Abstract
Matrix metalloproteinases (MMPs), A Disintegrin and Metalloproteinase (ADAM) and A Disintegrin and Metalloproteinase with Thrombospondin Motif (ADAMTS) are zinc-dependent endopeptidases that play a critical role in the destruction of extracellular matrix proteins and, the shedding of membrane-bound receptor molecules in various forms of arthritis and other diseases. Under normal conditions, MMP, ADAM and ADAMTS gene expression aids in the maintenance of homeostasis. However, in inflamed synovial joints characteristic of rheumatoid arthritis and osteoarthritis. MMP, ADAM and ADAMTS production is greatly increased under the influence of pro-inflammatory cytokines. Analyses based on medicinal chemistry strategies designed to directly inhibit the activity of MMPs have been largely unsuccessful when these MMP inhibitors were employed in animal models of rheumatoid arthritis and osteoarthritis. This is despite the fact that these MMP inhibitors were largely able to suppress pro-inflammatory cytokine-induced MMP production in vitro. A focus on ADAM and ADAMTS inhibitors has also been pursued. Thus, recent progress has identified the “sheddase” activity of ADAMs as a viable target and the development of GW280264X is an experimental ADAM17 inhibitor. Of note, a monoclonal antibody, GLPG1972, developed as an ADAMTS-5 inhibitor, entered a Phase I OA clinical trial. However, the failure of many of these previously developed inhibitors to move beyond the preclinical testing phase has required that novel strategies be developed that are designed to suppress both MMP, ADAM and ADAMTS production and activity.
Keywords: Arthritis, Cartilage, Matrix Metalloproteinases, Synthetic Inhibitors, Zinc-Dependent Endopeptidases
Graphical Abstract
1. Introduction
Matrix metalloproteinases (MMPs), A Disintegrin and Metalloproteinase (ADAM) and A Disintegrin and Metalloproteinase with Thrombospondin Motif (ADAMTS) are zinc-dependent endopeptidases that play an integral role in the maintenance of normal organ and tissue homeostasis [1]. MMPs, ADAMs and ADAMTS are also intimately involved in several pathologic conditions, including, various forms of cancer [2–6], rheumatoid arthritis (RA) [7–9], and osteoarthritis (OA) [10–12]. In that regard, RA is characterized by a state of chronic inflammation of large and small synovial joints that is accompanied by defects in both innate and adaptive arms of the immune system [13–16]. Abnormal proliferation of activated synovial fibroblasts is perpetuated by the migration of activated T-cells and B-cells, dendritic cells, macrophages and neutrophils into synovial tissue [17, 18]. The migration of these cell types into the synovial space leads to elevated levels of pro-inflammatory cytokines, most prominently, IL-1ß, IL-6, IL-15, IL-16,IL-17, IL-18, IL-22, IL-23 and TNF-α [15, 19] which, in turn, are responsible for increasing MMP, ADAMs/ADAMTS gene expression in RA and psoriatic arthritis (PsA). By contrast, the early stages of OA likely occurs as a function of ageing and through abnormal mechanical stressors which alter chondrocyte function [20]. However, as OA progresses over time (time is probably measured in decades) a state of what has been termed, “non-classical inflammation” ensues. This involves immune cell dysregulation, heightened prostaglandin production and synovial tissue activation the latter considered a factor responsible for elevating pro-inflammatory cytokines that results in elevated levels of MMP, ADAMs and ADAMTS gene expression [11, 21–23]. Thus, MMPs and ADAMTS mediate the degradation of cartilage extracellular (ECM) proteins resulting in the loss of cartilage integrity [22–26] whereas ADAMS act a “sheddases” and the removal of membrane-bound receptors (e.g. mIL-6R) [27, 28].
The MMP superfamily is comprised of the classical types of MMPs. These include MMP-1 (collagenase-1), MMP-8 (neutrophil collagenase), MMP-13 (collagenase-3), and gelatinases (MMP-2) [29] and (MMP-9), the stromelysins exemplified by MMP-3, MMP-10, MMP-11, the matrilysins (MMP-7/PUMP-1 and MMP-26), the membrane-type MMPs (MMP-14, MMP-17, MMP-24/−25), [30–32], the ADAMs, also referred to as adamlysins, including, ADAM-2, −7, −−10, −11, −17, −18, −19, −22, −23, −29, −32, 33 [33–36], ADAM soluble variants, 9-S, 12-S, 28-S and DEC-1 [31] and the ADAMTS, most notably, ADAMTS-2, −3, 4, −5, −7, −10, −12, −13, −17, −20, SL-2 and SL-4 [37–40] As such, these enzymes have been of considerable interest for developing agents that curtail MMP gene expression and/or MMP production using classical medicinal chemistry paradigms designed to inhibit enzymes [41–47], blocking signal transduction pathways, initiated by TNF-α [48–50] after anti-TNF-α receptor blockade in Crohn’s patients [51] and in experimentally-induced arthritis with Type II collagen [52], IL-17 [53], and IL-6 [54, 55], by exploiting epigenetic mechanisms [56], by preventing enzyme-protein interactions [57], and by employing microRNA (miRNA) technology [58, 59] or small interfering RNAs [60] or by using viral vectors [61] or glycosylated and non-glycosylate substrates [62].
One of the more important aspects for curtailing inflammation involves ADAM-17 also known as Tumor necrosis factor-α converting enzyme [63]. Thus inhibition of TACE could serve as an effective way of blocking the involvement of TNF-α in inflammation [64–67]. In that regard, the development of effective inhibitors of ADAM17 is ongoing [68].
At present, the lack of selectivity for MMPs and ADAMs seriously compromise their use in the clinical setting [69–71]. Thus, in part, this narrative review focuses on the search for effective selective MMP inhibitors that could be added to various treatment modalities for RA, OA, and PsA.
1. Selection of Literature
The PubMed database (www.ncbi.nlm.nih.gov/pubmed) was employed to select papers for coverage in this narrative review. The search terms, “MMP Gene Expression/Drugs”, “ADAMS”, “ADAMTS”, “ADAMTS/Drugs”, “ADAMs/Drugs” “Synthetic Aggrecanase Inhibitors” and “Tumor necrosis factor-α converting enzyme” were employed for this purpose. Although a few cited references were published prior to 2004, the majority of the cited references from the PubMed database were from 2004 to 2018.
2. MMP-13, ADAMTS and ADAMs
2.1. MMP-13
MMP-13 (along with MMP-1, MMP-8) are interstitial collagen-degrading enzymes that have a particular relevance to the degradation of articular cartilage in RA and OA because they aggressively breakdown Type II collagen [72]. Of note, the expression of MMP-13 (along with MMP-1) is increased in response to IL-1 and TNF-α suggesting transcriptional regulation of both MMP-13 and MMP-1 genes. Moreover, high levels of MMP-1 and MMP-13 have been found in arthritic tissues [72].
2.2. MMP-13 Inhibitors
As indicated above, the degradation of Type II collagen and aggrecan constitute major cellular events in the progression of RA and OA to joint failure. Several findings have also implicated MMP-13 as a suitable target for the development of selective MMP-13 inhibitors [73, 74]. Thus, medicinal chemistry produced an MMP-13 inhibitor, PF152 (N-(4-fluoro-3-methoxybenzyl)-6-(2-(((2S,5R)-5-(hydroxymethyl)-1,4-dioxan-2-yl)methyl)-2H-tetrazol-5-yl)-2-methylpyrimidine-4-carboxamide), which was shown to decrease human cartilage degradation ex vivo as well as possessing the capacity to reduce the severity of articular cartilage lesions in dogs with OA induced by partial medial meniscectomy [75]. However, additional preclinical testing of PF152 indicated significant nephrotoxicity which was believed to have been mediated by human organic anion transporter 3. Thus, a follow-up analysis produced a compound lacking this nephrotoxic property [76]. As of this writing, a search of the PubMed data base using the search term, “MMP-13 inhibitors/Osteoarthritis Clinical Trials” failed to reveal any human OA trials as yet in which PF152 or its successor was evaluated for clinical efficacy.
2.3. ADAMTS
ADAMTS and ADAMTS-like proteins are members of a superfamily of 26 secreted enzyme molecules comprising 2 related, but distinct families. ADAMTS are zinc-metalloproteinases with a thrombospondin motif, whereas ADAMTS-like molecules lack the thrombospondin motif [77]. ADAMTS-5 is the principle “aggrecanase” found in animal [78] and human OA articular cartilage [77]. In that regard, the degradation and diffusion of Type II collagen and aggrecan fragments from OA articular cartilage without the compensatory synthesis of these macromolecules to replace those lost through degradation significantly compromises the biomechanical properties of articular cartilage [79].
2.4. ADAMTS Inhibitors
ADAMTS-5 was validated as a drug target for OA and experimental ADAMTS-5 inhibitors were shown to reduce synovial joint damage in OA animal models. Thus, an active ADAMTS-5 drug development program has been established with the lead compound, GLPG1972, being assessed in a Phase I OA clinical trial (NCT03311009). In addition to GLPG1972, a humanized anti-ADAMTS-5 monoclonal antibody, GSK2394002 [80] which was shown to inhibit ADAMTS-5 catalytic activity with a Ki 0.08nM, has been earmarked as a potential OA therapeutic agent. However, as of April, 2018, GSK2394002 did not appear to have progressed beyond preclinical evaluation. ADAMTS-4 and −5 also abolish cartilage integrity in RA by degrading aggrecan [81].
Additional novel ADAMTS-5 inhibitors are in the process of development. In one such study a bias-selection of antibodies analysis targeting ADAMTS-5 was shown to block the catalytic site of ADAMTS-5 [82] resulting in selective “aggrecanase” inhibition.
2.5. ADAMs and ADAM Inhibitors
We previously proposed a biological role for soluble IL-6 receptor (IL-6R) in OA [28]. In that regard, sIL-6R was shown to stimulate MMP synthesis by activating the JAK-STAT and ERK-MAPK signaling pathways in human chondrocyte cultures [83]. The sIL-6R is generated by “ectodomain shedding” [84–86] mediated by the ADAM class of metzincin proteases [87]. In the present view, dysregulation of “ectodomain shedding” mediated by ADAM proteases has been associated with autoimmune and cardiovascular diseases, neurodegeneration, cancer, infection, and inflammation [85]. Regarding the removal of the membrane form of the IL-6 receptor (mIL-6), this is carried out either by ADAM10 or ADAM17 [88], where ADAM17 is mostly associated with sIL-6R arising from neutrophils during acute and chronic inflammation [89]. In the course of recognizing the role played by ADAM17, an inhibitor, GW280264X was developed wherein this agent was shown to block the constitutive release of mIL-6R in addition to blocking the release of chemokines CX3CL1/fractalkine, and chemokine C-X-C ligand 16 [90]. This finding was consistent with a previous report showing that ADAM17, and not ADAM10 was responsible for removing TNF-α and L-selectin from leukocyte membranes [89].
3. Signal Transduction Pathways: Pro-inflammatory Cytokines, NF-κB, MMPs and Apoptosis
Early on the JAK-STAT pathway was identified as a critical inducer of inflammation in RA and PsA because several of the pro-inflammatory cytokines (e.g. IL-6) and other soluble mediators (e.g. interferon-γ) activate STAT proteins via their interaction with specific receptors [91–93].
However, more recently, other pro-inflammatory cytokines exemplified by the activity of IL-29 [94] was shown to increase the production of other pro-inflammatory cytokines (e.g. IL-1ß, IL-6, TNF-α), growth factors (e.g. fibroblast growth factor) that are intimately associated with the maintenance of chronic inflammation (e.g. elevated production of IL-8, CRP), and MMP-3/ADAMTS gene expression in synovial tissue obtained from OA patients [95]. This response also involved the activation of the NF-κB complex (Figure 1). Although this and other evidence have implicated the MAPK pathway in MMP and ADAMTS gene expression as well as apoptosis (Figure 1), the finding that activation of JAK-STAT signaling also leads to MMP gene expression indicated that more than one signaling pathway participated in regulating pro-inflammatory cytokine-induced MMP gene expression. Thus, in that scenario one envisions that more than one signaling pathway may have to be inhibited for cytokine-induced MMP gene expression to be completely dampened. We had also previously contended that this was likely to be the case for preventing the progression of tissue destruction in various autoimmune and inflammatory diseases [96]. Despite this conjecture, the small molecule inhibitor tofacitinib [97, 98] was developed with the goal of inhibiting the progression of RA mainly through its ability to reduce the capacity of pro-inflammatory cytokine [99] and MMP gene expression [100–102] both of which sustain the chronic inflammatory state characteristic of RA and PsA.
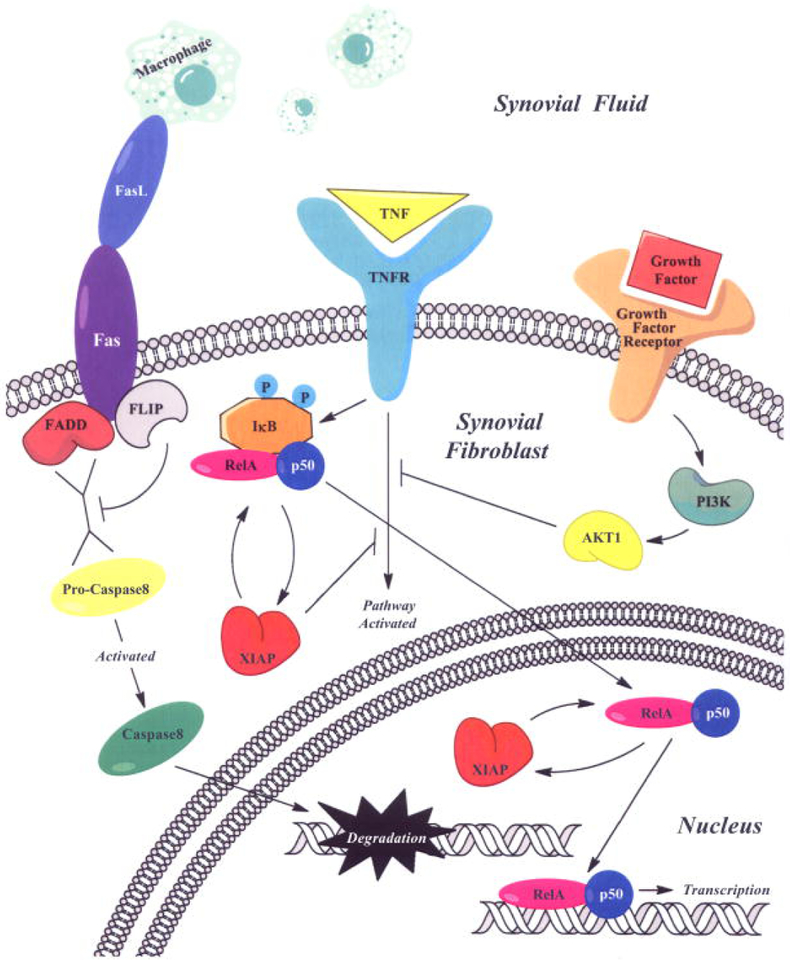
Figure 1. Pro-Inflammatory Cytokine Activation of NF-κB in Synovial Fibroblasts.
Figure 1 shows that pro-inflammatory cytokines (e.g. TNF-α) activates the NF-κB complex (IκB/ReLa/p50). The activation of the NF-κB complex can cause an enhancement of MMP gene expression [136]. However, as noted, TNF-α/TNFR does not alter ADAMTS activity [95]. Other factors such as Fas Ligand (FasL) produced by macrophages can lead to apoptosis through pro-caspase activation [137]. Growth factors can also activate the PI3K/AKT1/mTor pathway and “cross-talk” with the TNF-α/TNFR pathway [138]. This, and other evidence suggests a complex network of interacting ligand/receptor activities that modulates downstream synovial fibroblast pathways associated with inflammatory arthritis [136].
Figure 1 was previously published: C. J. Malemud. Intracellular signaling pathways in rheumatoid arthritis. J Clin Cell Immunol-Open Access 4 (2013) 160. DOI: 10.4172/2155-9899.1000160
4. Which If Any MMP Inhibitors Should Be Considered for Further Development?
4.1. In Vitro Cell Cultures Appear to be Integral for Assessing the Action of MMP Inhibitors
The association of periodontal inflammation and the development of RA has been reported [103–106] as well the crucial involvement of MMPs in the progression of periodontal disease [107, 108]. In that regard using periodontal ligament fibroblasts [107] was influential in assigning significance to MMPs in this disease process. Thus, these cells proved useful for identifying the main gelatinolytic activity in a band that migrated on gelatin-impregnated gels at 72kDa (MMP-2) whereas a less plentiful gelatinolytic band was observed at 92kDa (this was likely to be MMP-9). Moreover, several agents, namely, H7, staurosporine, cycloheximide and TGF-ß suppressed MMP-2 production, indicative of the utility of using these cells to potentially identify MMP-2 inhibitors. Platelets have also been identified as playing a role in arthritis and as such, the types of MMPs secreted by these cells is fundamentally important to developing inhibitors of platelet MMP activity [109].
Fibroblast-like synoviocytes [110], and cartilage “constructs” comprised of chondrocytes derived from pluripotential stem cells [111] have also been employed. In one such study [111], a readout of specific MMP activity in response to IL-1 was employed to identify agents that could neutralize this effect. In another study immortalized human chondrocytes [55] were employed to show that tocilizumab, a humanized monoclonal antibody that neutralizes the action of IL-6, suppressed MMP-9 production. Importantly, authentic mature chondrocytes [112] and other cell types were identified as target cells for determining the role of MMPs in disease processes. Additional studies used tumor cells [113] where a novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) was tested. Tumor cells that include human metastatic breast cancer cells [114–116] were employed to determine the effect of an MMP inhibitor reversion-inducing cysteine-rich protein with Kazal motifs (RECK) on levels of MMP-9, MMP-2, and membrane Type 1 [(MT1) MMP (MMP-14)] secretion [116]. Human cardiac smooth muscle cells [117], monocyte/macrophages [118], and rat astrocytes and microglia cultures [119] were among others that were employed as appropriate for testing experimental MMP inhibitors or for providing data on other strategies designed to limit the effects of MMPs. As previously mentioned, monoclonal antibodies [120] are also among several additional experimental MMP/ADAMs inhibitors undergoing pre-clinical testing in various inflammatory disorders and cancer. These include, MMP-14 [121], MMP-9 [122], and, ADAM17 [123].
5.2. In Vivo Studies
Many of these experimental strategies have emerged as a novel approach in basic and translational research that are designed to either inhibit MMP/ADAMTS gene expression or to reduce the pathologic changes brought about by these enzymes in various disorders where these cell types play an intimate role. A few of these MMP inhibition strategies have also been employed in preclinical animal models of arthritis [124, 125] and persistent inflammatory pain [126], the effect(s) of which are summarized in Table 1.
Table 1.
Effects MMP Inhibitors in Preclinical Animal Models of Arthritis and Persistent Pain
| Animal Model Animal |
Molecule/Targeted MMP/Effect(s) | Reference |
|---|---|---|
|
Collagen-induced Arthritis Mouse |
TGF-ß-inducible gene h3/MMP-1-cleavable composite peptide, MFK24/Reduced Inflammatory Mediators | 124 |
|
Collagen-Induced Arthritis Mouse |
PI3Kδ,γ inhibitor-IPI-145/MMP-3,-13/Reduced Arthritis Severity; Reduced MMP3/MMP13 gene expression Anti-CII IgG2a:Total IgG Modestly Reduced |
125 |
|
Persistent Inflammatory Pain CFA1-Rat |
Attenuation of Mechanically-Induced Allodynia | 126 |
Complete Freund Adjuvant;
Nephroblastoma Overexpressed (Also Known as CCN3)
5. Conclusions and Future Perspectives
From the foregoing narrative review we concluded that development of inhibitors to directly suppress MMP gene expression has given way to other novel pharmacological strategies designed to impair MMP gene expression and/or MMP activity. The reason for this development appears to be several-fold. Firstly, inhibition of MMP activity using administered synthetic MMP inhibitors generally failed in vivo because of poor individual MMP selectivity as well as unacceptable bioavailability [127]. Secondly, OA, cancer and cardiovascular clinical trials which were designed to test the efficacy of administering synthetic MMP inhibitors were not only poorly designed, but the use of these MMP synthetic inhibitors also caused undesirable side-effects [128]. Thirdly, restoring the balance between MMPs and Tissue Inhibitor of Metalloproteinases (TIMPs), which is skewed towards MMP in most inflammatory disorders and generally determines overall MMP activity [129] was also difficult to achieve. In fact, several studies have clarified the extent to which deficient TIMP activity provided the perfect microenvironment that allowed MMP activity to efficiently degrade neutral substrates [130–132]. Thus, strategies designed to determine the extent to which improving serum TIMP levels to regulate MMP activity [133] in pre-clinical models and thereafter in clinical trials remains critical for reestablishing the proper balance of MMP to TIMP.
With these issues in mind, it is now noteworthy that one of the experimental strategies shown in Table 1 utilized activated MMP-2 to develop a potentially novel approach to anti-arthritis therapy [124]. This formulation was designed to show clinical efficacy in a preclinical experimental model of arthritis and in the short run, resulted in a follow-up study. Thus, Nam et al [134] followed up their initial study of TGF-ß1-gene h3 (ßig-h3) [124] by designing ßig-h3 derivatives, which encompasses the 4th fas-1 domain truncated for H1 and H2 sequences of mouse and MMP-2 cleavable peptides complex, termed MFK902. Thus, MFK902 was specifically cleaved by activated MMP-2. Furthermore, MFK902 reduced arthritis severity in collagen-induced arthritic (CIA) mice that was accompanied by a reduction in the histopathologic changes in synovial joints associated with CIA. These results indicated that an MMP-2 cleavable peptide complex based on ßig-h3 structure could eventually become be a useful adjunctive anti-arthritis therapy. Another recent strategy was one in which the experimental design involved inhibiting the activity of MMP-13 and ADAMTS-5 after surgically-induced destabilization of the medical meniscus led to changes consistent with OA in male C57/BL6 mice [135]. This study employed intra-articular injection of silencing RNA (siRNA) directed at each enzyme or at the combination of MMP-13 and ADAMTS-5 [135]. The combination of siRNAs directed against MMP-13 and ADAMTS-5 resulted in just about the same inhibitory effect as MMP-13 siRNA alone. Moreover, the histological score of the OA mice improved as well.
It may be hard to believe but at the present time the antibiotic, doxycycline, is the only inhibitor of MMP activity approved by the US Food and Drug Administration for the treatment of musculoskeletal and other disorders where elevated levels of MMPs play a significant role in their pathogenesis and progression. Thus, there is ample room for assessing novel pharmacological strategies such as the ones reviewed herein which may prove to be useful in treating RA, OA and other musculoskeletal conditions going forward.
6. Acknowledgements:
The results and discussion of experimental studies from the Malemud research group at CWRU are provided in references 28, 55, 57, 93, 95, 99. These studies were supported, in part, by grants from the NIH and Genentech/Roche Group. I thank Ms. Jessica Thorpe, B.Sc. for helpful discussions and Mr. Evan C. Meszaros for rendering Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The author declares no conflict of interest.
8. References
- 1.Wojowcitz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs 15 (1997) 61–75. PMID: 9195290. [DOI] [PubMed] [Google Scholar]
- 2.Vihinen P, Ala-aho R, Kähäri VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 5 (2005) 203–220. PMID: 15892620 DOI: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 3.Lu X, Lu D, Scully M, Kakkar V. ADAM proteins – therapeutic potential in cancer. Curr Cancer Drug Targets 8 (2008) 720–732. PMID: 19075595 DOI: 10.2174/156800908786733478. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki I, Nabeshima N. Introduction: MMPs, ADAMs/ADAMTSs research products to achieve big dreams. Anticancer Agents Med Chem 12 (2012) 688–706. PMID: 22292751 DOI: 10.2174/187152012802650200. [DOI] [PubMed] [Google Scholar]
- 5.Rossello A, Nuti E, Ferrini S, Fabbi M. Targeting ADAM17 sheddase activity in cancer. Curr Drug Targets 17 (2016) 1908–1927. PMID: 27469341 DOI: 10.2174/1389450117666160727143618. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y, Lu YT, Sun Y, Shi ZH, Li NG, Tang YP, Duan JA. Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin Drug Discov 13 (2018) 75–87. PMID: 29088927 DOI: 10.1080/17460441.2018.1398732. [DOI] [PubMed] [Google Scholar]
- 7.Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC, Huizinga TW. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis 61 (2002) 975–980. PMID: 12379519 DOI: 1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dancevic CM, McCulloch DR. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res Ther. 16 (2014) 429 PMID: 25606593 DOI: 10.1186/s13075-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh Y. Metalloproteinases in rheumatoid arthritis: potential therapeutic targets to improve current therapies. Prog Mol Biol Trans Sci 148 (2017) 327–338. PMID: 28662826 DOI: 10.1016/bs.pmbts.2-2017/03.002. [DOI] [PubMed] [Google Scholar]
- 10.Troeberg L, Nagase H. Proteases involved in cartilage degradation in osteoarthritis. Biochim Biophys Acta 1824 (2012) 133–145. PMID: 21777704 DOI: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malemud CJ. Biological basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol 27 (2015) 289–294. PMID: 25784380 https://DOI: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis – looking beyond the ‘usual suspects’. Osteoarthritis Cartilage 25 (2017) 1000–1009. PMID: 28216310 DOI: 10.1016/j.joca.2017.02.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity 46 (2017) 183–196. PMID: 28228278 DOI: 10.1016/j.immunl/2017.02/066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toh ML, Moissec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol 19 (2007) 284–288. PMID: 17414957 DOI: 10.1097/BOR.0b013e3280e87e0 [DOI] [PubMed] [Google Scholar]
- 15.Karouzakis E, Neidhart M, Gay RE, Gay S. Molecular and cellular basis of rheumatoid arthritis destruction. Immunol Lett 106 (2006) 8–13. PMID: 16824621 DOI: 10.1016/j.imlet.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 16.Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta 455 (2016) 161–171. PMID: 26883280 DOI: 10.1016/j.cca/2016.02.010 [DOI] [PubMed] [Google Scholar]
- 17.Veale DJ, Orr C, Fearon U. Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol 39 (2017) 343–354. PMID: 28508153 DOI: 10.1007/ss00281-017-0633-1 [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Fox DA. Targeting IL-17 and Th17 cells in rheumatoid arthritis. Rheum Dis Clin N Am 36 (2010) 345–366. PMID: 20510238 DOI: 10.1016/j.rdc.2010/02.006 [DOI] [PubMed] [Google Scholar]
- 19.Fox DA, Gizinski A, Morgan B, Lundy SK. Cell-cell interactions in rheumatoid arthritis synovium. Rheum Dis Clin N Am 36 (2010) 311–323. PMID: 20510236 DOI: 10.1016/j.rdc.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hϋgle T, Geurts J, Nϋesch C, Mϋller-Gerbi M, Valderrabano V. Aging and osteoarthritis: an inevitable encounter? J Aging Res 2012 (2012) 950192 PMID: 22720159 DOI: 10.1155/2012/950192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malemud CJ, Islam N, Haqqi TM. Pathophysiologic mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs 174 (2003) 34–48. PMID: 12784040 DOI: 10.1159/000070573 [DOI] [PubMed] [Google Scholar]
- 22.Attur MG, Dave M, Akamatsu M, Katoh M, Amin AR. Osteoarthritis or osteoarthrosis: the definition of inflammation becomes semantic in the era of molecular medicine. Osteoarthritis Cartilage 10 (2002): 1–4. PMID: 11795977 DOI: 10.1053/joca.2001.0488 [DOI] [PubMed] [Google Scholar]
- 23.Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol 146 (2013) 185–196. PMID: 23360836 DOI: 10.1016/j.clim.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malemud CJ, Schulte ME. Is there a final common pathway for arthritis? Future Rheumatol 3 (2008) 253–268. DOI: 10.2217/17460816.3.3 [DOI] [Google Scholar]
- 25.Gargiulo S, Gamba S, Poli G, Leonarduzzi G. Metalloproteinases and metalloproteinase inhibitors in age-related diseases. Curr Pharm Des 20 (2014) 2993–3018. PMID: 24079771 [DOI] [PubMed] [Google Scholar]
- 26.Verma P, Delal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem 112 (2011) 3507–3514. PMID: 21815191 DOI: 10.1002/jcb.23298 [DOI] [PubMed] [Google Scholar]
- 27.Giebeler N, Zigrino PA. A disintegrin and metalloprotease (ADAM): historical overview of their functions. Toxins (Basel) 8 (2016) 122 PMID: 27120619 DOI: 10.3390/toxins8040122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akeson G, Malemud CJ. A role for soluble IL-6 receptor in osteoarthritis. J Funct Morphol Kinesiol 2 (2017) 27 PMID: 29276788 DOI: 10.3390/jfmk2030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puliti M, Momi S, Falcinelli E, Gresele P, Bistoni F, Tissl L. Contribution of matrix metalloproteinase 2 to joint destruction in group B Streptococcus-induced murine arthritis. Arthritis Rheum 64 (2012) 1089–1097. PMID: 22042442 DOI: 10.1002/art.33450 [DOI] [PubMed] [Google Scholar]
- 30.Malemud CJ. Matrix metalloproteinases and synovial joint pathology. Prog Mol Biol Trans Sci 148 (2017) 305–325. PMID: 28662824 https://doi: 10.1016/bs.pmbts.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Edwards DR, Hendsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 29 (2008) 258–259. PMID: 18762209 DOI: 10.1016/j.mam.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, Kiyonari H, Shioi G, Yamaguchi A, Tsumaki N, Akiyama H, Yoneda T. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem 287 (2012) 33179–33190. PMID: 22869368 DOI: 10.1074/jbc.M111.337063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alunno A, Falcinelli E, Luccioli F, Petito E, Bartoloni E, Momi S, Mirabelli G, Mancini GB, Gerli R, Gresele P. Platelets contribute to the accumulation of matrix metalloproteinase type 2 in synovial fluid in osteoarthritis. Thromb Haemost 117 (2017) 2116–2124. PMID: 28981555 DOI: 10.1160/TH17-06-0379 [DOI] [PubMed] [Google Scholar]
- 34.Qi B, Newcomer RG, Sang QX. ADAM19/adamalysin 19 structure, function and role as putative target in tumors and inflammatory diseases. Curr Pharm Des 15 (2009) 2336–2348. PMID: 19601835 [DOI] [PubMed] [Google Scholar]
- 35.Klein T, Bishhoff R. Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: biological function and structure. J Proteome Res 10 (2011) 17–33. PMID: 20849079 DOI: 10.1021/pr100556z. [DOI] [PubMed] [Google Scholar]
- 36.Takeda S. ADAM and ADAMTS family proteins and snake venom metalloproteases: a structural overview. Toxins (Basel) 8 (2016) pii: E155 PMID: 27196928 DOI: 10.3390/toxins8050155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelwick R, Desanlis J, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloprotease with thrombospondin motifs) family. Genome Biol 16 (2015) 113 PMID: 26025392 DOI: 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Lin J, Wei F. The function and roles of ADAMTS-7 in inflammatory diseases. Mediators Inflamm 2015 (2015) 801546 PMID: 26696755 DOI: 10.1155/2015/801546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis – looking beyond the usual suspects. Osteoarthritis Cartilage 25 (2017) 1000–1009. PMID: 28216310 DOI: 10.1016/j.joca.2017.02.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol 71–72 (2018) 225–239. PMID: 29885460 DOI: 10.1016/j.matbio.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fioravanti A, Tintl L, Pascarelli NA, Di Capua A, Lamboglia A, Cappelli A, Blava M, Giordani A, Niccolini S, Galeazzi M, Anzini M. In vitro effects of VA441, a new selective cyclooxygenase inhibitor, on human chondrocytes exposed to IL-1ß. J Pharmacol Sci 120 (2012) 6–14. PMID: 22878602. DOI: 10.1254/jphs.12016FP [DOI] [PubMed] [Google Scholar]
- 42.Higashi S, Hirose T, Takeuchi T, Miyazaki K. Molecular design of highly selective and strong protein inhibitor against matrix metalloproteinase-2 (MMP-2). J Biol Chem 288 (2013) 9066–9076. PMID: 23395821 DOI: 10.1074/jbc.M112.441758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stawikowska R, Cudic M, Giulianotti M, Houghten RA, Fields GB, Minold D. Activity of ADAM17 (a disintegrin and metalloproteinase 17) is regulated by its noncatalytic domains and secondary structure of substrates. J Biol Chem 288 (2013) 22871–22879. PMID: 23779109 DOI: 10.1074/jbc.M113.462267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romanchikova N, Trapencieris P, Zemītis J, Turks M. A novel matrix metalloproteinase-2 inhibitor triazolylmethyl azaridine reduces melanoma cell invasion, angiogenesis and targets ERK1/2 phosphorylation. J Enzyme Inhib Med Chem 29 (2014) 765–772. PMID: 24246091 DOI: 10.3109/14756366.2013.855207. [DOI] [PubMed] [Google Scholar]
- 45.Dreymueller D, Ludwig A. Considerations of inhibition approaches for proinflammatory functions of ADAM proteases. Platelets 28 (2017) 354–361. PMID: 27460023 DOI: 10.1080/09537104.2016.1203396 [DOI] [PubMed] [Google Scholar]
- 46.Nara H, Sato K, Kaieda A, Oki H, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori M. Design, synthesis, and biological activity of novel, potent, and highly selective fused pyrimidine-2-carboxamide-4-one-based matrix metalloproteinase (MMP)-13 zinc-binding inhibitors. Bioorg Med Chem 24 (2016) 6149–6155. PMID: 27825552 DOI: 10.1016/j.bmc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Xie XW, Wan RZ, Liu ZP. Recent research advances in selective matrix metalloproteinase-13 inhibitors as anti-osteoarthritis agents. ChemMedChem 12 (2017) 1157–1168. PMID: 28722301 DOI: 10.1002/cmdc.201700349. [DOI] [PubMed] [Google Scholar]
- 48.Chandran V, Shen H, Pollock RA, Pellett FJ, Carty A, Cook RJ, Gladman DD. Soluble biomarkers associated with response to treatment with tumor necrosis factor inhibitors in psoriatic arthritis. J Rheumatol 40 (2013) 866–871. PMID: 23637322 DOI: 10.3899/jrheum.121162. [DOI] [PubMed] [Google Scholar]
- 49.Obeng JA, Amoruso A, Camaschella GL, Sola D, Brunellesci S, Fresu LG. Modulation of human monocyte/macrophage activity by tocilizumab, abatacept and etanercept: An in vitro study. Eur J Pharmacol 780 (2016) 33–37. PMID: 26997366 DOI: 10.1016/j.ejphar.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Hattori Y, Kojima T, Kaneko A, Kida D, Hirano Y, Fujibayashi T, Yabe Y, Oguchi T, Kanayama Y, Miyake H, Kato T, Takagi H, Hayashi M, Ito T, Shioura T, Takahashi N, Ishikawa H, Funahashi K, Ishiquro N. High rate of improvement in serum matrix metalloproteinase-3 levels at 4 weeks predicts remission at 52 weeks in RA patients treated with adalimumab. Mod Rheumatol 28 (2018) 119–125. PMID: 28463029 DOI: 10.1080/14397595.2017.1317320. [DOI] [PubMed] [Google Scholar]
- 51.Di Sabatino A, Saarialho-Kere U, Buckley MG, Gordon JN, Biancheri P, Rovedatti L. Corazza GR, Macdonald TT, Pender SL. Stromelysin-1 and macrophage metalloelastase expression in the intestinal mucosa of Crohn’s disease patients treated with infliximab. Eur J Gastroenterol Hepatol 21 (2009) 1049–1055. PMID: 19357521 DOI: 10.1097/MEG.0b013e32832930f [DOI] [PubMed] [Google Scholar]
- 52.Kaneko K, Williams RO, Dransfield DT, Nixon AE, Sandison A, Itoh Y. Selective inhibition of membrane type 1 matrix metalloproteinase abrogates progression of experimental inflammatory arthritis: synergy with tumor necrosis blockade. Arthritis Rheumatol 68 (2016) 521–531. PMID: 26315469 DOI: 10.1022/art.39414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer JA, Hueber AJ, Wilson S, Gaim M, Baum W, Kitson C, Auer J, Lorenz SH, Moelleken J, Bader M, Tissot AC, Tan SL, Seeber S, Schett G. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol 67 (2015) 51–62. PMID: 25303306 DOI: 10.1002/art.38896. [DOI] [PubMed] [Google Scholar]
- 54.Mano Y, Shibata K, Sumigama S, Hayakawa H, Ino K, Yamamoto E, Kajiyama H, Nawa A, Kikkawa F. Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and −9 secretions from human amnion cells in preterm premature rupture of membranes, Gynecol Obstet Invest 68 (2009) 143–153. PMID: 19628948 DOI: 10.1159/000229021. [DOI] [PubMed] [Google Scholar]
- 55.Meszaros EC, Dahoud W, Mesiano S, Malemud CJ. Blockade of recombinant human IL-6 by tocilizumab suppresses matrix metalloproteinase-9 production in the C28/I2 immortalized human chondrocyte cell line. Integr Mol Med 2 (2015) 304–310. PMID: 26753098 DOI: 10.15761/IMM.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labrie M, St-Pierre Y. Epigenetic regulation of mmp-9 gene expression. Cell Mol Life Sci 70 (2013) 3109–3124. PMID: 23184252 DOI: 10.1007/s00018-012-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meszaros E, Malemud CJ. Prospects for treating osteoarthritis: enzyme-protein interactions regulating matrix metalloproteinase activity. Ther Adv Chronic Dis 3 (2012) 219–229. PMID: 23342237 DOI: 10.1177/2040622312454157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu M, Wang YZ. miR-133a suppresses cell proliferation, migration and invasion in human lung cancer by targeting MMP-14. Oncol Rep 30 (2013) 1398–1404. PMID: 23783274 DOI: 10.3892/or.2013.2548. [DOI] [PubMed] [Google Scholar]
- 59.Malemud CJ. MicroRNAs and osteoarthritis. Cells 7 (2018) pii: E92 PMID: 30071609 DOI: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo F, Tian J, Cui M, Fang M, Yang L. Downregulation of matrix metalloproteinase 9 by small interfering RNA inhibits the tumor growth of ovarian epithelial carcinoma in vivo and in vitro. Mol Med Rep 12 (2015) 753–759. PMID: 25738807 DOI: 10.3892/mmr.2015.3425. [DOI] [PubMed] [Google Scholar]
- 61.Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49 (2015) 563–573. PMID: 24026771 DOI: 10.1007/s12035-013-8538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minond D, Cudic M, Bionda N, Giulanotti M, Maida L, Houghten RA, Fields GB. Discovery of novel inhibitors of a distintegrin and metalloproteinase 17 (ADAM17) using glycosylated and non-glycosylated substrates. J Biol Chem 287 (2012) 36473–36487. PMID: 22927435 DOI: 10.1074/jbc.M112.389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto H, Koga H, iida M, Tarumi K, Fujita M, Haruma K. Blockade of tumor necrosis factor-alpha-converting enzyme improves experimental small intestinal damage by decreasing matrix metalloproteinase-3 production in rats. Scand J Gastroenterol 41 (2006) 1320–1329. PMID: 17060126 DOI: 10.1080/00365520600684571 [DOI] [PubMed] [Google Scholar]
- 64.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des 15 (2009) 2319–2335. PMID: 19601834. [DOI] [PubMed] [Google Scholar]
- 65.DasGupta S, Murumkar PR, Giridhar R, Yadav MR. Current perspective of TACE inhibitors: a review. Bioorg Med Chem 17 (2009) 444–459. PMID: 19095454 DOI: 10.1016/j.bmc.2008.11.067 [DOI] [PubMed] [Google Scholar]
- 66.Murumkar PR, DasGupta S, Chanani SR, Giridhar R, Yadav MR. Novel TACE inhibitors in drug discovery: a review of patented compounds. Expert Opin Ther Pat 20 (2010) 31–57. PMID: 20021284 DOI: 10.1517/13543770903465157 [DOI] [PubMed] [Google Scholar]
- 67.Rose-John S. ADAM17, shedding, TACE as therapeutics targets. Pharmacol Res 71 (2013) 19–22. PMID: 23415892 DOI: 10.1016/j.phrs.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 68.Moss ML, Minond D. Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm 2017 (2017) 9673537 PMID: 29230082 DOI: 10.1155/2017/9673537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgiadis D, Yiotakis A. Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem 16 (2008) 8781–8794. PMID: 18790648 DOI: 10.1016/j.bmc.2008.08.058 [DOI] [PubMed] [Google Scholar]
- 70.Nakai R, Salisbury CM, Rosen H, Cravatt BF. Ranking the selectivity of PubChem screening hits by activity-based protein profiling: MMP13 as a case study. Bioorg Med Chem 17 (2009) 1101–1108 PMID: 18364257 DOI: 10.1016/j.bmc.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahasenan KV, Ding D, Gao M, Nguyen TT, Sucknow MA, Schroeder VA, Wolter WR, Chang M, Mobashery S. In search of selectivity in inhibition of ADAM10. ACS Med Chem Lett 9 (2018) 708–713. PMID: 30034605 DOI: 10.1021/acsmedchemlett.8b00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 4 (2002) 157–164. PMID: 12010565. DOI: org/ 10.1186/ar401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther 19 (2017) 248 PMID: 29126436 DOI: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL, Qian LH, Qian DW, Duan JA. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem 18 (2011) 977–1001. PMID: 21254976 DOIi: 10.2174/092986711794940905. [DOI] [PubMed] [Google Scholar]
- 75.Settle S, Vickery L, Nemirovskiy O, Vidmar T, Bendele A, Messing D, Ruminski P, Schnute M, Sunyer T. Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis: confirmation by multivariate analysis that modulation of type II collagen and aggrecan degradation peptides parallels pathologic changes. Arthritis Rheum 62 (2010) 3006–3015. PMID: 20533541 DOI: 10.1002/art.27596. [DOI] [PubMed] [Google Scholar]
- 76.Ruminski PG, Massa M, J Strohbach, Hanau CE, Schmid M, Scholten JA, Fletcher TR, Hamper BC, Carroll JN, Shieh HS, Caspers N, Collins B, Grapperhaus M, Palmquist KE, Collins J, Baldus JE, Hitchcock J, Kleine HP, Rogers MD, McDonald J, Munie GE, Messing DM, Portolan S, Whiteley LO, Sunyer T, Schnute ME. Discovery of N-(4-Fluoro-3-methoxybenzyl)-6-(2-(((2S, 5R)-5-(hydroxymethyl)-1, 4-dioxan-2-yl) methyl)-2H-tetrazol-5-yl)-2-methylpyrimidine-4-carboxamide. A highly selective and orally bioavailable matrix metalloproteinase-13 inhibitor for the potential treatment of osteoarthritis. J Med Chem 59 (2016) 313–327. PMID: 26653735 DOI: 10.1021/acs.jmedchem.5b01434. [DOI] [PubMed] [Google Scholar]
- 77.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol 72 (2018) 225–239. PMID: 29885460 DOI: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanton H, Rogerson EM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434 (2005) 648–652. PMID: 15800625 DOI.org/ 10.1038/nature0317. [DOI] [PubMed] [Google Scholar]
- 79.Malemud CJ, Shuckett R. Impact loading and lower extremity disease In: Clinical Concepts in Regional Musculoskeletal Illness. Hadler NM (Ed), Grune & Stratton: Orlando, (1987) 109–135. [Google Scholar]
- 80.Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, Xue Y, Liu F, Genell C, Miller RE, Tran PB, Malfait AM, Maier CC, Metheny CJ. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage 23 (2015) 1254–1266. PMID: 25800415 DOI: 10.1016/j.joca.2015.02.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTs metalloproteinases. Biochem J 386 (2005) 15–27. PMID: 15554875 DOI: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apte SS. Anti-ADAMTS5 monoclonal antibodies: implications for aggrecanase inhibition in osteoarthritis. Biochem J 473 (2016) e1–e4. PMID: 26657033 DOI: 10.1042/BJ20151072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, Suzuki N, Shimizu O, Takeichi O, Makimura M, Maeno M. IL-6 and soluble IL-6 receptor stimulate the production of MMPs and their inhibitors via JAK-STAT and ERK-MAPK signalling in human chondrocytes. Cell Biol Int 36 (2012) 367–376. PMID: 22087578 DOI: 10.1042/CBI20110150. [DOI] [PubMed] [Google Scholar]
- 84.Bridges LC, Bowditch RD. ADAM-integrin interactions: potential integrin regulated ectodomain shedding activity. Curr Pharm Des 11 (2005) 837–847. PMID: 15777238 DOI: 10.2174/1381612053381747. [DOI] [PubMed] [Google Scholar]
- 85.Saftig P, Reiss K. The “Disintegrin and Metalloproteases” ADAM10 and ADAM17: Novel drug targets with therapeutic potential. Eur J Cell Biol 90 (2011) 527–535. PMID: 21194787 DOI: 10.1016/j.ejcb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 86.Hartmann M, Herrlich A, Herrlich P. Who decides when to cleave an ectodomain? Trends Biochem Sci 38 (2013) 111–120. PMID: 23298902 DOI: 10.1016/j.tibs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Giebeler N, Zigrino P. A disintegrin and metalloprotease (ADAM): Historical overview of their functions. Toxins (Basel) 8 (2016) 122 PMID: 27120619 DOI: 10.3390/toxins8040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble interleukin-6 receptor: Generation and its role in inflammation and cancer. Eur J Cell Biol 90 (2011) 484–494. PMID: 21145125 DOI: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Condon TP, Flournoy S, Sawyer GJ, Baker BF, Kishimoto TK, Bennett CF. ADAM17 but not ADAM10 mediates tumor necrosis factor-alpha and L-selectin shedding from leukocyte membranes. Antisense Nucleic Acid Drug Dev 11 (2001) 107–116. PMID: 11334139 DOI: 10.1089/108729001750171353. [DOI] [PubMed] [Google Scholar]
- 90.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloprotease inhibitors for the disintegrin and metalloproteases ADAM10 and ADAM17 that differentially block constitutive and phorbol-ester-inducible shedding from cell surface molecules. Comb Chem High Throughput Screen 8 (2005) 161–171. PMID: 15777180 DOI: 10.2174/1386207053258488 [DOI] [PubMed] [Google Scholar]
- 91.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol 32 (2005) 1650–1653. PMID: 16142855 [PubMed] [Google Scholar]
- 92.Malemud CJ. Recent advances in neutralizing the IL-6 pathway in arthritis. Open Access Rheumatol Res Rev 1 (2009) 133–150. PMID: 27789987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malemud CJ, Pearlman E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr Signal Transduct Ther 4 (2009) 201–221. [Google Scholar]
- 94.Xu L, Peng Q, Xuan W, Feng X, Kong X, Zhang M, Tan W, Xue M, Wang F. Interleukin-29 enhances synovial inflammation and cartilage degradation in osteoarthritis. Mediators Inflamm 2016 (2016) 9631510 PMID: 27433031 DOI: 10.1155/2016/9631510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sondergaard B-C, Schultz N, Madsen SH, Bay-Jensen AC, Kassem K, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation – divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthritis Cartilage 18 (2010) 279–288. [DOI] [PubMed] [Google Scholar]
- 96.Malemud CJ. Dysfunctional immune-mediated inflammation in rheumatoid arthritis dictates that development of anti-rheumatic disease drugs target multiple intracellular signaling pathways. Anti-Inflammatory Anti-Allergy Agents Med Chem 10 (2011) 78–84. PMID: 25182056 DOI: 10.2174/1871523011107020078 [DOI] [PubMed] [Google Scholar]
- 97.Tanaka Y, Yamaoka K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod Rheumatol 23 (2013) 415–424. PMID: 23212593 DOI: 10.1007/s10165-012-0799-2. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem 158 (2015) 173–179. PMID: 26152731 DOI: 10.1093/jb/mvv069. [DOI] [PubMed] [Google Scholar]
- 99.Malemud CJ. Suppression of pro-inflammatory cytokines via targeting of STAT-responsive genes. In: Discovery Drug. El-Shemy H (Ed), InTech Publishing: Rijeka, CROATIA, (2013) 373–411. [Google Scholar]
- 100.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, Falcone DJ. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol 192 (2014) 349–357. PMID: 24285838 DOI: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, Shumur R, Singhal AK, Wei N, Rosengren S, Kaplan I, Krishnaswami S, Luo Z, Bradley J, Firestein GS. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signaling in rheumatoid arthritis. Ann Rheum Dis 74 (2015) 1311–1316. PMID: 25398374 DOI: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis 75 (2016) 311–315. PMID: 26353790 DOI: 10.1136/annrheumdis-2014-207201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Araύjo VM, Melo IM, Lima V. Relationship between periodontitis and rheumatoid arthritis: review of the literature. Mediators Inflamm 2015 (2015) 259074 PMID: 26347200 DOI: 10.1155/2015/259074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of rheumatoid arthritis. J Clin Rheumatol 13 (2007) 134–137. PMID: 17551378 DOI: 10.1097/RHU.0b013e3180690616 [DOI] [PubMed] [Google Scholar]
- 105.Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, Askari A. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodont 80 (2009) 535–540. PMID: 19335072 DOI: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Da Silva AP, Bissada NP. Arthritis and periodontitis: an association debated for over two centuries. Curr Rheumatol Rev 12 (2016) 202–207. PMID: 26496784 DOI: 10.2174/1573397111666151026223058. [DOI] [PubMed] [Google Scholar]
- 107.Chang YC, Yang SF, Lai CC, Liu JY, Hsieh YS. Regulation of matrix metalloproteinase production by cytokines, pharmacological agents and periodontal pathogens in human periodontal ligament fibroblast cultures. J Periodontal Res 37 (2002) 196–203. PMID: 12113554 DOI: 10.1034/j.1600-0765.2002.00663-x [DOI] [PubMed] [Google Scholar]
- 108.Sorsa T, Tiäderhane L, Kontinnen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P. Matrix metalloproteinases, contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 36 (2006) 306–321. PMID: 16938801 DOI: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 109.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327 (2010) 580–583. PMID: 20110505 DOI: 10.1126/science.1181928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asif Amin M, Fox DA, Ruth JH. Synovial cellular and molecular markers in rheumatoid arthritis. Semin Immunopathol 39 (2017) 385–393. PMID: 28497350 DOI: 10.1007/s00281-017-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Willard VP, Diekman BO, Sanchez-Adams J, Christoforou N, Leong KW, Guilak F. Use of cartilage derived from murine induced pluripotential stem cells for osteoarthritis drug screening. Arthritis Rheumatol 66 (2014) 3062–3072. PMID: 25047145 DOI: 10.1002/art.38780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho H, Walker A, Williams J, Hasty KA. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res Int 2015 (2015) 595273 PMID: 26000299 DOIi: 10.1155/2015/595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rah B, Amin H, Yousuf K, Khan S, Jamwal G, Mukherjee D, Goswami A. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PloS One 7 (2012) e44039 PMID: 22962598 DOI: 10.1371/journal.pone.0044039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rhee JS, Coussens LM. RECKing MMP function: implications for cancer development. Trends Cell Biol 12 (2002) 209–211. PMID: 12062160 DOI: 10.1016/S0962-8924(02)02280-8 [DOI] [PubMed] [Google Scholar]
- 115.Takagi S, Hoshino Y, Osaki T, Okumura M, Fuginaga T. Expression of membrane-anchored matrix metalloproteinase inhibitor reversion inducing cysteine rich protein with Kazal motifs in murine cell lines. Exp Oncol 29 (2007) 30–34. PMID: 17431385 [PubMed] [Google Scholar]
- 116.Span PN, Sweep CG, Manders P, Beex LV, Leppert D, Lindberg RL. Matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer 97 (2003) 2710–2715. PMID: 12767082 DOI: 10.1002/cncr.11395 [DOI] [PubMed] [Google Scholar]
- 117.Gallego-Colon E, Klych-Ratuszny A, Kosowska A, Garczorz W, Aghdam MRF, Wozniak M, Francuz T. Exenatide modulates matrix metalloproteinase gene expression in human cardiac smooth muscle cells via the inhibition of Akt signaling pathway. Pharmacol Rep 70 (2018) 178–183. PMID: 29414148 DOI: 10.1016/j.pharep.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 118.Tauber S, Paulsen K, Wolf S, Synwoldt P, Pahl A, Schneider-Stock R, Ullrich O. Regulation of MMP-9 by a WIN-binding site in the monocyte-macrophage system independent of cannabinoid receptors. PloS One 7 (2012) e48272 PMID: 23139770 DOI: 10.1371/journal.pone.0048272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gramegna P, Latronico T, Branà MT, Di Bari G, Mengoni F, Belvisi V, Mascellino MT, Lichtner M, Vullo V, Mastroianni CM, Liuzzi GM. In vitro downregulation of matrix metalloproteinase-9 in rat glial cells by CCR5 antagonist maraviroc: therapeutic implication for HIV brain infection. PLoS One 6 (2011) e29499 PMID: 22174822 DOI: 10.1371/journal.pone.0028499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu Z, Li J, Ruan G, Wang G, Huang C, Ding C. Investigational drugs for the treatment of osteoarthritis, an update on recent developments. Expert Opin Investig Drugs 27 (2018) 881–900. PMID: 30345826 DOI: 10.1080/13543784.2018.1539075 [DOI] [PubMed] [Google Scholar]
- 121.Nam DH, Ge X. Generation of highly selective MMP antibody inhibitors. Methods Mol Biol 1731 (2018) 307–324. PMID: 29318563 DOI: 10.1007/978-1-4939-7595-2 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goffin L, Fagagnini S, Vicari A, Mamie C, Melhem H, Weder B, Lutz C, Lang S, Scharl M, Rogler G, Chvatchko Y, Hausmann M. Anti-MMP-9 antibody: a promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm Bowel Dis 22 (2016) 2041–2057. PMID: 27542125 DOI: 10.1097/MIB.00000000000863 [DOI] [PubMed] [Google Scholar]
- 123.Mullooly M, McGowan PM, Crown J, Duffy MJ. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol Ther 17 (2016) 870–880. PMID: 27115328 DOI: 10.1080/15384047.2016.1177684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nam EJ, Kang JH, Sung S, Sa KH, Kim KH, Seo JS, Kim JH, Han SW, Kim IS, Kang YM. A matrix metalloproteinase-1 cleavable composite peptide derived from transforming growth factor-ß inducible gene h3 potently inhibits collagen-induced arthritis. Arthritis Rheum 65 (2013) 1753–1763. PMID: 23508298 DOI: 10.1002/art.37932. [DOI] [PubMed] [Google Scholar]
- 125.Boyle DL, Kim HR, Topolewski K, Bartok B, Firestein GS. Novel phosphoinositide 3-kinase δ,γ inhibitor: potent anti-inflammatory effects and joint protection in models of rheumatoid arthritis. J Pharmacol Exp Ther 348 (2014) 271–280. PMID: 24244039 DOI: 10.1124/jpet.113.205955. [DOI] [PubMed] [Google Scholar]
- 126.Kular L, Rivat C, Lelongt B, Calmel C, Laurent M, Pohl M, Kitabgi P, Melik-Parsadaniantz S, Martinerie C. NOV/CCN3 attenuates inflammatory pain through regulation of matrix metalloproteinases −2 and −9. J Neuroinflammation 9 (2012) 36 PMID: 22353423 DOI: 10.1186/1742-2094-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cathcart JM, Cao J. MMP inhibitors: Past, present and future. Front Biosci (Landmark Ed) 20 (2015) 1164–1175. PMID: 25961551 DOI: 10.2741/4365 [DOI] [PubMed] [Google Scholar]
- 128.Liu J, Khalil RA. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog Mol Biol Transl Sci 148 (2017) 355–420. PMID: 28662828 DOI: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 147 (2017) 1–73. PMID: 28413025 DOI: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bokarewa M, Dahlberg L, Tarkowski A. Expression and functional properties of antibodies to tissue inhibitors of metalloproteinases (TIMPs) in rheumatoid arthritis. Arthritis Res Ther 7 (2005) R1014–R1022. PMID: 16207317 DOI: 10.1186/ar1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases (TIMPs): the biological functions and involvement in oral disease. J Dent Res 85 (2006) 1074–1084. PMID: 17122157 DOI: 10.1177/154405910608501202 [DOI] [PubMed] [Google Scholar]
- 132.Fiedorczyk M, Klimiuk PA, Sierakowski S, Gindzienska-Sieskiewicz E, Chwiecko J. Serum matrix metalloproteeinases and tissue inhibitors of metalloproteinases in patients with early rheumatoid arthritis. J Rheumatol 33 (2006) 1523–1529. PMID: 16881109 [PubMed] [Google Scholar]
- 133.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44–46 (2015) 247–254. PMID: 25805621 DOI: 10.1016/j.matbio.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 134.Nam EJ, Kang JH, Sa KH, Sung S, Park JY, Jo DG, Park JH, Kim IS, Kang YM. Robust therapeutic efficacy of matrix metalloprotease-2-cleavable Fas-1-RGD peptide complex in chronic inflammatory arthritis. PLoS One 11 (2016) e0164102 PMID: 27741237 DOI: 10.1371/journal.pone.0164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoshi H, Akagi R, Yamaguichi S, Muramatsu Y, Akatsu Y, Yamamoto Y, Sasaki S, Takahashi K, Sasho T. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res 368 (2017) 379–387. PMID: 28120109 DOI: 10.1007/s04441-016-2563-y [DOI] [PubMed] [Google Scholar]
- 136.Malemud CJ. Intracellular signaling pathways in rheumatoid arthritis. J Clin Cell Immunol 4 (2013) 160 PMID: 24619558 DOI: 10.4172/2155-9899.1000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wylie MA, Malemud CJ. Perspective: Deregulation of apoptosis in arthritis by altered signal transduction. Int J Clin Rheumatol 8 (2013) 483–490. DOI: 10.2217/IJR.13.31 [DOI] [Google Scholar]
- 138.Malemud CJ. PI3K/Akt/PTEN/mTOR signaling: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem 7 (2015) 1137–1147. PMID: 26132523 DOI: 10.4155/fmc.15.55 [DOI] [PubMed] [Google Scholar]