Abstract
Objective
Approximately 1 in 7 US adults have diabetes; and over 60% of deaths in patients with diabetes have cardiac disease as a principal or contributing cause. Both coronary and peripheral artery disease (PAD) identify high-risk cohorts among patients with diabetes. We have previously demonstrated improved cardiovascular outcomes with edetate disodium-based chelation in post-MI patients with diabetes, enrolled in the Trial to Assess Chelation Therapy (TACT). In these analyses we further studied the effect size of patients with diabetes and severe disease in 2 vascular beds; coronaries, and lower extremity arteries. We questioned whether greater atherosclerotic burden would attenuate the observed beneficial effect of edetate disodium infusions.
Research Design and Methods
The multicenter TACT used a double blind, placebo controlled, 2 × 2 factorial design with 1708 participants, randomly assigned to receive edetate disodium-based chelation, or placebo and high dose oral vitamins or placebo. There were 162 (9.5% of 1708) post-MI patients with a diagnosis of diabetes mellitus and PAD for this post hoc analysis. Patients received up to 40 double-blind intravenous infusions of edetate disodium-based chelation, or placebo. The composite primary endpoint of TACT consisted of death from any cause, myocardial infarction, stroke, coronary revascularization and hospitalization for angina.
Results
The median age was 66 years, 15% female, 5% non-Caucasian, and BMI was 31. Insulin was used by 32% of patients. Active infusions significantly reduced the primary endpoint compared with placebo infusions (HR, 0.52; 95% CI, 0.30–0.92; P = 0.0069), with a 30% absolute risk reduction in the primary endpoint. There was a marked reduction in total mortality from 24% to 11%, although of borderline significance (p=0.052).
Conclusion
Atherosclerotic disease in multiple vascular beds did not attenuate the beneficial effect of edetate disodium infusions in post MI patients with diabetes. Studies now in progress will prospectively test this post hoc finding.
Introduction
Treatment of atherosclerosis with edetate disodium (disodium ethylenediamine tetraacetic acid, Na2EDTA) infusions has been in use for coronary and peripheral artery disease (PAD) for over 60 years. The Trial to Assess Chelation Therapy (TACT) was the first large randomized double-blind placebo-controlled clinical trial performed to test this therapy. TACT demonstrated a reduction in combined cardiovascular events in the group assigned to edetate disodium infusions,1 particularly in patients with diabetes2. On that basis, chelation with edetate disodium was upgraded to a 2b indication in current Guidelines for Chronic Ischemic Heart Disease.3
PAD is an aggressive form of atherosclerotic vascular disease more prevalent in patients with diabetes. PAD was not a prespecified subgroup in TACT but collected on the study forms. These patients represent a particularly high-risk cohort.4 Based on the findings in the patients with diabetes and anecdotal reports of the benefits of edetate disodium-based chelation in PAD, we considered whether this therapy might maintain effectiveness in spite of the greater disease burden and higher risk of diabetes with both CAD and PAD, or whether efficacy of active therapy might be blunted due to extensive vascular disease.
Methods:
The methods and results of TACT have been previously reported.2,5 The study was a double blind 2 × 2 factorial design with 1708 participants, randomized to receive 40 intravenous (IV) infusions of edetate disodium-based chelation, or placebo. In addition, the patients were also assigned to an oral high dose vitamins and mineral regimen, or oral placebo.5 The allocation ratio was 1:1:1:1. The present analyses focus on chelation versus placebo infusions.
Treatment regimen
The 500 mL TACT intravenous infusion consisted of up to 3 g of edetate disodium, adjusted based on the estimated creatinine clearance; 7 g of ascorbic acid; 2 g of magnesium chloride; B-vitamins; and other components.5 The placebo solution consisted of 500 mL of 1.2% dextrose (2.5 g total) in normal saline. The first 30 infusions were administered weekly for 30 weeks and the next 10 biweekly to bimonthly for a total of 40 infusions. Edetate disodium also binds divalent cations such as magnesium and zinc which serve important physiological functions. Thus, all patients received a daily low dose vitamin and mineral regimen while they were receiving infusions consisting of vitamin B6, zinc; copper; manganese and chromium to prevent any possible depletion syndromes. Optimal medical therapy for post-MI patients was encouraged and monitored by the coordinating centers.
Safety Monitoring
Safety monitoring during the infusion phase included physical examination and laboratory assessments which included glucose, calcium, renal function, hepatic function and hematologic parameters.
Study Population:
Eligible patients were at least 50 years old.1,5 All had a history of MI ≥ 6 weeks before enrollment. Patients were ineligible if they had a serum creatinine > 2.0 mg/dL, platelet count < 100,000 per uL, abnormal liver function test, blood pressure > 160/100 mmHg, past allergies to chelation or vitamin components, chelation therapy within 5 years, revascularization within 6 months before enrollment, heart failure hospitalization within 6 months of enrollment, or inability to tolerate the 500 mL study infusion. Women of childbearing potential also were excluded. The study enrolled 1708 patients in 134 sites in the United States and Canada. The institutional review board at each clinical site approved the study with all patients providing informed consent. A Data and Safety Monitoring Board provided oversight for the study.
Peripheral Artery Disease and Diabetes Mellitus Definition:
The diabetes mellitus subgroup had self-reported diabetes mellitus, were taking oral or insulin treatment for diabetes mellitus, or had a fasting blood glucose of at least 126 mg/dL at the time of enrollment in the study. The presence of PAD was based on 1] patients’ self-reported diagnosis; or 2] a history of intermittent claudication on the baseline case report form; or 3] a history of prior lower extremity revascularization with either angioplasty or lower extremity bypass grafting as reported by the patient at baseline. There were 162 (9.5% of 1708) post-MI patients with a diagnosis of diabetes mellitus and PAD for this analysis.
Follow-Up:
The patients were seen at baseline and each infusion visit. Laboratory evaluations were performed during the screening visit and throughout the infusion phase, including fasting glucose levels, lipids, renal function, and clinical history of coronary revascularization. Once patients completed the infusion phase, they were followed by quarterly phone calls or clinic visits for up to five years.
Endpoints:
The composite primary endpoint consisted of death from any cause, myocardial infarction (MI), stroke, coronary revascularization and hospitalization for angina.1 The secondary endpoint consisted of a composite of cardiovascular (CV) death, non-fatal MI or stroke. All endpoint events except coronary revascularization were reviewed and adjudicated by a clinical events committee blinded to the randomized treatment assignments. Coronary revascularization was confirmed at the Data Coordinating Center.
Statistical Analysis:
Randomization for the study was accomplished using secure web base permuted block randomization stratified by the clinical site (neither diabetes mellitus nor PAD was a stratification factor). Baseline characteristics of patients were obtained during screening visits and summarized using the median and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. The baseline characteristics of patients with diabetes and PAD infused with EDTA or placebo were compared using the Wilcoxon rank-sum test for continuous variables and the conventional Chi-square test for categorical variables. The log-rank test was used for comparing EDTA chelation versus placebo with respect to the primary and secondary endpoints. Each patient was counted only once at the time of the first occurrence of a component of the primary endpoint. All treatment comparisons were performed using 2-sided statistical tests at the significance level of 0.05. Relative risks were expressed as hazard ratios (HR) with associated 95% confidence intervals (CI), calculated using the Cox proportional hazards model. The proportional hazards assumption was assessed graphically as well as using a Kolmogorov-type supremum test. Data management and statistical analyses were performed by the Data Coordinating Center at the Duke Clinical Research Institute. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results:
Baseline Characteristics of Diabetes Mellitus and Peripheral Artery Disease Patients Treated with Edetate disodium or Placebo:
Among patients with diabetes and PAD, 87 were randomly assigned to receive edetate disodium-based chelation infusion regimen and 75 assigned to receive placebo infusions. At baseline, patients with diabetes and PAD treated with edetate disodium chelation or placebo had similar demographics and clinical history. There were no differences in the use of concomitant post-MI medications or in diabetes treatment at baseline. Patients in the edetate disodium-assigned group had lower creatinine (1.0 vs 1.2 mg/dL, p = 0.001) compared with patients in the placebo group, with all other laboratory examinations similar between the two groups (Table 1), including use of statins. Therefore, creatinine was included in Cox models to be adjusted for along with the oral vitamin/placebo group assignment.
Table 1.
Baseline Characteristics of Patients with Diabetes and PAD by Infusion Arm*
| EDTA Chelation (n = 87) |
Placebo (n = 75) |
|
|---|---|---|
| Demographics Age, median (IQR), years |
65.8 (61.7, 71.7) | 67.9 (61.7, 73.7) |
| Female | 15 (17%) | 10 (13%) |
| Minority (Hispanic or non-Caucasian) | 6 (7%) | 6 (8%) |
| Caucasian | 82 (94%) | 72 (96%) |
| Body Mass Index, median (IQR) | 31.2 (27.0, 36.2) | 32.4 (28.4, 35.9) |
| Medical History Hypertension |
73 (84%) | 60 (80%) |
| Hypercholesterolemia | 74 (86%) | 67 (89%) |
| Atrial fibrillation | 11 (13%) | 14 (19%) |
| Former cigarette smoker | 57 (66%) | 41 (55%) |
| Coronary revascularization Coronary artery bypass grafting |
51 (59%) | 43 (57%) |
| Percutaneous coronary intervention | 49 (56%) | 36 (48%) |
| Concomitant Medications Beta-blocker |
69 (79%) | 55 (73%) |
| Statin | 69 (79%) | 60 (80%) |
| ACE or ARB | 66 (76%) | 53 (71%) |
| Clopidogrel | 21 (25%) | 19 (27%) |
| Warfarin | 10 (12%) | 11 (15%) |
| Aspirin or warfarin | 78 (91%) | 66 (88%) |
| Insulin | 26 (31%) | 25 (34%) |
| Multivitamin | 31 (36%) | 31 (43%) |
| Laboratory Examinations, median (IQR), mg/dL Glucose |
130.0 (104.0, 159.0) | 129.0 (103.0, 153.0) |
| Creatinine† | 1.0 (0.9, 1.3) | 1.2 (1.1, 1.4) |
| Total cholesterol | 160.0 (139.0, 188.0) | 163.5 (134.5, 197.5) |
| HDL-C | 42.0 (35.0, 49.0) | 40.0 (34.0, 48.0) |
| LDL-C | 81.0 (65.0, 105.0) | 81.5 (62.5, 113.5) |
| Triglycerides | 160.5 (112.0, 227.0) | 156.5 (110.5, 227.0) |
Abbreviations: PAD, peripheral artery disease; IQR, interquartile range; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol;
Data are expressed as No. (%) unless otherwise indicated.
P-value < 0.05. There were no other statistically significant differences between groups.
Outcome events:
Patients with diabetes, post-MI, and PAD:
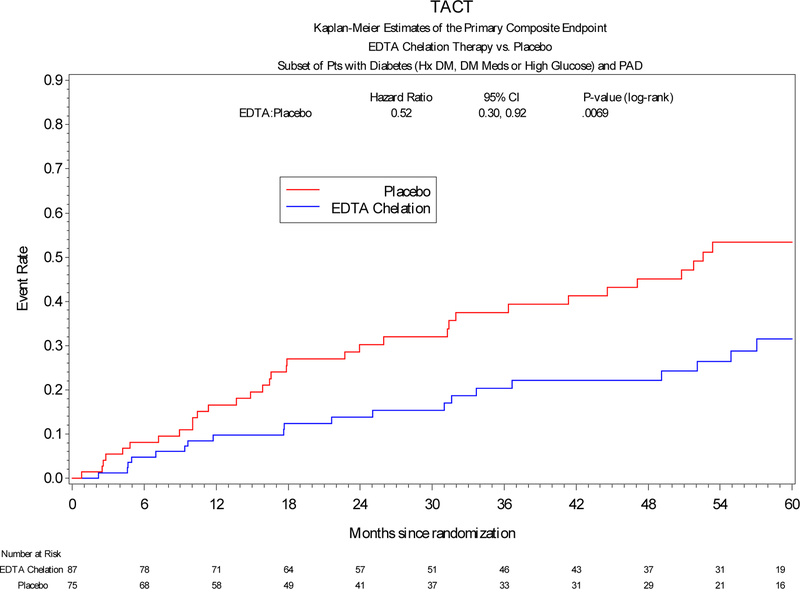
The incidence of the primary end-point was significantly lower in post-MI patients with diabetes and PAD treated with edetate disodium-based infusions compared with placebo (HR, 0.52; 95% CI, 0.30–0.92; log-rank P-value = 0.0069), with a 48% relative reduction in the combined endpoint (Figure 1). The number needed to treat to prevent a single event over 5 years was 3.3. Patients with diabetes and PAD randomized to edetate disodium-based chelation had a directionally consistent reduction in all-cause mortality (HR, 0.54; 95% CI, 0.25–1.18; log-rank P-value = 0.052). The incidence of myocardial infarction (HR, 0.48; 95% CI, 0.16–1.43; log-rank P-value = 0.078), and the pre-specified secondary endpoint of death, MI, or stroke (HR, 0.68; 95% CI, 0.32–1.42, log-rank P-value = 0.098) all demonstrated a trend towards reduction by active therapy (Table 2). An analysis of interaction of edetate disodium therapy with the presence of PAD demonstrated no significance (p = 0.442). An analysis of interaction of vitamin therapy (active versus placebo) with active treatment was not significant (p=0.235).
Figure 1.
Primary endpoint in patients with DM, MI and PAD assigned to edetate disodium or placebo
Table 2.
Clinical End Points of Patients with Diabetes and PAD by Infusion Arm
| Endpoint | EDTA Chelation (n = 87) |
Placebo (n = 75) |
Hazard Ratio*
(95% CI) |
P-value§ |
|---|---|---|---|---|
| Primary Endpoint† | 20 (23%) | 33 (44%) | 0.52 (0.30, 0.92) | 0.0069 |
| Death | 10 (11%) | 18 (24%) | 0.54 (0.25, 1.18) | 0.0523 |
| Myocardial infarction | 5 (6%) | 10 (14%) | 0.48 (0.16, 1.43) | 0.0775 |
| Coronary revascularization | 11 (13%) | 13 (17%) | 0.66 (0.29, 1.51) | 0.3212 |
| Hospitalization for angina | 2 (2%) | 3 (4%) | 0.52 (0.08, 3.28) | 0.4818 |
| Secondary Endpoint‡ | 12 (14%) | 18 (24%) | 0.68 (0.32, 1.42) | 0.0983 |
| Cardiovascular Death | 6 (7%) | 11 (15%) | 0.55 (0.20, 1.51) | 0.1289 |
| Non-fatal Myocardial infarction | 5 (6%) | 9 (12%) | 0.59 (0.18, 1.66) | 0.1333 |
Estimated by Cox proportional hazard model adjusted by vitamin group and creatinine.
By log-rank test
Primary endpoint=first occurrence of death from any cause, myocardial infarction, stroke, or hospitalization for unstable angina.
Secondary endpoint=first occurrence of death from a cardiovascular cause, myocardial infarction, or stroke.
Patients with diabetes, post-MI, without PAD:
In post-MI patients with diabetes, but without PAD, the primary end-point was significantly lower in patients treated with edetate disodium-based infusions compared with placebo (HR, 0.68; 95% CI, 0.48–0.97; log-rank P-value = 0.035), with a 32% relative risk reduction (Supplementary Figure 1). The effect size was consistent with prior analyses of post-MI patients with diabetes.2
Safety
There were 32 serious adverse events (non–end point) in the study population with diabetes and PAD, 17 placebo and 15 active (p=0.39).
Discussion
One of 7 adults in the US has diabetes- including almost 30% of Americans age 60 and over.6 Patients with diabetes have a 2 to 3-fold increased risk of vascular mortality compared to patients without diabetes. Pre-specified subgroup analyses in TACT showed that post-MI patients with diabetes accrued the greatest benefit from edetate disodium-based chelation therapy.2 This finding is being re-tested in the NIH supported TACT2 Trial. Since the combination of diabetes with established disease of two vascular territories- coronaries (post-MI), and peripheral arteries (PAD) - has been reported to convey extraordinarily high risks for adverse vascular complications, we tested whether the previously demonstrated beneficial effect of active treatment was still present in a population with diabetes and a greater burden of atherosclerotic disease.
The present study of edetate disodium-based chelation in patients with diabetes, a prior MI and PAD demonstrates a 48% relative, and 30% absolute risk reduction in combined cardiovascular events (P = 0.0069). Such a large effect size is unexpected, and merits explanation as well as further study.
PAD is an atherosclerotic occlusive disease affecting the lower extremities and confers very high vascular risk. In such patients, mortality is most often due to coronary events, placing the spotlight on the residual atherosclerotic risk of patients with diabetes and established atherosclerosis.7,8 Our hypothesis in embarking on the present analyses was that chelation and excretion of vasculotoxic metals, particularly cadmium and lead –metals preferentially chelated by edetate disodium-9 might still demonstrate a reduction in cardiovascular events in spite of the greater atherosclerotic burden of patients with diabetes and disease in multiple vascular territories.
There are robust epidemiologic data that support a causal association of toxic metals with peripheral artery disease.10–16 A recent meta-analysis encompassing over 300,000 individual patients demonstrated that lead and cadmium are cardiovascular risk factors.16,17 But within the associations teased out by epidemiologic studies, cadmium appears to have a stronger association with PAD. This is consistent with work from Tellez-Plaza et al, who demonstrated an association between cadmium exposure and incidence of PAD.11 And more recently, Zhuang et al in 2018 analyzed the NHANES data for predictors of PAD in an environment–wide analysis encompassing 417 measured and self-reported variables. Cadmium exposure was 1 out of only 4 independently predictive variables.10 Thus, our striking findings with a cadmium and lead chelator are consistent with the present science on low-level toxic metal exposure and cardiovascular disease.
In the present analyses, the edetate disodium-based chelation therapy regimen reduced the incidence of a composite cardiovascular end-point with a large effect size. This is of particular importance as patients were concurrently taking evidence-based post-MI medical therapy. We also demonstrated, in the full diabetes cohort, that randomization to active treatment did not improve glycemic control.2 Therefore, we are led to the internally consistent conclusion that the TACT infusions may be directly targeting additional modifiable risk factors – cadmium and lead.
The pharmacologic data on edetate disodium as used in TACT are supportive. Edetate disodium is an artificial amino acid synthesized in 1935 that functions as a chelating agent with a high affinity for lead and cadmium.18 A previous study demonstrated that post-MI patients treated with a single infusion of edetate disodium had a significantly higher urinary excretion of cadmium and lead following a 3 gram infusion compared with baseline (633% higher for cadmium and 3835% higher for lead, p < 0.001 for both compared with baseline urinary levels).9 Cadmium is a highly toxic metal widely distributed in the environment.19 Soil contamination of cadmium is a significant environmental problem because vegetables and grains bio-concentrate cadmium from soil resulting in major cadmium exposure through diet and smoking tobacco leaf. More recent data suggest that even in patients with coronary disease, the presence of cadmium in urine is highest when there is concomitant severe critical limb ischemia, supporting the underlying concept and results of this paper.20
Lead is more commonly recognized as a cardiovascular toxicant and lead abatement is recognized as desirable. In spite of this, lead exposure is still two orders of magnitude higher today than prior to the 17th century.21 To the point, a recent analysis of NHANES data reported over 250,000 cardiac deaths per year in the US attributable to low level lead contamination that persists to this day.22 Thus, both cadmium and lead are vasculotoxic. Our work suggests that even in patients with the highest atherosclerotic burden, treatment with edetate disodium-based chelation has downstream vascular benefits, as observed here.
There have also been various hypotheses on the effects of metals in diabetes. Diabetes increases the production of advanced glycation end products, which result in macrovascular complications.23 Advanced glycation end products interact with endothelial cells to induce release of pro-atherogenic molecules implicated in atherogenesis.24 Metals bind to glycation end-products, promoting reactive oxidative species, endothelial dysfunction and inflammation, characteristics of atherosclerosis. Advanced glycation end products require metal catalyzed oxygen chemistry for their formation, thus edetate disodium-based chelation may have specific benefit in patients with diabetes.25 PAD in patients with diabetes affects infrapopliteal vessels thus decreasing vasculotoxic metal burden may reduce endothelial dysfunction, reactive oxygen species and pro-atherosclerotic burden. This may be supported by the continued divergence of the active compared with placebo curves for the primary end-point well after infusions ended at about 18 months (Figure 1). Additionally, PAD atheroma cause symptoms by obstruction, not plaque rupture. Considering that the study was undertaken over many years and the active EDTA and placebo curves for the primary end-point continued to diverge, one might hypothesize that total atheromatous burden rather than plaque rupture was the entity reduced or prevented. Interestingly some current diabetes medications with positive cardiac effects may also have chelating properties.26,27
This post-hoc study has many limitations. First, the self-reported history of PAD, or the history of intermittent claudication, may be due to other factors other than vascular disease. An ongoing trial TACT3a, which includes critical limb ischemia, a severe stage of PAD, will help further investigate the benefit of edetate disodium-based chelation in this subgroup of patients with diabetes. Second, neither urine or blood levels of cadmium or lead were obtained during the infusion phase or follow-up. Both toxic metals have been associated with cardiovascular disease thus decreasing the total body burden of these metals may explain the benefit of edetate-disodium based chelation. The ongoing TACT2 trial in post-MI patients with diabetes is collecting urine and blood levels. Third, although site investigators were trained and monitored for the use of optimal medical therapy for post-MI patients, circulating LDL-C levels were not optimal for this high-risk population as understood in the present day. Finally, current smokers and patients with chronic kidney were excluded from the study which limits generalizability. Low environmental lead exposure has shown a possible acceleration in renal insufficiency in patients without diabetes28. Consequently, further studies are needed to investigate the benefit of edetate disodium-based chelation in this subgroup of patients.
Conclusions;
In spite of extreme atherosclerotic burden, post-MI patients with diabetes and PAD on medical therapy demonstrated a significant reduction in combined cardiovascular events with edetate disodium-based chelation therapy. When faced with patients with diabetes and disease in multiple vascular beds, clinicians may want to consider edetate disodium-based therapies. These analyses, however, do not yet constitute enough evidence to recommend routine use of chelation therapy for all post-MI patients with diabetes and PAD. An ongoing clinical trial that is still recruiting performance sites (www.tact2.org), and another yet to start (TACT3a) will provide a definitive answer in the near future.
Supplementary Material
Highlights.
Diabetic patients with peripheral artery disease arguably have the worst cardiovascular prognosis.
We published in 2014 that post-MI diabetic patients demonstrated a large risk reduction in combined coronary events over 5 years when treated with edetate disodium-based chelation infusions. In this paper, we ask whether those patients with atherosclerotic burden in multiple areas, coronaries and peripheral arteries (PAD), would demonstrate benefit, or whether the greater atherosclerotic burden of PAD in post-MI diabetic patients would attenuate the beneficial effect of edetate disodium-based infusions.
In spite of greater atherosclerotic burden, post-MI diabetic patients with PAD randomized to edetate disodium-based therapy demonstrated a significant reduction in combined cardiovascular events over 3 years.
Edetate disodium therapy should be considered for post-MI diabetic patients with PAD.
Acknowledgments
Source of Funding
The National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute provided funding and oversight, grant U01AT001156 and U01HL092607.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no conflicts.
References:
- 1.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Linbald L, Lewis EF, Drisko J, Lee KL; TACT Investigators. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA 2013;309(12):1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, Nahin RL, Ouyang P, Rozema T, Magaziner A, Nahas R, Lewis EF, Linbald L, Lee KL. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes 2014;7(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–67. [DOI] [PubMed] [Google Scholar]
- 4.Jude EB, Oyibo SO, Chalmers N, Boulton AJM. Peripheral artery disease in diabetic and nondiabetic patients. Diabetes Care 2001;24(8):1433–1437. [DOI] [PubMed] [Google Scholar]
- 5.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Drisko JA, Lee KL. Design of the Trial to Access Chelation Therapy. Am Heart J 2012;163(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Heart Disease and Stroke Statistics. 2018. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000558. Accessed August 19, 2018.
- 7.Iida O, Takahara M, Soga Y, Azuma N, Nanto S, Uematsu M. Prognostic impact of revascularization in poor-risk patients with critical limb ischemia. JACC: Cardiovasc Interv 2017; 10 (11):1147–57. [DOI] [PubMed] [Google Scholar]
- 8.Faglia E, Clerici G, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Lupattelli T, Morabito A. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J of Vascular and Endovasc Surg 2006;32(5):484–490. [DOI] [PubMed] [Google Scholar]
- 9.Arenas IA, Navas-Acien A, Ergui I, Lamas GA. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients following a single edetate disodium-based infusion. 2017. Environ Res;158:443–449. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang X, Ni A, Liao L, Guo Y, Dai W, Jiang Y, Zhou H, Hu X, Du Z, Wang X, Liao X. Environment-wide association study to identify novel factors associated with peripheral arterial disease: Evidence from the National Health and Nutrition Examination Survey (1999–2004) 2018; 269:172–177. [DOI] [PubMed] [Google Scholar]
- 11.Tellez-Plaza M, Guallar E, Fabsitz RR, Howard BV, Umans JG, Fransesconi KA, Goessler W, Devereux RB, Navas-Acien A. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes 2013;6:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking and increased risk of peripheral arterial disease. Circulation 2004;109(25):3196–201. [DOI] [PubMed] [Google Scholar]
- 13.Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation 2006;114(13):1347–9. [DOI] [PubMed] [Google Scholar]
- 14.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiology 2013;24(3):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect 2012;120(7):1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, Chowdhury S, Gobin R, Franco OH, Di Angelantonio E. Environmental toxic metal contaminants and risk of cardiovascular outcomes: systematic review and meta-analysis. BMJ 2018;362:k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellez-Plaza M, Guallar E, Navas-Acien A. Environmental metals and cardiovascular disease. BMJ 2018;362:k3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Münz F, inventor; General Aniline Works, Inc., assignee. Polyamino carboxylic acids and process for making same US patent 2,130,505. September 20, 1938. [Google Scholar]
- 19.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep 2013;15(10):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ujueta F, Arenas IA, Diaz D, Yates T, Beasley R, Navas-Acien A, Lamas GA. Cadmium level and severity of peripheral artery disease in patients with coronary artery disease. Eur J Prev Cardiol 2018. 10.1177/2047487318796585 [DOI] [PubMed] [Google Scholar]
- 21.Flegal AR, Smith DR. Lead levels in preindustrial humans. N Engl J Med 1992;326(19):1293–4. [PubMed] [Google Scholar]
- 22.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health 2018;3:e177–84. [DOI] [PubMed] [Google Scholar]
- 23.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab 2013;pII:S1043–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40:405–412. [DOI] [PubMed] [Google Scholar]
- 25.Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun 1998;250:385–389. [DOI] [PubMed] [Google Scholar]
- 26.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012;61:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol 2002;13:2478–2487. [DOI] [PubMed] [Google Scholar]
- 28.Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med 2003;348(4):277–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.