Abstract
Variants in FA2H have been associated with a wide range of phenotypes including hereditary spastic paraplegia type 35 (SPG35); however, genetically confirmed cases have not been reported in Africa. We report here the first African family with a variant in the FA2H gene causing SPG35.
Four affected siblings with consanguineous parents presented with walking difficulty at age 2-3 and progressive limb weakness. They became wheelchair-bound two years after disease onset. Neurological examination confirmed lower greater than upper limb weakness and atrophy, brisk reflexes throughout, and spasticity with scissor legs. The patients also had choking, urinary urgency and mental retardation. A brain MRI showed thin corpus callosum and periventricular leucodystrophy. Testing of 58 SPG genes showed a homozygous variant in FA2H at the exon 5 donor site c.786+1G>A, which has previously been shown to cause skipping of exons 5 and 6 of the gene transcript. This variant segregated with the disease in the family.
This variant has been reported previously with a similar phenotype and slow progression in a population with different background. Here we confirm its pathogenicity and expand its genetic epidemiology. Studying diverse populations may help to increase understanding of the disease mechanism and ultimately lead to therapeutic targets.
Keywords: SPG35, FA2H variant, genetic epidemiology, Mali, Africa
Introduction
Hereditary spastic paraplegias (HSPs) are heterogeneous neurodegenerative disorders due to progressive and length-dependent degeneration of the corticospinal tracts and posterior columns of the spinal cord. Abnormal development has also been implicated in the occurrence of the disease. They manifest with progressive lower extremity spasticity and hyperreflexia and mild lower limb weakness in their “pure” forms or are associated with other neurologic or non-neurologic symptoms in their “complicated” (or “complex”) forms (Harding, 1993). All modes of inheritance are seen; dominant HSPs are prevalent in northern Europe and North America, while recessive cases are mostly seen in North Africa, the Middle East, and Mediterranean regions (Harding, 1993; Erichsen et al., 2009), probably due to the high consanguinity rate in these areas. Reports of genetically-confirmed HSP cases in sub-Saharan Africa are rare (Landouré et al., 2013). SPG35 is a complex HSP caused by variants in the fatty acid 2-hydroxylase (FA2H) gene. The c.786+1G>A change was previously reported in two Arabic families (Edvardson S, 2008). Since then, no other family has been reported with this variant, though other pathogenic variants in the same gene causing different phenotypes have been identified (Kruer et al., 2010; Pierson et al., 2012). We report here a Malian family with early onset HSP caused by the c.786+1G>A variant in FA2H.
Methods
The patients underwent a thorough neurological examination and photographs were taken after giving informed consent. Brain MRI, HTLV-1 serology and blood chemistries including Vitamin B12 and E levels were performed. DNA was extracted from peripheral blood from the three patients, the parents and an unaffected brother for genetic analysis. A Spastic Paraplegia Next-Generation DNA Sequencing Test including a panel of 58 genes (Medical Neurogenetics, Atlanta, GA) was performed using the Illumina platform HiSeq 1500 using the Agilent SureSelect capture reagent with 99.9% coverage of the regions of interest. Uncovered regions with known pathogenic variants are sequenced in a targeted manner (List based on ClinVar Database: July 5, 2016 release). DNA from all available family members was sequenced for segregation analysis.
Results
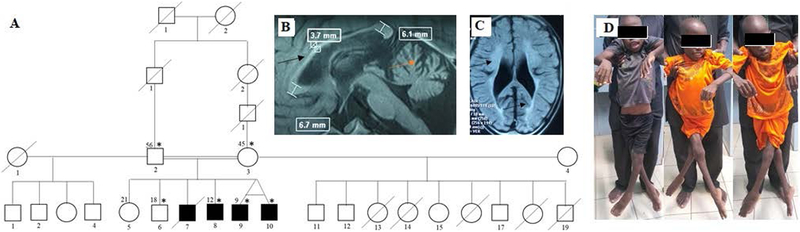
The patients are from a polygamous family of Mandingo ethnicity (Figure 1A), but only offspring of the couple with consanguinity presented with the disease. Four siblings, of whom one died at age 10, developed symptoms between 2 and 3 years of age, starting with progressive walking difficulty followed by frequent falls. After two years, the patients became wheelchair-bound, and they developed choking on food and bladder urgency.
Figure 1:
Pedigree and brain MRI of a subject with SPG35. A) Pedigree of the family showing affected individuals in the branch with consanguinity. Asterisks indicating those seen in clinic. Brain MRI of the oldest living patient showing B) T1 signal: thin corpus callosum (black arrow), and cerebellar atrophy (red arrow), and C) T2 FLAIR: periventricular leucodystrophy (black arrow). D) Patient photographs showing spasticity with scissor legs.
Clinical examination showed brisk reflexes, severe spasticity with scissor legs (Figure 1D), irreducible hyperextension of knees and bilateral equine foot, extensor plantar responses, and muscle atrophy and weakness more marked in lower extremities. Severe dysarthria with very few words if any, mental retardation, and a cat-like cry were also noted. Moreover, the patients had clinodactyly, and the oldest had scoliosis. There was no apparent sensory, visual, or hearing impairment. HTLV-1 serology was negative, and vitamin B12 and E levels were normal. A brain MRI showed thin corpus callosum and periventricular leucodystrophy (Figure 1B & C). Clinical and laboratory features are summarized in Table 1. HSP-targeted gene panel testing including SPG4 deletion and duplication and mitochondrial DNA sequencing in one affected individual identified a homozygous missense variant at the splice donor site of exon 5 of the FA2H gene at position c.786+1G>A (Supplemental Figure 1 A-C).
Table I:
Clinical and laboratory findings in the patients
| Findings╲Patients | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Current age | 12 | 9 | 9 |
| Age at onset | 2-3 | 3 | 3 |
| Initial symptom | Walking difficulty | Walking difficulty | Walking difficulty |
| Spasticity | + | + | + |
| Limb atrophy | + | + | + |
| Cerebellar signs | + | + | + |
| Cognitive impairment | + | + | + |
| Wheelchair-bound age | 7 | 5 | 5 |
| Dysarthria age | 5 | 4 | 4 |
| Skeletal deformity | + | + | + |
| MRI findings | TCC, CA, PVL | ND | ND |
| HTLV-1 | Negative | ND | ND |
| Vitamin B12 and E | Normal | ND | ND |
Age in years, +: present, −: absent, TCC: thin corpus callosum, CA: cerebellar atrophy, PVL: periventricular leucodystrophy, ND: not done
Sequencing of DNA from five other family members demonstrated that the c.786+1G>A variant co-segregates perfectly with the disease status; the other two affected siblings were homozygous for the variant while the parents and another unaffected sibling were heterozygous.
Discussion
To date, about 100 HSP types (SPG1–79, plus others) have been reported worldwide, and mutations have been identified in about 80 genes, including FA2H (de Souza et al., 2017). To our knowledge, pathogenic variants in FA2H have not been previously identified in Africa. Only a few African SPG families with molecular diagnosis have been reported (Landouré et al., 2013; Boukhris et al., 2009; Guinto et al., 2017), although studies have suggested that SPG is underreported in Africa, and new clinical and genetic entities might be found there (Landouré et al., 2013; Landouré et al., 2016). The low rate of SPG reporting could be due to limited access to genetic analysis and a tendency to attribute lower extremity spasticity to more common spinal disorders such as injury, infection (Roman, 2014), and tumors.
Although the major clinical symptoms and disease course of the cases we reported here are similar to those published previously (Edvardson et al., 2008), their ages of onset are different. Our data showed earlier onset of the disease, on average at 2–3 years of age, and patients became wheelchair-bound around 5–6 years old, while late onset is the rule for patients in published literature, with the first symptoms around 4–6 years and the patients remaining ambulatory until 11–14 years of age. Another particularity of the cases we reported here is the early occurrence of cognitive impairment and the high frequency of choking and bladder urgency. The phenotypic differences between the cases in our study and those published might be due to genetic or environmental factors or differences in the clinical care.
The c.786+1G>A was reported in two Arabic families (Edvardson et al., 2008) with a phenotype similar to what we reported here. The variant lies at the splice donor site of exon 5 of the FA2H gene and was shown to result in the skipping of exons 5 and 6, which has been predicted to abolish its catalytic activity (Edvardson et al., 2008). Other pathogenic variants in the FA2H gene has been involved in a wide range of phenotypes including leucodystrophy, neurodegeneration with brain iron accumulation, and spastic paraplegia, or a combination of these features with some degree of genotype-phenotype correlation (Edvardson et al., 2008; Kruer et al., 2010; Pierson et al., 2012; Dick et al., 2010). Most point mutations are associated with mild presentation while deletions and the splice site variants often lead to a moderate to severe phenotype as seen in the family studied here. The patients we studied do not have brain iron deposition as reported in other patients with FA2H variants.
In conclusion, we have identified a c.786+1G>A variant in the FA2H gene in black African patients for the first time, expanding the genetic epidemiology of this disease. Further studies may improve our understanding of the origin of the sequence variant, the phenotypic variability and the role of this gene in the function of the nervous system.
Supplementary Material
Supplemental Figure 1: Genetic data of the SPG35 sequence variant. Chromatogram showing a A) normal control B) heterozygous parent, C) one of the affected individuals.
Acknowledgement:
This work is supported by grant number U01HG007044 funded by the National Institute of Neurological Disorders and Stroke (NINDS) and administered by the National Human Genome Research Institute as part of the NIH Common Fund H3Africa Initiative, intramural funds from NINDS, the Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali, and the Centre Hospitalier Universitaire du Point G, Bamako, Mali.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Data Availability Statement: De-identified DNA and sequences from participants are deposited in H3Africa biorepositories and data archives, and are currently available upon request through a Data and Biospecimen Access Committee.
References
- Boukhris A, Stevanin G, Feki I, Denora P, Elleuch N, Miladi MI, Goizet C, Truchetto J, Belal S, Brice A, Mhiri C (2009). Tunisian hereditary spastic paraplegias: clinical variability supported by genetic heterogeneity. Clinical Genetics, 75, 527–536. doi: 10.1111/j.1399-0004.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- de Souza PVS, de Rezende Pinto WBV, de Rezende Batistella GN, Bortholin T, Oliveira ASB (2017). Hereditary spastic paraplegia: clinical and genetic hallmarks. Cerebellum, 16(2), 525–551. doi: 10.1007/s12311-016-0803-z. [DOI] [PubMed] [Google Scholar]
- Dick KJ, Eckhardt M, Paisán-Ruiz C, Alshehhi AA, Proukakis C, Sibtain NA, Maier H, Sharifi R, Patton MA, Bashir W, Koul R, Raeburn S, Gieselmann V, Houlden H, Crosby AH (2010). Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Human Mutation, 31(4), E1251–1260. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, Korman SH, Taustein I, Saada A, Elpeleg O (2008). Mutations in the fatty acid 2-Hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. American Journal of Human Genetics, 83, 643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen AK, Koht J, Stray-Pedersen A, Abdelnoor M, Tallaksen CM (2009). Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: a population-based study. Brain, 132, 1577–1588. doi: 10.1093/brain/awp056. [DOI] [PubMed] [Google Scholar]
- Guinto CO, Diarra S, Diallo S, Cissé L, Coulibaly T, Diallo SH, Taméga A, Chen K-L, Schindler AB, Bagayoko K, Simaga A, Blackstone C, Fischbeck KH, Landouré G (2017). A novel mutation in KIF5A in a Malian family with spastic paraplegia and sensory loss. Annals of Clinical and Translational Neurology, 4(4), 272–275. doi: 10.1002/acn3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AE (1993). Hereditary spastic paraplegias. Seminars in Neurology, 13, 333–336. [DOI] [PubMed] [Google Scholar]
- Kruer MC, Paisán-Ruiz C, Boddaert N, Yoon MY, Hama H, Gregory A, Malandrini A, Woltjer RL, Munnich A, Gobin S, Polster BJ, Palmeri S, Edvardson S, Hardy J, Houlden H, Hayflick SJ (2010). Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Annals of Neurology, 68(5), 611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landouré G, Zhu P-P, Lourenço CM, Johnson JO, Toro C, Bricceno KV, Rinaldi C, Meilleur KG, Sangaré M, Diallo O, Pierson TM, Ishiura H, Tsuji S, Hein N, Fink JK, Stoll M, Nicholson G, Gonzalez MA, Speziani F, Dürr A, Stevanin G, Biesecker G; NIH Intramural Sequencing Center, Accardi J, Landis DM, Gahl WA, Traynor BJ, Marques W Jr., Züchner S, Blackstone C, Fischbeck KH, Burnett BG (2013). Hereditary spastic paraplegia type 43 (SPG43) is caused by mutation in C19orf12. Human Mutation, 34, 1357–1360. doi: 10.1002/humu.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landouré G, Samassékou O, Traoré M, Meilleur KG, Guinto CO, Burnett BG, Sumner CJ, Fischbeck KH (2016). Genetics and genomic medicine in Mali: challenges and future perspectives. Molecular Genetics and Genomics Medicine, 4(2), 126–134. doi: 10.1002/mgg3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TM, Simeonov DR, Sincan M, Adams DA, Markello T, Golas G, Fuentes-Fajardo K, Hansen NF, Cherukuri PF, Cruz P, Mullikin JC, Blackstone C, Tifft C, Boerkoel CF, Gahl WA; NISC Comparative Sequencing Program. (2012). Exome sequencing and SNP analysis detect novel compound heterozygosity in fatty acid hydroxylase-associated neurodegeneration. European Journal of Human Genetics, 20(4), 476–479. doi: 10.1038/ejhg.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC (2014). Tropical myelopathies. Handbook of Clinical Neurology, 121, 1521–1548. doi: 10.1016/B978-0-7020-4088-7.00102-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Genetic data of the SPG35 sequence variant. Chromatogram showing a A) normal control B) heterozygous parent, C) one of the affected individuals.