Abstract
One successful class of cancer immunotherapies, immune checkpoint inhibitory antibodies, disrupt key pathways that regulate immune checkpoints, such as cytotoxic T lymphocyte–associated protein 4 (CTLA-4). These agents unleash the potency of antigen-experienced T cells that have already been induced as a consequence of the existing tumor. But only 20% of cancers naturally induce T cells. For most cancers, vaccines are require to induce and mobilize T effector cells (Teffs) to traffick into tumors. We evaluated the effects of anti–CTLA-4 given in combination with an antigen-specific dendritic cell vaccine on intratumoral Teffs in a murine pancreatic cancer model. The dendritic cell–targeted tumor antigen plus anti–CTLA-4 significantly increased the number of vaccine-induced CD4+ Teffs within the tumor. This increase was accompanied by a reduction in the size of the peripheral CD4+ Teff pool. We also found that IL-3 production by activated CD4+ T cells was significantly increased with this combination. Importantly, the CD4+ Teff response was attenuated in Il3−/− mice, suggesting mediation of the effect by IL-3. Finally, the induced T cell infiltration was associated with activation of the tumor endothelium by T cell–derived IL-3. Our findings collectively provide a new insight into the mechanism driving Teff infiltration and vascular activation in a murine pancreatic cancer model, specifically identifying a new role for IL-3 in the anticancer immune response.
Keywords: IL-3, cancer immunotherapy, anti-CTLA-4, dendritic cell–targeted cancer vaccine, tumor-infiltrating T cells
Graphical abstract
This paper demonstrates that combining anti–CTLA-4 antibody, an immune checkpoint inhibitory antibody used in cancer immunotherapy, with a dendritic cell vaccine triggers an increase in CD4+ effector T cell infiltration into tumors in a mouse pancreatic cancer model, and IL-3 was identified as a critical mediator of this effect. These findings offer a potential strategy to improve cancer vaccine efficacy.
Introduction
Immune checkpoint inhibitors (ICIs) provide durable antitumor immune responses for about 20% of patients with advanced cancers. Cancer vaccines are being evaluated in combination immunotherapy approaches to enhance the activity of ICIs in ICI-sensitive and currently ICI-unresponsive cancer patients. The development of an effective cancer vaccine requires the induction and mobilization of T effector cells (Teffs) into a tumor. Potent anti–tumor responses can then be achieved by modulating immune checkpoint molecules on the vaccine-induced Teffs. Among the growing list of checkpoint molecules, cytotoxicity T lymphocyte–associated antigen-4 (CTLA-4) plays a critical role in tumor tolerance 1,2. The first observations for a function of CTLA-4 in tumor immunology came from studies using Ctla4−/− mice, in which lymphoproliferative disorders were shown to be associated with early mortality 3. Subsequently, anti–CTLA-4 monoclonal antibodies (mAbs), which block the engagement of CTLA-4 with its ligands B7.1 and B7.2 on murine antigen presenting cells (APCs), were shown to have anti-tumor activity 4. One such mAb, ipilimumab, a fully human IgG1, obtained FDA approval in 2011 for treatment of metastatic melanoma. Multiple clinical trials have documented the efficacy of ipilimumab in improving overall survival in metastatic melanoma patients with or without previous standard therapy, as well as in the adjuvant setting after surgical resection 5–7. Ipilimumab was the first ICI to be approved for the treatment of cancer. As a result, significant efforts are underway to uncover the multiple mechanisms by which ICIs alter the tumor microenvironment in favor of an antitumor immune response.
There is a strong correlation between the presence of tumor-infiltrating T lymphocytes (TILs) and clinical outcomes in melanoma, breast, prostate, renal cell, esophageal, colorectal, urothelial, and ovarian carcinomas 8–15. In both human and mouse studies, a high Teff/Treg ratio in particular is associated with a more favorable prognosis 4,14. Regulatory T cells (Tregs that are CD4+CD25+FOXP3+ T cells) are generally immunsuppressive and impede the proliferation and function of Teffs. Teffs provide the antitumor activity and do not express FOXP3. Thus, depletion of the Treg cell subset is favorable for an antitumor response. In addition, anti–CTLA-4 increases the intratumoral Teff/Treg ratio and this also correlates with improved tumor rejection 4. Notably, although prior mouse studies had demonstrated that depletion of the Treg subset was one mechanism of anti-CTLA-4 activity 16, more recent studies on human samples treated with anti-CTLA-4 mAb have demonstrated that there is an increase in FOXP3+ Tregs rather than the expected decrease 17. Thus, the mechanism of anti-CTLA-4 therapy remains incompletely understood 18.
The Steinman group and others have emphasized the role of tumor vasculature in mediating effective immunotherapy 19,20. The tumor’s vascular endothelium plays a critical role in regulating T cell homing into tumors and in the outcome of immunotherapy. The tumor endothelial barrier, often prohibitive to vaccine-induced Teffs, is in turn regulated by intratumoral cytokines, endothelial growth factors, and adhesion molecules expressed by endothelial cells. The endothelin B receptor (ETBR) has, for example, been identified as an important barrier for the tumor endothelium 21. Amplifying signals mediated by the endothelin 1 (ET-1)/ETBR axis suppress the upregulation of intercellular adhesion molecule-1 (ICAM-1). ICAM-1 is critical for T cell adhesion and subsequent transmigration, both under steady-state and during inflammatory conditions 22. Thus, blocking ETBR has been found to increase ICAM-1 expression, T cell homing to tumors, and tumor protection in a mouse ovarian cancer model 22. IL-3 has also been shown to induce tumor angiogenesis and thus hypothesized to be tumor-promoting; however, to our knowledge, this effect has not been proven in vivo 23.
Interestingly, while initially described as a broad hematopoietic growth factor, IL-3 is now recognized as an endothelial cell modulator, and it plays a key role in acute and chronic inflammation. IL-3 also regulates endothelial cell differentiation, proliferation, and activation 24,25. It also functions in the delayed–type hypersensitivity response, which is reduced significantly in Il3−/− mice after sensitization, and importantly, is accompanied by decreased infiltration of immune cells 26. In contrast, overexpression of IL-3 in transgenic mice is associated with increased autoimmunity against motor neurons and causes a motor neuron disease 27.
Here, we have examined the effect of anti–CTLA-4 antibody in combination with a dendritic cell vaccine on TILs in a pancreatic cancer mouse model. Vaccination with dendritic cell-targeted tumor antigen plus anti–CTLA-4 triggered an increase in CD4+ Teff infiltration into tumors, reduced the peripheral Teff pool, increased activated T cell–derived IL-3 production, and enhanced IL-3–mediated activation of the tumor endothelium. In contrast, CD4+ Teff homing to tumors was attenuated in Il3−/− mice, identifying IL-3 as a critical mediator. Thus, our findings provide a hitherto uncharacterized mechanism for increased intratumoral CD4+ Teffs with vaccine plus anti-CTLA-4 antibody, thus offering a potential strategy to improve vaccine efficacy.
Materials and methods
Mice and cell lines
C57BL/6 mice (6–8 weeks old) were purchased from Jackson Laboratory. Il3−/− mice on a C57BL/6 background were kindly provided by Dr. Tomohiro Yoshimoto (Hyogo College of Medicine, Japan) 26. The mice were maintained under specific pathogen-free conditions and used in accordance with the guidelines of Animal Care and Use Committee at The Rockefeller University. The C57BL/6 syngeneic mouse pancreatic tumor cell line, Panc02 28, was maintained in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum (FBS) and antibiotics. Mycoplasma screening was performed at the Monoclonal Antibody Core Facility of Memorial Sloan Kettering Cancer Center. Tumor cells used for experiments tested negative for mycoplasma.
Antibodies and peptides
Anti-CD28 (clone 37N), anti-CD16/CD32 (clone 2.4G2), anti-CD4 (clone GK1.5), and rat control IgG (clone III/10) were purified from hybridoma culture supernatant with a Protein G column (all hybridoma cell lines were obtained from ATCC). Hamster anti–αCTLA-4 Ab (clone 9H10) and hamster control IgG were purchased from BioXcell. Anti-mouse DEC-205-MSLN (DEC-MSLN) chimeric mAb was produced by transient transfection of 293T cells and purified on a Protein G column. FITC- or Alexa Fluor 700-αCD4 (RM4–5), PerCP-Cy5.5-αCD8α (53–6.7), PE-αCD45 (30-F11), Pacific Blue-αCD3ε (17A2) and APC-αFOXP3 (FJK-16s) were purchased from eBioscience. PE-αKi-67 (B56), APC- or PECy7-αIFNγ (XMG1.2), purified αCD31 (MEC13.3), biotin-αICAM-1 (YN1/1.7.4), Alexa Fluor 488-goat αrat IgG Ab, Alexa Fluor 555-streptavidin, and Cytofix/Cytoperm Plus Kit were purchased from BD Bioscience. Live/Dead Fixable Aqua Viability Dye was obtained from Invitrogen. Overlapping (staggered by 4 amino acids) 15-mer peptides of MSN, HIV Gag p24 were synthesized in the Proteomic Resource Center of The Rockefeller University.
Immunization, tumor challenge and adoptive transfer
Mice were primed and boosted 4 weeks later with a DC-targeting vaccine composed of 5 μg DEC-MSLN, 50 μg poly-IC (InVivogen), and 25 μg agonistic anti-αCD40 Ab. Vaccine was administrated by i.p. injection in 200 μL PBS. For anti–CTLA-4 therapy, 100 μg anti–αCTLA-4 Ab was given i.p. at day −1, 0, and +3 of the immunization (day 0). Hamster control IgG was used as control IgG. For CD4+ T cell depletion, 250 μg of anti–αCD4 or rat isotype control IgG were given i.p. at day −3, 0, and +3 of boost immunization (day 0). Mice were challenged in the right flank with 1×106 Panc02 cells 14 days after the boost immunization.
Cell isolation
For isolation of TILs, mice were sacrificed one week or one month after tumor challenge. Tumor tissues were minced and incubated with Liberase CI (1.67 Wünsch U/ml) and DNAse I (0.2 mg/mL) (Roche) at 37 °C for 30 min with gentle shaking. Digested tissues were passed through a 70 μm filter. The resulting cell suspension was washed three times and a Percoll gradient separation was performed to eliminate tumor debris and dead cells. TILs were analyzed by FACS for the expression of CD4, CD8, CD25, and FOXP3. CD4+ T cells were purified from enriched TILs by positive selection with anti-CD4 microbeads (Miltenyi Biotech). CD11c+ DCs were purified from collagenase-treated spleens with anti-CD11c microbeads. For isolation of lymphocytes from peripheral tissues, mice were perfused with PBS with heparin (75 U/mL). Lymphocytes from the lamina propia and peritoneal cavity were isolated as previously described 29,30. Lung tissues were minced, incubated with 0.13 U/mL Liberase and 50 μg/ml DNase I (Roche) at 37 °C for 25 min and separated on a Percoll gradient. Liver tissue was mashed through stainless steel mesh and lymphocytes were separated on a Percoll gradient. Bone marrow cells were obtained by flushing two femurs with cold RPMI 1640. Cells from the resulting pellets were treated with ACK lysis buffer (Invitrogen) to remove red blood cells and washed before use. Peripheral blood lymphocytes were isolated using Lympholyte M (Cedarlane).
Quantification of serum cytokines
Peripheral blood was collected 4 days after boost immunization by submandibular bleeding. Sera were collected using serum gel tube (SARSTEDT). Serum IL-3 was measured by the BD OptEIA Set Mouse IL-3 ELISA kit (mice were not repeat-bled for serum IL-3 measurements). Serum IFN-γ was measured by the Ready-Set-Go ELISA set (eBioscience). Serum concentrations of TH1/TH2 cytokines (IFN-γ, TNF-α, IL-2, IL-4, and IL-10) were measured 0, 1, 4 and 14 days after immunization by the MSD mouse TH1/TH2 assay (Meso Scale Discovery).
Analysis of antigen–specific T cell responses
T cells were stimulated in vitro with MSLN or HIV gag p24 peptide mix (1 μg/mL) for 6 hours, and with BFA for the last 5 hours. Note that the mix contained overlapping MSN and HIV Gag p24 15-mer peptides staggered by 4 amino acids. Intracellular IFN-γ production was measured by intracellular cytokine staining. To measure the production of TH1/TH2 cytokines from in vitro stimulated TILs, supernatant from the DC:T cell co-cultures were collected 48 hours after stimulation and quantified by ELISA and MSD mouse TH1/TH2 assays.
Histology and immunofluorescent microscopy
Tumors were dissected and snap frozen in optimal cutting temperature solution. Sections (10 μm) were cut from frozen tumors using a cryomicrotome and then fixed in acetone/ethanol mix for 10 min and stained with anti-αCD4, anti-αCD31, and/or anti-αICAM-1-biotin Ab. Samples were analyzed with an inverted LSM 510 laser scanning confocal microscope (Zeiss) or A×70 upright brightfield microscope (Olympus) with a 20× water immersion objective.
Statistical analysis
Group sizes for T cell and serum responses varied between three and five biological replicates. Experiments were repeated independently two or three times. Two-tailed Student’s t-test was performed on cytokine and ELISA data sets using GraphPad Prism software. P values < 0.05 were considered statistically significant.
Results
Anti–CTLA-4 interacts with a DC-targeted vaccine to promote tumor infiltration by CD4+ Teffs
To examine the effect of anti–CTLA-4 on vaccine-induced CD4+ T effector cell infiltration into pancreatic tumors, we used a DC-targeted prophylactic vaccination protocol in a mouse pancreatic tumor model (Panc02 transplantable tumor cells) (Fig. 1A). Our previous study showed that targeting a tumor antigen, human mesothelin (MSLN), to the DEC-205 (DEC) receptor together with a DC maturation stimulus induced robust systemic CD4+ T cell responses in mice 31. Nevertheless, and despite the observed systemic CD4+ T cell responses, DC-targeted vaccines had only a small impact on CD4+ Teff infiltration into tumors.
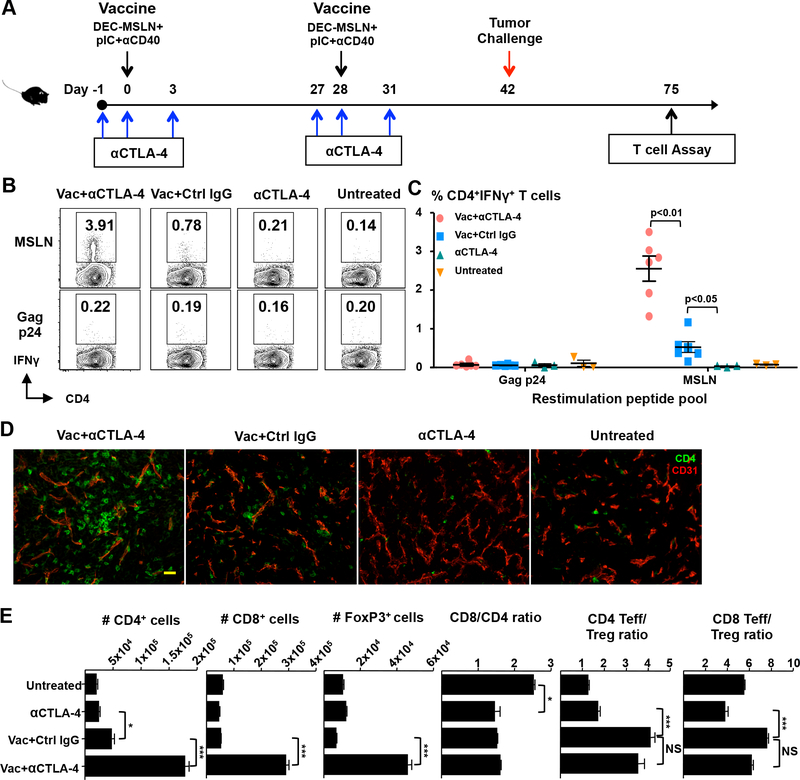
Figure 1.
Anti-CTLA–4 in combination with a protein vaccine increases intratumoral T effector cells. (A) Immunization and tumor challenge protocol. (B and C) Mice were challenged with pancreatic cancer cells (Panc02) two weeks after boost immunization. MSLN-specific CD4+ T cells were quantified in enriched TILs by intracellular IFN-γ staining ~30 days after tumor challenge. Representative FACS data (B) and quantified percentage of IFN-γ+ cells in the viable CD45+CD3+CD4+ population (C) are shown. Each dot represents tumor-infiltrating lymphocytes (TILs) pooled from 2 or 3 tumors. (D) Analysis of frozen tumor sections by immunofluorescent confocal laser microscopy. Tumors were dissected and fresh frozen in optimum cutting temperature solution (OCT), cut and stained for CD4 (green) and CD31 (red). Images were acquired with a 20× water immersion objective. Bar, 50 μm. (E) Quantification of the number of different T cell populations in TILs by flow cytometry. Data in B–E are representative of three (B and C) and two (D and E) individual experiments.
Mice were vaccinated according to the protocol shown in Figure 1A, and CD4+ Teff infiltration and function was evaluated 33 days following tumor challenge. Ex vivo re-stimulation of tumor-infiltrating CD4+ T cells with MSLN peptide or irrelevant peptide mix (HIV gag) revealed that the combinatorial strategy (anti–CTLA-4 plus vaccine) significantly enhanced the number of intratumoral MSLN-specific, IFN-γ–secreting CD4+ Teffs (Fig. 1B and C). The total number of tumor–infiltrating CD4+ T cells was also significantly increased (Fig. 1D and E). To exclude blood lymphocyte contamination from tumor-draining vessels, we separately performed a perfusion experiment, wherein the right ventricle was ruptured and the left ventricle perfused with a large volume of heparinized PBS, and this yielded similar results (data not shown). The data collectively show that anti–CTLA-4 therapy together with a DC-targeted vaccine promotes CD4+ Teff infiltration into Panc02 tumors. In addition, we found that vaccine plus anti–CTLA-4 significantly increased the total number of CD8+ Teffs and FOXP3+ Tregs in the tumor. However, while the CD8/CD4 T cell ratio remained unaffected, there was an increase in the ratio of CD4+ and CD8+Teff/Treg ratio with the vaccine (Fig. 1E). Anti–CTLA-4 when given with vaccine did not further increase these Teff/Treg ratios.
Teff mobilization from periphery into tumor
Accumulation of vaccine–induced CD4+ Teffs can result from either the expansion of the existing Teff pool and/or an increase in cell migration from peripheral tissues. We first investigated whether our vaccine, consisting of a mesothelin-targeted DEC-205 mAb in combination with anti-CD40 mAb and poly-IC as adjuvants, can expand Teffs in peripheral tissues, with or without anti–CTLA-4. We found that vaccine alone increased the frequency of MSLN-specific Teffs in vaccine-draining mesenteric lymph nodes, spleen, peritoneal cavity, liver, lamina propria, and peripheral blood 14 days after boost immunization and before tumor implantation (Fig. 2A). However, a greater expansion of Teffs was noted only in mesenteric lymph nodes when anti–CTLA-4 was combined with vaccine compared with vaccine alone (Fig. 2A).
Figure 2.
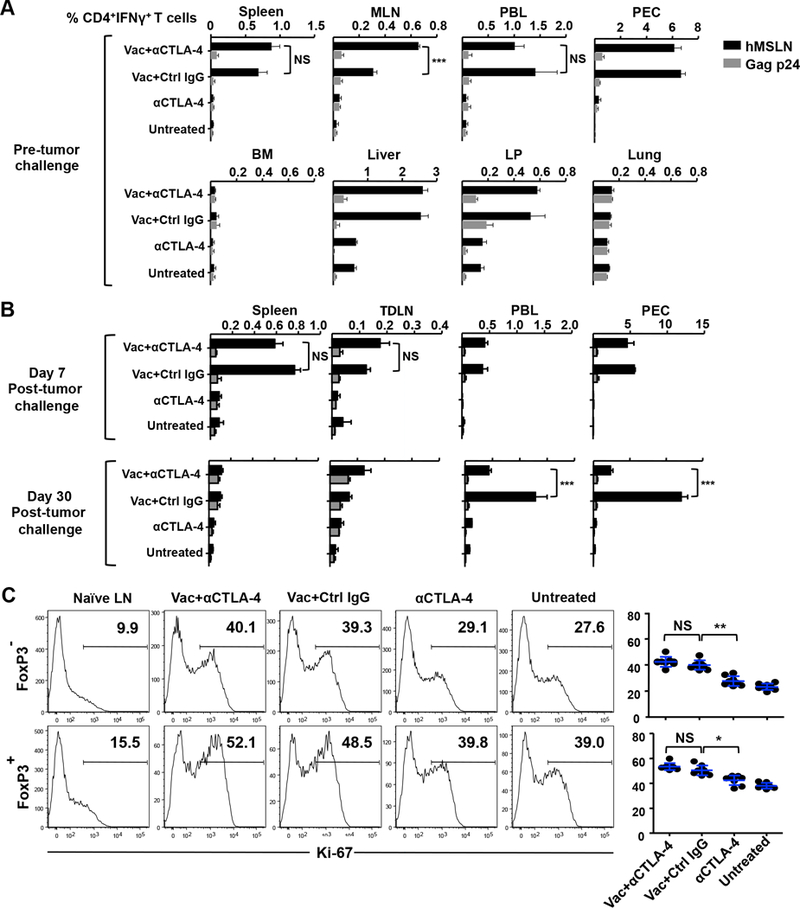
Increased mobilization of vaccine-induced T effector cells from the periphery to tumor with anti–CTLA-4. (A) Two weeks after boost immunization, vaccine-induced, MSLN-specific CD4+ T cells were quantified by intracellular IFN-γ staining in lymphocytes from spleen, mesenteric lymph node (MLN), peritoneal exudate (PEC), peripheral blood (PBL), bone marrow (BM), liver, lamina propria (LP), and lungs. Percentage of IFN-γ+ cells in viable CD3+CD4+-gated cells is shown. (B) Immunized mice were challenged with Panc02 tumor cells. Seven or 30 days after tumor challenge, MSLN-specific CD4+ T cells were quantified by intracellular IFN-γ staining in spleen, tumor-draining LN (TDLN), PBL, and PEC. (C) Expression of Ki-67 in intratumoral CD4+ Teff and Treg cells. Enriched TILs were stained for CD3, CD4, and Live/Dead Aqua, followed by permeabilization, fixation, and staining for Ki-67 and FOXP3. Representative FACS data are shown. Data are representative of two or three individual experiments (n = 4 mice per group). *P < 0.01, **P < 0.01, ***P < 0.001, NS: not significant.
T cell infiltration was also measured one week (day 7) or one month (day 30) after tumor challenge by quantifying the frequencies of MSLN-specific CD4+ T cells in spleen, tumor-draining lymph nodes, peripheral blood, and peritoneal cavity. No significant differences were noted in the vaccine plus anti–CTLA-4 Ab group versus vaccine alone group at the 7-day time point (Fig. 2B). However, at the 30-day time point, MSLN-specific CD4+ T cells in the peripheral blood and peritoneal cavity were reduced when anti–CTLA-4 was given together with vaccine, compared with vaccine alone (Fig. 2B). This reduction was associated with an increase in intratumoral CD4+ Teffs (Figs. 1E and2B), suggesting that these Teffs are mobilized from peripheral blood and the peritoneal cavity into the tumor.
To determine whether the increases in CD4+ Teffs arose from increased cell proliferation within tumors, we evaluated the expression of the proliferative marker Ki-67 (Fig. 2C). We noted that while our DC-targeted vaccine increased the Ki-67 labeling in both CD4+ Teff and Treg cells, its combination with anti–CTLA-4 displayed no additional effect. These latter data suggest that the increase in intratumoral Teffs by anti–CTLA-4 does not arise primarily from local T cell proliferation. Taken together, the results show that increases in intratumoral CD4+ Teffs are not the result of an expanded peripheral or tumor Teff pool, but rather arise from the active mobilization of vaccine-activated T cells from the periphery into tumor tissue.
Anti–CTLA-4 increases IL-3 production by CD4+ Teffs
To examine the lineage characteristics of the tumor-infiltrating Teffs, we purified intratumoral CD4+ T cells and measured cytokine production by stimulation in vitro with DCs pulsed with a MSLN or HIV gag p24 peptide mix (see Materials and Methods for details). Surprisingly, instead of solely inducing a TH1 (IL-2 and IFN-γ) response, the addition of anti–CTLA-4 to vaccine significantly increased both TH1 and TH2 (IL-4 and IL-10) cytokines (Fig. 3A). There was also a striking ~8-fold increase compared with vaccine alone in the levels of IL-3, a cytokine known to be produced by both TH1 and TH2 lineages 32. However, TNF-α levels were increased similarly in vaccine alone and vaccine plus anti–CTLA-4 groups.
Figure 3.
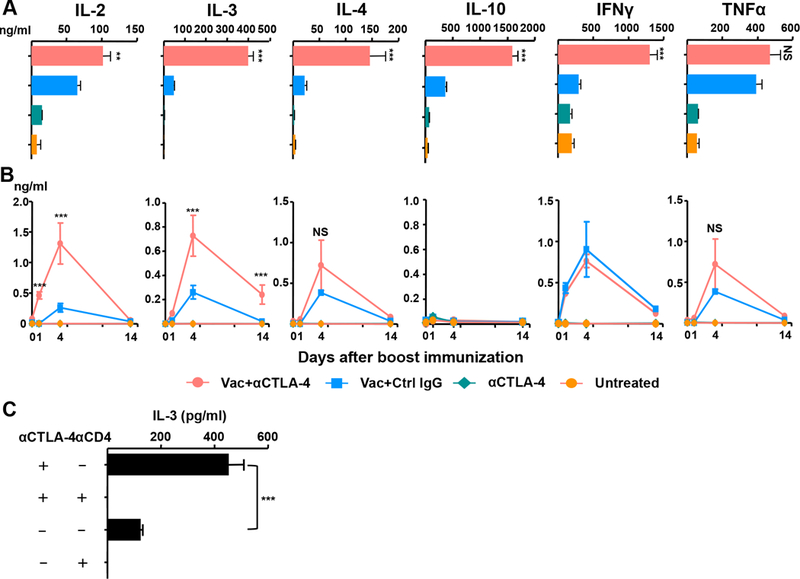
Anti–CTLA-4 increases IL-3 production by CD4+ T effector cells. (A) TH1/TH2 cytokine production by CD4+ TILs. Purified CD4+ TILs were re–stimulated with CD11c+ DCs pulsed with Gag p24 or human mesothelin (MSLN) peptide mix in vitro for 48 hours. Supernatants were analyzed for IL-3 levels by ELISA, and for other TH1/TH2 cytokines by MSD assay (see Materials and Methods). (B) Kinetics of serum TH1/TH2 cytokine production in vaccinated mice. Sera from immunized mice were harvested at 0, 1, 4 or 14 days after boost immunization. Serum TH1/TH2 cytokine levels were quantified as in A. (C) CD4-depleting Ab or control rat IgG was injected −3, 0, and 3 day of boost immunization. Serum IL-3 levels were measured by ELISA four days after the boost. Data in A–C are representative of two (C) or three (A and B) experiments (n = 3–4 mice per group). **P < 0.01, ***P < 0.001, NS: not significant.
To determine whether the vaccine induced mixed TH1/TH2 hypercytokinemia systemically, we quantified serum cytokine levels at different time points after boost immunization (Fig. 3B). Vaccine itself induced increases in serum cytokine levels to variable extents as early as 1 day after boost, with maximal increases noted at day 4, and reductions at day 14. Notably, there were further significant elevations in serum IL-2 and IL-3 levels upon the addition of anti–CTLA-4 compared with vaccine alone. This difference between vaccine alone and vaccine plus anti–CTLA-4 was not seen with serum IL-4, IFN-γ and TNF-α levels. To assess whether IL-3 in the serum was derived from vaccine-induced CD4+ T cells, we depleted CD4+ T cells using an anti–CD4 antibody; this completely abrogated the vaccine-induced serum IL-3 elevations (Fig. 3C). Thus, we conclude that the vaccine increases IL-3 production by CD4+ T cells, both systemically and locally.
IL-3–dependent accumulation of intratumoral Teffs through interactions with ICAM-1 in the vascular endothelium
Given that IL-3 is known to activate the vasculature 33,34, our finding of high IL-3 levels produced by tumor-infiltrating CD4+ Teff cells suggested that IL-3 may in fact promote T cell infiltration through interactions with the tumor endothelium. To confirm a function for IL-3 in T cell infiltration, two groups of mice were immunized with the DC–targeting vaccine, one with and one without anti–CTLA-4. One month after tumor challenge, lymphocytes were harvested from tumor, tumor-draining lymph nodes, spleen, peripheral blood, and peritoneal cavity. Quantification of vaccine-induced CD4+ Teffs showed that while anti–CTLA-4 collaborated with the vaccine to increase CD4+ Teffs into the tumors in wild type mice, there was no effect in Il3−/− mice (Fig. 4A). Instead, in Il3−/− mice, the addition of anti–CTLA-4 to vaccine led to an accumulation of tumor–reactive T cells in peripheral blood at 7 days and in tumor–draining lymph nodes at day 30 after tumor challenge (Fig. 4B).
Figure 4.
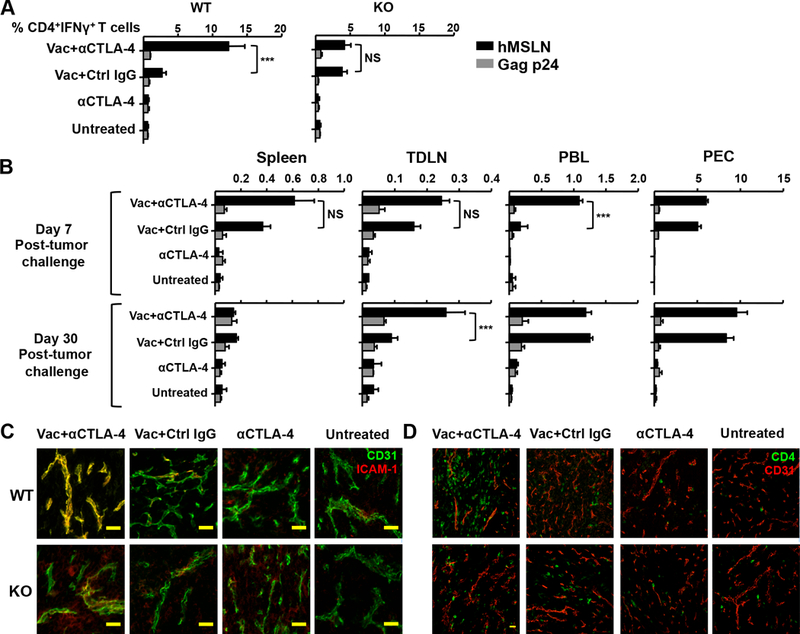
Increased intratumoral T effector cells with anti-CTLA-4 is dependent on IL-3. (A) Il3−/− mice or wild type (WT) littermates were vaccinated with CTLA-4–blocking Ab or control (Ctrl) IgG, and challenged with Panc02 tumor cells. Thirty days after tumor challenge, FACS-purified CD4+ TILs were re–stimulated with splenic CD11c+ cells pulsed with human mesothelin (MSLN) or Gag p24 peptide mix. MSLN-specific CD4+ T cells were quantified by intracellular IFN-γ staining. (B) MSLN-specific CD4+ T cells were quantified in lymphocytes from spleen, tumor-draining lymph nodes (TDLN), peripheral blood lymphocytes (PBL), and PEC in Il3−/− mice. (C) Tumors were dissected and flash frozen in optimum cutting temperature (OCT) solution, cut, and stained for CD4 (green) and CD31 (red). Images were acquired with a 20× water immersion objective. (D) Tumor sections were stained for the expression of ICAM-1 (green) and CD31 (red) 30 days after tumor challenge. Bar, 50 μm. Data in A–D are representative of three individual experiments (n = 3–4 mice per group). ***P < 0.001, NS: not significant.
As IL-3 is known to stimulate endothelial cell differentiation, proliferation, and activation 24,35,36, we tested whether the IL-3 produced in response to anti–CTLA-4 and DC-targeted vaccine results in the activation of tumor endothelial cells, which would, in turn, enhance T cell adhesion and transendothelial migration. Analysis of frozen tumor sections for endothelial cell activation markers by immunocytochemistry revealed a significant increase in the expression of ICAM-1 on tumor endothelial cells (CD31+ cells) in wild-type mice receiving vaccine plus anti–CTLA-4 (Fig. 4C). However, treatment with vaccine alone did not increase ICAM-1 expression on CD31+ endothelial cells. Neither was this increase observed in Il3−/− mice, indicating that ICAM-1 expression mediated by anti-CTLA-4 is dependent on IL-3 expression. Figure 4D further confirms the enhanced localization of CD4+ Teff cells in tumor tissue in wild type mice, but not in Il3−/− mice. Overall, therefore, the data suggest an IL-3–dependent activation of tumor endothelium after treatment with anti–CTLA-4, resulting in CD4+ Teff cell infiltration into tumors.
Discussion
Combination therapy using an antigen-specific, DC-targeted vaccine and anti–CTLA-4 uncovered a new mechanism for enhanced vaccine-induced CD4+ Teff infiltration into a mouse pancreatic cancer model. Specifically, this combination increases the expression of IL-3 by activated CD4 Teffs; the cytokine in turn facilitates Teff cell trafficking into tumors by upregulating ICAM-1 expression on endothelial cells. Our findings are the first to implicate IL-3 as a key mediator of increased intratumoral CD4 Teffs with anti–CTLA-4 therapy.
Our data show that while the DC-targeted vaccine increases intratumoral MSLN-specific, IFN-γ–producing CD4+ Teffs, a substantially greater response was noted when vaccine was used in combination with anti–CTLA-4. This is consistent with previous studies both in mouse models and in advanced cancer patients. For example, in a mouse melanoma model, anti–CTLA-4 in combination with a GM-CSF–producing vaccine, GVAX, significantly increased Teff cell infiltration into melanomas 19. Likewise, patients treated with anti–CTLA-4 increased T cell infiltration into human tumors 37,38. However, while an association between anti–CTLA-4 therapy and increased intratumoral Teff accumulation is now well established, the precise mechanisms underlying T cell accrual remains less well understood.
In addition, we also addressed two additional previously unanswered questions—where do the intratumoral Teffs originate from and what is the mechanism of their enhanced accumulation in response to anti–CTLA-4? First, our data provide compelling evidence for increased mobilization of Teffs from the periphery into tumor, rather than an expanded peripheral pool size, in situ expansion of intratumoral cells, or reduced cellular egress. Specifically, tracking studies over time revealed an increase in intratumoral Teff number in association with reduced peripheral blood and peritoneal cavity Teffs. These data thus suggest that Teff cells from the periphery have the ability to infiltrate tumors and that this infiltration can be promoted by anti–CTLA-4 treatment. Second, as abnormal tumor vasculature is a known barrier for T cell infiltration 21, we assumed that it was unlikely that intratumoral Teffs will reach equilibrium with the peripheral pool via blood and lymph circulation. Consistent with this, we did not observe sustained increases in the T cell pool size in major peripheral lymphoid tissues. While there was a small increase of Teffs in vaccine-draining lymph node before tumor challenge, the effect was transient. Thus, intratumoral Teff cells are unlikely to arise from an expanded pool of peripheral Teff cells. Interestingly, despite previous reports of proliferative effects of anti–CTLA-4 on intratumoral Teff cells 19, we did not observe increased Ki-67 expression with anti–CTLA-4 in this model when compared with the control vaccine group. This inconsistency may be a timing issue since previous studies used “prophylactic” protocols and our study used a protracted “treatment” protocol. Finally, we found virtually no expression of CD62L or CCR7 on the tumor-infiltrating CD4+ Teffs (unpublished data) that would suggest egress of intratumoral Teffs to distant lymph nodes. This may nonetheless reflect a loss of the ability of T cells to migrate out of the tumor tissue. The interaction of CTLA-4 with the natural ligands CD80/CD86 may hinder or promote T cell motility, depending upon the cellular context and animal models 39–42. Further studies are thus required to determine if anti–CTLA-4 impairs the motility of intratumoral Teffs, particularly when used in combination with vaccine.
We found that combination of vaccine with anti–CTLA-4 induced a mixed intratumoral and systemic inflammatory response involving both TH1 and TH2 cytokines. With the exception of TNF-α, anti–CTLA-4 plus vaccine enhanced the expression of both TH1 and TH2 cytokines compared with vaccine alone. Prominent among these was IL-3, which was also persistently elevated in the circulation. Furthermore, and importantly, anti–CTLA-4 alone, without vaccine, was unable to increase cytokine expression, pointing to the requirement of vaccine-activated T cells. Moreover, whereas vaccine triggered Teff infiltration in both Il3−/− and wild-type mice, the absence of IL-3 prevented further Teff accrual with the addition of anti-CTLA-4. This latter finding suggests that the effect of anti–CTLA-4, but not vaccine, is dependent on IL-3. We also found that CD4+ T cells were the dominant, albeit not the sole source of IL-3, as anti–CTLA-4–induced IL-3 expression was only partly attenuated in mice depleted of CD4+ T cells. It is therefore likely that CD8+ T cells also contribute to IL-3 production, and although we have not studied this directly, CD8+ T cells do accumulate intratumorally upon combined vaccine and anti–CTLA-4 therapy. Whereas, as noted before, IL-3 production can be regulated by CTLA-4 signals 32, this to our knowledge is the first report on the permissive function of intratumoral IL-3 in mediating the effect of anti–CTLA-4 therapy.
Mechanistically, we posit that the IL-3 produced in response to vaccine and anti–CTLA-4 activates the IL-3 receptor (IL-3R) expressed on tumor vasculature, and in doing so, modulates the expression of adhesion molecules. Indeed, a lack of T cell infiltration can arise from the loss of adhesion molecules on tumor endothelium 43,44. And, when combined with vaccines, anti–CTLA-4 has previously been shown to increase the expression of ICAM-1/VCAM-1 on endothelial cells 19, an effect linked to increased Teff ingress and tumor regression. Likewise, anti–CTLA-4 plus vaccine, and not vaccine or anti–CTLA-4 alone, increased ICAM-1 expression on CD31+ endothelial cells. That this effect was markedly reduced in tumors from Il3−/− mice suggests that IL-3 mediates the effect, and that this action occurs likely via IL-3R. It is also known that IL-3R expression can be induced by IFN-γ and TNF-α 33,34. While anti–CTLA-4 itself did not further enhance IFN-γ and TNF-α, our DC–targeted vaccination did elicit inflammation that could potentially increase endothelial cell IL-3R expression and thereby enhance IL-3 sensitivity. Indeed, a permissive relationship between anti–CTLA-4, the IL-3/IL-3R complex, and ICAM-1 is not unexpected as an increase in intratumoral IL-3 expression is known to increase the expression of ICAM-1, which then facilitates T cell ingress 45–47.
In summary, we find that while vaccine-induced Teff activation triggers IL-3 production, this is not enough to get peripheral T cells into tumors. IL-3–associated T cell infiltration also requires the addition of anti–CTLA-4 to upregulate adhesion molecules on tumor-resident endothelial cells via further IL-3 production. As a yet uncharacterized mechanism for intratumoral T cell accumulation, we highlight a gatekeeper role for IL-3. We further surmise that knowledge of IL-3 expression in clinical settings 37,38 may help establish a new biomarker for prognosis and therapeutic efficacy.
Acknowledgements
This work was supported by grants from the National Institute of Health, NIAID AI13013, P01A1081677 and AI051573; National Center for Advancing Translational Sciences, UL1 TR000043. N.Z. was a Howard Hughes Medical Institute (HHMI) Medical Research Fellow at the Steinman Laboratory at the time. We would like to thank H. Zebroski for synthesizing peptide libraries and J. Adams for graphic editing.
Footnotes
R.M. Steinman passed away on September 30th, 2011
Reference
- 1.Popovic A, Jaffee EM & Zaidi N Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest 128, 3209–3218, doi: 10.1172/JCI120775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi N & Jaffee EM Immunotherapy transforms cancer treatment. J Clin Invest 129, 46–47, doi: 10.1172/JCI126046 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterhouse P et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Quezada SA, Peggs KS, Curran MA & Allison JP CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermont AM et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 375, 1845–1855, doi: 10.1056/NEJMoa1611299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711–723, doi: 10.1056/NEJMoa1003466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526, doi: 10.1056/NEJMoa1104621 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Zhang L et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Schumacher K, Haensch W, Roefzaad C & Schlag PM Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer research 61, 3932–3936 (2001). [PubMed] [Google Scholar]
- 10.Marrogi AJ et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. International journal of cancer. Journal international du cancer 74, 492–501 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Vesalainen S, Lipponen P, Talja M & Syrjanen K Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30A, 1797–1803 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Halpern AC & Schuchter LM Prognostic models in melanoma. Seminars in oncology 24, S2–7 (1997). [PubMed] [Google Scholar]
- 13.Naito Y et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer research 58, 3491–3494 (1998). [PubMed] [Google Scholar]
- 14.Nakano O et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer research 61, 5132–5136 (2001). [PubMed] [Google Scholar]
- 15.Sharma P et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proceedings of the National Academy of Sciences of the United States of America 104, 3967–3972 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peggs KS, Quezada SA, Chambers CA, Korman AJ & Allison JP Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 206, 1717–1725, doi: 10.1084/jem.20082492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A et al. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers. Clinical cancer research : an official journal of the American Association for Cancer Research, doi: 10.1158/1078-0432.CCR-18-0762 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quezada SA & Peggs KS Lost in Translation: Deciphering the Mechanism of Action of Anti-human CTLA-4. Clinical cancer research : an official journal of the American Association for Cancer Research, doi: 10.1158/1078-0432.CCR-18-2509 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Quezada SA et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. The Journal of experimental medicine 205, 2125–2138 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzur M, Hamzah J & Ganss R Modulation of the “blood-tumor” barrier improves immunotherapy. Cell Cycle 7, 2452–2455 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Kandalaft LE, Facciabene A, Buckanovich RJ & Coukos G Endothelin B receptor, a new target in cancer immune therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 4521–4528, doi: 10.1158/1078-0432.CCR-08-0543 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckanovich RJ et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 14, 28–36, doi: 10.1038/nm1699 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Dentelli P, Rosso A, Olgasi C, Camussi G & Brizzi MF IL-3 is a novel target to interfere with tumor vasculature. Oncogene 30, 4930–4940, doi: 10.1038/onc.2011.204 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Korpelainen EI, Gamble JR, Vadas MA & Lopez AF IL-3 receptor expression, regulation and function in cells of the vasculature. Immunol Cell Biol 74, 1–7, doi: 10.1038/icb.1996.1 (1996). [DOI] [PubMed] [Google Scholar]
- 25.A, K., P, K., M, G., A, M. & S, S. Profile of Clients Tested HIV Positive in a Voluntary Counseling and Testing Center of a District Hospital, Udupi, South Kannada. Indian J Community Med 33, 156–159, doi: 10.4103/0970-0218.42051 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Mach N et al. Involvement of interleukin-3 in delayed-type hypersensitivity. Blood 91, 778–783 (1998). [PubMed] [Google Scholar]
- 27.Chavany C, Vicario-Abejon C, Miller G & Jendoubi M Transgenic mice for interleukin 3 develop motor neuron degeneration associated with autoimmune reaction against spinal cord motor neurons. Proceedings of the National Academy of Sciences of the United States of America 95, 11354–11359 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbett TH et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer research 44, 717–726 (1984). [PubMed] [Google Scholar]
- 29.Laky K et al. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer’s patches. J Exp Med 191, 1569–1580 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray A & Dittel BN Isolation of mouse peritoneal cavity cells. Journal of visualized experiments : JoVE, doi: 10.3791/1488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B et al. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann. N. Y. Acad. Sci. 1174, 6–17, doi:NYAS4933 [pii] 10.1111/j.1749-6632.2009.04933.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alegre M-L, Shiels H, Thompson CB & Gajewski TF Expression and Function of CTLA-4 in Th1 and Th2 Cells. Journal of Immunology 161, 3347–3356 (1998). [PubMed] [Google Scholar]
- 33.Korpelainen EI et al. The receptor for interleukin 3 is selectively induced in human endothelial cells by tumor necrosis factor alpha and potentiates interleukin 8 secretion and neutrophil transmigration. Proceedings of the National Academy of Sciences of the United States of America 90, 11137–11141 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korpelainen EI et al. Interferon-gamma upregulates interleukin-3 (IL-3) receptor expression in human endothelial cells and synergizes with IL-3 in stimulating major histocompatibility complex class II expression and cytokine production. Blood 86, 176–182 (1995). [PubMed] [Google Scholar]
- 35.Brizzi MF et al. Interleukin 3 stimulates proliferation and triggers endothelial-leukocyte adhesion molecule 1 gene activation of human endothelial cells. J Clin Invest 91, 2887–2892, doi: 10.1172/JCI116534 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin H et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320, 807–811 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Huang RR et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clinical cancer research : an official journal of the American Association for Cancer Research 17, 4101–4109, doi: 10.1158/1078-0432.CCR-11-0407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribas A et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin. Cancer Res. 15, 390–399 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Schneider H et al. Reversal of the TCR stop signal by CTLA-4. Science 313, 1972–1975 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Lozanoska-Ochser B, Klein NJ, Huang GC, Alvarez RA & Peakman M Expression of CD86 on Human Islet Endothelial Cells Facilitates T Cell Adhesion and Migration. Journal of Immunology 181, 6109–6116 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Poirier N et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Science translational medicine 2, 17ra10, doi: 10.1126/scitranslmed.3000116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruocco MG et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest 122, 3718–3730, doi: 10.1172/JCI61931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dirkx AEM et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer research 63, 2322–2329 (2003). [PubMed] [Google Scholar]
- 44.Clark RA et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of experimental medicine 205, 2221–2234, doi: 10.1084/jem.20071190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiang C-S, Hong J-H, Wu YC, McBride WH & Dougherty GJ Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Therapy 7, 1172–1178 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Chiang CS et al. Effects of IL-3 gene expression on tumor response to irradiation in vitro and in vivo. Cancer research 57, 3899–3903 (1997). [PubMed] [Google Scholar]
- 47.Sampson JH et al. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proceedings of the National Academy of Sciences 93, 10399–10404 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]