Abstract
Study Objectives:
Pregnant women are at risk for sleep-disordered breathing (SDB); however, screening methods in this dynamic population are not well studied. The aim of this study was to examine whether anthropometric measures can accurately predict SDB in pregnant women.
Methods:
Pregnant women with snoring and overweight/obesity were recruited in the first trimester. Anthropometric measures were performed according to the International Standards for Anthropometric Assessment, including a seated neutral and extended neck Mallampati class. Home sleep apnea monitoring was performed using a level III device after completion of anthropometric assessment. SDB was defined as an apnea-hypopnea index ≥ 5 events/h of sleep. Pearson and Spearman tests examined correlations between various measures. Generalized linear models, sensitivity, specificity, and area under the curve as well as odds ratios were performed to test the model.
Results:
A total of 129 participants were recruited, and 23 had SDB. Average gestational age was 10.6 ± 1.9 weeks. Due to concerns over multicollinearity, the final model included extended Mallampati class and upright neck circumference. Neck circumference was significantly higher in participants with Mallampati classes 2/3 and grade 4 compared to participants with Mallampati class 1 (P = .0005). Increasing neck circumference was associated with higher odds of SDB (P = .0022). In Mallampati class 1, odds ratio for SDB was 2.89 (1.19, 7.03) per unit increase in neck circumference.
Conclusions:
Modeling neck circumference while allowing for differences by Mallampati class showed a nearly threefold increase in the risk of SDB with increasing neck circumference in women with Mallampati class 1. Other potential sites of airway obstruction need to be investigated in future research.
Citation:
Bourjeily G, Chambers A, Salameh M, Bublitz MH, Kaur A, Coppa A, Risica P, Lambert-Messerlian G. Anthropometric measures and prediction of maternal sleep-disordered breathing. J Clin Sleep Med. 2019;15(6):849–856.
Keywords: anthropometric measures, Mallampati, neck circumference, obstructive sleep apnea, pregnancy, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Pregnancy is associated with physiological changes that may be transient; however, these changes may affect the pathogenesis of sleep-disordered breathing in pregnancy. This study examined the ability of clinical upper airway measurements to detect sleep-disordered breathing.
Study Impact: We found that a larger neck circumference was most predictive of sleep-disordered breathing in women with Mallampati class 1, despite this group having smaller average neck circumference compared to other Mallampati classes. Though counterintuitive, our findings suggest that physiological changes of pregnancy may affect the site of obstruction in pregnancy, begging for future studies examining measurements such as airway luminal areas and nasal resistance. A better understanding of this physiology can further the application of specific therapy of sleep-disordered breathing in this population.
INTRODUCTION
Sleep-disordered breathing (SDB) has been shown to affect almost 10% of premenopausal women based on data from the Wisconsin Sleep Cohort.1 Prospective data from pregnancy show that 4% of all women examined with home sleep monitors have SDB in early pregnancy.2 However, data from samples of pregnant women with obesity3 or medical4,5 or obstetric complications6,7 show that the prevalence of SDB is as high as 70% in some studies. To date, screening questionnaires that have been validated in the general nonpregnant population have failed to reliably predict objectively measured SDB in pregnancy.8,9 In addition, performance of these screening questionnaires appears to be influenced by gestational stage, with some screening tools performing better in the second and third trimester than in the first trimester.10 Models that include body mass index (BMI), age, snoring, and hypertension have been proposed in the general and high-risk pregnant populations9,11; however, these remain to be validated in other studies. Hence, the ability to predict SDB in pregnant women with obesity and those at risk for pregnancy complications12–15 remains quite limited.
Data from the Sleep Heart Health Study show that the most powerful predictors of SDB in the general population are age, BMI, neck and waist circumference, snoring frequency, and loudness.16 In addition, the change in neck circumference from the standing to the supine position is strongly predictive of obstructive sleep apnea (OSA).17 Waist circumference appears to be more predictive of OSA and OSA severity18 than BMI alone. Furthermore, visceral and abdominal fat appear to better correlate than BMI with indices of sleep apnea such as apnea-hypopnea index (AHI) and oxygen desaturation index.19,20 Though data in pregnancy suggest that early weight gain, biceps and triceps skinfolds,21 and waist circumference22 positively correlate with metabolic abnormalities associated with OSA, there are no studies evaluating anthropometric data other than BMI and gestational weight gain in predicting OSA in pregnancy. Our previously published cross-sectional data showed a correlation between SDB in the third trimester—as determined by questionnaires—and prepregnancy BMI, as well as BMI at delivery.13 Specific anthropometric measurements in pregnancy are not synonymous with the same measurements taken outside of pregnancy for many reasons. Weight gain and weight deposition differ by gestational age and although early weight gain may be due to fat deposition, weight gain later in pregnancy is related to an increase in amniotic fluid, plasma volume, and volume of products of conception. Furthermore, given the presence of sex hormone receptors in the upper airway, the multiple fold increase in circulating hormone levels in pregnancy may affect upper airway structure and site of obstruction.
Given the current limited ability to identify women at high risk for SDB to guide a referral for polysomnography or sleep apnea monitoring, an evaluation of the predictive ability of anthropometric measures is warranted. The aim of this study was to examine anthropometric measures that have been previously identified as predictors of SDB in a population of pregnant women with obesity in the first trimester of pregnancy. We hypothesized that these previously identified measures may perform differently in the pregnant population.
METHODS
Participants
Participants consisted of women screened in the first trimester of pregnancy. Participants were recruited from community-based practices and local hospitals that serve women in Rhode Island and southern Massachusetts. Pregnant women with overweight or obesity in their first trimester (gestational age less than 14 completed weeks) who were 18 years or older were recruited. Exclusion criteria of the parent studies included falling asleep while driving, nonsingleton pregnancies, cervical insufficiency, advanced cardiac disease or arrhythmias, chronic lung conditions, respiratory failure, severe prepregnancy hypertension, and history of treated OSA. This study was approved by the Lifespan Institutional Review Board.
Procedures
After informed consent to participate was obtained, women reported on past pregnancies and medical history. Women underwent a physical examination assessment and were instructed on the proper methods of using a home sleep apnea test.
Anthropometric Measures
Participants had anthropometric measurements performed by an experienced operator at the time of the screening visit, following guidelines by the International Standards of Anthropo-metric Assessment.23 Height in meters and weight in kilograms were measured and BMI calculated. Neck circumference was measured using a disposable measuring tape at the level of the hyoid bone in the upright seated position, with the head in a neutral position. The measuring tape was kept in place and the patient was asked to assume a supine position without neck support. The measurement of neck circumference was then repeated in the supine position.
Breast circumference was measured at the widest diameter of the chest area in the seated resting position. Waist circumference was measured in the upright position as follows: a measurement at the midaxillary line is taken between the lowest point of the rib cage and the highest point of the iliac crest. Halfway of that line, waist circumference was measured horizontally at end expiration. Hip circumference was measured in the upright position at the widest diameter over the buttocks.
A modified Mallampati class was measured in a resting seated position prior to the home test by a trained research nurse. A Mallampati class estimates the size of the tongue relative to the oral cavity and is related to tongue volume. The assessment records visibility of oral cavity structures and has good interobserver agreement.24 The patient had her neck in a neutral position, with mouth open wide at eye level of the examiner, and the tongue remaining inside the mouth.25 A Mallampati class of 1, 2, 3, or 4 was then assigned after inspiration. Class I is identified by complete visualization of the soft palate. Class II distinguished by complete visualization of the uvula and class III by visualization of only the base of the uvula. Class IV is determined by complete lack of visibility of the soft palate. In order to mimic a sleeping position, the patient was then asked to perform a craniocervical extension. Craniocervical extension is thought to improve the specificity and the positive predictive value of the Mallampati classification system while retaining its sensitivity.26 The upper airway was then examined similarly to Mallampati class measured in the neutral neck position. Mallampati classes of 1 through 4 were similarly recorded.
Home Sleep Study
The home sleep apnea test was performed using a level III recording device, Nox T3 (Carefusion, San Diego, California, United States), which uses built-in sensors to include a pressure transducer allowing recording of nasal pressure and snoring, a three-dimensional acceleration sensor for measuring body position and activity, and a microphone for true audio-recording capabilities. The external sensor options used included electrocardiography and dual abdominal/thoracic respiratory inductance plethysmography belts, the latter being the preferred noninvasive technology in the measurement of respiratory effort. The T3 device also supports wireless Bluetooth connectivity, allowing it to record signals from a Bluetooth pulse oximeter. Nox T3 autoscore has been validated against in-laboratory polysomnography and found to correctly identify 100% of individuals who do not have OSA and 88% of individuals who have OSA.27 In addition, autoscore T3 respiratory event index (REI) strongly relates to AHI derived from polysomnography (r = .93). OSA is defined in this study as REI ≥ 5 events/h, and severity defined as 5–14.9, mild; 15–29.9, moderate; and ≥ 30, severe. Measurements of oxygen saturation parameters were also collected. Hypopnea was defined based on the recommended American Academy of Sleep Medicine rule of 3% desaturation.28 Sleep studies were performed within 12 to 24 hours of the anthropometric assessment and scored by the same experienced polysomnography technician, and supervised by the investigative team.
Statistical Methods
Demographics of Study Sample and Anthropomorphic Measure Correlations
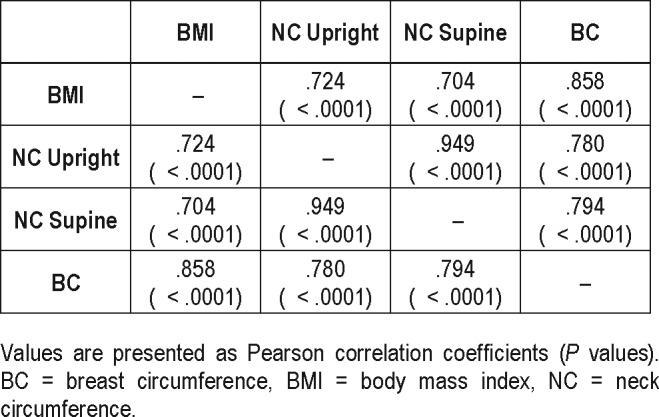
During preliminary analysis, Pearson and Spearman correlation coefficients were used to test the relationship between morphologic characteristics and AHI. Significant correlations among the anthropomorphic variables were found (BMI, breast circumference, and waist to hip ratio, neck circumference, Mallampati class). Upright neck circumference and supine neck circumference were tightly correlated (r = .95, P < .0001; Table 1). Similarly, upright and extended Mallampati classes were highly correlated (r = .98, P < .0001). Therefore, only one of the two measures was included in further analyses. Although the association of each anthropomorphic measure with SBD status was considered, given concerns over nonindependence of predictor variables (multicollinearity), the final model focused on upper airway anthropometric measures (extended Mallampati class and upright neck circumference) to predict SDB status.
Table 1.
Pearson correlation coefficients for anthropomorphic measures.
Upright Neck Circumference and Extended Mallampati Class
A general linear model (proc glimmix) was used to test the relationship between extended Mallampati class and upright neck circumference. No difference in upright neck circumference was found between extended Mallampati classes of 2 and 3 (P = .6854). For that reason, the two classes of Mallampati were grouped as one category (2/3) for subsequent analysis. Neutral Mallampati class and supine neck circumference were also considered as possible predictors for modeling but preliminary analysis showed extended Mallampati class and upright neck circumference had marginally stronger relationships to apneic outcome and were used for the remaining analysis.
Apnea Prediction
A generalized linear model for binary outcomes (proc glimmix) was used to model the proportion of subjects with SDB (defined as REI ≥ 5 events/h) versus no SDB (defined as REI < 5 events/h) by their upright neck circumference and extended Mallampati class. An interaction term was included in the model to allow for differences in the relationship between neck circumference and apnea with the level of extended Mallampati class. Familywise alpha was maintained at 0.05 using the Holm adjustment for comparisons across extended Mallampati class. Odds ratios, receiver operating characteristic curves, and optimal cutoffs were also determined from the model. Sensitivity, specificity, and area under the curve (AUC or accuracy) were then reported for these cutoffs. All statistical analyses were performed using SAS version 9.4 (The SAS Institute; Cary, North Carolina, United States).
RESULTS
Demographics and Maternal Characteristics
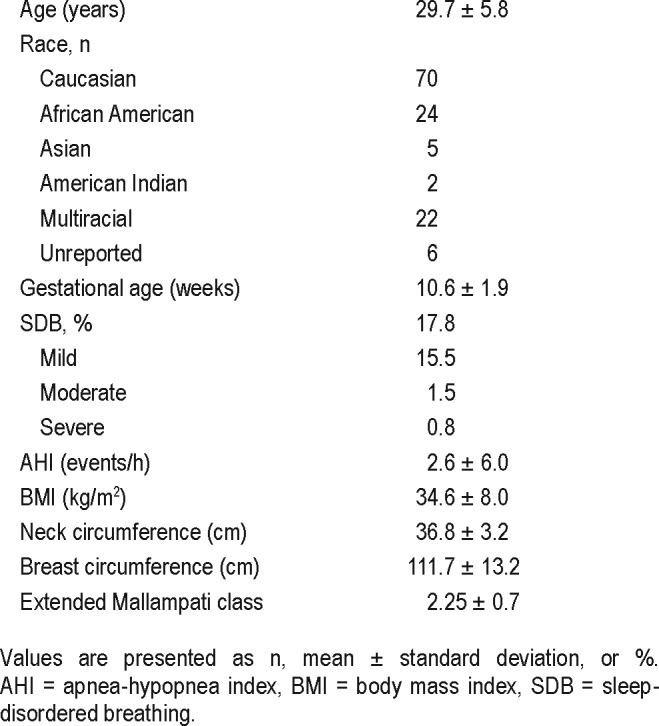
A total of 129 pregnant participants with overweight or obesity were recruited in the first trimester of pregnancy. Average REI of the sample was 2.6 ± 6.04 events/h. Twenty-three women received a diagnosis of SDB based on an REI ≥ 5 events/h of sleep. Of those, most (87%) had mild SDB, 8.7% had moderate disease, and 4.3% had severe disease.
Mean age of participants was 29.7 ± 5.8 years (Table 2). Mean BMI was 34.7 ± 7.8 kg/m2. Average neck circumference in the upright position was 36.8 ± 3.2 cm.
Table 2.
Participant demographics.
SDB Prediction
Given concerns over multicollinearity, the final model included only extended Mallampati class and upright neck circumference to predict SDB status. Neck circumference was significantly higher in participants with Mallampati class 2/3 (neck circumference = 37.85 ± 0.42 cm) and class 4 (neck circumference = 36.8 ± 0.43 cm) compared to participants with Mallampati class 1 (35.05 ± 0.55 cm), (P = .0005). These results are illustrated in Figure 1. In general, the odds of SDB increased with the Mallampati class.
Figure 1. Neck circumference by Mallampati class.
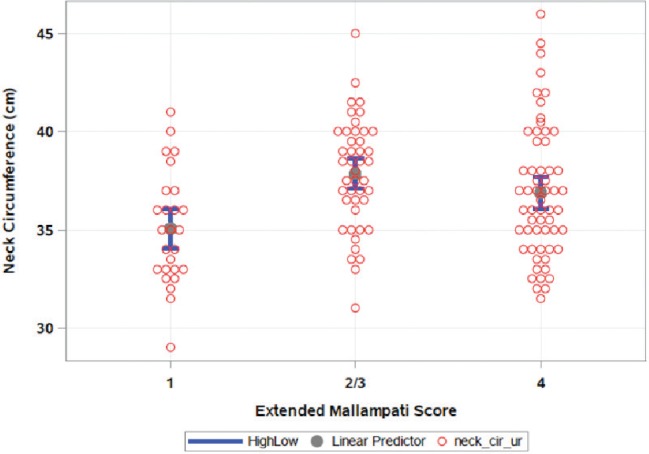
Gray circles represent mean neck circumference and blue bars represent 95% confidence intervals.
Figure 2 shows the final model and the effect of increasing neck circumference on SDB status and how the relationship changes depending on level of Mallampati class. Table 3 shows SDB probability at various neck circumference cutoffs in different Mallampati classes. In general, increasing neck circumference was associated with higher odds of SDB (P = .0022). The risk of SDB increased significantly with increasing neck circumference in women with Mallampati class 1. Considering Mallampati class 1, the odds ratio was 2.89 (1.19, 7.03), P = .0207. For Mallampati classes 2/3, there was a trend to -ward statistical significance at 1.32 (0.99, 1.77), P = .0565. Though the risk was higher than 1 for Mallampati class 4, the increased risk did not reach statistical significance at 1.2 (0.95, 1.54), P = .1192. It should also be noted that the relationship between neck circumference and SDB for the Mallampati class 1 group achieved statistical significance despite this group of subjects having the smallest neck circumference (Figure 1 and Figure 2).
Figure 2. Probability of obstructive sleep apnea by neck circumference in three Mallampati class categories.
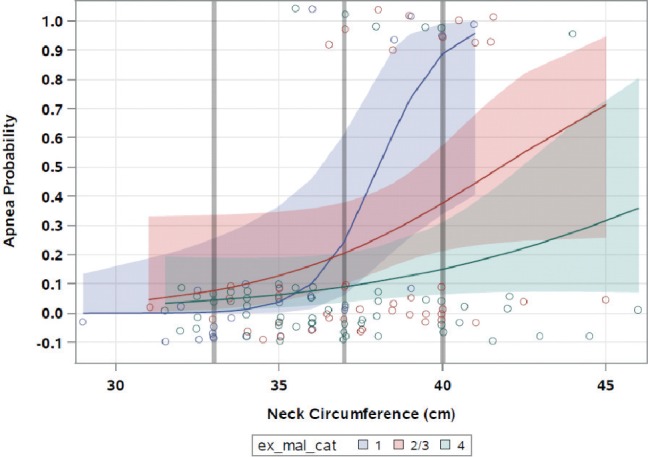
Shaded red, blue, and green areas represent 95% confidence intervals. Note: Grey lines indicate where estimated probability was performed for varying neck circumference and Mallampati classes (also see Table 2).
Table 3.
Estimated SDB probability at neck circumferences 33, 37, 40 cm for extended Mallampati classes of 1, 2/3, and 4.
Figure 3 shows sensitivity and specificity of the model at the different levels of Mallampati classes. The cutoff with best sensitivity and specificity for neck circumference was 36.8 cm in those with Mallampati class 1 (see Figure 3A), 39.5 cm in those with Mallampati class 2/3 (see Figure 3B), and 40.7 cm in those with Mallampati class 4 (see Figure 3C). The AUC or accuracy for the model was 0.815 (81.5% accuracy of the combination of neck circumference and Mallampati class to discriminate between absence of SBD and presence of SBD).
Figure 3. Sensitivity and specificity versus increasing neck circumference by Mallampati class.
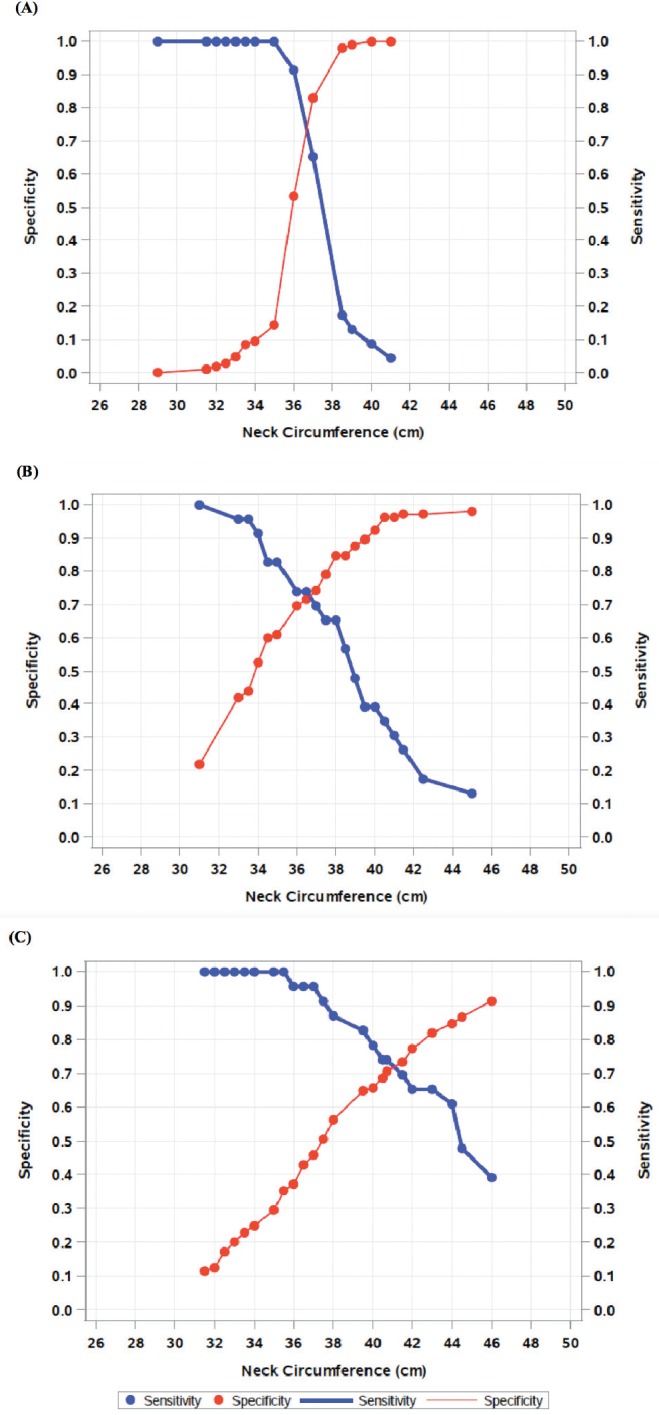
Sensitivity and specificity versus increasing neck circumference for (A) Mallampati class 1, (B) Mallampati class 2/3, and (C) Mallampati class 4.
DISCUSSION
We found a relationship between higher BMI, neck circumference, breast circumference, and waist-to-hip ratio with SDB. However, because of concerns about multicollinearity with the anthropomorphic measures, our approach incorporated the measures that were physiologically most closely associated with airway obstruction. Our final model tested the specific effects of upper airway anthropometrics (Mallampati class and neck circumference) on SDB. In general, we found increased risk of SDB with increased neck circumference. However, modeling neck circumference and allowing for differences by level of Mallampati class, the risk of SDB was higher with larger neck circumference, in women with Mallampati class 1 specifically, and the probability of having SDB was mainly driven by increases in neck circumference. Women with Mallampati class 1 saw a higher risk of having SDB by more than twofold for every unit increase in neck circumference. Mallampati classification has been shown to be a good predictor of SDB in the general nonpregnant population. Numerous studies have shown an increased prevalence and risk for SDB29–31 with higher Mallampati classes, as well as evidence of a positive correlation between AHI and more pharyngeal crowding.30 This association was also demonstrated in samples that consisted exclusively of women.32 However, Mallampati class only explained 1.7% of the variability in AHI in one large retrospective study (64% male),30 and one study demonstrated an association only in subgroups of patients with nasal obstruction.33 Neck circumference has also been demonstrated to be a valuable tool in screening for OSA34 and has been included in various predictive models in the nonpregnant population.35,36 Predictive ability of these measures to detect SDB in pregnancy may relate to physiologic changes that are specific to pregnancy and that may play a role in upper airway patency and the anatomic location of airflow limitation.
Airway patency depends on numerous factors including characteristics that are inherent to sex, weight, and weight distribution, as well as length of the airway, and obstruction leading to airflow limitation can occur at different levels in the airway. Mallampati class and neck circumference measure different areas of the upper airway, and despite BMI contributing to and affecting both measurements in our sample, the two measurements did not correlate with one another. The Mallampati classification system, originally described to predict difficult intubations,37 is dependent on tongue volume, mandibular size, and pharyngeal crowding. However, neck circumference is a reproducible measure38,39 that assesses a more distal area in the upper airway. Both Mallampati class and neck circumference are influenced by fat deposition and fluid shifts. Even though obesity is an important contributing factor to OSA because of peripharyngeal fat accumulation and an increase in neck circumference,40 it only accounts for a small portion of the variability of the frequency of SDB.41,42 Wasicko et al. have shown that systemic vasodilation in anesthetized cats reduces pharyngeal cross-sectional area and increases pharyngeal collapsibility, whereas systemic vasoconstriction has the opposite effect.43 Human studies have suggested that rostral redistribution of fluids from the lower extremities to the upper airway influences neck circumference and pharyngeal resistance,44 pharyngeal cross-sectional area,45 and airway collapsibility.46
Pathobiology of SDB in pregnancy may also be different because of sex and pregnancy-specific factors that may affect airway patency. Though men and women have similar amounts of fluid movement from the legs, more fluid accumulates in the thorax and the neck area in men.47,48 Sex hormones have been implicated in the pathogenesis of SDB and may play either a predisposing role or a protective role, possibly depending on the anatomical level in question. It has been proposed that progesterone, acting as a respiratory stimulant, may lead to a vacuum effect on the upper airway, predisposing to airway collapsibility.49 Both estrogens and progesterone have been implicated in fluid retention and regulation.50,51 As levels of these hormones are significantly increased compared to pre-pregnancy levels, a hormonal contribution to airway patency would be extremely relevant in the pregnant population. As measurements in our study occurred in early pregnancy at an average of 10.3 weeks of gestation, changes related to plasma volume and fluid retention would likely have played some role, though plasma volume does not peak until the second half of pregnancy.
Estrogens may also act to predispose to nasal obstruction— which has been linked to reduced upper airway patency and SDB52,53—via histamine receptors in the nasal epithelium and microvascular endothelial cells.54 Nasal obstruction may occur due to direct hormonal changes such as nasal vascular smooth muscle relaxation by progesterone, or may be mediated via changes in systemic blood volume and increased concentration of neurotransmitter substance P, altered by both estrogen and progesterone.55
Nonvascular effects of sex steroid hormones on upper airway patency cannot be discounted and may also affect the location of upper airway obstruction. As previously noted, progesterone is a respiratory stimulant, and stimulates upper airway dilator muscles.56 Similarly, estrogens such as 17-beta estradiol accentuate contractility of the rat genioglossus muscle,57 reverse the effect of chronic intermittent hypoxia in ovariectomized rats,58 and can directly modulate the output of respiratory motor neurons.59 Furthermore, estrogen has been reported to inhibit hypoxia-inducible factor 1-alpha expression, thereby exerting protective effects on the genioglossus muscle in chronic intermittent hypoxia.60
Our findings are somewhat counterintuitive in showing that changes in neck circumference have the greatest effect in patients with Mallampati class 1, among whom average neck circumference was smallest, compared to other Mallampati classes. Hence, previously cited literature examining the contribution of sex hormones to upper airway patency and our current findings suggest that the location of upper airway narrowing and airflow limitation may be different in pregnancy due to pregnancy physiology. This understanding of the effect of female sex hormones suggests that pregnant women may be protected by increased contractility of the genioglossus muscle but may be more prone to fluid retention and to having higher anatomical levels of obstruction in the nasal passages. Future studies examining physiological data regarding airway collapsibility and impedance, including nasal obstruction and resistance as they relate to SDB and female sex hormones, are sorely needed, as a better understanding of these pathophysio-logic mechanisms can affect the application of specific therapy.
Strengths of this prospective study include the objective identification of SDB and the performance of anthropometric measures by a trained operator. Measurements of all anthropometric measures were blinded to SDB status. A limitation of this study is the use of a home sleep apnea study rather than in-laboratory polysomnography. The lack of recording of electroencephalogram to measure sleep and arousals may lead to an underestimation of hypopneas. Hypopneas associated with arousals and without significant desaturations could have been missed. However, level III devices have been validated in the evaluation of SDB in pregnant women.61 Additionally, because our population consisted of women with obesity and overweight, our results may not be generalizable to the normal weight population suspected of OSA, although excess body weight is a major driver of SDB in pregnancy.11 Furthermore, our study was designed to examine these markers in the first trimester to standardize the effect of pregnancy; however, because pregnancy is a dynamic state, our current findings may not apply to later stages of gestation and future studies should examine the predictive ability of these measures in late gestation. Similarly, as lung volumes and their associated changes across pregnancy may be a confounding factor, future research should examine the effect of lung volume and its changes on SDB prediction. Last, for logistical reasons, neck circumference measures could not be performed at the same time of day in all participants; hence, variability across patients could have occurred depending on time of day of measurement and may change based on day-to- day variability.
DISCLOSURE STATEMENT
Work for this study was performed at the Women's Medicine Collaborative at The Miriam Hospital, Providence, RI. All authors have seen and approved this manuscript. National Institutes of Health R01HD078515 and R01HL130702 funded this work, as well as P20 GM103652. Ghada Bourjeily has received research equipment from Respironics. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge all the women who agreed to participate in this study.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BMI
body mass index
- OSA
obstructive sleep apnea
- REI
respiratory event index
- SDB
sleep-disordered breathing
REFERENCES
- 1.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 2.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202(3):261.e1–261.e5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 4.Bublitz MH, Monteiro JF, Caraganis A, et al. Obstructive sleep apnea in gestational diabetes: a pilot study of the role of the hypothalamic-pituitary-adrenal axis. J Clin Sleep Med. 2018;14(1):87–93. doi: 10.5664/jcsm.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reutrakul S, Zaidi N, Wroblewski K, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–4202. doi: 10.1210/jc.2013-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pamidi S, Marc I, Simoneau G, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–725. doi: 10.1136/thoraxjnl-2015-208038. [DOI] [PubMed] [Google Scholar]
- 7.Fung AM, Wilson DL, Lappas M, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7):e68057. doi: 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivarez SA, Ferres M, Antony K, et al. Obstructive sleep apnea screening in pregnancy, perinatal outcomes, and impact of maternal obesity. Am J Perinatol. 2011;28(8):651–658. doi: 10.1055/s-0031-1276740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8(4):389–394. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tantrakul V, Sirijanchune P, Panburana P, et al. Screening of obstructive sleep apnea during pregnancy: differences in predictive values of questionnaires across trimesters. J Clin Sleep Med. 2015;11(2):157–163. doi: 10.5664/jcsm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis JM, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1–521.e12. doi: 10.1016/j.ajog.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourjeily G, Danilack VA, Bublitz MH, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57. doi: 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1–487.e9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep. 2014;37(5):843–849. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caffo B, Diener-West M, Punjabi NM, Samet J. A novel approach to prediction of mild obstructive sleep disordered breathing in a population-based sample: the Sleep Heart Health Study. Sleep. 2010;33(12):1641–1648. doi: 10.1093/sleep/33.12.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MK, Martinez D, Cassol CM, Rahmeier L, Vieira LR. Immediate and overnight recumbence-dependent changes of neck circumference: relationship with OSA severity in obese and nonobese subjects. Sleep Med. 2012;13(6):650–655. doi: 10.1016/j.sleep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Rivera C, Abad J, Fiz JA, Rios J, Morera J. Usefulness of truncal obesity indices as predictive factors for obstructive sleep apnea syndrome. Obesity (Silver Spring) 2008;16(1):113–118. doi: 10.1038/oby.2007.20. [DOI] [PubMed] [Google Scholar]
- 19.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122(3):829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241(1):11–18. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomedi LE, Simhan HN, Chang CC, McTigue KM, Bodnar LM. Gestational weight gain, early pregnancy maternal adiposity distribution, and maternal hyperglycemia. Matern Child Health J. 2014;18(5):1265–1270. doi: 10.1007/s10995-013-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingras V, Tchernof A, Weisnagel SJ, Robitaille J. Use of glycated hemoglobin and waist circumference for diabetic screening in women with a history of gestational diabetes. J Obstet Gynaecol Can. 2013;35(9):810–815. doi: 10.1016/s1701-2163(15)30837-9. [DOI] [PubMed] [Google Scholar]
- 23.Marfell-Jones M, Olds T, Stewart A, Carter L. International Standards for Anthropometric Assessment. Potchefstroom, South Africa: International Society for the Advancement of Kinanthropometry; 2006. [Google Scholar]
- 24.Rosenstock C, Gillesberg I, Gatke MR, Levin D, Kristensen MS, Rasmussen LS. Inter-observer agreement of tests used for prediction of difficult laryngoscopy/tracheal intubation. Acta Anaesthesiol Scand. 2005;49(8):1057–1062. doi: 10.1111/j.1399-6576.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 25.Manabe Y, Iwamoto S, Miyawaki H, Seo K, Sugiyama K. Mallampati classification without tongue protrusion can predict difficult tracheal intubation more accurately than the traditional Mallampati classification. Oral Sci Int. 2014;11(2):52–55. [Google Scholar]
- 26.Mashour GA, Sandberg WS. Craniocervical extension improves the specificity and predictive value of the Mallampati airway evaluation. Anesth Analg. 2006;103(5):1256–1259. doi: 10.1213/01.ane.0000237402.03329.3b. [DOI] [PubMed] [Google Scholar]
- 27.Cairns A, Wickwire E, Schaefer E, Nyanjom D. A pilot validation study for the NOX T3(TM) portable monitor for the detection of OSA. Sleep Breath. 2014;18(3):609–614. doi: 10.1007/s11325-013-0924-2. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiremath AS, Hillman DR, James AL, Noffsinger WJ, Platt PR, Singer SL. Relationship between difficult tracheal intubation and obstructive sleep apnoea. Br J Anaesth. 1998;80(5):606–611. doi: 10.1093/bja/80.5.606. [DOI] [PubMed] [Google Scholar]
- 30.Hukins C. Mallampati class is not useful in the clinical assessment of sleep clinic patients. J Clin Sleep Med. 2010;6(6):545–549. [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi H, Nakata S, Tsuge H, et al. Morphological examination of upper airway in obstructive sleep apnea. Auris Nasus Larynx. 2009;36(4):444–449. doi: 10.1016/j.anl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Polesel DN, Hirotsu C, Nozoe KT, et al. Waist circumference and postmenopause stages as the main associated factors for sleep apnea in women: a cross-sectional population-based study. Menopause. 2015;22(8):835–844. doi: 10.1097/GME.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 33.Liistro G, Rombaux P, Belge C, Dury M, Aubert G, Rodenstein DO. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J. 2003;21(2):248–252. doi: 10.1183/09031936.03.00292403. [DOI] [PubMed] [Google Scholar]
- 34.Cizza G, de Jonge L, Piaggi P, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Metab Syndr Relat Disord. 2014;12(4):231–241. doi: 10.1089/met.2013.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duarte RLM, Rabahi MF, Magalhaes-da-Silveira FJ, de Oliveira-E-Sá TS, Mello FCQ, Gozal D. Simplifying the screening of obstructive sleep apnea with a 2-item model, no-apnea: a cross-sectional study. J Clin Sleep Med. 2018;14(7):1097–1107. doi: 10.5664/jcsm.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 37.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29(7):903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 38.Schwab RJ, Leinwand SE, Bearn CB, et al. Digital morphometrics: a new upper airway phenotyping paradigm in OSA. Chest. 2017;152(2):330–342. doi: 10.1016/j.chest.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gjevre JA, Taylor-Gjevre RM, Reid JK, Skomro R, Cotton D. Inter-observer reliability of candidate predictive morphometric measurements for women with suspected obstructive sleep apnea. J Clin Sleep Med. 2013;9(7):695–699. doi: 10.5664/jcsm.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(2):260–265. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 41.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5 Pt 1):1228–1231. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 42.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122(3):840–851. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 43.Wasicko MJ, Hutt DA, Parisi RA, Neubauer JA, Mezrich R, Edelman NH. The role of vascular tone in the control of upper airway collapsibility. Am Rev Respir Dis. 1990;141(6):1569–1577. doi: 10.1164/ajrccm/141.6.1569. [DOI] [PubMed] [Google Scholar]
- 44.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174(12):1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 45.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62(10):868–872. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su MC, Chiu KL, Ruttanaumpawan P, et al. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol. 2008;161(3):306–312. doi: 10.1016/j.resp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Su MC, Chiu KL, Ruttanaumpawan P, et al. Difference in upper airway collapsibility during wakefulness between men and women in response to lower-body positive pressure. Clin Sci (Lond) 2009;116(9):713–720. doi: 10.1042/CS20080321. [DOI] [PubMed] [Google Scholar]
- 48.Yadollahi A, Singh B, Bradley TD. Investigating the dynamics of supine fluid redistribution within multiple body segments between men and women. Ann Biomed Eng. 2015;43(9):2131–2142. doi: 10.1007/s10439-015-1264-0. [DOI] [PubMed] [Google Scholar]
- 49.Edwards N, Middleton PG, Blyton DM, Sullivan CE. Sleep disordered breathing and pregnancy. Thorax. 2002;57(6):555–558. doi: 10.1136/thorax.57.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stachenfeld NS. Hormonal changes during menopause and the impact on fluid regulation. Reprod Sci. 2014;21(5):555–561. doi: 10.1177/1933719113518992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawai A, Tochigi Y, Kavaliova N, et al. MRI reveals menstrually-related muscle edema that negatively affects athletic agility in young women. PLoS One. 2018;13(1):e0191022. doi: 10.1371/journal.pone.0191022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99(2):S757–S762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 53.Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;161(12):1514–1519. doi: 10.1001/archinte.161.12.1514. [DOI] [PubMed] [Google Scholar]
- 54.Hamano N, Terada N, Maesako K, et al. Expression of histamine receptors in nasal epithelial cells and endothelial cells--the effects of sex hormones. Int Arch Allergy Immunol. 1998;115(3):220–227. doi: 10.1159/000023904. [DOI] [PubMed] [Google Scholar]
- 55.Bowser C, Riederer A. [Detection of progesterone receptors in connective tissue cells of the lower nasal turbinates in women] Laryngorhinootologie. 2001;80(4):182–186. doi: 10.1055/s-2001-13870. [DOI] [PubMed] [Google Scholar]
- 56.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 57.Hou YX, Jia SS, Liu YH. 17beta-Estradiol accentuates contractility of rat genioglossal muscle via regulation of estrogen receptor alpha. Arch Oral Biol. 2010;55(4):309–317. doi: 10.1016/j.archoralbio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Liu YH, Huang Y, Shao X. Effects of estrogen on genioglossal muscle contractile properties and fiber-type distribution in chronic intermittent hypoxia rats. Eur J Oral Sci. 2009;117(6):685–690. doi: 10.1111/j.1600-0722.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 59.Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience. 2005;130(3):725–734. doi: 10.1016/j.neuroscience.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Liu Y, Lu Y, Zhao B. Inhibitory effects of 17beta-estradiol or a resveratrol dimer on hypoxia-inducible factor-1alpha in genioglossus myoblasts: Involvement of ERalpha and its downstream p38 MAPK pathways. Int J Mol Med. 2017;40(5):1347–1356. doi: 10.3892/ijmm.2017.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharkey KM, Waters K, Millman RP, Moore R, Martin SM, Bourjeily G. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnography in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497–502. doi: 10.5664/jcsm.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]


