With Obstructive Sleep Apnea, Identifier: NCT02696629, URL: https://clinicaltrials.gov/show/NCT02696629
Citation:
Li Y, Ye J, Han D, Zhao D, Cao X, Orr J, Jen R, Deacon-Diaz N, Sands SA, Owens R, Malhotra A. The effect of upper airway surgery on loop gain in obstructive sleep apnea. J Clin Sleep Med. 2019;15(6):907–913.
Keywords: hypoxemia, loop gain, lung, obstructive sleep apnea, upper airway surgery, ventilatory control
BRIEF SUMMARY
Current Knowledge/Study Rationale: Unstable ventilatory control (high loop gain) is an important non-anatomical risk factor for obstructive sleep apnea (OSA). Controversy exists as to whether elevated loop gain is a cause or consequence of OSA. Studies have shown high loop gain may be acquired from long-term intermittent hypoxemia due to OSA and can decrease after continuous positive airway pressure therapy.
Study Impact: Unstable ventilatory control in patients with OSA can be improved by upper airway surgery, supporting the notion that ventilatory control abnormalities may be acquired in some patients. The results may guide future therapeutic approaches by providing insight into potential mechanisms of surgical improvement and/or failure.
INTRODUCTION
Obstructive sleep apnea (OSA) is common throughout the world, including in China. Upper airway surgery is commonly performed in Asian patients with OSA who have failed continuous positive airway pressure (CPAP) therapy and who are thought to be anatomically predisposed to OSA. By enlarging the velopharyngeal/retroglossal airway, soft tissue surgery likely improves pharyngeal collapsibility and/or mechanical load.1 However, the benefits of upper airway surgery for OSA are highly variable,2 leading to some focus on mechanisms of improvement in those who benefit.
Unstable ventilatory control (high loop gain) is an important non-anatomical risk factor for OSA.3 Loop gain is a term used to define the stability of a negative feedback control system, quantified as the ratio of the ventilatory response over the ventilatory disturbance (eg, reduced ventilation from apneas and/or hypopneas). High loop gain promotes ventilatory overshoot and instability whereas a low loop gain is intrinsically more stable. Loop gain is often elevated in OSA4 and contributes to repetitive apnea through variable central pattern generator output to the upper airway muscles.5 However, controversy exists as to whether elevated loop gain is inherent or acquired in OSA.6–9
Exaggerated ventilatory chemoreflex responsiveness (the controller component of loop gain) is ameliorated with nasal CPAP treatment in patients with OSA.9–11 This finding suggests elevated loop gain is an inducible trait that may be amenable to treatments which prevent obstructive events and exposure to hypoxemia.
Although nasal CPAP does lower loop gain in small studies,11–13 whether upper airway surgery (structural change) could improve loop gain is not known. Theoretically, upper airway surgery could change loop gain via a number of mechanisms. Reducing the severity of OSA and preventing exposure to intermittent hypoxemia may normalize maladaptive chemoreflex control abnormalities. If so, the change of OSA severity should be associated with a change in loop gain. However as loop gain is quantified by the ventilatory response to a stimulus, improving the mechanics of the upper airway may alter chemoresponsiveness, ie, increasing ventilation for a given chemoreflex stimulus. Based on this conceptual framework, we tested the hypothesis that elevated loop gain would be reduced by upper airway surgery. We also assessed predictors of any observed loop gain change to understand better the mechanisms of OSA improvement with upper airway surgery. We view such efforts as critical to defining a priori predictors of surgical success.
METHODS
Study Design
This study was an observational cohort of patients who underwent upper airway surgery to treat OSA and a control group who remained untreated. The study population who underwent surgery was a selective population since the surgical treatment group was considered to be suitable candidates for upper airway surgery in China. In-laboratory overnight polysomnography (PSG) was performed preoperatively and postoperatively to assess sleep apnea severity and were used to assess the stability of the ventilatory control system.
This study was approved by the institutional review board of Beijing Tongren Hospital, Capital Medical University with written informed consent obtained from all patients. No contact of participants occurred at University of California, San Diego and all data received were de-identified so the study was deemed exempt from local IRB oversight.
The number of observations needed was estimated based on the minimal detectable difference in loop gain of 0.2. With a 90% power to reject the null hypothesis and alpha = .05, the estimated minimum sample size was 18 for each group.
Study Population
All participants were 18–70 years of age and had a history of untreated OSA with apnea-hypopnea index (AHI) > 5 events/h and clinical symptoms (such as snoring, witnessed apneas, and daytime sleepiness). CPAP treatment was recommended for all participants, but was refused or not tolerated. In the surgical treatment group, no other surgical procedures were performed prior to or during the current treatment.
Patients with OSA who had a severe coexisting lung, neurological, cardiovascular, psychiatric disorder or who were on chronic medications that could affect sleep/breathing were excluded. Patients with severe maxillofacial or mandibular deformities were excluded.
All the patients were evaluated by one surgeon and admitted as candidates for surgical treatment. Patients who underwent other upper airway surgical procedures prior to the current treatments were excluded.
Upper Airway Surgery
Surgical procedures included revised UPPP with uvula preservation (Han-UPPP)14 with and without concomitant transpalatal advancement pharyngoplasty, genioglossus advancement or hyoid suspension).15
All surgeries were performed under general anesthesia by one surgeon. Patients with severe OSA were encouraged to use CPAP immediately preoperatively for safety reasons.
The definition of responders for surgical treatment, defined as a ≥ 50% reduction in baseline AHI and a final AHI of < 20 events/h, was used. Moreover, a postoperative AHI below 10 events/h with a postoperative Epworth Sleepiness Scale score ≤ 9 and no residual sleep apnea symptoms was considered cured.
Polysomnography
Standard clinical PSG was performed and analyzed according to the guidelines of American Academy of Sleep Medicine Task Force 2007 criteria.16 Electroencephalograms, electrooculograms, and surface electromyograms were applied to score arousals, leg movements and sleep stage. Abdominal and chest movements, pulse oxygen saturation and nasal pressure airflow were recorded to detect respiratory events. An apnea episode was scored when there was a complete cessation of airflow or a ≥ 90% reduction in the peak thermal sensor signal for at least 10 seconds. A hypopnea episode was scored when there was a ≥ 50% reduction in the nasal pressure signal for at least 10 seconds in association with oxygen desaturation of ≥ 3% or an arousal. The AHI was defined as the sum of the numbers of apneas and hypopneas per hour of sleep. Postoperative PSG studies were performed approximately 4–6 month after surgery (cases) or the baseline visit (controls).
Loop Gain Analyses
The stability of the ventilatory control system (loop gain) was quantified by fitting a simplified but validated mathematical model to the spontaneous ventilatory pattern of OSA.17,18 Standard raw PSG data (European data format) and scoring were imported into MATLAB (MathWorks, Natick, Massachusetts, United States). All available 7-minute windows of non-rapid eye movement (NREM) sleep were used for calculations. Ventilatory fluctuations are estimated using the square-root transformed nasal pressure waveform. Briefly, loop gain is estimated according to the magnitude and time course of the ventilatory “response” (increased ventilatory effort) that follows the ventilatory “disturbance” (apnea/hypopnea). For each 7-minute window, a ventilatory control model was fit to obtain the parameters that define the gain, time constant and delay. Loop gain was reported as the ventilatory response to a 1 cycle/min disturbance as used previously.17 The median value for loop gain in all the 7-minute windows was used to provide a single value for each individual for each sleep study.
Analysis
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, Illinois, United States). Sleep apnea severity and loop gain before and after surgery/baseline and follow up were compared using the Wilcoxon signed ranks test. Spearman correlation coefficient was used to identify significant associations. Statistical significance was set at P < .05.
RESULTS
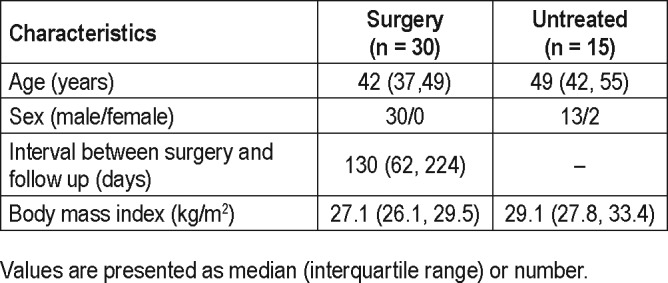
The baseline characteristics of the participants are listed in Table 1. In the 30 patients who underwent upper airway surgery, 13 patients underwent Han-UPPP alone; 15 patients underwent Han-UPPP with concomitant transpalatal advancement pharyngoplasty; 2 patients underwent velopharyngeal and retroglossal surgery (Han-UPPP and concomitant genioglossus advancement or hyoid suspension). No patient suffered from severe complications related to surgery during the postoperative follow-up. Compared with the treated patients, the 15 patients who were untreated had similar baseline AHI, but relatively higher baseline body mass index (BMI) and lower baseline loop gain.
Table 1.
Demographic data.
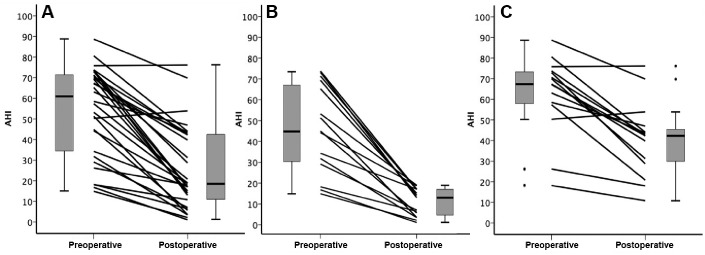
The severity of OSA improved significantly after surgery. In the treated group, a > 50% reduction in AHI to a final AHI of < 10 events/h and < 20 events/h was achieved in 8 (26.7%) and 15 (50%, responders) of patients respectively. Figure 1 shows individual AHI change from before to after surgery.
Figure 1. Preoperative and postoperative AHI.
The distribution of group AHI values are shown in the box-and-whiskers plots (group median, upper and lower quartile, upper and lower extreme, and outliers). (A) Preoperative and postoperative AHI in the whole group. (B) Individual preoperative and postoperative AHI in responders (n = 15). (C) Preoperative and postoperative AHI in nonresponders (n = 15). AHI = apnea-hypopnea index.
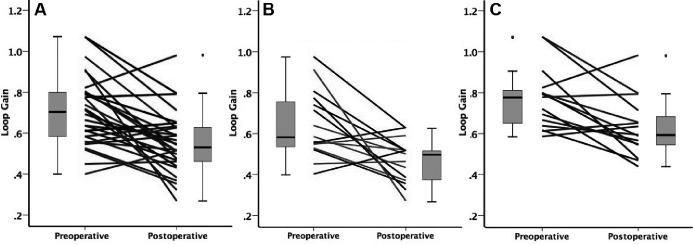
Table 2 shows the change in loop gain, BMI and other PSG parameters. An average of 121 ± 50 (25–189) windows per participant was analyzed. Overall loop gain and loop gain in supine position decreased after surgery (0.70 [0.58, 0.80] versus 0.53 [0.46, 0.63], P < .001). In both surgical responders and nonresponders, loop gain decreased significantly after surgery (P < .05). The loop gain did not change significantly in the untreated group. Figure 2 shows individual loop gain change from before to after surgery.
Table 2.
Baseline and follow-up sleep apnea severity.
Figure 2. Preoperative and postoperative loop gain.
The distribution of group loop gain values are shown in the box-and-whiskers plots (group median, upper and lower quartile, upper and lower extreme, and outliers). (A) Individual preoperative and postoperative loop gain. (B) Individual preoperative and postoperative loop gain in responders (n = 15). (C) Preoperative and postoperative loop gain in nonresponders (n = 15).
In the surgical treatment group, there were positive associations between the change of loop gain and the change of AHI and NREM AHI (r = .409, P = .025; r = .429, P = .018). There was a positive association between the change of loop gain and the change of ≥ 3% oxygen desaturation index after surgery (r = .381, P = .045). The change of REM AHI, BMI and the change of nadir oxygen desaturation were not correlated with the change of loop gain (P > .05).
DISCUSSION
Summary of the Findings
Our study adds to the existing literature in several important ways. We have observed that loop gain in patients with OSA was reduced by upper airway surgery. A substantial decrease of loop gain was observed in the study (median decreased by 0.17). The loop gain change appears similar to the effect of O 2 (0.14) or acetazolamide (0.25) in patients with OSA in the literature.17
Our findings support the concept that high loop gain is induced at least to some extent in Chinese patients with OSA. Moreover, loop gain could be improved with structural changes in anatomy and ameliorated hypoxemia. Reduction of loop gain correlated with the improvement of hypoxemia and severity of OSA as measured by AHI. This observation supports a potential role for intermittent hypoxemia inducing chemoreflex changes that increase loop gain.
Although CPAP has been shown to improve loop gain when used to treat OSA, CPAP has other effects that might alter control of breathing, such as changes in end-expiratory lung volume. Our findings suggest that therapies that eliminate OSA might have the potential to improve loop gain.
However, a recent study found that loop gain did not change in a Caucasian group undergoing upper airway surgery, which is in contrast to our findings here.18 This inconsistency may be explained by differences in baseline OSA severity and the observed reduction of AHI after treatment between studies (39.1 versus 26.5 events/h postoperatively reported by Joosten et al. and 60.8 versus 18.4 events/h in the present study). Moreover, recent research in different racial/ethnic groups suggests that OSA may have different underlying causes in various groups.19 Considering the potential for a dominant role of anatomical factors in Asian patients with OSA, the high upper airway mechanical load may initiate sleep apnea and the elevated loop gain might be partially “acquired” over time in these patients.
Upper airway anatomical abnormality is a prominent risk factor in Asian patients with OSA, which might be improved by surgical strategies. However, surgery shows variable clinical effectiveness. One potential reason for patients' responding poorly to a single treatment procedure is that multiple non-anatomical physiological traits (such as high loop gain) contribute to OSA.20 In the future, individualized therapy in OSA may be improved by better understanding the reversibility of increased loop gain. The new results could provide a rationale for a combined treatment approach for those patients with both elevated loop gain and anatomical risk factors in an Asian population, ie, to address residual sleep apnea shortly after upper airway surgery, using oxygen or acetazolamide; such adjunct treatments could then theoretically be withdrawn over time as loop gain falls. Moreover, one might predict that mild to moderate elevations in loop gain might be ameliorated by surgical treatment alone.
Elevated Loop Gain as a Cause and Consequence of OSA
Patients with OSA exhibit elevated loop gain that improves with nasal CPAP in small studies to date.6,11–13,21,24,26 Of note, the calculation of loop gain may reflect upper airway dynamic effects in addition to changes in chemical drive. With improved upper airway mechanics, we might expect a greater ventilatory overshoot to be observed (due to reduced upper airway resistance) with a given ventilatory disturbance. However, such effects would explain an increased rather than the reduced loop gain we observed with surgery. We also note that studies have previously found no relationship between this measure of loop gain and pharyngeal collapsibility (Pcrit),17 and a recent study which improved collapsibility (Pcrit) with a single night of an investigational drug found no effect on loop gain.27 Thus we believe it is likely that the lowered loop gain truly reflects a reduced chemical drive response to changes in ventilation rather than via surgical effects on upper airway patency per se. Reduction of loop gain following surgery in the present study strengthens the assertion that intermittent hypoxemia may have led to increased loop gain prior to surgery.
Elevated loop gain appears to both contribute to causing repetitive apneas and also be induced by OSA itself, which may develop in a feedforward manner. The potential mechanisms that lead to loop gain changes (eg, neuroplasticity11 or interactions from peripheral and central chemoreceptor drive28) may not contribute to long-term progressive worsening of OSA. In aggregate, studies have shown that progression of OSA over time is relatively modest in the absence of weight gain.29 Although studies have shown that pharmacological reduction of loop gain (using either oxygen or acetazolamide) can improve AHI,30,31 this finding does not confirm or refute whether the elevated loop gain is intrinsic to OSA or acquired from the condition. Thus, a better understanding of the mechanisms causing elevated loop gain in patients with OSA will be necessary to optimize therapy and to predict surgical responses.
Limitations
Despite a number of strengths, we acknowledge a number of limitations. First, our study population in China is a selective population since the surgical treatment group was considered to be suitable candidates for upper airway surgery, and the untreated group was not matched with the surgical treatment group in some of the PSG parameters and physiological traits. Thus, we emphasize that our findings are relevant to the population studied and we cannot be confident regarding the generalizability of our findings in other clinics/populations. However, our findings are consistent with the bulk of the existing literature regarding OSA-induced elevation in loop gain. Moreover, the absence of morbid obesity in the present study helps to confirm that high loop gain is seen in OSA itself not just as a function of obesity. Second, although we acquired new mechanistic insights, we still cannot truly answer questions regarding the “chicken or egg” regarding OSA and high loop gain. Moreover, the patients were followed up 2–11 month after surgery. The time course of changes may have relevance since further reductions in loop gain over time may contribute to progressive improvements in OSA in longer term follow-up. However, the variation of follow-up time after surgery is unlikely to affect the change in loop gain in the current study. We failed to find any correlation between the follow-up days and the change in loop gain (r = −.141, P = .458, Spearman correlation test), or with the change in AHI (r = .015, P = .936). Nonetheless, we believe that our new findings provide reasonable motivation to design further rigorous longitudinal studies. Third, we did observe a small but significant reduction in BMI following upper airway surgery. Thus, we cannot be confident regarding which of our findings were a function of improved body weight. We believe this weight change is not a limitation per se but rather might help to explain some of the variability in outcomes following upper airway surgery. Moreover, such weight changes represent the reality of upper airway surgery and not an artifact of our research. Fourth, in patients with major reductions in AHI (“cured” patients), our technique may not allow reliable estimates of loop gain since the method relies on spontaneous respiratory disturbances—that is, residual sleep apnea. Thus, some methodological variance may be present in our loop gain estimates for this small subset of patients who were cured of OSA. In this study, we still obtained over 10 sample windows for loop gain analysis in the few patients who had postoperative AHI < 5 events/h (n = 4). Thus we think our methods are unlikely to cause a significant deviation in loop gain estimation. Fifth and finally, our validated loop gain estimation technique relies on nasal pressure recordings and thus downplays the impact of oral breathing which may have occurred on occasion. Because our goal was to analyze real world data using clinically accessible techniques, while minimizing adverse effects of instrumentation on sleep quality, we did not use sealed oronasal masks or other such instrumentation as we typically do in our physiology laboratory. Despite these limitations, we believe that our findings provide important food for thought and will definitely lay the groundwork for subsequent studies by our group and others.
CONCLUSIONS
Our study shows that unstable ventilatory control in Chinese patients OSA can be improved by upper airway surgery, supporting the notion that ventilatory control abnormalities may be acquired in the specific group of patients. The results may guide future therapeutic approaches by providing insight into potential mechanisms of surgical improvement and/or failure.
DISCLOSURE STATEMENT
Work for this study was performed at Beijing Tongren Hospital, Capital Medical University, Key Laboratory of Otolaryngology Head and Neck Surgery (Ministry of Education of China) Beijing, China and data analysis was done at University of California at San Diego, California, United States. All authors have seen and approved the article. Yanru Li, Jingying Ye, Demin Han, Xin Cao, Di Zhao, Jeremy Orr, Rachel Jen, Naomi Deacon, and Atul Malhotra report no conflicts of interest. Robert L Owens has received travel and honoraria from ResMed and Itamar Medical. Scott Sands has consulted for Cambridge Sound Management, Nox Medical, Merck and receives project support from Apnimed. This study was supported by the National Key R&D Program of China 2017YFC0112500, National Natural Science Foundation of China (81200735), and Priming Scientific Research Foundation (2017-YJJ-GGL-006). Dr. Malhotra is PI on NIH R01 HL085188, K24 HL132105, and co-investigator on R21 HL121794, R01 HL119201, R01 HL081823. As an Officer of the American Thoracic Society, Dr. Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to the UC San Diego. Dr. Yanru Li is PI on The National Natural Science Foundation of China (81200735) Scientific Research and Cultivation Program of Beijing Subordinate Hospital (PX2019005) and co-investigator on the National Key R&D Program of China No. 2017YFC0112504). Dr. Sands was supported by the American Heart Association (15SDG25890059) and American Thoracic Society Foundation.
ACKNOWLEDGMENTS
The authors thank the study participants, technicians and physicians at Beijing Tongren Hospital and University of California, San Diego for their help with this study. Author contributions: Yanru Li, contributed to study design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; Xin Cao and Di Zhao contributed to acquisition of the data; Jingying Ye and Demin Han contributed to acquisition of the data and interpretation of data; Robert L Owens, contributed to interpretation of the data and revision of the manuscript; Naomi Deacon, contributed to study conception and design, and interpretation of the data and revision of the manuscript; Scott Sands, contributed to analysis of the loop gain data and gave insights into control of breathing; Jeremy Orr and Rachel Jen contributed to analysis and interpretation of the data; Atul Malhotra contributed to study conception and design, and preparation and revision of the manuscript for intellectual content.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–532. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea--a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 7.Orr JE, Edwards BA, Malhotra A. Crosstalk opposing view: loop gain is not a consequence of obstructive sleep apnoea. J Physiol. 2014;592:2903–2905. doi: 10.1113/jphysiol.2014.271841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler JP, Owens RL, Malhotra A, Wellman A. Crosstalk opposing view: the human upper airway during sleep does not behave like a starling resistor. J Physiol. 2013;591:2233–2234. doi: 10.1113/jphysiol.2012.242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes M. Crosstalk proposal: elevated loop gain is a consequence of obstructive sleep apnoea. J Physiol. 2014;592:2899–2901. doi: 10.1113/jphysiol.2014.271833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacon NL, Catcheside PG. The role of high loop gain induced by intermittent hypoxia in the pathophysiology of obstructive sleep apnoea. Sleep Med Rev. 2015;22:3–14. doi: 10.1016/j.smrv.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Spicuzza L, Bernardi L, Balsamo R, Ciancio N, Polosa R, Di Maria G. Effect of treatment with nasal continuous positive airway pressure on ventilatory response to hypoxia and hypercapnia in patients with sleep apnea syndrome. Chest. 2006;130:774–779. doi: 10.1378/chest.130.3.774. [DOI] [PubMed] [Google Scholar]
- 12.Loewen A, Ostrowski M, Laprairie J, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han D, Ye J, Lin Z, Wang J, Wang J, Zhang Y. Revised uvulopalatopharyngoplasty with uvula preservation and its clinical study. ORL J Otorhinolaryngol Relat Spec. 2005;67:213–219. doi: 10.1159/000087390. [DOI] [PubMed] [Google Scholar]
- 15.Jingying Y, Biao Y, Jingming L, et al. Combination of transpalatal advancement pharyngoplasty and H-uvulopalatopharyngoplasty for obstructive sleep apnea. Oper Tech Otolayngol Head Neck Surg. 2009;20:152–158. [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosten SA, Leong P, Landry SA, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnoea: ventilatory control abnormalities predict surgical responsiveness. Sleep. 2017;40(7) doi: 10.1093/sleep/zsx094. [DOI] [PubMed] [Google Scholar]
- 19.Lee RWW, Sutherland K, Sands SA, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22:1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 20.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 2010;108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pialoux V, Hanly PJ, Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 24.Brugniaux JV, Pialoux V, Foster GE, et al. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans. Eur Respir J. 2011;37:880–887. doi: 10.1183/09031936.00156009. [DOI] [PubMed] [Google Scholar]
- 25.Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol. 2009;587:5451–5467. doi: 10.1113/jphysiol.2009.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol (1985) 2004;96:1197–1205. doi: 10.1152/japplphysiol.00573.2003. discussion 1196. [DOI] [PubMed] [Google Scholar]
- 27.Taranto-Montemurro L, Edwards BA, Sands SA, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194(7):878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen M, Smith H. Studies on the regulation of respiration in acute hypoxia; preliminary report. Acta Physiol Scand. 1951;22:44–46. doi: 10.1111/j.1748-1716.1951.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 29.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 30.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]