Abstract
Purpose
Residual newborn screening dried bloodspots (DBS) are a valuable resource for research but the extent, type, and nature of uses are unknown. The objective of this research was to systematically review the published literature about secondary research uses of residual DBS using a scoping review protocol.
Methods
A total of 654 publications meeting the inclusion criteria with a 94% interrater reliability were identified. A coding template was created with input from expert advisory board to summarize the data. Electronic literature search of Ovid MEDLINE, Embase (via Embase.com), CINAHL (EBSCO),and Science and Social Sciences Citation Indices (via Web of Science) was conducted.
Results
A large proportion of the secondary research with DBS was conducted within the United States (30%). The number of published studies utilizing DBS are increasing each year, primarily with observational or case–control designs. Only a small number of studies reported whether or not consent was obtained and if the DBS were identifiable or not.
Conclusion
Outcomes of this research indicate that residual DBS are well utilized worldwide for research addressing individual and public health issues. Future analyses will summarize outcomes of disease-specific research and provide evidence of the use of residual DBS in research on health outcomes.
Keywords: newborn screening, dried bloodspots, scoping review, evidence synthesis
INTRODUCTION
Newborn bloodspot screening programs (NBS) are collectively the largest application of genetic testing in medicine.1 Approximately 4 million infants in the United States are screened for more than 30 disorders each year.2 NBS tests are run using a few drops of blood drawn from the newborn’s heel, and although the amount of blood taken is small, there is usually blood left over after NBS tests. The remaining blood drops, referred to as dried bloodspots (DBS), are retained, stored, and made available for research and investigation by a number of state programs.3 For the most part, the storage and use of the NBS DBS has occurred without explicit parental permission. The leftover blood, or the residual dried bloodspots, are stored for variable lengths of time by different state-based NBS programs. Some states only retain the DBS for long enough to complete the clinical screening activities (about 3–6 months). Six states store DBS indefinitely, eight states store them for 21–30 years, and three states store them for 10–20 years.4
In 2009, the Minnesota and Texas state public health programs were sued by parents who objected to use of residual DBS without parental knowledge or permission. The primary argument for the Minnesota lawsuit was the premise that secondary research use of DBS without parents’ permission violated the state's genetic privacy act. In Texas, the lawsuit was based on a constitutional argument that retention violated the Fourth Amendment as a form of illegal search and seizure. While the legal theories differed substantially in these two cases, the results indicate both that parents have legitimate concerns about the retention and use of residual DBS without consent/parental permission and that there can be legal grounds for objecting to this practice.
In response to these concerns, the US Government enacted amendments to the Public Health Service Act. The Newborn Screening Saves Lives Reauthorization Act of 2014 characterizes the use of DBS as human subject research and, as such, requires informed consent for storage or use (https://www.govtrack.us/congress/bills/113/hr1281/text). It highlights that biospecimens collected in other clinical settings are different than those obtained through newborn screening because most clinical and research biospecimens are obtained with consent rather than through state mandated public health programs.5 However, the recent updates to the Common Rule supersede the Reauthorization Act and will not require consent for the use of de-identified biospecimens, including DBS from newborn screening programs.
In the aftermath of the legal controversy over DBS and the Reauthorization Act of 2014, a number of states have implemented a consent process for the retention and research use of DBS. Implementing consent for DBS has raised concerns in the research and health policy communities. Specifically, there are concerns that the number of available DBS samples will decrease substantially, leading to a loss of population representativeness. However, to date, there has not been an evidence synthesis of how DBS have been used in secondary research. More specifically, a major concern among stakeholders is how DBS have been used for research not directly related to NBS. This project sought to identify and describe the types and extent of research using DBS related and unrelated to NBS to provide decision makers—scientists, policy makers, parents, and others—with a comprehensive overview of the nature and extent of secondary research uses of DBS. The following research questions were used to guide this evidence synthesis:
How much research has been undertaken using DBS?
What type of research has been conducted using DBS?
What study designs are employed in research using DBS?
MATERIALS AND METHODS
A scoping review is a type of research evidence synthesis that aims to “map the literature on a particular topic or research area and provide an opportunity to identify key concepts; gaps in the research; and types and sources of evidence to inform practice, policymaking, and research.”6 Using Arksey and O’Malley’s framework6 with methodological and definitional refinements suggested by Daudt and Colqhoun, a scoping review protocol was created by systematic review methodologists, librarians, and an expert advisory committee.7 The expert advisory committee was comprised of experienced professionals in NBS, laboratory methods, and public health.
Eligibility criteria
Studies describing secondary research utilizing residual dried bloodspots from newborn screening programs were the primary criterion. Secondary research is defined as research unrelated to the original purpose of bloodspot collection. All study designs, as well as quality assurance or quality improvement studies, using DBS not directly related to current NBS modalities were included. Conference abstracts, posters, and non-English reports were excluded.
Search methods (identifying the evidence)
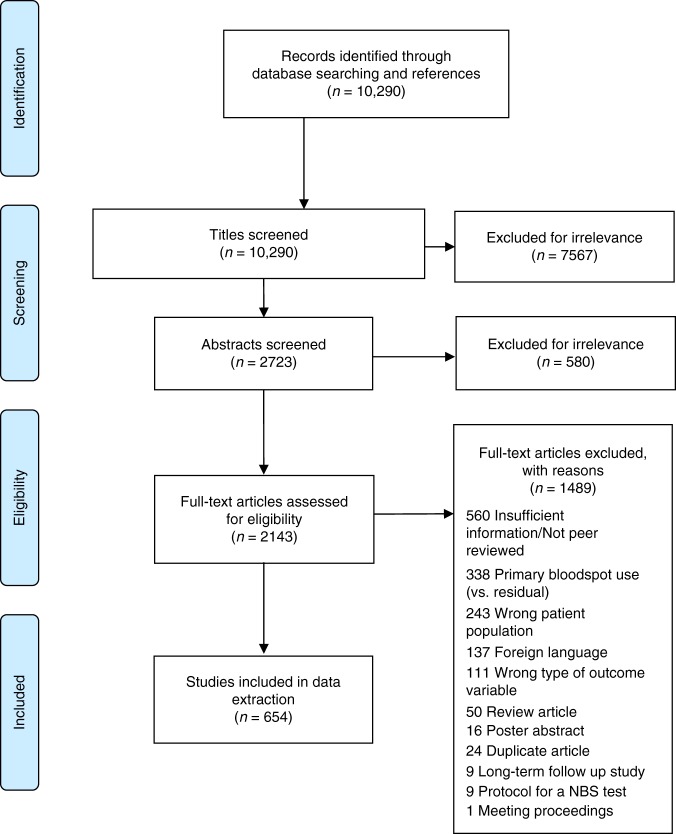
An information specialist with experience in systematic reviewing was employed to assist with the development of methodologically sound, sensitive search strategies to interrogate the published literature using the following databases: Ovid MEDLINE, Embase (via Embase.com), CINAHL (EBSCO), and Science and Social Sciences Citation Indices (via Web of Science). Search strategies were reviewed by a second librarian using the PRESS Checklist.8 Any lists of included and excluded studies from related systematic reviews or meta-analyses identified during database searches were also evaluated. Search terms used included (1) bloodspot OR bloodspots OR bloodspot OR blood samples OR Guthrie, AND (2) archived OR archive OR dried OR residual. A draft Ovid MEDLINE strategy is provided as an appendix. No contact with authors was initiated and there was no inclusion of unpublished abstracts/studies. This resulted in 10,290 unique citations after duplicates were removed (see Fig. 1).
Fig. 1.
PRISMA 2009 flow dagram. NBS newborn screening.
Data management
The 10,290 citations were exported to Covidence for an abstract review. Two trained research assistants independently assessed the titles of the publications for inclusion/exclusion. Based on the titles, the citations were coded as relevant, not relevant, and unknown based on established inclusion criteria. Citations categorized as relevant and unknown (n = 2723) were then exported into Covidence systematic review software for an abstract review. Then two PhD prepared research assistants independently assessed the abstracts and voted to include or exclude. Throughout the process, a senior investigator conducted quality checks to ensure consistency between the two coders and resolved discrepancies in their inclusion/exclusion determinations. These steps occurred between August 2015 and December 2016. In spring 2017, an updated search of the literature resulted in 878 additional citations, published from August 2015 to May 2017. Finally, 2143 publications underwent full-text review by the two PhD prepared research assistants, and 654 publications were identified as meeting the inclusion criteria with a 94% interrater reliability. Discrepancies were resolved by the lead author.
Data extraction
Data was extracted from the included 654 publications by one of the PhD research assistants in the software program RedCap. Consistency and accuracy were reviewed by two independent investigators for 10% of the data, and no major discrepancies were found. Coding categories for data extraction were created through an iterative process with the expert advisory board. After the first coding template was created, it was pilot tested among 50 publications and the results were presented back to the expert advisory board. Additional refinements to the coding template were made and then the official data extraction was initiated (the original 50 publications were recoded). The data coding template included year of publication, type of the research, study design, geographic location, funding source, anonymized or de-identified DBS, parental permission, population of interest, quality improvement/assurance, pilot study, target of analysis within the DBS, number of DBS used, and disease/condition targeted. For definitions, refer to the protocol located in online supplemental material.
RESULTS
The results presented below are categorized by an overview of all DBS research including international and US-only DBS research. The results are further categorized for DBS research conducted in the United States.
Study-specific data
The dates of publications for the 654 articles ranged from 1973 to 2017. Approximately 55.21% of all of the studies were published between 2008 and 2017, and 26.23% between 1998 and 2007 (see Fig. 2). Of the US studies (n = 192), 66.67% were conducted between 2008 and 2017, and 18.75% between 1998 and 2007.
Fig. 2.
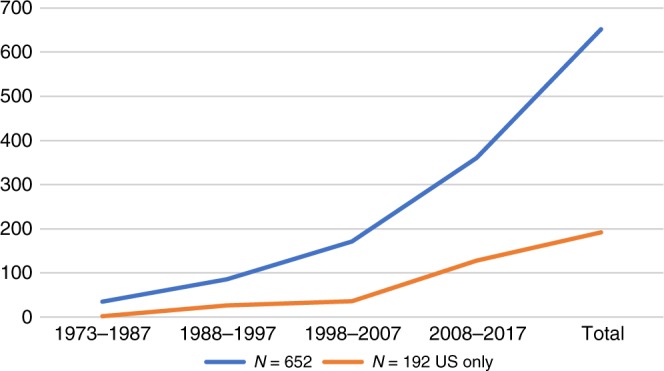
Number of dried bloodspot (DBS) studies by year.
There were no randomized controlled or clinical trials, and the majority were observational study designs (42.6%) or case–controls (38.8%); 15.1% were cross-sectional and 3.5% were case reports. Study designs were defined from the National Library of Medicine’s (NLM) Medical Subject Headings. For US studies, 46.7% were observational, 39% were case–control, 12.6% were cross-sectional, and 1.7% were case reports.
Country of origin was also included in the analyses. Some of the DBS reported more than one country for DBS and as such there are more countries listed than actual research conducted. About 55% of the studies originated outside the United States; 7.4% were from the United Kingdom, 7.2% were from Denmark, and 6.1% were from Italy, followed by Australia (5.8%), Germany (5.5%), and Sweden (5.0%). Of the studies from the United States (n = 192), 13.8% were from California, 12% were from multiple states, 11.7% were from New York, followed by Washington (6.0%), North Carolina (5.7%), and Texas (5.1%). For the DBS collected in the United States that received federal funding, 67.2% were funded by the National Institutes of Health (NIH), 16.4% were funded by multiple federal agencies (often including the NIH), 13.7% were funded by the Centers for Disease Control and Prevention, and 2.7% by other federal agencies. There were three studies that used DBS from the United States but the research was funded and conducted outside of the United States.
Consent and identification of DBS
The publications were also evaluated to document if consent or parental permission was obtained. In 33.8% of the studies, consent or parental permission was obtained as part of the research process, 4.1% stated no consent, and in 62.1% of the cases consent was unknown because it was not reported. For US studies, 21.9% reported consent or parental permission was obtained for the research, 2.6% reported no consent, and 75.5% were unknown.
These publications were further analyzed regarding the de-identified or anonymous nature of the DBS. Overall, only 36.9% reported using anonymized or de-identified data while 10.6% reported using identified data and 52.6% did not report whether the DBS were identified, de-identified, or anonymous. For US studies, 55.7% reported that de-identified or anonymized DBS were used, 7.3% stated DBS were identified, and 37% did not report this information. These data are further analyzed by consent/parental permission and identification status of DBS for US-only studies (See Table 1).
Table 1.
Identification and consent for dried bloodspots (DBS)
| US studies only (n = 192) | ||||
|---|---|---|---|---|
| Parental permission/consent obtained | ||||
| De-identified/anonymized | Yes (n = 42) | No (n = 5) | Unknown (n = 145) | |
| Yes | 13 (30.1%) | 5 (100%) | 89 (61.4%) | |
| No | 6 (14.6%) | 0 (0%) | 8 (5.9%) | |
| Unknown | 23 (54.8%) | 0 (0%) | 48 (33.1%) | |
Type of method targeted and disease for DBS
The type of method used to analyze the DBS also was assessed. Three target methods categories were used: analytes (54.2%), DNA (40.1%), and enzymes (5.7%). This was similar to US studies: analytes (54%), DNA (40.3%), and enzyme (5.8%). The population used in the research was defined as targeted or general population. A targeted population was defined as specific spots, based on individuals presenting with a particular condition, to be pulled from the biobank and analyzed. A general population sample did not identify the individuals prior to the research. Most of the research used a targeted population (61.6%). For US studies, 61.5% used a targeted population. For the types of medical conditions studied, see supplemental Table S1.
Quality improvement data
The publications were also coded to include information about secondary uses of DBS for quality improvement and non-NBS related research. One code, called “quality improvement,” designated studies that tested improvements to preexisting tests. This could be an improvement in the processing time, an improvement in output, automating part of the test, etc. For example, if the published research goal was to speed up the elution phase in a time-consuming test for tyrosinimia, this would be coded as quality improvement on a preexisting test. In contrast, if the research was to develop a new approach such as using tandem mass spectrometry (MS/MS) before its established use for that NBS test, then this was considered a “novel” approach and not quality improvement. Of the 654 publications, 18.2% (n = 119) were for quality improvement. For US studies, 19.8% (n = 38) were quality improvement and 80.2% (n = 154) were not. See supplemental Table S2.
The studies were also coded to assess whether the published research was for a disease that was or was not already part of the NBS program at the time of the study. If the study’s targeted disease was included on the panel for NBS, such as assessing incidence or prevalence of a disorder such as phenylketonuria (PKU), then this was coded as part of the NBS program. In comparison, many studies were designed to determine if conditions not on the current panel could be evaluated with NBS bloodspots; these studies were marked accordingly. In total, 26.3% (n = 172) were studies that assessed a disease that was already part of the NBS program. Most of the studies aimed to assess a new disease or method that could be potentially included into the NBS program (73.7%). This was consistent with the US-only studies, in which 27.2% included diseases already on the NBS panel for their state, and 72.8% did not. Examples of new conditions at the time of the research include tyrosinemias, cystic fibrosis, cytomegalovirus, and maple syrup urine disease (MSUD). Examples of methods evaluated include MS/MS, liquid chromatography–mass spectrometry (LC-MS), and polymerase chain reaction (PCR).
Non-NBS related research with DBS for US research
Quality improvement is typically an acceptable use of research with DBS. However, the results also identified how DBS were used outside of NBS related research for US studies. Of the US-based studies (n = 192), 101 (52.6%) were conducted that were not directly related to NBS. The target methods most commonly used include analyte (52.5%) and DNA (45.5%). The study designs most utilized were observational (40.6%), case–control (36.6%), and cross-sectional (20.8%). The type of medical conditions targeted are listed in Tables S3–S6 in the online supplementary material.
Consent was obtained for 66.3% of the studies and was unknown for 29.7%. None of the studies reported using identifiable DBS without consent. Of the 5 studies that used identifiable samples, these studies were conducted by the states of Washington, Texas, Louisiana, Georgia, and California and parental permission/consent was obtained. The number of samples by state for non-NBS research are presented in Table 2 and the most-cited US studies are listed in Table 3.
Table 2.
Total number of US studies and DBS used by state for NBS and non-NBS research
| State | # of NBS studies (number of DBS used) | # Non-NBS studies (number of DBS used) | Unknowna | Total studies in each state |
|---|---|---|---|---|
| California | 13 (1,475,042) | 19 (18,367) | 32 | |
| Colorado | 1 (279,399) | 1 | ||
| Florida | 1 (3101) | 1 | ||
| Georgia | 2 (36,301) | 1 (6904) | 3 | |
| Iowa | 1 (762) | 1 | ||
| Louisiana | 1 (71) | 1 | ||
| Maryland | 5 (6864) | 1 (31,273) | 6 | |
| Massachusetts | 2 (13) | 2 (49,111) | 4 | |
| Michigan | 2 (362) | 6 (557) | 8 | |
| Minnesota | 6 (100,940) | 7 (3559) | 13 | |
| Missouri | 1 (43,701) | 1 | ||
| Multiple states | 21 (178,279) | 19 (1,469,434) | 1 | 40 |
| New Jersey | 1 (319) | 1 | ||
| New York | 11 (159,528) | 19 (25,932) | 1 | 30 |
| North Carolina | 4 (417) | 3 (89) | 1 | 7 |
| Ohio | 1 (30,582) | 1 (18) | 2 | |
| Oregon | 1 (1) | 1 | ||
| Pennsylvania | 1 (21) | 1 | ||
| Rhode Island | 1 (49) | 1 | 1 | |
| Texas | 4 (1956) | 8 (1877) | 12 | |
| Unknown | 2 (24) | 3 (7749) | 2 | 5 |
| Utah | 1 (10,000) | 1 | ||
| Virginia | 2 (296) | 2 | ||
| Washington | 9 (43,532) | 6 (1679) | 15 | |
| Wisconsin | 3 (810,790) | 3 | ||
| Total | Total | Total | ||
| 91 (3,171,148) | 101 (1,627,751) | 192 |
DBS dried bloodspots, NBS newborn screening.
aCould not be determined from article.
Table 3.
Frequency of citations of published US-based residual dried bloodspot (DBS) research
| Number of citations | Number of articles | Titles of top cited articles (# of citations) |
|---|---|---|
| >300 | 1 | • Neonatal cytokines and coagulation factors in children with cerebral palsy (368) |
| 201–300 | 4 |
• Prevalence of HIV infection in childbearing women in the United States. Surveillance using newborn blood samples (243) Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal bloodspots by tandem mass spectrometry (240) • Evidence-based path to newborn screening for Duchenne muscular dystrophy (219) • Rapid diagnosis of MCAD deficiency: quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn bloodspots by tandem mass spectrometry (208) |
| 100–200 | 11 |
• Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group (200) • Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA (192) • Development of population-based newborn screening for severe combined immunodeficiency (166) • In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia (166) • Rapid diagnosis of maple syrup urine disease in bloodspots from newborns by tandem mass spectrometry (161) • Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry (132) • Molecular genetic diagnosis of sickle cell disease using dried blood specimens on blotters used for newborn screening (129) • Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations (116) • Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor-blocking antibodies in over one million babies (104) • Prevalence of K329E mutation in medium-chain ACYL-COA dehydrogenase gene determined from Guthrie cards (104) • Rapid diagnosis of homocystinuria and other hypermethioninemias from newborns’ bloodspots by tandem mass spectrometry (100) |
| 51–99 | 16 | |
| 31–50 | 24 | |
| 11–30 | 85 | |
| <10 | 51 |
DISCUSSION
Outcomes of this research indicate that residual DBS are well utilized worldwide for research addressing individual and public health issues. A large proportion of the secondary research with DBS was conducted within the United States (30%) although many other countries actively support the use of this resource. Within the United States, California and New York demonstrate high occurrence of use perhaps because the states are large, enabling the evaluation of rare conditions, and both states store DBS indefinitely and are supportive of research collaborations. As expected, the number of published studies utilizing DBS is increasing each year, primarily with observational or case–control study designs. Finally, most of the published research is biomedical research that involves diseases that were not part of the newborn screening program at the time of the research.
Because the use of secondary research with DBS increases annually, consideration regarding what resources are needed for maintaining their sustainability is important. At this time, it is unknown what the future uptake will be especially in the context of challenges to public support and trust and state-based funding from departments of health for secondary DBS research. Watson et al. defined a three-dimensional model of biobank sustainability that included financial, operational, and social dimensions.9 Although it is not clear what financial resources are needed for storage and research use of DBS, this model does include the importance of social values such as acceptability by the public and accepted standards of practice for secondary research with DBS. As policymakers and state departments of health reflect on the benefits and costs of secondary DBS research, these results provide evidence of the extent, type, and nature of uses. These data can be used to seek further public input and ensure social values are taken into consideration for sustainability of this important resource.
Only a small percentage of research projects obtained consent or parental permission. This is permissible under the regulations if the DBS are de-identified to the investigator or if a waiver of consent is approved by the institutional review board (IRB). Unfortunately, the majority of studies we identified did not report whether DBS were identified or de-identified and whether consent/parent permission was obtained. Given the controversy over research uses of residual DBS, we encourage investigators in this domain to document elements relevant to human subject projections. Of the studies that did obtain parental permission (n = 42), 6 used identifiable samples. It is notable that there have been no known adverse events resulting in individual harm or breaches of privacy or confidentiality from research with DBS.
Given the controversies in the United States regarding the use of these samples for research, the data were coded to identify how they have been used. The majority of the research conducted with DBS was not for quality improvement efforts. Of the research conducted not directly related to NBS, much of it focused on improving understanding of an inborn genetic disease, or infectious, toxicological, or other biomedical research related to relevant childhood diseases. Most of the research utilized de-identified or anonymous samples, and consent was obtained for the majority of this research. Further, these studies also included the actual number of DBS used in this research. A total of 1,627,751 DBS were used for non-NBS related research, which is only a small percentage of the actual DBS available for research. Multiple-state collaborations were utilized the most, followed by California, and New York.
The State of Michigan in 2010 implemented a parental permission process for the storage and research use of DBS. Michigan also requires additional, study-specific consent for research that proposes to use identifiable DBS. The state developed the Michigan BioTrust for Health as a way for researchers to access stored DBS for research. The Michigan BioTrust has been successful in obtaining documented consent choices from approximately 86% of new parents with the use of a brochure and shortened consent form with 66% agreeing to the BioTrust, 19% declining, and 14% providing no signature.10 However, it is unknown how representative this sample is of the entire population with these uptake rates. Some have argued that informed consent would limit the number of DBS for development of new screening tests due to low uptake, and the potential for varying participation rates by hospitals that may exclude representative segments of the population. Further, the adequacy of informed consent is questioned in this context due to the timing of newborn screening in the hectic postnatal environment.11 Other arguments against broad consent for DBS include the difficulty in communicating what types of studies will use DBS, who will use them, and how long they will be stored. Now that the extent, type, and nature of secondary research using DBS is better understood, and with the revised Common Rule changes occurring soon, revisiting which type of consent approach (opt-in or opt-out) best supports representativeness, sustainability, and social values of secondary DBS research should be evaluated.
Research indicates that parental permission is supported by the public because it creates more transparency, options for education, and a choice that may improve trust in newborn screening programs.11 Our evidence synthesis maps the types of secondary research uses with DBS, potentially useful for NBS programs, investigators, and parents as an illustration of the value of DBS and the potential to improve public health and newborn screening through research. Further, it gives examples of how the DBS have been used in research that may promote more transparency and education about this important resource for biomedical research.
Limitations
Although this study used established guidelines, experienced systematic review experts, librarians, and independent coders, some articles may have been missed. The large number of published papers included in this review is not typical of evidence syntheses, but significantly captures the nature, type, and extent of use of DBS in the literature. Also, the coding template was purposively broad due to the number of articles, and more detailed extraction of a more specific evidence synthesis (e.g., type of disease) will allow more in-depth information about the research use of DBS in these domains. As such, next steps are to conduct meta-analyses in more focused areas with more detailed coding templates to assess effectiveness, as well as to examine how this resource can link with other data sources so that use of residual DBS research can have a positive impact on health outcomes.
Electronic supplementary material
Acknowledgements
Special thanks to our expert advisory board: Nicola Longo, Marci Sontag, Kim Hart, Marzia Pasquali, Michele Fiander, Mike Watson, Rebecca Anderson, and Beth Tarini. This research was supported by National Institute of Child Health and Development (NIH/NICHD HD082148). It was partly supported by the University of Utah Center for Clinical and Translational Science (NIH/NCATS 1UL1TR001067), and the Utah Center in Excellence for Ethical, Legal and Social Implications Research (NIH/NHGRI HG009037).
Disclosure
The authors declare no conflicts of interest.
Electronic supplementary material
The online version of this article (10.1038/s41436-018-0387-8) contains supplementary material, which is available to authorized users.
References
- 1.Botkin JR. Research for newborn screening: developing a national framework. Pediatrics. 2005;116:862–871. doi: 10.1542/peds.2004-2571. [DOI] [PubMed] [Google Scholar]
- 2.Therrell BL, Johnson A, Williams D. Status of newborn screening program in the United States. Pediatrics. 2006;117:212–252. doi: 10.1542/peds.2005-2633C. [DOI] [PubMed] [Google Scholar]
- 3.Association of Public Health Laboratories. APHL position statement: newborn screening residual dried blood spot specimens. 2013. https://www.aphl.org/policy/Position_Documents/NBS_2013_Newborn_Screening_Residual_Dried_Blood_Spot_Specimens.pdf. Accessed 6 December 2018.
- 4.Association of Public Health Laboratories. Data retention profile by state. 2015. https://data.newsteps.org/newsteps-web/reports/profile/dataRetention.action. Accessed 9 September 2015.
- 5.Harty-Golder B. Retention and ownership of blocks. MLO Med Lab Obs. 2004;36:37. [PubMed] [Google Scholar]
- 6.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 7.Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13:48. doi: 10.1186/1471-2288-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Watson PH, Nussbeck SY, Carter C, et al. A framework for biobank sustainability. Biopreserv Biobank. 2014;12:60–68. doi: 10.1089/bio.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michigan Department of Health and Human Services. Michigan Department of Health and Human Services newborn screening follow up program quarterly report. Michigan Department of Health and Human Services 2016;2nd Quarter. https://www.michigan.gov/documents/mdhhs/NBS_Fall_2016_Newsletter_543388_7.pdf. Accessed 6 December 2018.
- 11.Rothwell E, Wong B, Anderson RA, Botkin JR. The influence of education on public trust and consent preferences with residual newborn screening dried blood spots. J Empir Res Human Res Ethics. 2016;11:231–236. doi: 10.1177/1556264616656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.