Abstract
There is a growing interest in the effectiveness of mindfulness meditation for sleep disturbed populations. Our study sought to evaluate the effect of mindfulness meditation interventions on sleep quality. To assessTo assess for relative efficacy, comparator groups were restricted to specific active controls (such as evidenced-based sleep treatments) and nonspecific active controls (such as time/attention-matched interventions to control for placebo effects), which were analyzed separately. From 3303 total records, 18 trials with 1654 participants were included. We determined the strength of evidence using four domains (risk of bias, directness of outcome measures, consistency of results, and precision of results). At post-treatment and follow-up, there was low strength of evidence that mindfulness meditation interventions had no effect on sleep quality compared with specific active controls (ES 0.03 [95% CI −0.43–0.49]) and (ES −0.14 [95% CI −0.62–0.34]) respectively. Additionally, there was moderate strength of evidence that mindfulness meditation interventions significantly improved sleep quality compared with nonspecific active controls at post-intervention (ES 0.33 [95% CI 0.17–0.48]) and at follow-up (ES 0.54 [95% CI 0.24–0.84]). These preliminary findings suggest that mindfulness meditation may be effective in treating some aspects of sleep disturbance. Further research is warranted.
Keywords: dose–response, insomnia, MBCT, MBSR, meditation, mindfulness, sleep
Graphical abstract:
There is a growing interest in the effectiveness of mindfulness meditation for sleep disturbed populations. Our study sought to evaluate the effect of mindfulness meditation interventions on sleep quality. To assess for relative efficacy, comparator groups were restricted to specific active controls, such as evidenced-based sleep treatments and nonspecific active controls, such as time/attention-matched interventions to control for placebo effects, which were analyzed separately.
Introduction
Sleep disturbance is a common health complaint affecting an estimated 10–25% of the general population.1 Accumulated sleep deficiency can increase the risk for mood and anxiety disorders,2–4 cognitive impairment,5 and a variety of medical conditions, including cardiovascular disease6 and obesity.7 Pharmaceutical sleep aids remain the first-line treatment for insomnia. While effective, they have the potential for abuse, cross-reactivity with other medications, and side effects including memory loss, abnormal thoughts, behavioral changes, and headaches.8,9 Alternatively, behavioral treatments, such as cognitive behavioral therapy for insomnia (CBT-I), can be expensive and inaccessible.10 While the risks are attenuated with CBT-I, some of the therapeutic components, such as intensive sleep restriction, may exacerbate comorbid psychiatric symptoms and thus compromise adherence.11–13 Taken together, there is a need for complementary health interventions, which increase patient choice and may be offered as a second-line treatment option when first-line treatments are not viable or are intolerable.
In recent years, mindfulness meditation has gained interest as an alternative treatment for sleep disturbance. Mindfulness means paying attention in a particular way: on purpose, in the present moment, and non-judgmentally—this attention is curious and kind.14 Cultivating present moment awareness, in lieu of reinforcing past or future reactivity, may function to transform engrained cognitive patterns and subsequent maladaptive behaviors.15 Mindfulness meditation is hypothesized to target multiple cognitive and emotional processes that contribute to poor sleep quality. It has been shown to decrease ruminative thoughts,16 diminish emotional reactivity,17,18 and promote impartial reappraisal of salient experiences, which together may facilitate sleep.19
The effect of mindfulness meditation on sleep quality has also been the topic of recent meta-analyses. However, findings were inconsistent and ranged from no effect to a moderate positive effect in favor of mindfulness meditation. Two of the four meta-analyses were not restricted to randomized control trials (RCTs).20,21 A third meta-analysis, restricted its investigation to RCTs;22 however, due to the small number of included trials investigators were unable to analyze the active control and waitlist control trials separately. This made it difficult to parse nonspecific effects (e.g., attention and expectancy bias) from the effect of the intervention. A fourth meta-analysis compared the effect of mindfulness meditation to active controls independently;23 however, the small number of included trials limited its generalizability. The objective of this meta-analysis is to build on prior meta-analyses by only including RCTs that employed a mindfulness meditation intervention in populations with clinically significant sleep disturbance. Furthermore, To assess for relative efficacy, comparator groups were restricted to specific active controls (such as evidenced-based sleep treatments) and nonspecific active controls (such as time/attention-matched interventions to control for placebo effects), which were analyzed separately. We aim to examine the following three questions: (1) Does mindfulness meditation improve sleep quality when compared with specific active controls or nonspecific active controls; (2) Does the effect persist long-term; and (3) Is there a dose-response effect.
Methods
Systematic search
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement.24 PubMed, EBSCO, Embase, and The Cochrane Library databases were searched for articles through May 2018, with no start date restriction. For search terms, two main subject-heading domains were combined with the AND operator: one to designate the intervention (meditation, mindfulness, mindfulness-based stress reduction (MBSR), mindfulness-based cognitive therapy (MBCT), or Vipassanā), and the second to designate the outcome (sleep or insomnia). No language restrictions were placed on the search. The bibliography of identified trials and germane review articles were manually searched for additional references.
Inclusion and exclusion criteria
We included published reports of RCTs in populations with clinically significant sleep disturbance that employed a mindfulness meditation intervention with multiple treatment sessions and assessment of baseline and post-intervention sleep quality. Validated sleep measures included both objective and subjective measurements, for example, actigraphy, self-reported sleep quality questionnaires, and diary-reported sleep quality. Evidence-based sleep treatments were determined by an American Academy of Sleep Medicine 2006 report25 and updated with a recent meta-analysis of 19 trials reporting medium to large effects of physical activity on subjective measures of sleep.26 Trials were excluded that compared mindfulness meditation to an experimental sleep treatment (e.g., transcendental meditation, tai chi, and yoga), or compared novice meditators to experienced meditators. All other populations with clinically significant sleep disturbance, excluding children and adolescents, were eligible. Table 1 includes a detailed summary of the inclusion and exclusion criteria.
Table 1.
Detailed inclusion and exclusion summary.
| Inclusion | Exclusion | |
| Population | Adult populations with clinically significant sleep disturbance (i.e., ICD insomnia diagnosis or met symptom severity threshold defined by sleep quality questionnaires) | Children, adolescents, and experienced meditators |
| Intervention | In-person, structured mindfulness meditation (e.g., mindfulness-based stress reduction and Vipassanā) | Mantra-based meditation and movement-based therapies like, tai chi and yoga, internet administration |
| Comparator | Specific active controls: evidence-based sleep treatments | Waitlist or usual care controls |
| Nonspecific active controls: time/attention-matched interventions | ||
| Outcome | Assessment of a pre-intervention and post-intervention validated subjective or objective measure of sleep | No validated measure of sleep or only a baseline measurement |
| Study Design | Randomized controlled trials | Nonrandomized controlled trials |
| Other | All languages and dates through May 2018 | Abstracts, reviews, and nonpublished trials, as well as duplicate participant samples |
Data extraction
Two investigators independently screened the title and abstract of each record to assess eligibility. The full-text article was obtained for all potentially eligible trials and screened for inclusion. Of the included trials, three investigators independently extracted data relating to author, publication year, population type, sample size, mindfulness meditation intervention, control intervention, control type, intervention weeks, in-class meditation hours, retreat meditation hours, at-home meditation hours, criteria for sleep disturbance, sleep quality outcome measure, assessment time-points, assessment data, and risk of bias criteria. Discrepancies in the eligibility and data extraction were resolved through further contact with corresponding authors, discussion, and consensus.
The strength of the body of evidence
The methods for determining the strength of the body of evidence were replicated from our prior meta-analysis.27 Briefly, three investigators graded the strength of evidence for each outcome, independently and then by consensus, using the grading scheme recommended by the Methods Guide for Conducting Comparative Effectiveness Reviews.28 In assigning evidence grades, we considered four domains: risk of bias, directness of outcome measures, consistency of results, and precision of results. Evidence was classified into the following four categories: (1) high (indicating high confidence that the estimate of effect lies close to the true effect for this outcome, and further studies would not change the conclusion); (2) moderate (indicating moderate confidence that the estimate of effect lies close to the true effect for this outcome, and findings are likely to be stable, but some doubt remains); (3) low (indicating limited confidence that the estimate of effect lies close to the true effect for this outcome, and that additional evidence is needed); and (4) insufficient (indicating no evidence or inability to estimate an effect for this outcome).28
Risk of bias scoring was used to evaluate the methodological quality of the included trials. Four major and four minor criteria were determined based on a system implemented in a prior comprehensive U.S. Agency for Healthcare Research and Quality review of meditative practices.23 Two points were given for meeting each major criterion and one point was given for meeting each minor criterion. A low risk of bias was assigned to trials with a score between 9 and 12 points. A medium risk of bias was assigned to trials with a score between 6 and 8 points. Any trial with five or fewer points was assigned a high risk of bias (Table 2). In assessing the directness of measures, both objective and subjective sleep measures were considered direct if they were validated to assess a sleep quality dimension. The consistency of results was evaluated by comparing the overall direction of effect. Lastly, the precision of results was based on the CI range from the meta-analysis. If the CI range was wide due to a large heterogeneity (which was attributed to the inconsistency of results) the evidence was not scored as imprecise as well.28
Table 2.
Major and minor criteria in assessing risk of bias
| Major Criteria | Minor Criteria |
|---|---|
| Was the control matched for time and attention by the instructors? | Was the randomization procedure described? |
| Were evaluators blinded to participant allocation? | Was allocation concealed? |
| Was there a description of withdrawals and dropouts? | Was an intent-to-treat analysis used? |
| Was attrition less than 20% at post-intervention assessment? | Did the trial evaluate the credibility, and if so, was it comparable? |
Outcome measures
Objective measures of sleep quality included the actigraphy. Subjective measures with established validity included the insomnia sleep index (ISI), the medical outcomes study-sleep scale (MOS-SS), and the Pittsburgh sleep quality index (PSQI). Due to the high overlap in content validity between the three sleep quality scales, they were pooled in the meta-analysis.
Data synthesis and analysis
Quantitative data were analyzed with the Cochrane Collaborative Review Manager Software (RevMan 5.3).29 All essential data that were not reported in the original papers were requested and received from the trial authors. Since sleep quality measures differed between trials (e.g., ISI, MOS-SS, and PSQI), the between-group standardized mean difference was used as the summary effect estimate of sleep quality and was calculated as Hedges’ g. Two trials used multiple sleep quality measures (ISI and PSQI).30,31 In this instance, the PSQI score was included in the meta-analysis since it was the most common measure used across all trials. Outcomes were analyzed on change from baseline to post-intervention to evaluate between-group percent change and the consistency of results across trials. A meta-analysis was used to estimate long-term effects of trials with a follow-up assessment between 5 to 12 months from baseline. To test for relative efficacy, all analyses were stratified by control type (i.e., specific active control or nonspecific active control). Spearman’s correlation was used to examine a dose-response between in-class meditation hours and standardized sleep quality change scores. Heterogeneity was evaluated using the I2 statistic, whereby an I2 ≤ 25% was considered low, an I2 = 50% was considered moderate, and an I2 ≥ 75% was considered high.32 Effect sizes were interpreted based on Cohen’s recommendation.33 P-values of <0.05 were considered significant.
Results
Search results
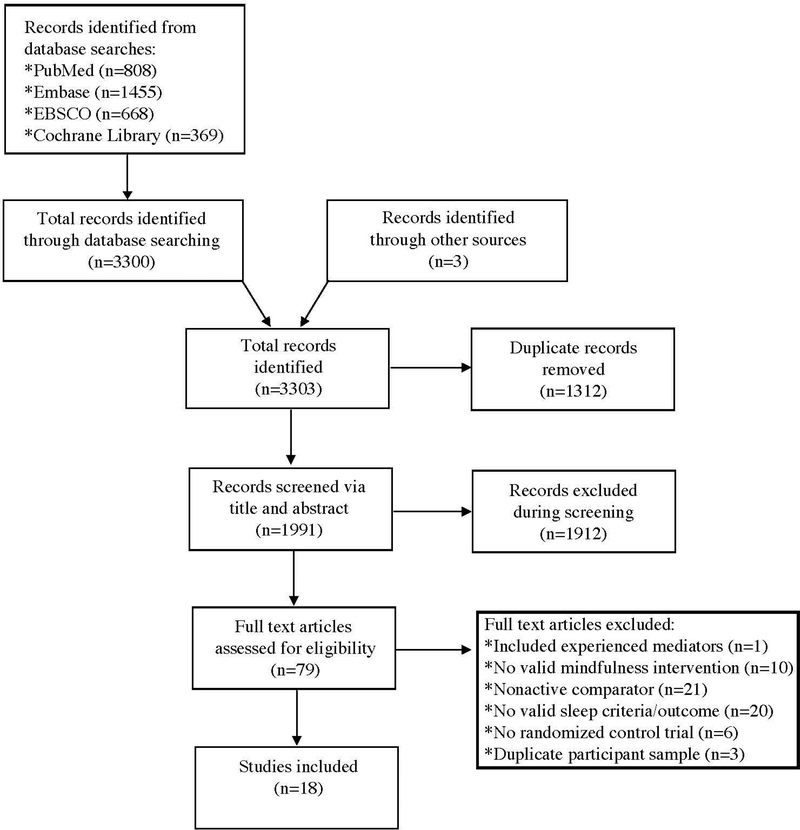
A total of 3303 records were initially identified for inclusion in the review. After adjusting for duplicates (n = 1312), another 1912 records were further excluded based on title and abstract. A full-text review of the remaining 79 articles was conducted and 18 trials with 1654 participants were included in the final analysis (see CONSORT flow diagram in Fig. 1).
Figure 1.
Flow diagram from record identification to a final study inclusion.
Characteristics of included trials
Publication dates ranged from 2010 to 2018. MBSR was the most prevalent intervention (9/18), followed by MBCT (3/18), and mind-body bridging (MBB) (3/18). Weekly in-class meditation sessions ranged from 1 to 2.5 h for 2 to 16 weeks. At-home meditation practice was encouraged in all 18 trials; however, 12 trials recommended a specific daily practice time, which ranged from 15 to 60 minutes. Seven trials included a one-day meditation retreat, and one trial offered an in-class booster session at 2 months post-intervention. All 18 trials included at least one subjective measure of sleep quality and two trials used an objective measure (e.g., actigraphy). Detailed characteristics of the 18 included trials are presented in Table 3.
Table 3.
Study characteristics.
| Authorref | Control type |
Population | Subject, n | Control, n | Meditation intervention |
Comparison intervention | Meditation duration, weeks |
Meditationa hours |
Scale /b V0 mean | AssessmentTime-point | Post- interventionc |
Follow-upd (5–12 months) |
Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adler,et al.34 | SAC | Obesity | 100 | 94 | MBSR | Progressive muscle relaxation | 16 | 42 | PSQI5.95 | 6-month12-month | Ø | ↑ | L (9) |
| Garland,et al.30 | SAC | Cancer with insomnia | 64 | 47 | MBSR | CBT-I | 8 | 18 | PSQI12.51 | Post-intervention5-month | - | - | M (7) |
| Gross,et al.31 | SAC | Insomnia | 20 | 10 | MBSR | Drug | 8 | 26 | PSQI11.56 | Post-intervention5-month | ↑ | ↑ | M (8) |
| Schmidt,et al.35 | SAC | Fibromyalgia syndrome | 53 | 56 | MBSR | Progressive muscle relaxation | 8 | 27 | PSQI11.34 | Post-intervention | Ø | M (8) | |
| van der Zwan,et al.36 | SAC | High stress | 27 | 23 | MM | Exercise | 5 | NR | PSQI5.74 | Post-intervention | ↑ | M (7) | |
| Vanhuffel,et al.37 | SAC | Insomnia | 16 | 13 | MBCT | CBT-I | 8 | 14 | PSQI13.07 | Post-intervention | ↑ | H (5) | |
| Wong,et al.38 | SAC | Insomnia | 101 | 95 | MBCT-I | Sleep psycho-education with exercise | 8 | 20 | ISI17.96 | Post-intervention8-month | + | Ø | L (9) |
| Black,et al.39 | NSAC | Older adults with sleep disturbance | 24 | 25 | MAPs | Sleep hygiene education | 6 | 12 | PSQI10.2 | Post-intervention | + | L (11) | |
| Dykens,et al.40 | NSAC | Mothers withdisabled children | 94 | 108 | MBSR | Positive adult development | 6 | 9 | ISI12.34 | Post-intervention7.5-monthe | + | Ø | M (8) |
| Gross,et al.41 | NSAC | Organ transplant | 71 | 66 | MBSR | Health education | 8 | 26 | PSQI7.77 | Post-intervention12-month | + | + | M (7) |
| Hoge,et al.42 | NSAC | General anxiety disorder | 48 | 41 | MBSR | Stress management education | 8 | 20 | PSQI8.26 | Post-intervention | + | M (8) | |
| Johns,et al.43 | NSAC | Breast and colorectal cancer | 35 | 36 | MBSR | Psychoeducation support group | 8 | 16 | ISI16.35 | Post-intervention6-month | Ø | Ø | L (12) |
| Malarkey,et al.44 | NSAC | Cardiovascular disease risk | 93 | 93 | MBSR-low dose | Lifestyle education | 8 | 10 | PSQI8.55 | Post-intervention | ↑ | L (9) | |
| Nakamura,et al.45 | NSAC | Posttraumatic stress disorder with sleep disturbance | 35 | 28 | MBB | Sleep hygiene education | 2 | 3 | MOS-SS57.73 | Post-intervention | + | M (6) | |
| Nakamura,et al.46 | NSAC | Cancer with insomnia | 19 | 18 | MBB | Sleep hygiene education | 3 | 6 | MOS-SS56.52 | Post-intervention | + | M (8) | |
| Nakamura,et al.47 | NSAC | Gulf war illnesswith sleep disturbance | 33 | 27 | MBB | Sleep hygiene education | 3 | 6 | MOS-SS64.2 | Post-intervention | ↑ | L (9) | |
| Oken,et al.48 | NSAC | Dementia caregivers | 10 | 11 | MM+CBT-I | Caregiver education | 6 | 9 | PSQI8.33 | Post-intervention | Ø | M (8) | |
| Van Gordon,et al.49 | NSAC | Fibromyalgia syndrome | 74 | 74 | MAT | Cognitive behavioral theory-group | 8 | 18 | PSQI14.10 | Post-intervention6-month | + | + | L (9) |
Meditation hours were reported as expected in-class hours per intervention, including the retreat.
The v0 sleep scale mean (e.g., baseline weighted average) was used to determine that the study cohort had clinically relevant sleep disturbance based on established cutoff scores.
Direction of effect is based on the relative difference in change analysis.
Follow-up findings were reported for studies with a follow-up assessment between 5 and 12 months.
Inability to obtain the Dykens, et al.,40 follow-up data precluded inclusion in the meta-analysis.
CBT-I, cognitive behavioral therapy-insomnia; ISI, insomnia severity index; MAPs, meditation awareness practices; MAT, meditation awareness training; MBB, mind-body bridging; MBCT, mindfulness-based cognitive therapy; MBCT-I, mindfulness-based cognitive therapy for insomnia; MBSR, mindfulness-based stress reduction; MM, mindfulness meditation; MOS-SS, medical outcomes study-sleep scale; NR, mediation was encouraged, but no specific time was reported; NSAC, nonspecific active control; PSQI, Pittsburgh sleep quality index; RCTS, randomized controlled trials; SAC, specific active control; h, high risk of bias; m, medium risk of bias; l, low risk of bias; +, favors meditation (>5%) and is statistically significant; –, favors control (<−5%) and is statistically significant; ↑, favors meditation (>5%) and is not statistically significant; ↓, favors control (<−5%) and is not statistically significant; ø, no effect (within –5% to 5%).
Quality of included trials
All or most trials included a description of withdrawals and dropouts (18/18), described the randomization procedure (17/18), matched the control group for time and attention to the meditation group (16/18), and reported attrition rates less than 20% at post-intervention (13/18). Quality limitations included a failure to evaluate the intervention credibility (3 did/18), conceal allocation (8/18), blind evaluators to participant allocation (9/18), and include an intent-to-treat analysis (10/18). The majority of trials had a moderate risk of bias (10/18), seven had a low risk of bias, and one had a high risk of bias. There were no significant differences in risk bias scores between the specific active control and nonspecific active control groups. Certified meditation instructors were included in 16 trials, trait mindfulness was assessed in 11 trials, and prior meditation was explicitly excluded in 10 trials.
Specific active controls
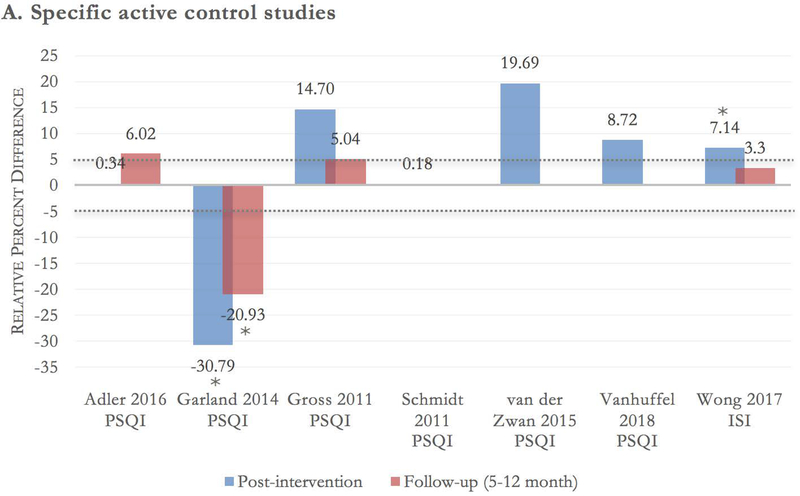
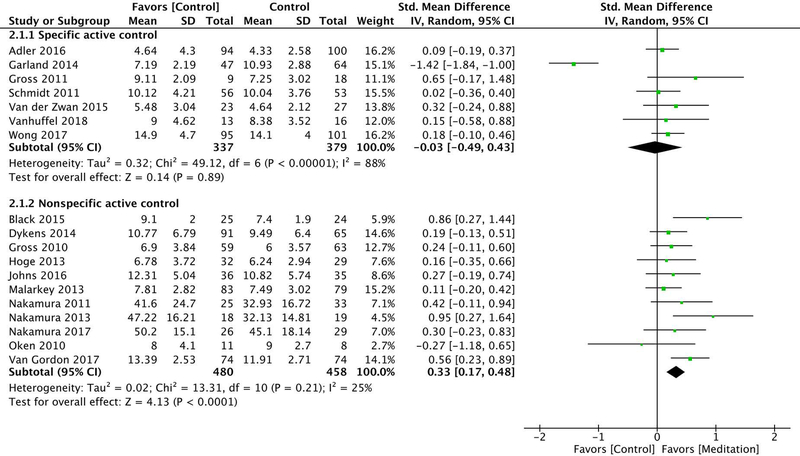
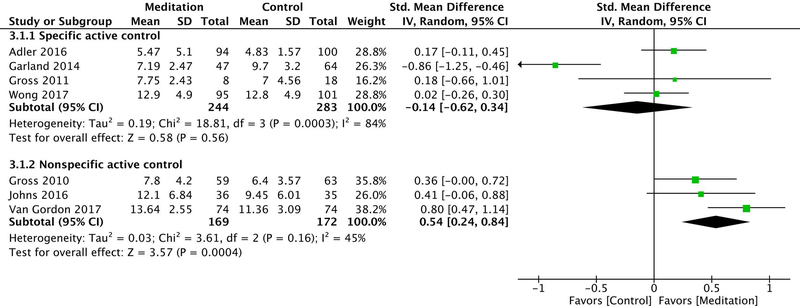
Seven of the included trials used specific active control groups,30,31,34–38 with a total of 716 participants. There was low strength of evidence that mindfulness meditation interventions had no effect on sleep quality compared with specific active controls (i.e., evidence-based sleep treatments) at post-intervention (ES −0.03 [95% CI −0.49–0.43]) and at a 5- to 12-month follow-up (ES −0.14 [95% CI −0.62–0.34]). This grading was based on an overall medium risk of bias, directness of measure, inconsistency of results (due to high heterogeneity at post-intervention [I2 = 88%] and a follow-up [I2 = 84%]), and precision of results (see Fig. 2A and Figs. 3 and 4).
Figure 2.
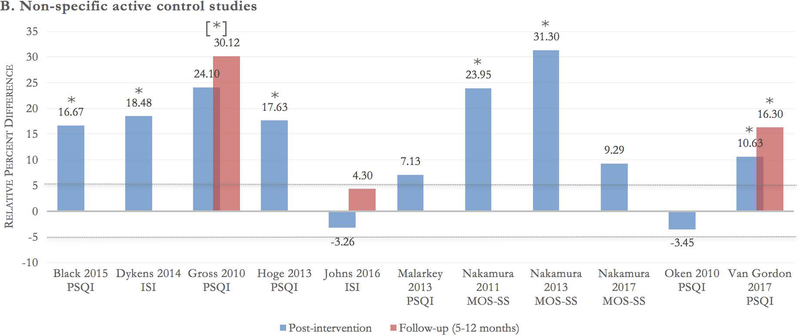
A-B Between-group relative percent difference in change score. Author, year, and sleep scale are noted at the bottom of each cluster bar. Follow-up scores are reported for trials with a follow-up assessment between 5 and 12 months from baseline. Percent change in sleep score was calculated using the formula: {[(postintervention mean^control – baseline mean^control) – (postintervention mean^meditation – baseline mean^meditation)] / (baseline mean^meditation)} × 100. Positive scores should be interpreted as relative percent change in favor of meditation. For example, a change score of 20% indicates the meditation group had a 20% higher improvement in sleep quality score compared with the control group. Dotted lines at −5% and 5% demarcate the effect threshold and do not indicate statistical significance. *The result is statistically significant per manuscript. [*] overall group effect is statistically significant; the effect for individual time points was not reported.
Figure 3.
Random effects meta-analysis of the effect of mindfulness meditation on sleep quality at post-intervention, stratified by control type. The standardized mean difference was used as the summary effect estimate and was calculated as Hedges’ g. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Total, number of participants.
Figure 4.
Random effects meta-analysis of the effect of mindfulness meditation on sleep quality at a 5- to 12-month follow-up, stratified by control type. The standardized mean difference was used as the summary effect estimate and was calculated as Hedges’ g. Abbreviations: CI, confidence interval; IV, inverse variance; SD, standard deviation; Total, number of participants.
Nonspecific active controls
Eleven of the included trials used nonspecific active control groups,39–49 with a total of 939 participants. There was moderate strength of evidence that mindfulness meditation interventions significantly improved sleep quality compared with nonspecific active controls (i.e., time/attention matched controls) at post-intervention (ES 0.33 [95% CI 0.17–0.48]) and at a 5- to 12-month follow-up (ES 0.54 [95% CI 0.24–0.84]). This grading was based on an overall medium risk of bias, directness of measure, consistency of results (due to low heterogeneity at post-intervention [I2 = 0%] and follow-up [I2 = 45%]), and precision of results (see Fig. 2B and Figs. 3–4).
Dose–response effect
Seventeen trials reported on total in-class meditation hours for the intervention, which ranged from 3 to 42 h (15.6 M, 9.8 SD), including the one-day retreat. No significant correlation was found between in-class meditation hours and standardized sleep quality change scores (rs = 0.1, P = 0.704). Six trials assessed a dose-response relationship between at-home practice minutes and sleep quality improvements from baseline to post-intervention. Three trials identified no relationship,31,38,41 while another three trials identified a significant positive correlation.34,36,49 One trial investigated a long-term dose–response effect and found a significant positive correlation between continued at-home practice minutes and additional sleep quality improvements at 18-month follow-up.34
Sensitivity analysis
We conducted a sensitivity analysis to confirm that our conclusions were not dependent on the updated evidenced-based sleep treatment determination. The specific active control group (minus the two exercise control trials36,38) had similar results at post-intervention (ES −0.14 [95% CI −0.80–0.53]) [I2 = 91%] and at a 5- to 12-month follow-up (ES −0.19 [95% CI −0.96–0.58]) [I2 = 89%]). The nonspecific active control group (plus the two exercise control trials) also had similar and significant results at post-intervention (ES 0.30 [95% CI 0.17–0.43]) [I2 = 14%] and did not report additional 5- to 12-month follow-up data. A second sensitivity analysis was conducted by including trials with a PSQI score greater than 10, and an ISI score greater than 14, which are indicative of severe sleep disturbance.50,51 The specific active controls had a similar effect size at post-intervention (ES −0.12 [95% CI −0.80–0.57]) [I2 = 91%]) and a decreased effect size at a 5 - to 12-month follow-up (ES −0.26 [95% CI −0.93–0.42]) [I2 = 85%]), which remained nonsignificant. Meanwhile, the nonspecific active controls had an increased effect size at post-intervention (ES 0.52 [95% CI 0.32–0.72]) [I2 = 0%]) and a similar effect size at a 5- to 12-month follow-up (ES 0.64 [95% CI 0.26–1.02]) [I2 = 44%]), which remained significant. Lastly, a leave-one-out sensitivity analysis determined that the significant heterogeneity in the specific active control meta-analysis was the result of a single CBT-I trial.30 The heterogeneity was reduced from 88% and 84% to 0% at post-intervention and at a 5- to 12-month follow-up. Results were similar at post-intervention (ES 0.15 [95% CI −0.01–0.31]) and at a 5- to 12-month follow-up (ES 0.10 [95% CI −0.09–0.29]), with a change in direction of effect.
Discussion
The evidence suggests that mindfulness meditation can improve sleep quality in a variety of clinical populations with sleep disturbance. While our results indicated no effect of mindfulness meditation on sleep quality when compared with evidenced-based sleep treatments, the strength of evidence was low and further studies are needed to elucidate these findings. Results also indicated that mindfulness meditation significantly improved sleep quality compared with nonspecific active controls. This meta-analysis only included RCTs with an active comparator group, so there is greater confidence that the reported benefits are not attributed to placebo effects commonly observed in usual care and waitlist control trials.
At a 5- to 12-month follow-up, mindfulness meditation did not differ in effect from evidence-based sleep treatments and significantly improved sleep quality compared with nonspecific active controls. These findings provide preliminary evidence for a long-term effect. The maintenance of intervention effects may be attributed to learned techniques that reduce sleep-interfering cognitive processes,20 changes in sleep architecture,52 as well as morphometric and connectivity alterations in sleep-related brain regions.53,54 Despite these advances, additional evidence is needed to clarify the conditions and mechanisms that drive the maintenance of intervention effects.
The evidence did not support a dose-response relationship between in-class meditation hours and sleep quality scores. This finding is consistent with a meta-analysis of 20 trials that assessed the relationship between in-class meditation hours and psychological distress.55 The link between at-home practice minutes and sleep quality scores was inconclusive due to the limited number of trials that assessed this relationship. Dose–response relationships are arguably one of the most challenging measures in meditation research. It’s difficult to accurately assess how mindful (versus mind wandering) an individual is during meditation practice.56 Studies with tailored curriculums, expert instructors, and different patient populations may result in larger effects with shorter course durations. Moreover, the nonlinear trajectory of mediation progress is often misunderstood.57 Traditionally, success is defined by increased awareness and equanimity, whereby positive states are a byproduct. When symptom change over a short period is utilized as a benchmark of success, meditation progress and its potential effect on well-being may be veiled.
Of the 10 trials that reported on adverse events, there was no evidence of increased risk of harm. Two trials reported a worsening of sleep quality in 3% and 7% of the meditation groups, compared with 24% and 12% in the comparator groups.45,47 Another trial reported one case of muscle soreness in the meditation group and one case of sleep disruption in the control group.42 It’s not uncommon for symptoms to worsen, particularly in the early weeks of the intervention.58 Feelings of anger, sadness, or fear, may appear stronger as practice develops since present moment awareness can highlight emotions.59 A history of trauma, mental instability, addiction, or major life changes, may heighten emotional reactivity and require additional clinical monitoring.60
Limitations
There are several limitations that reduced our ability to draw robust conclusions from these results. At the meta-analysis level, a leave-one-out sensitivity analysis indicated substantial heterogeneity due to the inclusion of a single CBT-I trial.30 This may be attributed to the large positive effects CBT-I is estimated to have on sleep quality when compared to other evidenced-based sleep treatments.61 It might also be due to the 50% attrition rate in the MBSR group (verses 14% in the CBT-I group). Participants who withdrew typically did so within the first three weeks and had higher levels of baseline insomnia severity, which may have attenuated effects.30 Additional heterogeneity might have been introduced by combining scores from the ISI, MOS-SS, and PSQI to create a single global sleep quality score. At the study level, the most common drawbacks were a failure to evaluate the intervention credibility and conceal allocation, which may lead to expectation bias. The lack of comprehensive reporting of treatment adherence and adverse events limited our ability to rigorously examine the effect of a dose–response and assess for safety. Moreover, only two trials included an objective measure of total sleep time via actigraphy. One trial identified a statistically significant between-group effect in favor of MBSR at a 5-month follow-up, but not at post-intervention.30 The other trial did not report between-group effects.31
Future directions
These findings support continued research exploring the clinical application of mindfulness meditation and provide a foundation for healthcare providers to consider these interventions in sleep-disturbed populations. Future research in mindfulness meditation would benefit from addressing the outstanding methodological limitations, as well as incorporating adherence measures, such as mobile applications, so participants can easily record at-home practice time. Future research should include systematic reporting of adverse events, which can help identify factors of increased risk. Researchers should use a combination of objective and subjective sleep outcomes to better understand if improved sleep quality is due to reduced sleep onset latency, improved total sleep time, or some other factor. The effectiveness of web- and app-based mindfulness meditation interventions should be investigated to increase accessibility, especially for low-income minorities with poor health and barriers to access.
Acknowledgments
We are grateful to Dr. Madhav Goyal for being an invaluable asset to the team and to the design and execution of this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The work was performed at the National Institutes of Health (NIH), Bethesda, MD, USA. The NIH had no role in the study design, collection, analysis, or interpretation of the data, or the writing of the manuscript. The manuscript received publication clearance by the NIH.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Morin C & Jarrin D 2013. Epidemiology of Insomnia : Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin: 281–297. [DOI] [PubMed] [Google Scholar]
- 2.Baglioni C et al. 2011. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 135: 10–19. [DOI] [PubMed] [Google Scholar]
- 3.Ritter PS, Marx C, Bauer M, Leopold K & Pfennig A 2011. The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord 13: 227–237. [DOI] [PubMed] [Google Scholar]
- 4.Rusch HL, Guardado P, Baxter T, Mysliwiec V & Gill JM 2015. Improved sleep quality is associated with reductions in depression and PTSD arousal symptoms and increases in IGF-1 concentrations. J. Clin. Sleep Med 11: 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durmer JS & Dinges DF 2005. Neurocognitive consequences of sleep deprivation. Semin Neurol 25: 117–129. [DOI] [PubMed] [Google Scholar]
- 6.Sofi F et al. 2014. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 21: 57–64. [DOI] [PubMed] [Google Scholar]
- 7.Taheri S, Lin L, Austin D, Young T & Mignot E 2004. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buscemi N et al. 2007. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J. Gen. Intern. Med 22: 1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holbrook AM, Crowther R, Lotter A, Cheng C & King D 2000. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ 162: 225–233. [PMC free article] [PubMed] [Google Scholar]
- 10.Koffel E, Bramoweth AD & Ulmer CS 2018. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J. Gen. Intern. Med 33: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong JC, Kuo TF & Manber R 2008. Who is at risk for dropout from group cognitive-behavior therapy for insomnia? Journal of psychosomatic research 64: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent N, Lewycky S & Finnegan H 2008. Barriers to engagement in sleep restriction and stimulus control in chronic insomnia. J Consult Clin Psychol 76: 820–828. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez E, Salem D, Swift JK & Ramtahal N 2015. Meta-analysis of dropout from cognitive behavioral therapy: Magnitude, timing, and moderators. J Consult Clin Psychol 83: 1108–1122. [DOI] [PubMed] [Google Scholar]
- 14.Kabat-Zinn J, Wherever You Go, There You Are (Hyperion, 2004). [Google Scholar]
- 15.Davis JH & Thompson E, From the Five Aggregates to Phenomenal Consciousness: Toward a Cross- Cultural Cognitive Science (Wiley-Blackwell, 2014). [Google Scholar]
- 16.Jain S et al. 2007. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med 33: 11–21. [DOI] [PubMed] [Google Scholar]
- 17.Desbordes G et al. 2012. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Frontiers in human neuroscience 6: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vugt MK, Hitchcock P, Shahar B & Britton W 2012. The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Frontiers in human neuroscience 6: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong JC, Ulmer CS & Manber R 2012. Improving sleep with mindfulness and acceptance: a metacognitive model of insomnia. Behav. Res. Ther 50: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winbush NY, Gross CR & Kreitzer MJ 2007. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore (NY) 3: 585–591. [DOI] [PubMed] [Google Scholar]
- 21.Kanen JW, Nazir RF, Sedky K & Basant PK 2015. The Effects of Mindfulness-Based Interventions on Sleep Disturbance: A Meta-Analysis. Adolescent Psychiatry 5: 105–115. [Google Scholar]
- 22.Gong H et al. 2016. Mindfulness meditation for insomnia: A meta-analysis of randomized controlled trials. J. Psychosom. Res 89: 1–6. [DOI] [PubMed] [Google Scholar]
- 23.Goyal M et al. 2014. Meditation programs for psychological stress and well-being. Comparative Effectiveness Review 124: 1–194. [Google Scholar]
- 24.Liberati A et al. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 25.Morgenthaler T et al. 2006. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep 29: 1415–1419. [PubMed] [Google Scholar]
- 26.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW & Otto MW 2015. The effects of physical activity on sleep: a meta-analytic review. J. Behav. Med 38: 427–449. [DOI] [PubMed] [Google Scholar]
- 27.Goyal M et al. 2014. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med 174: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens DK et al. 2010. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health-care program. J. Clin. Epidemiol 63: 513–523. [DOI] [PubMed] [Google Scholar]
- 29.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen. 2014.
- 30.Garland SN et al. 2014. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J. Clin. Oncol 32: 449–457. [DOI] [PubMed] [Google Scholar]
- 31.Gross CR et al. 2011. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore (NY) 7: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ & Altman DG 2003. Measuring inconsistency in meta-analyses. Bmj 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J, Statistical Power Analysis for the Behavioral Sciences (Routledge Academic, 1988). [Google Scholar]
- 34.Adler E et al. 2016. Impact of a Mindfulness-Based Weight-Loss Intervention on Sleep Quality Among Adults with Obesity: Data from the SHINE Randomized Controlled Trial. J. Altern. Complement. Med 23: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt S et al. 2011. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain 152: 361–369. [DOI] [PubMed] [Google Scholar]
- 36.van der Zwan JE, de Vente W, Huizink AC, Bogels SM & de Bruin EI 2015. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: a randomized controlled trial. Appl. Psychophysiol. Biofeedback 40: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanhuffel H, Rey M, Lambert I, Da Fonseca D & Bat-Pitault F 2018. [Contribution of mindfulness meditation in cognitive behavioral therapy for insomnia]. Encephale 44: 134–140. [DOI] [PubMed] [Google Scholar]
- 38.Wong SY et al. 2017. Comparing the Effects of Mindfulness-Based Cognitive Therapy and Sleep Psycho-Education with Exercise on Chronic Insomnia: A Randomised Controlled Trial. Psychother. Psychosom. 86: 241–253. [DOI] [PubMed] [Google Scholar]
- 39.Black DS, O’Reilly GA, Olmstead R, Breen EC & Irwin MR 2015. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med 175: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dykens EM, Fisher MH, Taylor JL, Lambert W & Miodrag N 2014. Reducing distress in mothers of children with autism and other disabilities: a randomized trial. Pediatrics 134: e454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross CR et al. 2010. Mindfulness-based stress reduction for solid organ transplant recipients: a randomized controlled trial. Altern. Ther. Health Med 16: 30–38. [PMC free article] [PubMed] [Google Scholar]
- 42.Hoge EA et al. 2013. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry 74: 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns SA et al. 2016. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support. Care Cancer 24: 4085–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malarkey WB, Jarjoura D & Klatt M 2013. Workplace based mindfulness practice and inflammation: a randomized trial. Brain. Behav. Immun 27: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura Y, Lipschitz DL, Landward R, Kuhn R & West G 2011. Two sessions of sleep-focused mind-body bridging improve self-reported symptoms of sleep and PTSD in veterans: A pilot randomized controlled trial. J. Psychosom. Res 70: 335–345. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Lipschitz DL, Kuhn R, Kinney AY & Donaldson GW 2013. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: a pilot randomized controlled trial. J. Cancer Surviv 7: 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura Y et al. 2017. Investigating Clinical Benefits of a Novel Sleep-Focused Mind-Body Program on Gulf War Illness Symptoms: A Randomized Controlled Trial. Psychosom. Med 79: 706–718. [DOI] [PubMed] [Google Scholar]
- 48.Oken BS et al. 2010. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J. Altern. Complement. Med 16: 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Gordon W, Shonin E, Dunn TJ, Garcia-Campayo J & Griffiths MD 2017. Meditation awareness training for the treatment of fibromyalgia syndrome: A randomized controlled trial. Br. J. Health Psychol 22: 186–206. [DOI] [PubMed] [Google Scholar]
- 50.Okun ML et al. 2009. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J. Clin. Sleep Med 5: 41–51. [PMC free article] [PubMed] [Google Scholar]
- 51.Gagnon C, Belanger L, Ivers H & Morin CM 2013. Validation of the Insomnia Severity Index in primary care. J. Am. Board Fam. Med 26: 701–710. [DOI] [PubMed] [Google Scholar]
- 52.Nagendra RP, Maruthai N & Kutty BM 2012. Meditation and its regulatory role on sleep. Front. Neurol 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox KC et al. 2014. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev 43C: 48–73. [DOI] [PubMed] [Google Scholar]
- 54.Hasenkamp W & Barsalou LW 2012. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmody J & Baer RA 2009. How long does a mindfulness-based stress reduction program need to be? A review of class contact hours and effect sizes for psychological distress. J. Clin. Psychol 65: 627–638. [DOI] [PubMed] [Google Scholar]
- 56.Davidson RJ & Kaszniak AW 2015. Conceptual and methodological issues in research on mindfulness and meditation. Am. Psychol 70: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Britton WB, Lindahl JR, Cahn BR, Davis JH & Goldman RE 2014. Awakening is not a metaphor: the effects of Buddhist meditation practices on basic wakefulness. Ann. N. Y. Acad. Sci 1307: 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creswell JD 2017. Mindfulness Interventions. Annu. Rev. Psychol 68: 491–516. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig DS & Kabat-Zinn J 2008. Mindfulness in medicine. JAMA 300: 1350–1352. [DOI] [PubMed] [Google Scholar]
- 60.Crane RS & Kuyken W 2013. The Implementation of Mindfulness-Based Cognitive Therapy: Learning From the UK Health Service Experience. Mindfulness (N Y) 4: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.2016. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Annals of internal medicine 165. [DOI] [PubMed] [Google Scholar]