Abstract
Advanced glycation end products (AGEs) represent a family of protein, peptide, amino acid, nucleic acid and lipid adducts formed by the reaction of carbonyl compounds derived directly or indirectly from glucose, ascorbic acid and other metabolites such as methylglyoxal. AGE formation in diabetes is of growing importance for their role as markers and potential culprits of diabetic complications, in particular retinopathy, nephropathy and neuropathy. Development of sensitive and specific assays utilizing liquid chromatography mass spectrometry with isotope dilution method has made it possible to detect and quantitate non-UV active AGEs such as carboxymethyl-lysine and glucosepane, the most prevalent AGE and protein crosslink of the extracellular matrix. Below we review studies on AGE formation in two skin biopsies obtained near the closeout of the Diabetes Control and Complications Trial (DCCT), one of which was processed in 2011 for assay of novel AGEs. The results of these analyses show that while several AGEs are associated and predict complication progression, the glucose/fructose-lysine/glucosepane AGE axis is one of the most robust markers for microvascular disease, especially retinopathy, in spite of adjustment for past or future average glycemia. Yet overall little biological and clinical information is available on glucosepane, making this review a call for data in a field of growing importance for diabetes and chronic metabolic diseases of aging.
Keywords: carbonyl stress, glycemia, HbA1c, nephropathy, neuropathy, retinopathy, methyglyoxal
Introduction
More than 35 years have elapsed since the landmark discovery of the clinical significance of hemoglobin A1c [1], a discovery that has unequivocally helped establish the causal relationship between hyperglycemia, the pathogenesis of microvascular disease and the accelerated progression of selected cardiovascular disease endpoints in both type 1 and type 2 diabetes [2–6]. Concomitant research during these years has successfully implicated glucose as a pathogenetic factor for diabetic retinopathy, nephropathy, neuropathy and a co-factor in cardiovascular disease in various animal models. In a nutshell, it is widely accepted that diabetic complications are the result of an intricate combination of oxidative and carbonyl stresses and inflammatory signals that can be pharmacologically efficiently prevented in the experimental animal [7]. Paradoxically however, except for sustained lowering of glycemia over many years (DCCT, UKPDS), few to none of the prevailing pharmacological anti-complication paradigms [aldose reductase activation, oxidative stress, advanced glycation end products (AGE) formation, protein kinase C (PKC) activation, inflammation] have so far convincingly succeeded delaying complications in the human [7].
Multiple reasons can be invoked as a reason why a given drug was found unable to normalize the oxidant and carbonyl stress or their biological consequences in diabetic humans. These may include poor bioavailability, poor therapeutic window and thus inability to achieve the necessary therapeutic dose, insufficient complication event rate linked to overall improved management of diabetes, yet unrecognized pathogenetic mechanisms and genetic factors, or simply the inability to carry out the drug trial long enough until efficacy is demonstrable. The latter proposition is supported by observations which show that euglycemia for 5 years was insufficient for improvement of morphological lesions of diabetic nephropathy and, instead, 10 years of euglycemia were needed [8].
Below, we develop arguments in support of the hypothesis that glycation damage to the extracellular matrix by glucose itself, and in particular glucosepane crosslinks, are among the factors that may explain the progression and slow reversibility of diabetic complications in the human. The latter emphasis is based on the belief that diabetic rodents, while adequate models of hyperglycemic cellular stress are not adequate for probing the intrinsic role of the glucose-damaged extracellular matrix (ECM) in diabetic complications, because of the duration of the glycemic stress is too short for quasi irreparable damage to occur.
The first part of this review explores the molecular properties of glucosepane, its in vitro and in vivo mechanism of formation and inhibition, its distribution in animal and human tissue and how diabetes affects its levels. The second focuses on the recently uncovered clinical relationship between glucosepane, other skin AGEs and the presence of diabetic complications, the results of which lead us to propose a key role for glucosepane in the pathogenesis of diabetic complications.
Glucosepane: chemistry and mechanism of formation
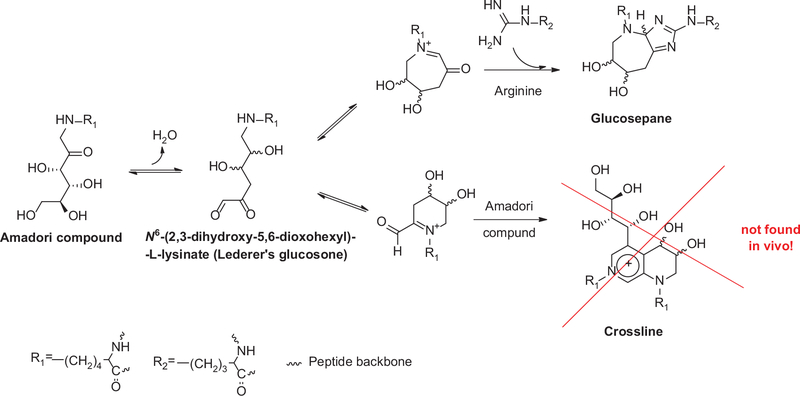
Glucosepane was discovered and so named by Lederer and colleagues, who isolated it from a model reaction of N-α-t-boc-L-lysine and N-α-t-boc-L-arginine and D-glucose at 37°C [9]. The compound has a molecular weight of 647 Da and constitutes a mixture of four diastereoisomers with a 7-membered ring made of glucose that crosslinks the two amino acid side chains of lysine and arginine (Figure 1). Glucosepane is labile to conventional acid hydrolysis and therefore can only be quantitated in glucose exposed proteins upon exhaustive mild enzymatic digestion at 37°C to release the free molecule. The fact that glucosepane absorbs UV light only at short wavelengths (λmax= 251 and 253 nm) makes it inconvenient for assay by HPLC using absorption spectroscopy, and therefore the method of choice is liquid chromatography mass spectrometry with isotope dilution technique (LC/MS) [9].
Figure 1.
Mechanism of formation of glucosepane from N6-(2,3-dihydroxy-5,6-dioxohexyl)-L-lysinate precursor.
During in vitro incubation with free fructosyl-lysine in phosphate buffer significant amount of crossline are formed that are not detected under physiological conditions with proteins.
At first, the mechanism of glucosepane formation appears to be rather straight forward, proceeding via the key intermediate, N6-(2,3-dihydroxy-5,6-dioxohexyl)-L-lysinate (Lederer’s glucosone [10]) that can be trapped with ortho-phenylenediamine (OPD) [11]. We recently proposed that this intermediate is common to both glucose-derived crosslinks, i.e., crossline and glucosepane (Figure 1). Crosslines are isomeric lysine-lysine crosslinks that have been described several years ago and immunochemically detected in glucose exposed proteins and in vivo [12, 13]. However, our investigations using mass spectrometry failed to detect these in protein digest from biological tissue, making it highly unlikely that crosslines are physiological crosslinks [14]. Similarly to the recently described AGE glucold, crosslines represent a class of glucose-derived AGEs that can form only in vitro under very high glucose concentrations from free but not protein-bound amino acids.
One important point to emphasize about glucosepane is that it forms entirely non-oxidatively from glycated lysine residues (Amadori products) via a series of water elimination reactions. Thus, this form of carbonyl stress would proceed anaerobically in diabetes and be insensitive to antioxidants! Interestingly, Nasiri et al. found, based on theoretical considerations, that glucosepane might possibly originate from a combination of methylglyoxal and glyceraldehyde [15]. In unpublished preliminary studies, we have found that glyceraldehyde, but not methylglyoxal, generates glucosepane-like m/z species in our LC/MS chromatogram. This supports the notion that some glucosepane might originate from this triose sugar and explain why non-carboxymethyl-lysine (CML) immuno-reactive epitopes were detected in diabetes when glyceraldehyde modified protein is used as an immunogen [16]. However, given the small quantities of free glyceraldehyde found in vivo [normal urine contains 0.04 mg glyceraldehyde/mg creatinine, corresponding to 20–80 mg eliminated per day (data extracted from [17])], it appears highly unlikely that glyceraldehyde would significantly contribute to the glucosepane pool forming in vivo. Both this question and the potential cross-reactivity of “non- CML” antibodies cross-reacted with native glucosepane will need to be clarified.
Glucosepane in biological systems: effects of diabetes on tissue levels and protein turnover rate
Relatively little information is yet available on tissue levels of glucosepane (Table 1) and sites of formation in proteins. In the collagen-rich extracellular matrix, glucosepane increases with age [18]. In normal human skin levels increase curvilinearly to reach about 2000 pmol/mg collagen at 100 years. However, in purified glomerular basement membrane (GBM), levels are in steady state at 200–400 pmol/mg collagen, reflecting ongoing synthesis and degradation of the GBM. To date these levels are the highest of any known AGE. In lens, however, while levels also increase with age, they are 4–5-fold lower than in collagen, reflecting overall lower glucose levels than in the extracellular matrix [19].
Table 1.
Glucosepane levels in various human and animal tissues.
| Tissue | Species condition | Glucosepane levels | References |
|---|---|---|---|
| Skin collagen | Human (age-related increase) | Control: up to 2000 pmol/mg at 100 years | Sell [18, 19] |
| Diabetes: up to 4000 pmol/mg | |||
| Skin collagen | Human (30–40 years old) | Control mean: 1500 pmol/mg | Monnier [20] |
| Diabetes mean: 2700 pmol/mg | |||
| Diabetic mean: 2400 pmol/mg (good control) | |||
| Skin collagen | Mole rat | Up to 250 pmol/mg at 10 years | Dammann [21] |
| Tendon collagen | Rat (12 months) | Control mean: 100 mg/pmol | Mustata [22] |
| STZa diabetes mean: 300 pmol/mg | |||
| Lens crystallins | Human | Up to 400 pmol/mg protein at 100 years | Fan [19] |
| 132–241 pmol/mg | Biemel [23] | ||
| Kidney (glomerular basement membrane collagen) | Human | Mean: 250 pmol/mg collagen (no increase with age) | Sell et al. [18] |
| Diabetes: up to 900 pmol/mg | |||
| Serum proteins | Human | Control: 12–20 pmol/mg protein Up to 43 pmol/mg (diabetes) | Biemel et al. [23] |
STZ, streptozotocin.
Tissue levels of glucosepane are expected to be strongly dictated by glucose levels and turnover rate of the protein, as previously reported by Verzjil et al. [24]. However, yet poorly understood is the actual role of glucosepane in the expected age- and diabetes-related decrease of collagen digestibility that was observed by Schnider and Kohn [25, 26]. The levels encountered in diabetes represent about 1%−1.2% (mol/mol) modifications of total arginine and lysine residues, respectively. However, assuming a molecular mass of 100,000 kDa for a single strand of triple helical collagen, this translates into approximately one crosslink for every two and five collagen molecules in diabetic and non-diabetic individuals, respectively. From the work of Vater et al. [27], it is estimated that this degree of crosslinking should impose a 30- and 45-fold decrease in collagen digestibility in diabetic skin. The clinical relevance of this estimate is discussed later.
Other important factors that dictate the extent of glucosepane formation include: 1) the number of lysine glycation hot spots at which the Amadori compound are preferentially formed in the protein; 2) the availability of target arginine residues at a distance of less than 7.5Ǻ [28] and preferentially at 5Ǻ [11] from the ε-amino group of lysine; and 3) the extent of surface exposure of target residues. Using ribonuclease as a model, Watkins et al. showed that only selective residues are glycated under physiological conditions [29] and that some of the site specificity is dictated by the microenvironment, in particular, the ability of a catalytic phosphate group to facilitate the Amadori rearrangement. In similar studies, we found that increasing phosphate and glucose concentration during in vitro incubation broadly increases fructose-lysine and glucosepane formation [14, 30]. Surprisingly, however, the low surface availability of K41 in ribonuclease A did not prevent intramolecular glucosepane formation with R39, although the very high surface accessibility of K1 allowed it to form multiple intermolecular glucosepane and DODIC (3-deoxyglucosone dimer imidazole) crosslinks with R39 and R85 [30]. Thus, the extent of surface exposure of lysine residues is of relative importance.
Inhibition of glucosepane formation
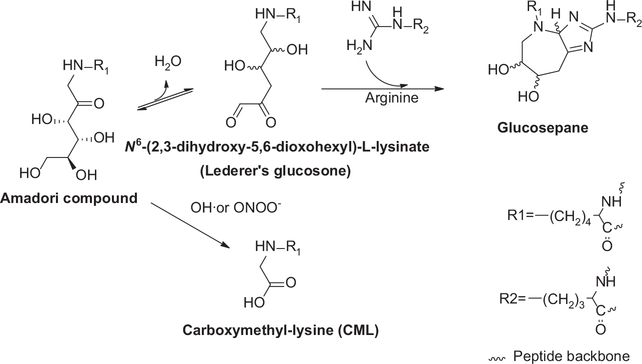
A potent physiological inhibitor of protein crosslinking is the oxidation of the Amadori product. This proposition was originally made by Baynes and colleagues who suggested that CML formation would effectively prevent crosslinking [31]. Thus, incubation of proteins in high glucose in presence of chelating agents to suppress glucose autoxidation and glyoxal formation may tilt the reaction from CML (or glyoxal hydroimidazolone) to glucosepane (Figure 2), whereby it remains to be seen which form of damage is worse for a given target protein [32].
Figure 2.
Glucosepane formation can conceivably be blocked under oxidative conditions, whereby attack of fructose-lysine by hydroxyl radical or peroxinitrite generates carboxymethyl-lysine (CML) incapable of engaging arginine residues.
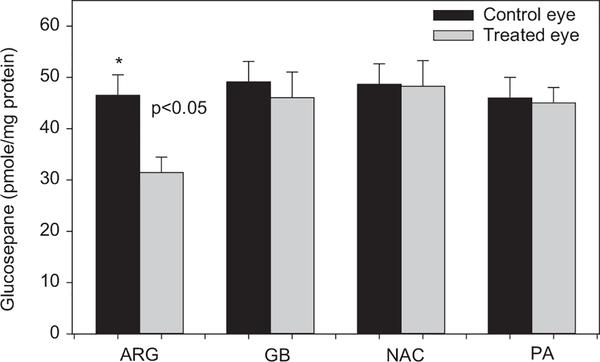
The necessity of a guanidino group for glucosepane formation suggests that pharmacological agents with such functional groups should be able to act as inhibitors. Formation of glucosepane analog during incubation of fructosyl-ε-aminocaproic acid with γ-guanidino butyric acid was most potently inhibited by o-phenylene diamine (OPD) (IC50 << 1 mM), an inhibitor of the glucosepane precursor N6-(2,3-dihydroxy-5,6-dioxohexyl)-L-lysinate (Lederer’s glucosone) [33]. However, while OPD itself is a useful reagent for trapping and investigation of Maillard reactive intermediates [10, 11, 34], it is too toxic for in vivo applications. Interestingly, aminoguanidine and pyridox-amine, both of which have beneficial effects in rodent models of diabetes [35, 36] were inhibitors of glucosepane at low mM concentrations [33]. It is not known whether any of these drugs inhibit glucosepane in vivo. However, we have shown that topical application of arginine to the eye for several weeks resulted in 30% inhibition of glucosepane formation in lens crystallins from non-diabetic hSVCT2 mice [37] (Figure 3). Thus, the feasibility of inhibiting glucosepane formation in vivo is clearly established.
Figure 3.
Inhibition of glucosepane formation in lens crystallins from mice treated with 2% arginine drops in the eye for 8 weeks. ARG, arginine; GBA, gamma aminobutyric acid; NCA, N-acetyl cysteine; PA, penicillamine. Lens crystallins were extracted and processed for AGE assay by LC/MS as described (Fan et al. [37]).
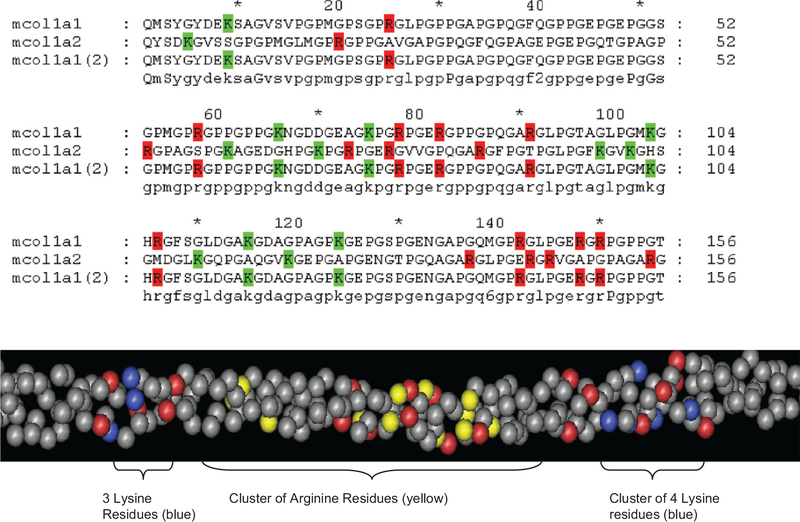
While the above results implicate free arginine as physiological inhibitor of glucosepane mediated protein crosslinking, an investigation of potential glucosepane formation sites in triple helical collagen has revealed a highly unexpected finding. Figure 4 shows a partial sequence of triple helical type 1 collagen in which we have highlighted lysine and arginine residues in blue(green) and yellow(red) color, respectively. The stunning outcome of this analysis is that evolution seems to have disfavored clusters of positive charges made of mixed lysine-arginine residues, suggesting that such proximity (<8Ǻ!) would have resulted in glucosepane- and perhaps methylglyoxal-derived MODIC crosslinks that would not be compatible with life. In that regard, Dobler et al. noted that methylglyoxal-modified arginine residues in collagen are associated with anoikis [38]. Therefore, the nagging question emerging from the above finding is how to explain the very high levels of glucosepane in the highly insoluble collagen fraction of human skin. Is glucosepane mostly in the telopeptide region that was not digested by pepsin? Does glucosepane form mostly in interfibrillar strands of collagen, acting therefore primarily as an intermolecular instead of intramolecular crosslink? Clearly these important questions may need to be addressed in the broader context of glucose toxicity in the complications of diabetes and aging.
Figure 4.
Clustering of lysine and arginine residues In type I collagen.</P/> Top: Sequence alignment of type 1 collagen showing lysyl and arginyl residues highlighted with green and red color, respectively. Surprisingly, separate clustering of lysyl and arginyl residues is observed throughout the sequences such that no lysines are sufficiently close to arginines to allow formation of glucosepane crosslinks. Bottom: Spacial molecular model of mouse type collagen I triple helix showing clustering of lysine (blue) separately from arginine (yellow) residues at distances > 5–8 Ǻ, making it virtually impossible for glucose to form intramolecular crosslinks. Red color indicates negatively charged residues.
Clinical significance of glucosepane
Very little clinical information on glucosepane is yet available. In 1993 we participated in an ancillary study of the Diabetes Complications and Control Trial (DCCT) that included 216 individuals with Type I diabetes, 122 of which underwent intensive control of glycemia, while 93 were treated conventionally over a period of 7–10 years. Two biopsies from this cohort were obtained in 1993, the first of which was immediately analyzed and the second of which was stored for 16 years at −80°C under N2. In the initial study, acid hydrolysis was used to release furosine (fructose-lysine), CML, and pentosidine that were quantitated by HPLC [20]. Collagen-linked fluorescence at 440 nm, acid and pepsin solubility of collagen were also determined. In 2011, the second biopsy was processed by enzymatic digestion to release and determine the newly discovered acid labile AGEs glucosepane, methylglyoxal and glyoxal hydroimidazolones (MG-H1 and G-H1), as well as carboxyethyl-lysine (CEL), by LC/MS with isotope dilution essentially according to Thornalley et al. [39]. Thus, the initial set of data from 1999 included six variables [20] to which an additional four were added in 2012 [40]. Below, we review these studies in light of the new data stemming from second biopsy, in particular the question of how glucosepane and the other added AGEs impact on the association between collagen AGEs, mean and cumulative glycemia and the progression of diabetic complications.
Correlation between AGE and long-term cumulative glycemia
The original analyses revealed strong and highly significant associations between each collagen AGE/solubility parameter and mean cumulative glycemia measured by averaging HbA1c values over the entire DCCT duration (Table 2) [20]. Among the new glycation products, only fructose-lysine and glucosepane were associated with past glycemia. The data show a higher R2 value for fructose-lysine (FL) than furosine, suggesting enzymatic hydrolysis to release FL is better than acid hydrolysis, which destroys 30%−40% fructose-lysine during its conversion to furosine. Vice-versa, we would have expected much higher R2 values for the more stable glucosepane than FL, but it is likely that some glucosepane was incompletely released and lost in the highly insoluble, undigestible collagen fraction that was discarded after centrifugation. Nevertheless, these data clearly show that the fructose-lysine/glucosepane tandem are the strongest modifications associated with past cumulative glycemia. This could be highly relevant for the observation of glycemic memory, i.e., the persistent progression of complications in spite of prolonged good control of glycemia after a previous period of greater hyperglycemia.
Table 2.
Correlation between collagen AGE, solubility and past cumulative glycemia measured by averaging HbA1c values over the entire duration of the DCCT.
| Collagen parameter | R2, % | p-Value |
|---|---|---|
| Fructose-lysinea | 36.8 | <0.0001 |
| Furosine | 19.4 | <0.0001 |
| Glucosepanea | 23.5 | <0.0001 |
| CML | 16.1 | <0.0001 |
| Pepsin-soluble collagen | 15.0 | <0.0001 |
| Pentosidine | 8.9 | <0.0001 |
| Fluorescence | 6.5 | <0.0001 |
| MG-H1a | 0.4 | NS |
| CELa | 0.2 | NS |
| G-H1a | 0.1 | NS |
New AGEs measured in enzymatic collagen digest. NS, not significant.
Effect of controlling glycemia on levels of glucosepane and other AGEs
In the original skin biopsy, intensive control of glycemia markedly suppressed skin levels of furosine, CML, pentosidine, fluorescence, pepsin and acid solubility of collagen in both the primary (no retinopathy at baseline) and secondary (background retinopathy present at baseline) DCCT cohort, except for acid soluble collagen that was not improved in the secondary cohort. Of the new markers, both fructose-lysine and glucosepane were improved by lowering glycemia, while no change was observed in MG-H1, G-H1 and CEL levels. However, the improvement of glucosepane in the secondary cohort did not reach significance. This latter finding may be due in part to the limited number of participants. However, it may also reflect the longer diabetes duration and glucose exposure of the secondary DCCT cohort, whereby the slow reversibility may represent an important mechanism for the glycemic memory of molecular damage in diabetic complications [41].
Relationship between AGE levels and past progression of diabetic microvascular disease
Below we are comparing the originally published set of AGE and collagen markers [20] with those recently obtained [40].
Retinopathy
Among the original markers, retinopathy in the conventional DCCT group was associated with CML and pepsin soluble collagen (p < 0.05). When all six collagen variables were investigated “as a complete set”, they were moderately associated with all three retinopathy metrics, i.e., sustained ≥ 3 step progression, sustained ≥ 3 microaneurysms and rate of change of worsening on the Early Treatment of diabetic Retinopathy Scale EDTRS (p < 0.03). Following adjustment for mean DCCT HbA1c only sustained ≥ 3 microaneurysms remained significant (p < 0.001). In the recent study, the odds ratio of having a sustained ≥ 3-step microaneurysms progression was associated with higher glucosepane levels (2.47 nmol/mg increase, p=0.003). Moreover, when backward selection was used to investigate the association between the new AGEs with retinopathy, glucosepane was selected as being the AGE most strongly associated with sustained ≥ 3-step progression (p=0.003) and sustained ≥ 3 microaneurysms (p<0.001). This finding applied only to the primary cohort though, i.e., the cohort that had no retinopathy or renal complications at DCCT baseline.
Nephropathy
The original study showed that nephropathy was associated with fluorescence, furosine and pentosidine (each p< 0.05). In the conventional treatment group, the original set of markers was associated with previous progression of nephropathy in the conventional treatment group, i.e., an association that remained robust despite adjustment for DCCT mean HbA1c. In the new study the odds of developing neuropathy were 5.31 per nmol/mg increase in glucosepane (p<0.0001). Backward model reduction selected glucosepane, G-H1, and MG-H1 hydroimidazolones together as the most significant risk factors for nephropathy (p<0.0001) [40].
Neuropathy
The original study revealed that neuropathy was associated with almost all of the markers, i.e., acid-soluble collagen, fluorescence, furosine, pentosidine and CML. As for nephropathy, the combined set of markers was robustly associated with neuropathy, even after adjusting for HbA1c (p<0.004). The odds ratio per nmol/mg increase of skin glucosepane was 4.42 per nmol/mg increase (p=0.015) for neuropathy progression.
AGEs as long-term predictors of microvascular disease
Five of six of the markers in the original set, i.e., furosine, CML, fluorescence and pepsin soluble collagen were predictors of the subsequent 10-year risk of retinopathy and microalbuminuria progression at p<0.0001–0.0008. Multiple logistics regression analysis selected furosine and CML, alone and combined, as the most robust predictors of retinopathy progression in spite of adjustment for mean glycemia during the DCCT and Epidemiology of Diabetes Interventions and Complications (EDIC). Moreover, the combination of skin furosine and HbA1c above the third tertile (Q3) increased four-fold the odds of developing retinopathy compared to skin furosine levels below Q3 [42].
Nephropathy progression was similarly predicted by the combination of furosine and CML, both of which explained most of the variation in risk attributable to the whole collagen panel and independent of the mean DCCT and EDIC A1c.
Preliminary studies presented at the American Diabetes Association meeting of 2012 revealed that addition of the four new collagen markers to the existing set of six markers strengthened the ability of skin AGEs to predict progression of all complications in spite of adjustment for DCCT and EDIC A1c.
Interdependence between tissue AGEs and glycemia (HbA1c) as risk predictors of microvascular disease
The strong association between glucose and the development of diabetic complications is solidly established both clinically and in a multitude of experimental animal models. The above data, however, raised an important question of which covariables most strongly influence the association between AGEs and complications in a hypothetical regression function:
Complication progression = f ( tissue AGE, HbA1c, age, diabetes duration, gender, pre-existing complications, metabolic factors, inflammatory factors....).
Below we summarize the major results of the effects of adjustments of the mean DCCT glycemia, i.e., glycemia measured up to the biopsy when assessing the past progression of complications, and the mean DCCT and EDIC HbA1c when assessing the future complication progression risk in the years post-biopsy. We also looked at the question of whether the well-documented association between HbA1c and complication progression is mediated by the effects of skin AGEs. This was done using multivariate regression analyses to see if the association between A1c and complication was weakened after adjustment for AGEs.
Importantly all AGEs data used in these analyses are already adjusted for age and diabetes duration.
The data in Table 3 present the effects of adjustment of AGEs for mean glycemia during the DCCT (for past and future progression) and EDIC (for future progression). At first glance, it is readily apparent that very few of the AGEs as risk factors were weakened by adjustment for mean DCCT HbA1c. This means that the skin AGEs were risk factors independent from mean glycemia during the DCCT and possibly lying downstream from HbA1c in the pathogenetic sequence. Only four of these associations became non-significant (NS). Surprisingly, adjustment for the mean DCCT HbA1c abolished the originally significant association between glucosepane/fructose-lysine and neuropathy. This suggests that A1c may be a mediator in the relationship of AGEs with neuropathy complication. However, the original set of six collagen markers (furosine, CML etc) was not weakened by the DCCT HbA1c adjustment. Similarly, adjustment for the EDIC A1c had no major impact on the ability of skin AGEs to predict the prospective risk.
Table 3.
AGEs as risk markers of complications: effect of adjustment for mean HbA1c during the DCCT and EDIC on associations between AGE and past or future 10-year progression of complications.
| Complication | Risk marker | p-Value after adjustment for HbA1c during DCCT (past progression) | p-Value after adjustment for HbA1c during DCCT (future 10-year progression) | p-Value after adjustment for HbA1c during EDIC (future 10-year progression |
|---|---|---|---|---|
| Retinopathy | 6 original collagen variablesa | < 0.001 (≥ 3-step MA) | <0.0001 | <0.0001 |
| NS (ΔRate ETDRS) | ||||
| Furosine | <0.0001 | <0.0001 | <0.0001 | |
| CML | <0.0001 | <0.0001 | <0.0011 | |
| Glucosepane | 0.025 | 0.025b | pending | |
| Fructose-lysine | NS | NS | pending | |
| Nephropathy | 6 original collagen variables | <0.006 (AER> 40 mg/24h) | N/A | |
| Furosine and CML | <0.019 (ΔRate of AER) | 0.0016 | 0.0079 | |
| Furosine | 0.0014 | 0.0079 | 0.0076 | |
| CML | 0.0123 | 0.0011 | ||
| Glucosepane | 0.001 | pending | ||
| Fructose-lysine | 0.005 | pending | ||
| Neuropathy | 6 collagen variables | < 0.001 clinical neuropathy | N/A | pending |
| <0.004 median | ||||
| Glucosepane | NCV | |||
| Fructose-lysine | NS | |||
| NS |
The original markers include furosine, CML, pentosidine, fluorescence, acid and pepsin collagen solubility. Pending: the 13–16 year prospective data are currently being investigated
Significant for ≥ 3-step MA in DCCT. NS, not significant.
However, the strong associations between mean DCCT glycemia and complications, were abolished or weakened by adjustment for skin fructose-lysine and CML (Table 4), strongly suggesting that the effects of glycemia on the subsequent 10 years of complications progression were more strongly mediated by the AGEs rather than past average glycemia. Not surprisingly, skin AGEs were not able to weaken the effects of future mean glycemia on complications as represented by mean EDIC A1c (Table 4). Studies are in progress to find out whether adjustments for glucosepane, MG-H1, G-H1 and CEL can abolish the effects on long-term glycemia on complication progression.
Table 4.
HbA1c as a 10-year prospective risk factor of complications: effect of adjustment for skin collagen AGE.
| Complicationa | Risk marker | Before adjustment for AGE | After adjustment for AGEb |
|---|---|---|---|
| Retinopathy | DCCT mean A1c | <0.0001 | NS: Furosine |
| NS: Furosine and CML | |||
| <0.0001: CML | |||
| EDIC mean A1c | <0.0001 | <0.0001: Furosine, CML | |
| <0.0001: Furosine | |||
| <0.0001: CML | |||
| Nephropathy | DCCT mean A1c | 0.0012 | NS: Furosine: |
| <0.0001 | NS: Furosine and CML | ||
| DCCT mean A1c, log AER | 0.0002 | 0.0287: CML | |
| 0.0046 (Furosine, CML) | |||
| EDIC mean A1c | <0.0001 | <0.0001: Furosine, CML | |
| <0.0001: Furosine | |||
| <0.0001: CML | |||
| Neuropathy | DCCT A1c | N/A | N/A |
| EDIC A1c |
For complication subcategories, consult original publication.
A significant p-value indicates the adjustment has no impact on the association. N/A, not available. NS, not significant.
Conclusions
Various studies reviewed elsewhere [43–46] have pointed to the strong association between tissue and serum AGEs and the complications of diabetes. Our own studies described above have shown that the determination of AGEs in the collagen-rich matrix of the skin provides robust and often glycemia-independent information on the risk of developing microvascular disease in Type I diabetes of long duration. Glucosepane now emerges as a strong likely mediator of complications by virtue of decreasing the turnover rate and impairing the renewal of the damaged extracellular matrix proteins wherever it forms. By blocking critical arginine residues, it may decrease the ability of cells to attach to the ECM. It may impart increased ECM stiffness and hamper interaction with cells, as well as contribute to the pathogenesis of diabetes-related joint disorders. Many questions need to be addressed. Does glucosepane explain accelerated cardiovascular disease in diabetes? Does it participate in intimal media thickening, impaired arterial plaque stability, thrombus formation, and impaired revascularization of occluded vessels? How much of glycemic chemical memory from glucosepane translates into biological memory? Does glucosepane explain the increased incidence of Alzheimer in diabetes by making plaques and neurofibrillary tangles less susceptible to turnover? By all accounts, much more research is needed to understand the role of glucosepane in diabetes and chronic degenerative disease, i.e., the single most prevalent AGE of the extracellular matrix found to date.
Acknowledgments
This work was supported by grants from NIDDK (R21 DK-79432 to DRS), JDRF (#2008-058) and NEI (EY-07099 to VMM) and NIH and non-governmental grants to The DCCT/EDIC Research Group. We thank Christopher Strauch for assistance with mass spectrometry analyses.
Research funding: None declared.
Biography
 Vincent M. Monnier, MD Dr. Monnier’s research focuses on the role of the Maillard reaction as a cause of protein damage during the formation of age-related cataract and to the extracellular matrix in diabetes and aging. His early contributions helped shape the field of the Maillard Reaction in vivo through the introduction of fluorescence as surrogate marker of advanced glycation endproducts, and the discovery of pentosidine as the first specific crosslink of the Maillard reaction in vivo. He was the Founding President of the International Maillard Reaction Society.
Vincent M. Monnier, MD Dr. Monnier’s research focuses on the role of the Maillard reaction as a cause of protein damage during the formation of age-related cataract and to the extracellular matrix in diabetes and aging. His early contributions helped shape the field of the Maillard Reaction in vivo through the introduction of fluorescence as surrogate marker of advanced glycation endproducts, and the discovery of pentosidine as the first specific crosslink of the Maillard reaction in vivo. He was the Founding President of the International Maillard Reaction Society.
 Wanjie Sun Dr Wanjie Sun is a Lead Research Scientist/Biostatistician at the Biostatistics Center of the George Washington University. She has been working on a landmark Type 1 diabetes study, the Diabetes Complication and Control Trial /Epidemiology of Diabetes Intervention and Complication (DCCT/EDIC) since 2002. Dr Sun is a named or contributing author of over 20 peer-reviewed publications, including NEJM, Archives of Internal Medicine, etc. Dr Sun has collaborated with Dr. Monnier and Dr. Genuth in the clinical AGE field for years.
Wanjie Sun Dr Wanjie Sun is a Lead Research Scientist/Biostatistician at the Biostatistics Center of the George Washington University. She has been working on a landmark Type 1 diabetes study, the Diabetes Complication and Control Trial /Epidemiology of Diabetes Intervention and Complication (DCCT/EDIC) since 2002. Dr Sun is a named or contributing author of over 20 peer-reviewed publications, including NEJM, Archives of Internal Medicine, etc. Dr Sun has collaborated with Dr. Monnier and Dr. Genuth in the clinical AGE field for years.
 David R. Sell, PhD Dr. Sell is an Assistant Professor of Pathology at Case Western Reserve University. His research focuses on the study amino-carbonyl reactions of sugars with long-lived proteins as the basis for posttranslational modifications of macromolecules during aging and diabetes He has played a key role in the discovery of pentosidine and the characterization of several protein adducts of the Maillard Reaction and is an active participant in the study of glycation products as markers of complications in the DCCT/EDIC. He is currently the Treasurer of the International Maillard Reaction Society.
David R. Sell, PhD Dr. Sell is an Assistant Professor of Pathology at Case Western Reserve University. His research focuses on the study amino-carbonyl reactions of sugars with long-lived proteins as the basis for posttranslational modifications of macromolecules during aging and diabetes He has played a key role in the discovery of pentosidine and the characterization of several protein adducts of the Maillard Reaction and is an active participant in the study of glycation products as markers of complications in the DCCT/EDIC. He is currently the Treasurer of the International Maillard Reaction Society.
 Xingjun Fan, PhD Xingjun Fan is an Assistant Professor in Department of Pathology at Case Western Reserve University. He earned his PhD degree in Chemistry from the Chinese Academy of Sciences. His research is focusing on the role oxidation and protein posttranslational modification in aging and age-related disease, such as cataract and neurodegenerative disorders.
Xingjun Fan, PhD Xingjun Fan is an Assistant Professor in Department of Pathology at Case Western Reserve University. He earned his PhD degree in Chemistry from the Chinese Academy of Sciences. His research is focusing on the role oxidation and protein posttranslational modification in aging and age-related disease, such as cataract and neurodegenerative disorders.
 Ina Nemet, PhD Dr. Ina Nemet is a biochemist who has made semantic contributions in various fields of the Maillard reaction, in particular the biochemical pathways of protein modifications by methylglyoxal, glucose and vitamin C, for which she has developed mass spectrometry-based methods. She is currently pursuing a new direction of research studying the mechanisms involved in retinal photoreceptor biochemistry and biology.
Ina Nemet, PhD Dr. Ina Nemet is a biochemist who has made semantic contributions in various fields of the Maillard reaction, in particular the biochemical pathways of protein modifications by methylglyoxal, glucose and vitamin C, for which she has developed mass spectrometry-based methods. She is currently pursuing a new direction of research studying the mechanisms involved in retinal photoreceptor biochemistry and biology.
 Saul Genuth, MD Dr. Genuth is Professor of Medicine and Endocrinology at Case Western Reserve University, Cleveland, OH, USA. His research interests focus on the pathogenesis and treatment of diabetic complications. He was vice chairman of the DCCT trial and is a co-chairman of the observational follow-up EDIC study, as well as an active investigator in several ancillary DCCT/EDIC studies, including those on the role of advanced glycation end products as predictors of complications, such as diabetic retinopathy and nephropathy.
Saul Genuth, MD Dr. Genuth is Professor of Medicine and Endocrinology at Case Western Reserve University, Cleveland, OH, USA. His research interests focus on the pathogenesis and treatment of diabetic complications. He was vice chairman of the DCCT trial and is a co-chairman of the observational follow-up EDIC study, as well as an active investigator in several ancillary DCCT/EDIC studies, including those on the role of advanced glycation end products as predictors of complications, such as diabetic retinopathy and nephropathy.
Footnotes
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article. Research funding played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Employment or leadership: None declared.
Honorarium: None declared.
Statement: The research reviewed in this publication fully complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects and animals.
Contributor Information
Vincent M. Monnier, Department of Biochemistry, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Wanjie Sun, Biostatistics Center, George Washington University, Rockville, MD, USA; and Case Western Reserve University School of Medicine, Cleveland, OH, USA.
David R. Sell, Department of Pathology, Case Western Reserve University School of Medicine, Cleveland, OH, USA
Xingjun Fan, Department of Pathology, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Ina Nemet, Department of Pathology, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Saul Genuth, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
References
- 1.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med 1976;295:417–20. [DOI] [PubMed] [Google Scholar]
- 2.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- 3.UKPDS33. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 4.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348: 2294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 7.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nature Rev Drug Disc 2009;8:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998;339:69–75. [DOI] [PubMed] [Google Scholar]
- 9.Lederer MO, Buhler HP. Cross-linking of proteins by Maillard processes - characterization and detection of a lysine-arginine cross-link derived from D-glucose. Bioorg Med Chem 1999;7:1081–8. [DOI] [PubMed] [Google Scholar]
- 10.Gobert J, Glomb MA. Degradation of glucose: reinvestigation of reactive alpha-dicarbonyl compounds. J Agric Food Chem 2009;57:8591–7. [DOI] [PubMed] [Google Scholar]
- 11.Biemel KM, Lederer MO. Site-specific quantitative evaluation of the protein glycation product N(6)-(2,3-Dihydroxy-5,6-dioxohexyl)-l-lysinate by LC-(ESI)MS peptide mapping: evidence for its key role in AGE formation. Bioconjug Chem 2003;14:619–28. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Hasegawa T, Fukunaga Y, Ienaga K. Crosslines A and B as candidates for the flurophores in age- and diabetes-related cross-linked proteins, and their diacetates produced by Maillard reaction of alpha-N-acetyl-L-lysine with D-glucose. J Chem Soc Chem Comm 1992:992–4. [Google Scholar]
- 13.Aoki S, Hasegawa G, Shigeta H, Obayashi H, Fujii M, Kimura F, et al. Crossline levels in serum and erythrocyte membrane proteins from patients with diabetic nephropathy. Diabetes Res Clin Pract 2000;48:119–25. [DOI] [PubMed] [Google Scholar]
- 14.Nemet I, Strauch CM, Monnier VM. Favored and disfavored pathways of protein crosslinking by glucose: glucose lysine dimer (GLUCOLD) and crossline versus glucosepane. Amino Acids 2011;40:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasiri R, Zahedi M, Jamet H, Moosavi-Movahedi AA. Theoretical studies on models of lysine-arginine cross-links derived from alpha-oxoaldehydes: a new mechanism for glucosepane formation. J Mol Model 2012;18:1645–59. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med 2000;6:114–25. [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas AJ, Lin SN, Conley SB, Schneider JA, Williams JC, Caprioli RC. Urine glyceraldehyde excretion is elevated in the renal Fanconi syndrome. Kidney Int 1989;35:99–104. [DOI] [PubMed] [Google Scholar]
- 18.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem 2005;280:12310–5. [DOI] [PubMed] [Google Scholar]
- 19.Fan X, Sell DR, Zhang J, Nemet I, Theves M, Lu J, et al. Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: a comparative analysis. Free Radic Biol Med 2010;49:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dammann P, Sell DR, Begall S, Strauch C, Monnier VM. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell’s mole-rat (Fukomys anselli). J Gerontol A Biol Sci Med Sci 2012;67:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustata GT, Rosca M, Biemel KM, Reihl O, Smith MA, Viswanathan A, et al. Paradoxical effects of green tea (camellia sinensis) and antioxidant vitamins in diabetic rats: improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes 2005;54:517–26. [DOI] [PubMed] [Google Scholar]
- 23.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major Maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem 2002;277:24907–15. [DOI] [PubMed] [Google Scholar]
- 24.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027–31. [DOI] [PubMed] [Google Scholar]
- 25.Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest 1981;67:1630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility of collagen from human skin, tracheal cartilage and dura mater. Exp Gerontol 1982;17:185–94. [DOI] [PubMed] [Google Scholar]
- 27.Vater CA, Harris ED Jr., Siegel RC. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J 1979;181:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Z, Nemet I, Shen W, Monnier VM. Isolation, purification and characterization of histidino-threosidine, a novel Maillard reaction protein crosslink from threose, lysine and histidine. Arch Biochem Biophys 2007;463:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins NG, Neglia-Fisher CI, Dyer DG, Thorpe SR, Baynes JW. Effect of phosphate on the kinetics and specificity of glycation of protein. J Biol Chem 1987;262:7207–12. [PubMed] [Google Scholar]
- 30.Dai Z, Wang B, Sun G, Fan X, Anderson VE, Monnier VM. Identification of glucose-derived cross-linking sites in ribonuclease A. J Proteome Res 2008;7:2756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem 1986;261:4889–94. [PubMed] [Google Scholar]
- 32.Chetyrkin S, Mathis M, Pedchenko V, Sanchez OA, McDonald WH, Hachey DL, et al. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 2011;50:6102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monnier VM, Sell DR, Dai Z, Nemet I, Collard F, Zhang J. The role of the amadori product in the complications of diabetes. Ann N Y Acad Sci 2008;1126:81–8. [DOI] [PubMed] [Google Scholar]
- 34.Nemet I, Monnier VM. Vitamin C degradation products and pathways in the human lens. J Biol Chem 2011;286:37128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986;232:1629–32. [DOI] [PubMed] [Google Scholar]
- 36.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch Biochem Biophys 2003;419:41–9. [DOI] [PubMed] [Google Scholar]
- 37.Fan X, Liu X, Potts B, Strauch C, Nemet I, Monnier V. Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice. Mol Vis 2011;17:2221–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 2006;55:1961–9. [DOI] [PubMed] [Google Scholar]
- 39.Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, et al. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 2003;375:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monnier VM, Sell DR, Strauch C, Sun W, Lachin JM, Cleary PA, et al. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J Diabetes Complications 2013;27:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pop-Busui R, Herman WH, Feldman EL, Low PA, Martin CL, Cleary PA, et al. DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep 2010;10:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005;54:3103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci 2005;1043:567–81. [DOI] [PubMed] [Google Scholar]
- 44.Bos DC, de Ranitz-Greven WL, de Valk HW. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther 2011;13:773–9. [DOI] [PubMed] [Google Scholar]
- 45.Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res 2012;159:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beisswenger PJ. Glycation and biomarkers of vascular complications of diabetes. Amino Acids 2012;42:1171–83. [DOI] [PubMed] [Google Scholar]