Abstract
Background/Objectives:
With aging, people commonly develop motor slowing (bradykinesia). Although this slowness with aging may be entirely related to degradation of the cerebral networks important in motor programing, it is possible that, at least in part, it may be a learned procedure for enhancing the accuracy and/or precision of movements. The goal of this study is to test these contradictory hypotheses.
Methods:
Twenty-four healthy adults, 12 younger than age 26 and 12 older than age 65 were asked to make alternative marks with a pen between a card centered in front of them and a series of circles distributed across a page. Performance was timed, and participants were instructed to complete the task as quickly as possible while not sacrificing accuracy for speed. The circle sizes and hand used varied by trial.
Results:
The older adults performed the task more slowly for all target circle diameters. As the circles decreased in size, the younger adults performed the task more rapidly than did the older participants, but the younger participants also had a greater decline in accuracy.
Conclusions:
During this aiming task, healthy older adults were less likely than younger adults to sacrifice accuracy for speed. Thus, at least in part, their slowing may be a learned adaptive strategy.
Keywords: Aging, Precision, Open-looped movements, Closed-looped movements, Hemispheric specialization
1. Introduction
As people age, there are both structural and functional alterations of the brain. These changes are often viewed as being pathological, existing on a spectrum with neurological diseases. For example, a study by Ross et al. (2004)demonstrated that in older adults without Parkinson disease there was a significant association between increasing number of Parkinson’s signs including bradykinesia with decreasing neuron density in the substantia nigra (SN). Low SN neuron density may be the basis for parkinsonian signs in the elderly without PD. Alternatively, age-associated brain changes and alterations in cognitive function may also be adaptive, enhancing the ability of the older individuals to perform at optimal levels. Slowing of motor activities(bradykinesia) typically occur with advancing age (Jiménez-Jiménez et al., 2011, Salthouse, 2000). Whereas this slowing can be viewed as a decrement related to aging of the brain, if this motor slowing is associated with preserved or even improved movement precision (i.e., variance) or accuracy (i.e., target aiming error), then this slowing may be adaptive and reflect a strategy to maintain or even improve high levels of performance. In contrast, slowing that is associated with impaired precision or accuracy may be more likely to represent a pathological condition. The purpose of this study is to test the contradictory hypotheses that, with normal aging in the absence of known neurological disease, motor slowing is associated with increased accuracy when performing aiming movements, or alternatively that both speed and accuracy decline even with healthy aging.
Fitts (1954) tested the hypothesis that the duration of a motor response is related to the amount of information required to program and perform the action. For example, actions with higher demands for spatial precision take more time to perform, presumably because these tasks require greater amounts of sensory information to guide motor programing and execution. Fast movement toward a target requires an initial open loop movement followed by a closed loop movement (Haaland & Harrington, 1989). Open loop movements utilize sensory information at the planning stage in order to program the action, but once the action is initiated there is no further modification. Conversely, closed loop movements are continuously modified by sensory input in order to allow for precise movement control throughout the action. Researchers, however, have noted that this conceptualization of distinct open and closed loop portions of movements is somewhat of an over-simplification, and kinesthetic feedback is present throughout both types of movements (Haaland & Harrington, 1989). Kinematic changes with healthy aging may reflect alterations in the brain networks that process sensory input and program open and closed loop movements.
It has been reported that when older adults perform movements toward targets, their movements are characterized by less smoothness and continuity, although performance precision is maintained (Morgan et al., 1994). If motor slowness with aging is adaptive, the reduction of motor speed should facilitate improved performance, as predicted by Fitts’s Law.
When compared to healthy participants, people with Parkinson’s disease, when performing aiming movements, often demonstrate both reduced precision and increased slowness as task demands increase (Sanes, 1985). With aging healthy older adults also have a loss of neurons in their substantia nigra and it has been posited that this dopamine depletion may contribute to changes in motor function associated with aging (Buchman et al., 2012). Thus, when performing motor activities that require precision and speed older adults, when compared to younger adults, would be expected to have both a slowing of their activities as well as reduced precision. There are also studies that suggest older people have a decrement in visuospatial functions (Staudinger, Fink, Mackay, & Lux, 2011) and based on a reduction of visuospatial abilities when compared to younger adults, the older adults would be expected to make more errors. Thus, the goal of this study is to learn about the spatial control and speed of older versus younger healthy participants’ upper limb aiming movements.
Previous research has demonstrated that, when tracing a path with a stylus, a visual motor task that requires continuous visual feedback and closed loop movement, younger adults were faster when performing the task with their dominant writing hand, while the older adults demonstrated no performance asymmetry between the hands (Raw, Wilkie, Culmer, & Mon-Williams, 2012). This loss of motor asymmetry with aging parallels the age related reduction of hemispheric specialization noted in cognitive domains (Cabeza, 2002, Dolcos et al., 2002). In addition, studies of patients with left hemisphere strokes(Hanna-Pladdy, Mendoza, Apostolos, & Heilman, 2002) and a lesion of the corpus callosum (Acosta, Bennett, & Heilman, 2014) suggest that the left hemisphere is critical for programming deft-precise movement for both hands. Since with aging there is a reduction of the corpus callosum (Jäncke, Mérillat, Liem, & Hänggi, 2015), it is also possible that with aging the left hand will show more changes than the right hand.
To our knowledge, there have been no previous studies of how asymmetries of aiming movements performed by the right versus the left hand change with healthy aging. If the motor networks that control aiming movements are lateralized to one hemisphere, this may induce an asymmetry of performance between the right and left hands in young adults with typical hemispheric specialization. However, this between hand asymmetry of performance may be reduced as hemispheric specialization decreases with healthy aging.
2. Materials and methods
2.1. Participants
We recruited 12 older adults and 12 younger adults. Each group had 6 women and 6 men. Since one goal of this study was to compare changes between right and left hand performance with aging, all participants recruited for this study were right handed as determined by the Benton Handedness Inventory (Varney & Benton, 1975). Mean age of the younger adults was 22.3 years (SD 1.6 years). Mean age of the older adults was 76 years, (SD 4.5 years). The mean level of education was 16.3 years (SD 1.3 years) for the younger adults and 18.1 years (SD 3.0 years) for the older adults. The difference in level of education did not reach statistical significance (t(22) = −1.946, p = 0.065).
Participants for this study were recruited from the community. Most of the younger adults were students or friends of students at the University of Florida. Older adults had taken part in other research at the University of Florida Institute on Aging, enrolled in a research registry, and had agreed to be contacted for participation in studies. Although we did not perform medical or neurological screening, participants were aware of our study inclusion and exclusion criteria that all participants must be in good health and without any medical, neurological, or psychiatric conditions that could affect their ability to perform the tasks administered for this study. This study was approved by the University of Florida Institutional Review Board and all participants provided written informed consent. Each participant received a $25 gift card to a local grocery store for participation in this study.
2.2. Apparatus and procedures
A blank index card (3″ by 5″) was taped to the proximal edge of the table, and this card was placed directly in front of the participant (in the participant’s midsagittal plane). Each stimulus page (8.5″ by 11″) contained 16 circles. The circles on each page were the same size and three sized circles were used, 1 cm, 2 cm, or 4 cm in diameter. For each participant 36 pages with circles were used, 18 used to test the right hand and 18 for the left hand. For each hand there were 6 pages used with each of the three sized circles. The examiner positioned each stimulus page so that the distal edge of the page was an arm’s length from the participant.
Each participant was seated and instructed to extend her or his arm on the table to a comfortable distance so that the elbow was slightly flexed and almost fully extended. They were instructed to repeat this procedure until each circle on the page was marked once with the pen, and the pen was then brought back to mark the index card for the final time on that trial. The right and left hands were tested separately, and the hand tested first was counterbalanced between participants.
The participants were instructed to begin each trial by holding the tip of the pen on the index card and were told that the task was to bring the pen from the index card to the stimulus page, to mark inside one of the circles on the page, and then bring the pen back to mark the index card. These circles were evenly distributed across the stimulus sheet for each trial, and the location of the circles varied between trials (see Fig. 1 for an example sheet for each size target circle). A mark within a target circle is counted as a hit, whereas a mark outside was counted as a miss, with the sum of hits (out of a maximum of 12) recorded for each trial. Hit count is predominantly a measure of accuracy, however precision contributes to hit count as well. Participants were not specifically instructed to aim for the center of the circles and the test was not structured to measure the variability of mark locations.
Fig. 1.
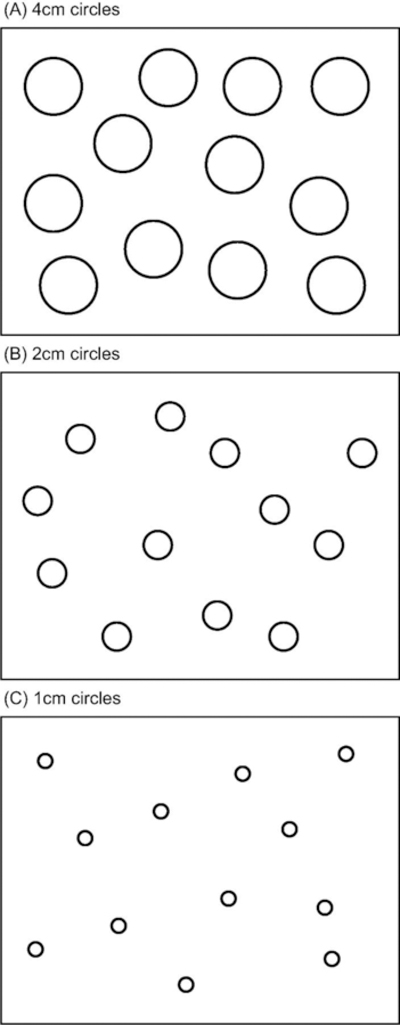
Schematic examples of target stimuli on US letter size sheets. (A) 4 cm circles. (B) 2 cm circles. (C) 1 cm circles.
Each participant’s performance was timed with a stopwatch by an investigator, beginning when the participant was instructed to start and ending when all circles were marked and the pen was brought back to the index card. When marks were not placed within the circles on the page, participants were not allowed to repeat these trials. Participants were instructed to perform this task as quickly as possible, but do not sacrifice accuracy for speed. Beyond this instruction, there was no restriction on how quickly or slowly each participant chose to perform the task.
Our instructions included the phrase “do not sacrifice accuracy for speed,” a common instruction in neuropsychological experiments. However, in the absence of any factor that would cause a systematic overestimation or underestimation of movement length or direction, the number of times a participant’s aiming movement does not reach the target area reflects both random error and targeting inaccuracy. Thus, throughout this manuscript, we will consider this spatial control outcome variable as primary a measure of accuracy, although it incorporates a precision component.
2.3. Analysis
Our two primary outcome measures were (1) time to complete the task measured in seconds and (2) hits, defined as the number of marks that were within the circles on each trial. Each participant completed the task six times for each condition (3 target circle diameters as well as left and right upper limb performance). We used a mixed-effects model with a dependent variable of completion time, a covariate of hits, a random effect of subject, and fixed effects of age group, hand used, circle size to evaluate main and 2-way interaction effects using SPSS v22. Reported means are estimated marginal means and 95% confidence intervals (95% CI). Values are rounded to two decimal places, three for p values.
3. Results
We found a significant main effect of group (F(1, 22.02) = 9.181, p = 0.006), hand (F(1, 830.99) = 343.65, p < 0.001), circle size (F(2, 831) = 537.1, p < 0.001), and the covariate hits (F(1, 836.17) = 15.44, p < 0.001), as well as an interaction between, group and circle size (F(2, 831.20) = 5.25, p = 0.005) and hand and circle size (F(2, 830.99) = 14.4, p < 0.001), but not an interaction between group and hand (F(1, 831) = 0.241, p < 0.624) (see Table 1 and Fig. 2).
Table 1.
Type III tests of fixed effects for mixed effects model with shown fixed factors and a random effect of subject. Time to complete task is dependent variable and number of hits is a covariate.
| Fixed effects | Numerator df | Denominator df | F | P |
|---|---|---|---|---|
| Intercept | 1 | 208.56 | 76.79 | <0.001 |
| Age Group | 1 | 22.03 | 9.18 | 0.006 |
| Circle Size | 2 | 831.49 | 537.1 | <0.001 |
| Hand | 1 | 830.99 | 343.65 | <0.001 |
| Hits | 1 | 836.17 | 15.44 | <0.001 |
| Age Group * Circle Size | 2 | 831.21 | 5.25 | 0.005 |
| Age Group * Hand | 1 | 831 | 0.24 | 0.624 |
| Circle Size * Hand | 2 | 830.99 | 14.4 | <0.001 |
Fig. 2.
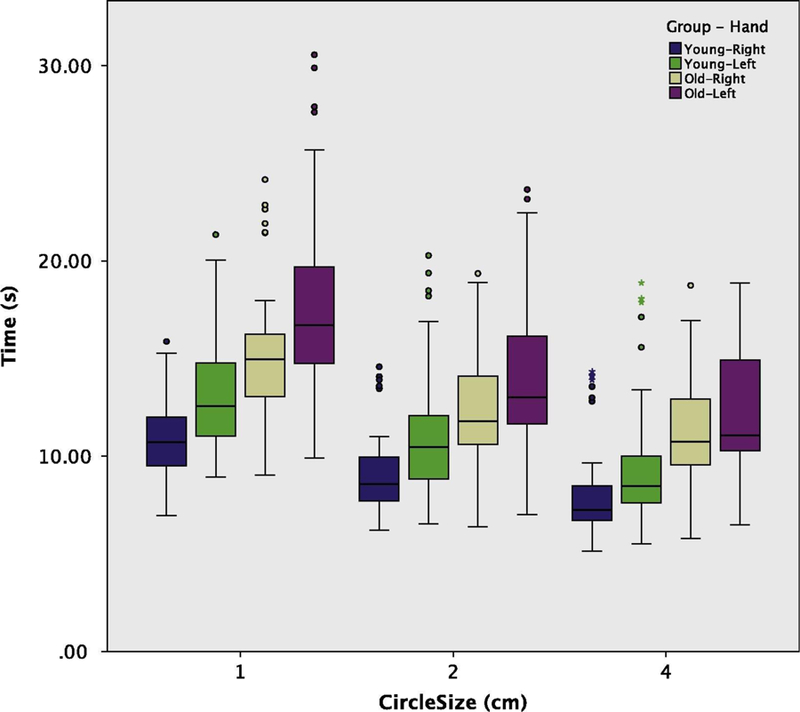
Box and Whiskers plot of task completion time (s) by circle size comparing younger and older groups and hand used.
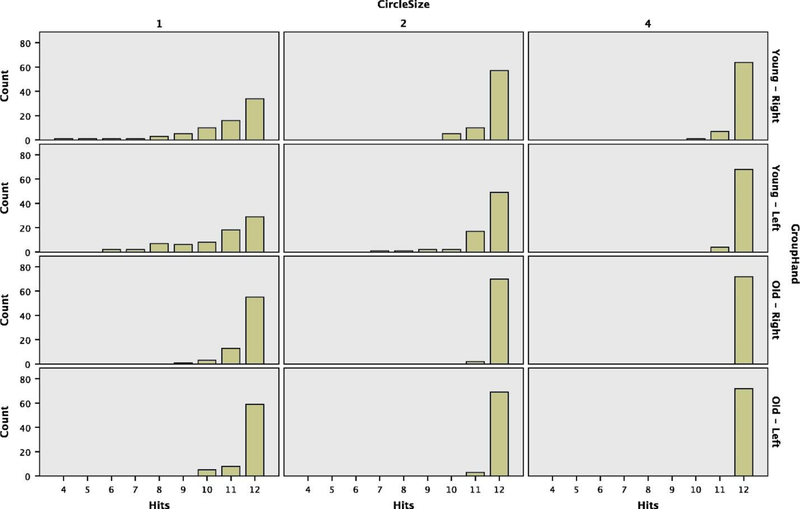
The overall estimated marginal mean for the time to complete the task was 11.93 s (95% CI: [10.8, 13.06]). Younger participants completed the task faster than older participants (mean difference of −3.29 s, 95% CI: [−5.56, −1.04]). Using the right hand resulted in a faster completion time than the left hand (mean difference of −1.8 s, 95%CI: [−1.99, −1.6]). The smaller the circle, the slower subjects completed the task (1 cm circles mean 14.27 s, 95%CI: [13.13, 15.4]; 2 cm circles mean 11.46, 95% CI: [10.32, 12.56]; 4 cm circles mean 10.05, 95% CI: [8.913, 11.185]) (see Table 2 for pairwise comparisons). Note that the younger subjects, while completing the task faster on average, also committed the majority of errors. For the older group, participants successfully landed 12 out of 12 hits in 91.9% of trials, but only 69.7% of trials by younger participants were errorless (12 hits out of 12). This is accounted for as our statistical model includes hits as a covariate (estimated effect of 0.279 s, 95% CI: [0.14, 0.42]). Also, as expected, the majority of misses occurred with the smallest circle and when participants were using their left hand, although older participants had very few trials with low hit counts (see Table 2 and Fig. 3).
Table 2.
Pairwise comparisons for fixed effects.
| Pairwise comparisons | Mean Δ | Std. Err. | df | P | 95% C1 | |
|---|---|---|---|---|---|---|
| Age Group | ||||||
| Young | Old | −3.23 s | 1.09 | 22.03 | 0.006 | [−5.56,1.04] |
| Hand used | ||||||
| Right | Left | −l.8 s | 0.1 | 831 | <0.001 | [−1.99,1.60] |
| Circle Size (Diameter) | ||||||
| 1 cm | 2 cm | 2.81 s | 0.13 | 831.6 | <0.001 | [2.50, 3.10] |
| 4 cm | 4.22 s | 0.13 | 831.92 | <0.001 | [3.90, 4,53] | |
| 2 cm | 4 cm | 1.41 s | 0.12 | 831.04 | <0.001 | [1.13,1.70] |
Note: Reported p is Bonferrom adjusted for mulhple comparisons.
Fig. 3.
Histograms of hits (out of maximum of 12 per trial) by circle size comparing younger and older groups and hand used.
Turning our attention to the significant interactions (see Table 3), we find that the older participants not only were slower overall and that smaller circles resulted in slower task completion times, but that the slowing of older participants, when compared to the younger participants, increased with greater demands for precision and accuracy (i.e., smaller circle diameter). As mentioned above, both groups performed slower with their non-dominant hand, but there was no interaction between age group and hand, with the mean difference between hands being 1.84 s (95% CI: [1.57, 2.11]) and 1.75 s (95% CI: [1.48, 2.02]) for younger and older age groups, respectively. Finally, the more challenging the task the more the use of the non-dominant left hand slowed the task completion, with mean Right-Left differences of −2.46 s, −1.74 s, and −1.19 s for 1, 2, and 4 cm circles, respectively.
Table 3.
Pairwise comparisons for significant interactions (Bonferroni corrected). Redundant rows removed for brevity.
| Pairwise comparisons | Mean Δ | Std. Err. | df | P | 95% C1 | ||
|---|---|---|---|---|---|---|---|
| Age Croup | Circle Size | ||||||
| Young | 1 cm | 2 cm | 2.49 s | 0.18 | 831.73 | <0.001 | [2.05, 2.92] |
| 4 cm | 3.85s | 0.19 | 832.16 | <0.001 | [3.4, 4.31] | ||
| 2 cm | 4 cm | 1.36 s | 0.19 | 831.07 | <0.001 | [0.96, 1.77] | |
| old | 1 cm | 2 cm | 3.12s | 0.17 | 831.05 | <0.001 | [2.72, 3.53] |
| 4 cm | 4.59 s | 0.17 | 831.06 | <0.001 | [4.13, 4.99] | ||
| 2 cm | 4 cm | 1.47s | 0.17 | 830.99 | <0.001 | [1.06, 1.87] | |
| Circle Size | Age Croup | ||||||
| 1 cm | Young | Old | −3.76 s | 1.1 | 22.39 | 0.002 | [−6.03,−1.48] |
| 2 cm | Young | Old | −3.12 s | 1.1 | 22.705 | 0.009 | [−5.39,−0.85 |
| 4 cm | Young | Old | −3.02 s | 1.1 | 22.683 | 0.011 | [−5.29,−0.75 |
| Circle Size | Hand Used | ||||||
| 1 cm | Right | Left | −2.46 | 0.17 | 830.99 | <0.001 | [−2.79,−2.13] |
| 2 cm | Right | Left | −1.74 | 0.17 | 831 | <0.001 | [−2.07,−1.41] |
| 4 cm | Right | Left | −1.19 | 0.17 | 830.99 | <0.001 | [−1.52,−0.86] |
| Hand Used | Circle Size | ||||||
| Right | 1 cm | 2 cm | 2.45 | 0.17 | 831.34 | <0.001 | [2.03, 2.86] |
| 4 cm | 3.59 | 0.I8 | 831.44 | <0.001 | [3.17, 4.01] | ||
| 2 cm | 4 cm | 1.14 | 0.17 | 831 | <0.001 | [0.74, 1.54] | |
| Left | 1 cm | 2 cm | 3.16 | 0.17 | 83I.28 | <0.001 | [2.75, 3.58] |
| 4 cm | 4.85 | 0.I8 | 831.56 | <0.001 | [4.43, 5.23] | ||
| 2 cm | 4 cm | 1.69 | 0.17 | 831.05 | <0.001 | [1.29, 2.11 | |
4. Discussion
Regardless of age, participants exhibited a speed-spatial control trade-off, such that both groups performed the task more slowly as the circle diameter decreased in size and the demand for precision and accuracy increased. This relationship is consistent with Fitts’s law. Whereas overall the older participants took more time to perform these target directed movements, when compared to young adults, the older adults demonstrated greater slowing as the target circle diameter decreased in size. This increase of slowing of the older participants, however, was associated with better performance than that exhibited by younger adults, who did not slow their actions as much as the older participants and had greater declines in their hit rate. This finding suggests that older adults derived benefit from slower responses during the more demanding task involving aiming movements toward circles with small target diameters. In addition, all participants were instructed that they should “not sacrifice accuracy for speed.” Therefore, it is possible that, as compared to the younger adults, the older adults adhered more strongly to the instruction to maintain precision at the expense of speed.
This may reflect a risk bias with age. For example, West, Tiernan, Kieffaber, Bailey, and Anderson (2014) examined age-related differences in the spatiotemporal distribution of event-related brain potentials (ERPs) related to feedback processing in a virtual blackjack game. Their data revealed that older adults were less risk seeking than younger adults both within and across trials. The older adults were using a “playing it safe strategy” (Van Halewyck et al., 2015). West et al. also found age-related differences in the amplitude of several ERP components that were localized to the anterior and cingulate and the inferior and medial frontal cortices. As mentioned above, movement toward a target is typically composed of an initial open loop movement followed by a closed loop movement (Haaland & Harrington, 1989). Thus, in our task an open loop movement probably occurred as the participants initially brought the pen, held by the left or right hand, from the index card toward the circle on the stimulus page. However, as the pen approached the target, a closed loop portion of the movement may have permitted greater movement control to bring the pen to the target circle. The demand for more precise movement control increased as the diameter of the circle decreased. Marsden (1989) noted that in order to produce a ballistic movement, the initial agonist muscle necessary for the movement must be activated with the appropriate amplitude and duration, and this initial agonist activation must be followed by a precise sequence of antagonist and agonist muscle activations.
Pratt, Chasteen, and Abrams (1994) studied the ability of healthy older and younger adults to perform aiming movements toward a target. The older adults performed the initial portion of the movement with reduced amplitude, and this was followed by a subsequent component of the movement with relatively increased duration and amplitude. We did not perform kinematic analysis to measure the amplitude and duration of the subcomponents of movement in our study. However, it is possible that the older adults in our study had reduced amplitude of the initial component followed by a longer duration of the second component of the movement toward the target circle, and this may explain why the older adults performed the task more slowly than the younger adults. Furthermore, since the final portions of the movement include the closed loop corrective component necessary to reach the target, increased duration of these later components of the movements may be related to increased precision by the older adults. In support of this postulate is the study of Warabi, Noda, and Kato (1986) who also demonstrated that healthy older adults revealed an increased duration of the final closed loop portion of their movements.
For those people who are right handed, the left hemisphere is dominant for praxis and thus controls “how” we perform purposeful skilled movements. In contrast, the right hemisphere appears to be dominant for action-intention and thus primarily controls “when” we start, continue, stop, and inhibit actions (Heilman, Valenstein, Rothi, & Watson, 2008, Chapter 10). Schaefer, Haaland, and Sainburg (2007) demonstrated that people with right hemisphere damage have reduced precision and impaired motor response deceleration during a task that requires performance of rapid finger movements toward a visual target. Although previous research has demonstrated a decline in right hemispheric function with aging (Coppi et al., 2014, Lapidot, 1987), we did not observe an impairment of final position precision in the older adults in our study. However, older adults may have adapted a compensatory strategy of increased slowing at the completion of the movement in order to maintain precision, and kinematic analysis is needed to test this hypothesis.
In Parkinson’s disease, reduced activity in the agonist muscle during the initial component of a ballistic movement may be one potential mechanism of bradykinesia (Hallett & Khoshbin, 1980). With aging, there is a tendency to produce aiming movements as sequential smaller movements rather than a single continuous movement, and this kinematic change may contribute to slowness of open loop movements with healthy aging (Yan, 2000).
Left hemisphere damage is associated with reduced speed and increased error during the initial component of an open loop movement (Haaland & Harrington, 1989). This study by Haaland and Harrington provides evidence for a role of the left hemisphere in the control of the initial open loop component of ballistic movements. A left hemisphere dominant mechanism for performance of open loop movements may explain the faster performance of open loop aiming movements with the right hand by both older and younger adults in our study. However, greater experience holding and using a pen with the right hand is another mechanism for the hand performance asymmetry that we observed. This right hand advantage increased as target circle diameter decreased, although performance with both hands slowed as target circle diameter decreased. Regarding changes in hand dominance with aging, prior studies have revealed both an attenuation of right hand dominance (Kalisch, Wilimzig, Kleibel, Tegenthoff, & Dinse, 2006) and an increase in right hand dominance (Mitrushina et al., 1995, Weller and Latimer-Sayer, 1985). We however, did not detect an age related decline in this intermanual asymmetry during performance of aiming movements and when Poston, Van Gemmert, Barduson, and Stelmach (2009) performed an aiming test in older and younger participants they also did not detect a change in intermanual asymmetry.
Aiming movements, such as the task we used in our study, include an initial open loop component and a closed loop corrective component. The primary difference between the two is that during the corrective component of closed loop movement, there is visual feedback to fine-tune the movement as the participant approaches the desired target. Previous researchers have suggested that a mesial frontal system controls endogenously evoked movements while a lateral frontal system controls movements produced in response to external stimuli (Goldberg, 1985, Nadeau and Heilman, 2007). However, mesial and lateral frontal systems may be differentially affected by healthy aging. Compared to younger adults, older adults have demonstrated greater reliance on visual feedback for motor performance after practicing an aiming task (Seidler-Dobrin & Stelmach, 1998). Changes in mesial versus lateral frontal networks with aging may help to provide another explanation for why, in our study, older adults maintained performance precision while younger adults sacrificed precision for speed during a motor task that requires attention to visual targets.
In addition another factor that may have altered performance with aging is changes in connectivity. Boisgontier (2015) wrote that, “As we age, movements become slower and inconsistent… These hallmarks of aging suggest a switch from predictive to reactive motor control”. Boisgontier examined evidence supporting the hypothesis that “motor aging is primarily determined by the early death of neurons in the cerebellum, a critical structure for predictive motor control.” In our study we did find that the movement of the older participants were slower, but we found that the older participants made fewer errors. In addition, in those patients who have disorders of the cerebellum one of their major problems is ataxia and ataxia would interfere with movement accuracy. Patients with a cerebellar lesion often have the most severe ataxia with rapid open looped movements and when they slow their actions they may become more accurate. Therefore, if forced to make movements at the same speed as the younger participants, it is possible that our older participants may have been less accurate than the younger participants. Van Halewyck et al. (2015) did test this postulate. They instructed younger and older adults to make discrete aiming movements under varying speed and accuracy constraints. Their results showed that older adults were physically able to produce fast primary sub-movements and that they demonstrated similar movement-programming capacities as young adults. Therefore, these results would appear not to be consistent with the cerebellar degeneration hypothesis. In addition, whereas many studies have found a reduction of connectivity with aging, Seidler et al., 2015 reported that that connectivity between the motor cortex and cerebellar lobule VIII with the putamen increased with age, providing evidence of greater interactivity across these motor networks with age.
The mesial frontal system not only initiates endogenously evoked movements, it also inhibits action. Previous research has demonstrated that right-sided mesial frontal brain lesions are associated with impaired control of motor inhibition (Floden & Stuss, 2006). Our study did not specifically test inhibitory control. However, the younger adults’ tendency to sacrifice precision for speed in our study may have been related to impulsivity or reduced inhibitory control. Frontal networks that control inhibition undergo development throughout the first three decades of life, and reduced inhibitory control in younger adults may reflect incomplete development of inhibitory networks (Sowell, Thompson, Holmes, Jernigan, & Toga, 1999). Conversely, some previous research has demonstrated a decrement in the control of inhibition in older adults (Schlaghecken, Birak, & Maylor, 2011). imaging research has demonstrated that with aging, mesial frontal systems are activated during both internally evoked actions and actions guided by external visual feedback (Heuninckx, Wenderoth, & Swinnen, 2010). Further research is needed to learn if this loss of functional specialization between mesial and lateral frontal lobe networks with aging represents a pathological process associated with mesial frontal dysfunction or if the increased mesial frontal lobe activation promotes enhanced inhibitory control during tasks that require action in response to external stimuli.
5. Conclusions
Starns and Ratcliff (2010) studied older versus younger participants using a perceptual task where the participants in each age group performed tasks such as letter and brightness discriminations. These investigators found that young participants attempted to balance speed and accuracy to achieve the most correct answers per unit time, whereas older participants attempt to minimize errors even if they must respond quite slowly to do so.
Since Jiménez-Jiménez et al. (2011) found a decrement of basic motor performance with age, in our study we want to examine the relation between spatial control and speed, but the participants were instructed, “Do not sacrifice accuracy for speed.” We found that our healthy older adults were less likely than younger adults to sacrifice accuracy for speed. Thus, at least in part, the older adults’ slowing may be a learned adaptive strategy. Further research is needed to learn if this improved performance with aging is the result of adaptive learning or an incidental benefit of age-induced reduced movement speed and the relative contribution of the open-and closed-loop components.
Acknowledgments
We would like to acknowledge all of the people who volunteered to take part in our study. This research is supported by grants and/or partial support from the Lawrence M. Goodman Scholarship, State of Florida Department of Elder Affairs, Alzheimer’s Disease Initiative, University of Florida Disorder Clinic and the McKnight Brain Research Foundation.
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- Acosta LM, Bennett JA, & Heilman KM (2014). Callosal disconnection and limb-kinetic apraxia. Neurocase, 20(6), 599–605. 10.1080/13554794.2013.826683. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP (2015). Motor aging results from cerebellar neuron death. Trends in Neurosciences, 38(3), 127–128. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC,Schneider JA, & Bennett DA (2012). Nigral pathology and parkinsonian signsin elders without Parkinson disease. Annals of Neurology, 71(2), 258–266. 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in old adults: the HAROLD Model. Psychology and Aging, 17, 85–100. Retrieved from: <http://www.ncbi.nlm.nih.gov/pubmed/11931290>. [DOI] [PubMed] [Google Scholar]
- Coppi E, Houdayer E, Chieffo R, Spagnolo F, Inuggi A, Straffi L, Comi G, & Leocani L (2014). Age-related changes in motor cortical representation and interhemispheric interactions: A transcranial magnetic stimulation study. Frontiers in Aging Neuroscience, 6, 209 10.3389/fnagi.2014.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, & Cabeza R (2002). Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neuroscience & BiobehavioralReviews, 26(7), 819–825. Retrieved from: <http://www.ncbi.nlm.nih.gov/pubmed/12470693>. [DOI] [PubMed] [Google Scholar]
- Fitts PM (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47(6), 381–391. 10.1037/h0055392. [DOI] [PubMed] [Google Scholar]
- Floden D, & Stuss DT (2006). Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience, 18(11),1843–1849. 10.1162/jocn.2006.18.11.1843 [DOI] [PubMed] [Google Scholar]
- Goldberg G (1985). Supplementary motor area structure and function: Review and hypothesis. The Behavioral and Brain Sciences, 8, 567–616. 10.1017/S0140525X00045167. [DOI] [Google Scholar]
- Haaland KY, & Harrington D (1989). Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia, 27(7),961–969. 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Hallett M, & Khoshbin S (1980). A physiological mechanism of bradykinesia. Brain,103(2), 301–314. 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Mendoza JE, Apostolos GT, & Heilman KM (2002). Lateralised motor control: Hemispheric damage and the loss of deftness. Journalof Neurology, Neurosurgery & Psychiatry, 73(5), 574–577.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E, Rothi LJG, & Watson RT (2008). Upper limb action-intentional and cognitive apraxic disorders. In Bradley WG, Daroff RB,Fenechel GM, & Jankovic J (Eds.),Neurology in clinical practice(5th ed.,pp. 121–132). Phila PA: Butterworth Heineman Elsevier. [Google Scholar]
- Heuninckx S, Wenderoth N, & Swinnen SP (2010). Age-related reduction in thedifferential pathways involved in internal and external movement generation. Neurobiology of Aging, 31(2), 301–314. 10.1016/j.neurobiolaging.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Jäncke L1, Mérillat S, Liem F, & Hänggi J (2015). Brain size, sex, and the aging brain. Human Brain Mapping, 36(1), 150–169. 10.1002/hbm.2261. Epub 2014 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Calleja M, Alonso-Navarro H, Rubio L, Navacerrada F,Pilo-de-la-Fuente B, Plaza-Nieto JF, Arroyo-Solera M, García-Ruiz PJ,García-Martín E, & Agúndez JA (2011). Influence of age and gender in motor performance in healthy subjects. Journal of Neurological Science, 302(1–2),72–80. 10.1016/j.jns.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, & Dinse HR (2006). Age-related attenuation of dominant hand superiority. PLoS ONE, 1,e9. Lapidot, M. B. (1987). Does the brain age uniformly? Evidence from effects of smooth pursuit eye movements on verbal and visual tasks .Journal of Gerontology, 42(3), 329–331. 10.1093/geronj/42.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD (1989). Slowness of movement in Parkinson’s disease. Movement Disorders, 4(1), S26–S37. 10.1002/mds.870040505. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Fogel T, D’Elia L, Uchiyama C, & Satz P (1995). Performance on motor tasks as an indication of increased behavioral asymmetry with advancing age. Neuropsychologia, 33(3), 359–364. [DOI] [PubMed] [Google Scholar]
- Morgan M, Phillips JG, Bradshaw JL, Mattingley JB, Iansek R, & Bradshaw JA (1994). Age-related motor slowness: Simply strategic? Journal of Gerontology,49(3), M133–M139. 10.1093/geronj/49.3.M133. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, & Heilman KM (2007). Frontal mysteries revealed. Neurology, 68(18), 1450–1453. 10.1212/01.wnl.0000264557.73283.a5. [DOI] [PubMed] [Google Scholar]
- Poston B, Van Gemmert AW, Barduson B, & Stelmach GE (2009). Movement structure in young and elderly adults during goal-directed movements of the left and right arm. Brain and Cognition, 69(1), 30–38. 10.1016/j.bandc.2008.05.002. Epub 2008 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL, & Abrams RA (1994). Rapid aimed limb movements: Age differences and practice effects in component submovements. Psychology and Aging, 9(2), 325–334. 10.1037/0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- Raw RK, Wilkie RM, Culmer PR, & Mon-Williams M (2012). Reduced motor asymmetry in older adults when manually tracing paths. Experimental Brain Research, 217(1), 35–41. 10.1007/s00221-011-2971-x. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D,Hardman J, Launer L, Masaki K, Tanner CM, & White LR (2004).Parkinsonian signs and substantia nigra neuron density in decendent elders without PD. Annals of Neurology, 56(4), 532–539. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2000). Aging and measures of processing speed. Biological Psychology, 54(1–3), 35–54. Retrieved from: <http://www.ncbi.nlm.nih.gov/pubmed/11035219>. [DOI] [PubMed] [Google Scholar]
- Sanes JN (1985). Information processing deficits in Parkinson’s disease during movement. Neuropsychologia, 23(3), 381–392. 10.1016/0028-3932(85)90024-7. [DOI] [PubMed] [Google Scholar]
- Schaefer S, Haaland KY, & Sainburg R (2007). Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain, 130(8), 2146–2158. Retrieved from: <http://www.ncbi.nlm.nih.gov/pubmed/17626039>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaghecken F, Birak KS, & Maylor EA (2011). Age-related deficits in low-level inhibitory motor control. Psychology and Aging, 26(4), 905–918. 10.1037/a0023832. [DOI] [PubMed] [Google Scholar]
- Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Mérillat S, & Jäncke L (2015). Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage, 108, 47–59. 10.1016/j.neuroimage.2014.12.023. Epub 2014 Dec 13. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, & Stelmach GE (1998). Persistence in visual feedback controlby the elderly. Experimental Brain Research, 119, 467–474. 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, & Toga AW (1999). In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience, 2(10), 859–861 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Starns JJ, & Ratcliff R (2010). The effects of aging on the speed–accuracy compromise: Boundary optimality in the diffusion model. Psychology and Aging,25(2), 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Fink GR, Mackay CE, & Lux S (2011). Gestalt perception and the decline of global precedence in older subjects. Cortex, 47(7), 854–862. [DOI] [PubMed] [Google Scholar]
- Van Halewyck F, Lavrysen A, Levin O, Boisgontier MP, Elliott D, & Helsen WF (2015). Factors underlying age-related changes in discrete aiming. Experimental Brain Research, 233(6), 1733–1744. [DOI] [PubMed] [Google Scholar]
- Varney NR, & Benton AL (1975). Tactile perception of direction in relation to handedness and familial handedness. Neuropsychologia, 13(4), 449–454. 10.1016/0028-3932(75)90068-8. [DOI] [PubMed] [Google Scholar]
- Warabi T, Noda H, & Kato T (1986). Effect of aging on sensorimotor functions of eye and hand movements. Experimental Neurology, 92(3), 686–697. 10.1016/0014-4886(86)90309-2. [DOI] [PubMed] [Google Scholar]
- Weller MP, & Latimer-Sayer DT (1985). Increasing right hand dominance with age on a motor skill task. Psychological Medicine, 15, 867–872. [DOI] [PubMed] [Google Scholar]
- West R, Tiernan BN, Kieffaber PD, Bailey K, & Anderson S (2014). The effects of age on the neural correlates of feedback processing in a naturalistic gambling game. Psychophysiology, 51(8), 734–745. [DOI] [PubMed] [Google Scholar]
- Yan JH (2000). Effects of aging on linear and curvilinear aiming arm movements. Experimental Aging Research, 26(4), 393–407. 10.1080/036107300750015778. [DOI] [PubMed] [Google Scholar]