Abstract
Introduction:
Pyloric drainage during minimally invasive esophagectomy (MIE) may be more technically challenging than during the open approach and alternatives to the classic surgical drainage approach have increased in popularity. However, data are lacking to demonstrate whether one technique is superior in MIE. The purpose of this study was to compare postoperative outcomes after MIE between different pyloric drainage methods.
Methods:
We performed a retrospective review of a prospectively maintained database of patients undergoing MIE at a single academic institution. Patients were divided into three groups for analysis: no drainage, intrapyloric Botulinum Toxin injection, and surgical drainage (pyloroplasty or pyloromyotomy). The primary outcome was any complication within 90 days of surgery; secondary outcomes included reported symptoms and need for pyloric dilation at 6 and 12 months postoperatively. Comparisons among groups were conducted using Kruskal Wallis
Results:
There were 283 MIE performed between 2011 and 2017; of these, 126 (45%) had drainage (53 Botulinum injection and 73 surgical). No significant difference in the rate of postoperative complications, pneumonia or anastomotic leak was observed between groups. At 6 and 12 months, patients that received Botulinum Toxin and surgical drainage had significantly more symptoms than no drainage (p<.0001) and had higher need for pyloric dilation at 6 months (p=0.007).
Conclusions:
Pyloric drainage was not significantly associated with lower postoperative complications or long-term symptoms. While Botulinum injection appears safe postoperatively, it was associated with increased morbidity long-term. Pyloric drainage in MIE may be unnecessary.
Keywords: esophagectomy, pyloric drainage, postoperative complications, pyloric stricture
INTRODUCTION
Increasing emphasis on improvement of postoperative outcomes and quality of life in conjunction with evolution of surgical technologies has led to growing utilization of minimally invasive esophagectomy (MIE). MIE has been associated with reduced postoperative blood loss and fewer major complications compared to open surgery. [1,2]
Despite the benefits of MIE, certain morbidities are inherent to esophagectomy regardless of the operative approach. Vagal nerve resection commonly necessary for oncologic quality may result in conduit dysmotility and pyloric dysfunction with subsequent postoperative delayed gastric emptying (DGE) reported in 4 to 50% of patients.[3] Resultant stasis within the conduit may result in symptoms (i.e. nausea, vomiting and early satiety) which complicate eating, and increase risk of aspiration pneumonia, anastomotic stricture and anastomotic leak. [4]
Pyloric drainage procedures are often used to minimize the negative sequelae attributed to DGE; however, available data are inconsistent about associated benefitsbenefits.[3,5,6] Some surgeons argue against intervention as DGE may impact only a minority of patients and nerve regeneration gradually allows recovery of gastric function.[7] Additionally, drainage has been associated with complications including increased gastric dumping, esophagitis and biliary reflux.[8,9]
Surgical drainage with pyloromyotomy or pyloroplasty has historically been the most common pyloric intervention; however, these approaches are more technically challenging and take more time in the minimally invasive setting. Intrapyloric injection with Botulinum toxin has become a popular alternative since it was first introduced in 2007 as a less invasive, simpler approach to drainage in MIE. Additional proposed benefits included the short term nature of this therapy - improved gastric emptying may be useful in the immediate postoperative period but the effects mitigate as gastric function recovers and therefore, negative long-term effects of drainage are minimized.[10] Current data comparing Botulinum injection as an alternative to surgical drainage procedures (pyloroplasty or pyloromyotomy) is inconclusive as to whether one approach is superior in reducing associated morbidity.[9,11]
Although previous studies have questioned the role of pyloric drainage following open esophagectomy, there is a paucity of data in MIE. As surgeon experience with MIE rises, it is important to determine whether pyloric drainage has a benefit in this setting and whether one approach is safer with fewer side effects. Therefore, the purpose of this study was to compare outcomes between pyloric intervention group (none, surgical, and Botulinum Toxin injection) in patients undergoing MIE.
MATERIALS AND METHODS
After approval was obtained from the institutional review board, consecutive patients that underwent minimally invasive esophagectomy (MIE) for esophageal malignancy between January 2011 and December 2017 were identified from a prospectively maintained institutional database of esophagectomies performed at Memorial Sloan Kettering Cancer Center. Data on patient demographics, esophageal pathology, treatment selection and postoperative outcomes were abstracted from patient charts. Postoperative clinical notes and endoscopic reports were reviewed for patient reported symptoms and subsequent interventions. This study was exempt by the Institutional Review Board.
Patients were divided into three groups by pyloric intervention, including: no drainage, Botulinum Toxin injection, and surgical drainage. Patients that received pyloroplasty or pyloromyotomy were analyzed together within the surgical group. Only patients that underwent esophagectomy for malignancy and dysplasia were included. We excluded patients that underwent pharyngolaryngoesophagectomy to eliminate confounders linked to case complexity. Additionally, we excluded patients that had conversion of the abdominal portion from minimally invasive to open. Patients that had conversion of the thoracic portion to thoracotomy were still analyzed as our primary interest was in a minimally invasive approach to the abdominal portion.
Surgical Technique
Each patient underwent clinical staging using a combination of endoscopy, endoscopic ultrasound, positron emission tomography and computed tomography scan, as is the standard at our institution. Neoadjuvant therapy with chemoradiation was given to all patients with node-positive disease and/or ≥T2 disease. Resection was performed 5-8 weeks after completion of therapy. Patients with in situ/T1N0 tumors or other pathology (GIST) were offered MIE alone without neoadjuvant therapy. All operations were performed by surgeons within the Department of Thoracic Surgery.
Surgical approach to MIE was selected by surgeon preference and tumor location. We prefer a transthoracic approach for cancer to better enable mediastinal lymphadenectomy, with anastomosis in the chest (Ivor Lewis) or neck (3-hole). Most patients in the United States present with distal esophageal adenocarcinoma, and consequently, Ivor Lewis is most commonly performed. All patients received a bronchoscopy and endoscopy prior to initiation of surgical resection. For Ivor Lewis, the procedure generally begins with a laparoscopic or robotic approach to the abdomen. A complete lymphadenectomy of the celiac branches is performed. The stomach and distal esophagus are mobilized at the hiatus, and the dissection is continued as far as proximally into the mediastinum as possible. Adjunct procedures, including feeding jejunostomy and pyloric drainage, are then performed. The stomach is tubularized to create a conduit approximately 5 cm in width. Thoracoscopic mobilization and lymphadenectomy is then performed via a right posterolateral approach with subsequent intrathoracic anastomosis using either a circular or a linear stapler. In the 3-hole (McKeown) approach, the procedure begins with en block dissection of the esophagus and lymphadenectomy. Laparoscopic gastric mobilization and nodal dissection is then performed. Finally, a cervical anastomosis is performed via a left neck incision using a stapled or hand-sewn approach.
Intraoperative Pyloric Interventions
The decision of whether and which type of pyloric intervention to use was dependent upon surgeon preference. Pyloric drainage included intraoperative pyloroplasty, pyloromyotomy or Botulinum Toxin A (Botox) injection. Botulinum injection was first used at our institution in 2016; as such, use was more frequent in the last 2 years of the study.
The pyloric intervention is performed after creation of the gastric conduit. Pyloromyotomy is performed using hook electrocautery in a longitudinal fashion across the pylorus without entering the gastric mucosa. Patients that were noted to have full thickness perforation into the gastric lumen are converted to pyloroplasty. During pyloroplasty the entire muscle and the gastric mucosa are divided. The pylorus is then closed in a Heineke-Mikulicz transverse fashion using an endosuture device in an interrupted fashion. Our preference for Botulinum Toxin injection is well described, but involves a transabdominal approach utilizing a 7 inch 22-gauge spinal needle into the pyloric musculature.[13] By decreasing the insufflation pressure, the pylorus is easily reached in each case.
Postoperative Evaluation and Follow-Up
All patients received a nasogastric tube postoperatively. Radiologic studies for DGE were not routinely performed.. Operative data collected included operative procedure type, operative time, estimated blood loss (EBL) in ml, and drainage procedure used.
Postoperative data included length of stay (LOS) in days, any complications, serious complications, anastomotic leak, pneumonia, tracheoesophageal fistula (TEF), empyema, readmission, reoperation and death within 90 days of surgery. Serious complications were defined as complications classified as Clavien-Dindo grade ≥ 3.[13] Leak and TEF were diagnosed by swallow study, computed tomography or endoscopic assessment. Pneumonia was diagnosed by combination of clinical symptoms suggestive of the diagnosis, leukocytosis, and infiltrates on imaging. Diet was advanced according to surgeon preference. High nasogastric tube output on postoperative day 3, defined as > 300 ml, was assessed as a measure of DGE. However, as an additional measure of DGE, we determined time in days to liquid diet and removal of jejunostomy tube as the ability to tolerate oral diet as these measures may be more objective than NGT removal, which may be a function of surgeon preference. Lastly, we identified whether any patients required pyloric dilation within 90 days of surgery. The decision for postoperative endoscopy with pyloric or anastomotic dilation was based on symptoms of conduit dysfunction or gastric outlet obstruction (dysphagia, early satiety, nausea, vomiting, bloating). Patients that had not received any intraoperative intervention but required subsequent postoperative dilation were maintained in the no drainage group for intent to treat.
Late outcomes included symptoms and need for endoscopic dilation (pyloric or anastomotic) at 6 months and 12 months. Patients completed a standard review of symptoms. Gastrointestinal symptoms of interest included reflux, coughing, dysphagia, early satiety, poor PO tolerance, regurgitation, nausea, vomiting, diarrhea, aspiration, and postprandial pain. Dumping included other commonly associated symptoms associated with dumping including flushing, sweating, light-headedness, or tachycardia. Aspiration events were deemed to have occurred in patients that had either been hospitalized or treated for aspiration related pulmonary complications.
Statistical Analysis
Demographic data was summarized and compared between the three pyloric intervention groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test or Chi Square test as appropriate for categorical variables. Odds ratios (OR) from logistic regression models were used to estimate the association between pyloric intervention groups and selected binary endpoints. Outcomes included symptoms at 6 and 12 months, need for pyloric dilation at 6 and 12 months and selected symptoms with sufficient event numbers (at least 10 events) to provide reliable estimates. All multivariable logistic models were adjusted for clinically relevant variables decided upon a priori: age and BMI at time of surgery. [14] Full multivariable selection procedure was not possible due to limited numbers of events. Two-sided p<0.05 was considered significant. Data was analyzed using Stata 15.0 (StataCorp, College Station, TX).
RESULTS
Demographics
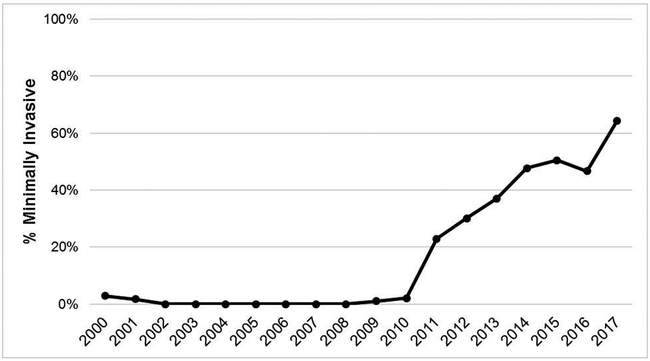
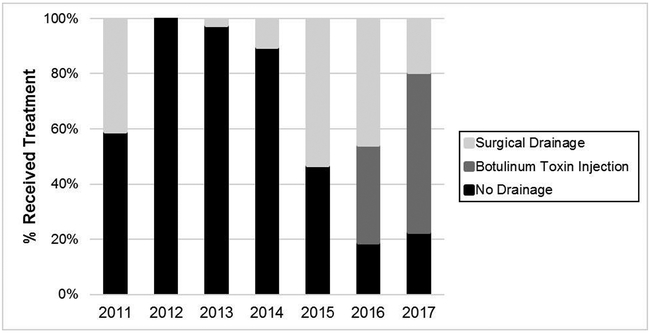
Of 1738 esophagectomies performed during the study period, 299 were MIE (17.2%). Changes in use of MIE at our institution during the study period are presented in Figure 1. Use of the MIE approach increased during the study period from 22.9% in 2011 to 64.4% in 2017. After excluding patients with esophagopharyngeal anastomosis (n=1) and conversion of the abdominal portion to laparotomy (n=15), 283 patients met inclusion criteria. The majority (55%) of patients did not have any pyloric drainage procedure performed at the index surgery. Amongst remaining patients, 73 (26%) underwent surgical drainage and 53 (19%) received Botulinum Toxin. Changes in trends of pyloric intervention strategies over the study period are demonstrated in Figure 2. Botulinum Toxin first came into use at our institution in 2016.
Figure 1.
Institutional use of MIE over time
Figure 2.
Institutional variability in selection of pyloric intervention group over time
Median patient age was 62 years (25th-75th percentile, 56-69). Adenocarcinoma was the most common pathology (250/283; 82.8%) and most patients received neoadjuvant treatment (209/283; 69.2%). Overall 90-day complication rate was 61.3% (n=185), with serious complication rate of 28.6% (n=81) and mortality 3% (9/283). Comparison of key factors between pyloric intervention groups are presented in Table 1 and 2. There was no difference in age, BMI, ASA score, or neoadjuvant therapy use between groups. Patients who received Botox had significantly longer median operative time (528 minutes vs 478 surgical drainage and 439 no drainage; p<0.0001) but lower median estimated blood loss (EBL) (150 ml vs 200 for surgical and no drainage; p=0.01). (Table 2) There was no significant difference in length of stay between groups.
Table 1.
Characteristics of 283 patients that underwent MIE compared by pyloric intervention group
| No Drainage (N=157; 55%) |
Botox (N=53; 19%) |
Pyloroplasty (N=73; 26%) |
p | |
|---|---|---|---|---|
| Age, years | 62.0 (55.0, 68.0) | 59.0 (54.0, 70.0) | 62.0 (58.0, 70.0) | 0.10 |
| Male | 123 (78%) | 44 (83%) | 62 (85%) | 0.5 |
| Comorbid Conditions | ||||
| Pulmonary | 15 (10%) | 9 (17%) | 6 (8.2%) | 0.3 |
| Cardiac | 70 (45%) | 33 (62%) | 40 (55%) | 0.058 |
| Diabetes | 26 (17%) | 12 (23%) | 9 (12%) | 0.3 |
| ASA | ||||
| 2 | 24 (15%) | 7 (13%) | 9 (12%) | 0.5 |
| 3 | 126 (80%) | 40 (75%) | 59 (81%) | |
| 4 | 7 (4.5%) | 6 (11%) | 5 (6.8%) | |
| BMI | 28.7 (24.6, 32.2) | 29.4 (24.9, 34.2) | 27.9 (25.3, 31.0) | 0.5 |
| Preoperative | 119 (76%) | 42 (79%) | 52 (71%) | 0.6 |
| Chemotherapy | ||||
| Preoperative Radiation | 114 (73%) | 40 (75%) | 48 (66%) | 0.4 |
| Procedure Type | ||||
| Ivor Lewis | 144 (92%) | 48 (91%) | 63 (86%) | 0.4 |
| 3-hole | 13 (8.3%) | 5 (9.4%) | 10 (14%) | |
| EBL, ml | 200.0 (125.0, 350.0) | 150.0 (100.0, 230.0) | 200.0 (100.0, 300.0) | 0.010 |
| Operative Time, minutes | 439.0 (384.0, 518.0) | 528.0 (477.0, 577.0) | 478.0 (435.0, 555.0) | <0.0001 |
| Histology | ||||
| Adeno | 139 (89%) | 45 (85%) | 66 (90%) | 0.8 |
| SCC | 13 (8.3%) | 7 (13%) | 6 (8.2%) | |
| Other | 5 (3.2%) | 1 (1.9%) | 1 (1.4%) | |
| Tumor Location | ||||
| Proximal | 0 (0%) | 1 (1.9%) | 0 (0%) | 0.001 |
| Mid | 1 (0.6%) | 5 (9.4%) | 4 (5.5%) | |
| Distal | 156 (99%) | 47 (89%) | 69 (95%) |
Values presented as N(%) or median (25th, 75th percentile).
Table 2.
90-day postoperative outcomes of 283 patients that underwent MIE compared by pyloric intervention group
| No Drainage (N=157; 55%) |
Botox (N=53; 19%) |
Pyloroplasty (N=73; 26%) |
p | |
|---|---|---|---|---|
| Length of Stay, days | 9.0 (8.0, 11.0) | 8.0 (7.0, 12.0) | 8.0 (7.0, 11.0) | 0.2 |
| Complications | ||||
| Any | 104 (66%) | 34 (64%) | 47 (64%) | 1 |
| Seriousa | 47 (30%) | 14 (26%) | 18 (25%) | 0.7 |
| Readmission | 35 (22%) | 10 (19%) | 12 (16%) | 0.6 |
| Reoperation | 6 (3.8%) | 4 (7.5%) | 7 (10%) | 0.2 |
| Leak | 35 (22%) | 8 (15%) | 11 (15%) | 0.3 |
| Tracheoesophageal Fistula (TEF) | 6 (3.8%) | 1 (1.9%) | 4 (5.5%) | 0.6 |
| Empyema | 15 (10%) | 5 (9.4%) | 7 (10%) | 1 |
| Pneumonia | 31 (20%) | 7 (13%) | 15 (21%) | 0.5 |
| Days to Liquid Diet (N=269) | 7.0 (6.0, 8.0) | 6.0 (6.0, 8.0) | 10.0 (7.0, 14.0) | <0.0001 |
| Days to Jejunostomy Removal | 26.0 (22.0, 47.0) | 25.0 (20.0, 34.0) | 36.5 (23.0, 57.0) | 0.009 |
| Days to Nasogastric Tube Removal | 6.0 (4.0, 7.0) | 4.0 (4.0, 5.0) | 3.0 (2.0, 4.0) | <0.0001 |
| High Nasogastric Tube Output (≥ 300 ml), Postoperative Day 3 | 103 (66%) | 36 (68%) | 62 (85%) | 0.007 |
| Postoperative Pyloric Dilation | 10 (6.4%) | 2 (3.8%) | 1 (1.4%) | 0.30 |
| Mortality | 5 (3.2%) | 0 (0%) | 4 (5.5%) | 0.20 |
| In-Hospital Mortality | 2 (1.3%) | 0 (0%) | 4 (5.5%) | 0.069 |
Values presented as N(%) or median (25th, 75th percentile).
Clavien-Dindo Classification ≥3
Postoperative Outcomes
Postoperative 90-day outcomes are compared between intervention groups in Table 2. There were no differences in complication, serious complication, reoperation or readmission rates between groups. Similarly, observed rates of leak, pneumonia or TEF did not differ. Patients with surgical drainage had slower median time to starting liquid diet (10 days vs 6 for Botox and 7 for no drainage, p<.0001) and removal of jejunostomy tube (36.5 days vs 25 Botox and 26 no drainage, p=0.009). Patients without drainage had the longest median time to removal of nasogastric tube (6 days vs 4 Botox and 3 surgical drainage) (p<.0001); however, patients with surgical drainage had a significantly higher rate of high nasogastric tube output on postoperative day 3 compared to no drainage and Botulinum Toxin (p=0.007).
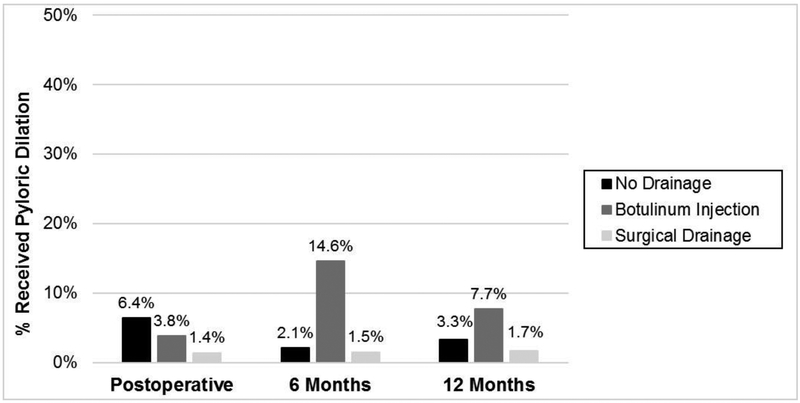
There were 10 (6.4%) patients without drainage that required pyloric dilation within 90 days of surgery compared to 2 (3.8%) and 1 (1.4%) in the Botulinum and surgical drainage groups, respectively (p=0.30). Patients that received surgical drainage displayed slightly higher rate of in-hospital mortality following the index procedure but was not statistically significant (5.5% vs 0% Botulinum injection and 1.3% no drainage, p=0.069). Of the 4 patients with surgical drainage, 3 died from pneumonia/aspiration and 1 from cardiac complications. There were 2 in hospital deaths in the no drainage group, 1 due to pneumonia/aspiration and the other due to cardiac issues. Three additional patients without drainage died within 90 days after surgery following hospital discharge because of aspiration/pneumonia (n=1), complications from anastomotic leak (n=1) and failure to thrive (n=1).
6 and 12 Month Postoperative Outcomes
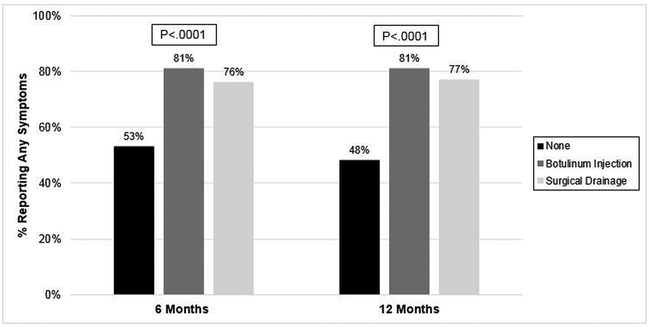
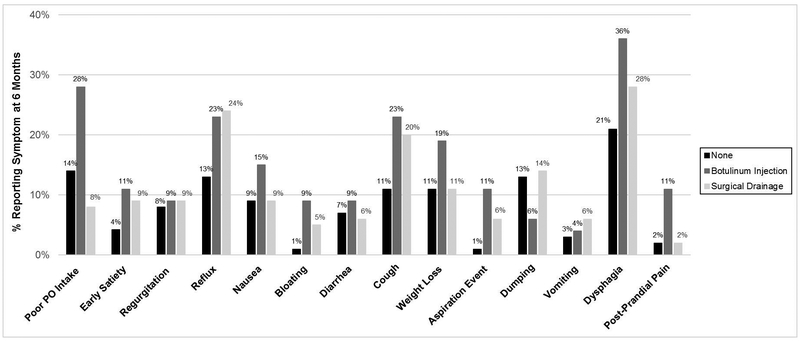
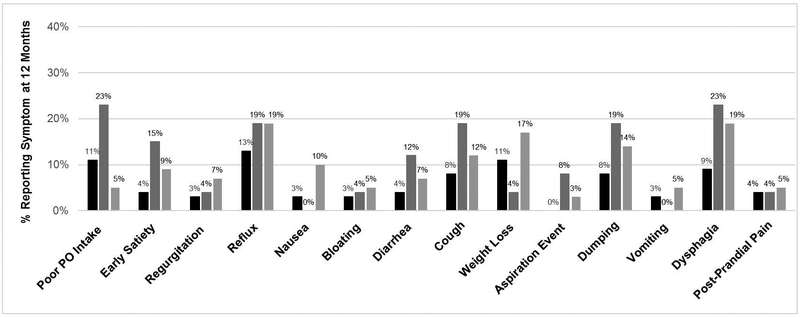
There were 257 (91%) patients that had a 6-month postoperative visit and 203 (80%) with a 12-month visit. Patients with pyloric drainage procedures reported significantly larger proportion of patients with any symptoms at 6 months (81 % Botox and 75% surgical drainage) and 12 months (81% and 77%, respectively) compared to patients without drainage (53% at 6 and 52% at 12 months) (p<0001). (Figure 3) Reported symptoms by group at 6 and 12 months are presented in Figures 4 and 5. At 6 months, patients with Botox had higher rates of poor PO tolerance (28%), post prandial pain (11%), and aspiration events (11%) compared to the other groups (p<0.02). Although not significant, a larger proportion of patients that received Botox or surgical drainage reportedly experienced reflux, cough and dysphagia compared to no drainage. Patients that received Botox received pyloric dilation more often than other groups at 6 months (14.6% vs 1.5% surgical drainage and 2.1% no drainage) (p<002). Although not significant, patients that received Botox underwent pyloric dilation more than the other groups at 12 months (7.7% Botox vs 3.3% no drainage and 1.7% surgical drainage, p=0.40).
Figure 3.
Frequency of reported symptoms by pyloric intervention group at 6 and 12 months
Figure 4.
Comparison of reported symptoms by pyloric intervention group at: A. 6 months B. 12 months
Figure 5.
Pyloric dilation by pyloric intervention group
Logistic regression was performed to estimate the association between pyloric intervention group and selected outcomes. As demonstrated in Table 3, univariable analysis demonstrated that patients that received Botox and surgical drainage had greater odds of reporting any symptoms at 6 months compared to no drainage (OR 3.93 (95% CI, 1.77 – 8.71; p=0.001 and 2.83 (1.48 – 5.44); p=0.002, respectively). At 12 months, patients that underwent surgical drainage were more likely to report symptoms compared to patients without drainage procedures (p=0.023). Patients that received Botox were also more likely to require pyloric dilation at 6 months compared to no drainage (p=.009); however, no difference was observed between surgical drainage and no drainage. Patients with Botox were more likely to report poor PO tolerance compared to both no drainage and surgical drainage at 6 and 12 months. Multivariable analysis is presented in Table 3. Adjusted odds ratio demonstrated that the observed increased odds of reporting any symptoms persisted at 6 months in both the Botox (OR=4.14; 95% CI 1.83-9.35; p=0.001) and surgical drainage groups (OR 3.16; 95% CI 1.62-6.16; p=0.001) compared to no drainage and persisted at 12 months in patients with surgical drainage (2.15; 95% CI 1.12-4.14; p=0.021). Patients with surgical drainage had lower odds of undergoing pyloric dilation at 6 months compared to Botox (0.07; 95% CI 0.01-0.70; p=0.023) and had lower odds of poor PO tolerance at 6 and 12 months (p<0.05)
Table 3.
Univariable and Multivariable logistic regression for any and specific symptoms reported at 6 and 12 Months
| Univariable | Multivariable** | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Any Symptoms | |||||
| 6 Months | Botox vs No drainage | 3.93 (1.77 – 8.71) | 0.001 | 4.14 (1.83 – 9.35) | 0.001 |
| Surgical vs No drainage | 2.82 (1.48 – 5.44) | 0.002 | 3.16 (1.62 – 6.16) | 0.001 | |
| Surgical vs Botox | 0.72 (0.29 – 1.81) | 0.5 | 0.76 (0.30 – 1.95) | 0.6 | |
| 12 Months | Botox vs No drainage | 2.23 (0.92, 5.38) | 0.08 | 2.26 (0.93, 5.52) | 0.07 |
| Surgical vs No drainage | 2.08 (1.09, 3.95) | 0.026 | 2.15 (1.12, 4.14) | 0.021 | |
| Surgical vs Botox | 0.93(0.35, 2.46) | 0.9 | 0.95 (0.36, 2.54) | 0.9 | |
| Pyloric Dilation | |||||
| 6 Month | Botox vs No drainage | 7.97 (1.97, 32.2) | 0.004 | 8.68 (2.1, 35.8) | 0.003 |
| Surgical vs No drainage | 0.72 (0.07, 7.0) | 0.8 | 0.63 (0.06, 6.2) | 0.7 | |
| Surgical vs Botox | 0.09 (0.01, 0.76) | 0.027 | 0.07 (0.01, 0.6) | 0.017 | |
| 12 Months | Botox vs No drainage | 2.46 (0.43, 14.2) | 0.3 | 2.45 (0.42, 14.2) | 0.3 |
| Surgical vs No drainage | 0.52 (0.06, 4.74) | 0.6 | 0.57 (0.06, 5.30) | 0.6 | |
| Surgical vs Botox1 | 0.21 (0.02, 2.43) | 0.3 | 0.23 (0.02, 2.74) | 0.2 | |
| Dumping | |||||
| 6 Months | Botox vs No drainage | 0.44 (0.12, 1.55) | 0.2 | 0.39 (0.11, 1.42) | 0.2 |
| Surgical vs No drainage | 1.01 (0.43, 2.38) | 1 | 1.12 (0.47, 2.67) | 0.8 | |
| Surgical vs Botox | 2.32 (0.59, 9.06) | 0.2 | 2.89 (0.7, 11.9) | 0.14 | |
| 12 Months | Botox vs No drainage | 1.37 (0.27, 7.0)) | 0.7 | 1.02 (0.17, 6.14) | 1 |
| Surgical vs No drainage | 1.90 (0.61, 5.92) | 0.3 | 2.45 (0.75, 8.05) | 0.14 | |
| Surgical vs Botox | 1.38 (0.26, 7.37) | 0.7 | 2.39 (0.36, 15.81) | 0.4 | |
| Poor PO Tolerance | |||||
| 6 Months | Botox vs No drainage | 2.33 (1.05, 5.16) | 0.037 | 2.33 (1.05, 5.20) | 0.038 |
| Surgical vs No drainage | 0.50 (0.18, 1.40) | 0.2 | 0.51 (0.18, 1.43) | 0.2 | |
| Surgical vs Botox | 0.21 (0.07, 0.65) | 0.007 | 0.22 (0.07, 0.67) | 0.008 | |
| 12 Months | Botox vs No drainage | 3.03 (1.00, 9.12) | 0.049 | 3.04 (1.00, 9.25) | 0.050 |
| Surgical vs No drainage | 0.55 (0.15, 2.05) | 0.4 | 0.60 (0.16, 2.29) | 0.5 | |
| Surgical vs Botox | 0.18 (0.04, 0.80) | 0.024 | 0.20 (0.04, 0.88) | 0.034 | |
| Early Satiety | |||||
| 6 Months | Botox vs No drainage | 2.70 (0.78, 9.29) | 0.12 | 2.6 (0.75,9.04) | 0.13 |
| Surgical vs No drainage | 2.27 (0.70, 7.32) | 0.2 | 2.41 (0.74, 7.86) | 0.14 | |
| Surgical vs Botox | 0.84 (0.24, 2.93) | 0.8 | 0.93 (0.26, 3.34) | 0.9 | |
| 12 Months | Botox vs No drainage | 4.25 (1.06, 17.11) | 0.041 | 3.94 (0.94, 16.54) | 0.061 |
| Surgical vs No drainage | 2.21 (0.61. 7.95) | 0.2 | 2.34 (0.62, 8.64) | 0.2 | |
| Surgical vs Botox | 0.52 (0.13, 2.12) | 0.4 | 0.59 (0.14, 2.55) | 0.5 | |
OR, odds ratio
Multivariable models were adjusted for age and BMI at the time of surgery.
DISCUSSION
Despite advances in surgical technique and morbidity benefits attributed to use of a minimally invasive approach to esophagectomy, DGE and pyloric hypertonia resulting from vagal disruption are an inherent part of esophagectomy for malignancy regardless of operative approach. Pyloric drainage was adopted into practice based on studies from the 1990s that demonstrated reduced DGE, postoperative complications and symptoms in patients that underwent surgical drainage procedures for open esophagectomy [5,6,15]; however, more recent literature has failed to demonstrate similar benefits and even demonstrated increased complications with drainage leaving the role of these techniques unclear. [4,8] As MIE has increased in use, Botulinum Toxin was also introduced as an alternative approach to the pylorus with the potential for improved morbidity and technical ease compared to surgical drainage techniques. As use of MIE will continue to rise, identification of the optimal approach to the pylorus with this surgical approach will better facilitate management of DGE and associated sequelae following esophagectomy.
This study presents the largest comparison series of pyloric interventions in MIE alone to date, and the only study to evaluate postoperative outcomes and long-term symptoms. Our results demonstrated no difference in postoperative complications between patients that received no drainage, Botox or surgical drainage; and furthermore, patients that received drainage (Botox and surgical drainage) had increased reported symptoms compared to no drainage. Our findings support previous studies in open esophagectomy that demonstrated no improvement in postoperative complications in patients that underwent pyloric drainage.[4,8,11,16]
Early studies with use of Botox demonstrated no clinical evidence for DGE in the postoperative period.[10,17] A 2009 comparative study demonstrated improved postoperative DGE with use of Botox compared to surgical drainage approaches; however, the authors found no difference in morbidity and these results have not been replicated.[11] In fact, a subsequent study found that patients that received Botox may even have increased risk of pneumonia and postoperative mortality when compared to pyloroplasty. [9] Although we failed to demonstrate a difference in morbidity, it is interesting to note that patients that underwent surgical drainage and no drainage had a trend towards increased pneumonia. It is possible that in the immediate postoperative period, patients that receive surgical drainage techniques have edema at the pylorus which may impede function and result in stasis. This hypothesis is further supported by the observation that patients in this group had a significantly longer period to postoperative jejunostomy removal compared to the other groups, perhaps as a reflection of poor oral feeding tolerance as a result of DGE. Additionally, it is interesting to note that the 90-day reoperation rate in the no drainage group was almost 2x lower than the Botox group and 2.6x lower than the surgical drainage group. None of the reoperations in the no drainage group were indicated for DGE. Postoperative endoscopic dilation for early DGE is a safe, short procedure and the demonstrated 90-day reintervention dilation rate in the no drainage group was low compared to previously published studies. [3, 9]
Results of long-term outcomes are less conclusive. Our study demonstrated that patients in both drainage groups had higher reported long-term symptoms at 6 and 12 months, and increased need for pyloric dilation at 6 months. Patients with Botox had a trend towards higher reported symptoms and need for pyloric drainage at all time points compared to surgical drainage, although there were too few patients at 12 months to demonstrate a significant result. Drainage has previously been associated with increased bile reflux and esophagitis. [8] Studies comparing long-term outcomes between approaches have demonstrated conflicting findings regarding reflux and dumping symptoms and need for pyloric dilation.[9,11] In a study comparing drainage techniques in an exclusively MIE group of 146 patients, Giugliano et al failed to demonstrate superiority of any approach in reducing need for subsequent postoperative pyloric dilation.[18] Although patients with no drainage required the highest rate of pyloric dilation in the immediate postoperative period, we demonstrated increased long-term symptoms and need for pyloric dilation in patients that receive Botox, even at 12 months after the effects would have certainly worn off. A possible explanation for this finding may be consequent fibrosis of the pylorus, as has been postulated in achalasia treatment, resulting in continued dysfunctional emptying. Similarly, it is possible that surgical drainage techniques result in stricturing of the pylorus in some patients leading to continued problems with gastric emptying despite recovery of nerve function. In a propensity matched comparison of patients with open esophagectomy that received surgical drainage against MIE without any drainage, Mehran and colleagues demonstrated no difference in reported dumping, reflux or overall satisfaction at a median of 12-month follow-up.[16] Similarly, a series of 145 patients comparing pyloroplasty versus no drainage in MIE demonstrated that drainage could be safely omitted with minimal difference in gastric emptying and reduced operative time.[19] In the perioperative and long-term period neither Botox nor surgical drainage has been shown to provide a definitive benefit over no drainage; as such, foregoing pyloric drainage may be a safe, reasonable option in MIE.
Our study was composed of 283 patients with more than 50 patients in each pyloric intervention group which supports the strength of our findings. However, the retrospective design of this study yields several limitations. We attempted to minimize confoundingthrough multivariable analysis. There were 11 surgeons that performed MIE during the study period. There were too few events to adjust for surgeon as a variable in our analysis. Although differences in operative technique and perioperative patient management may impact immediate short-term postoperative outcomes, the primary focus for this study was presence of symptoms and need for dilation long-term at 6 and 12 months by which point these perioperative differences should no longer have an impact. Additionally, given that the decision to perform pyloric drainage is based upon specific surgeon practice rather than patient characteristics, it is less likely that there was selection bias in treatment allocation. Also, most of Botox injections were performed in the last 2 years of the study. Although we did not account for relative experience with MIE in this study, variation in surgeon experience should have had minimal to no impact on observed outcomes given that it is standard for junior attendings or attendings with minimal MIE experience to have two attendings perform the procedure. Lastly, the subjective assessment of symptoms does complicate the ability to quantify symptoms into broader categories, such as reflux or dumping; however, this method is consistent with other studies on this subject.[9,11] These findings highlight the need for development of a unified system for classification of postoperative symptoms following esophagectomy. A currently ongoing prospective randomized study comparing Botulinum Toxin and no intervention in MIE may yield further insight into the current controversies surrounding the role of pyloric drainage. [20]
In conclusion, this study demonstrated that use of surgical drainage and Botulinum Toxin in MIE does not reduce postoperative morbidity and has increased long-term symptoms and need for pyloric dilation.
FUNDING:
This study was supported, in part, by the National Institutes of Health /National Cancer Institute Cancer Support Grant P30 CA008748. Tamar Nobel is supported, in part, by a grant from the American Cancer Society.
Footnotes
DISCLOSURES:
Tamar Nobel, Kay See Tan, Arianna Barbetta, Prasad Adusumilli, Manjit Bains, Matthew Bott, David Jones and Daniela Molena have no conflict of interest to disclose.
REFERENCES
- 1.Lazzarino AI, Nagpal K, Bottle A, Faiz O, Moorthy K, Aylin P (2010) Open Versus Minimally Invasive Esophagectomy. Ann Surg 252:292–298. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Shen Y, Feng M, Zhang Y, Jiang W, Xu S, Tan L, Wang Q (2015) Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score–matched comparison of operative approaches. J Thorac Cardiovasc Surg 149:1006–1015. [DOI] [PubMed] [Google Scholar]
- 3.Antonoff MB, Puri V, Meyers BF, Puri V, Meyers BF, Baumgartner K, Bell JM, Broderick S, Krupnick SA, Kreisel D, Patterson GA, Crabtree TD (2014) Comparison of pyloric intervention strategies at the time of esophagectomy: is more better? Ann Thorac Surg 97:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arya S, Markar SR, Karthikesalingam A, Hanna GB (2015) The impact of pyloric drainage on clinical outcome following esophagectomy: a systematic review. Dis Esophagus 28:326–335. [DOI] [PubMed] [Google Scholar]
- 5.Mannell A, McKnight A, Esser JD (1990) Role of pyloroplasty in the retrosternal stomach: results of a prospective, randomized, controlled trial. Br J Surg 77:57–59. [DOI] [PubMed] [Google Scholar]
- 6.Fok M, Cheng SW, Wong J (1991) Pyloroplasty versus no drainage in gastric replacement of the esophagus. Am J Surg;162:447–452. [DOI] [PubMed] [Google Scholar]
- 7.Chen K-N (2014) Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis 6 Suppl 3:S355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmes D, Weilinghoff M, Colombo-Benkmann M, Senninger N, Bruewer M (2007) Effect of pyloric drainage procedures on gastric passage and bile reflux after esophagectomy with gastric conduit reconstruction. Langenbeck’s Arch Surg 392:135–141. [DOI] [PubMed] [Google Scholar]
- 9.Eldaif SM, Lee R, Adams KN, Kilgo PD, Gruszunski MA, Force SD, Pickens A, Fernandez FG, Luu TD, Miller DL (2014) Intrapyloric Botulinum Injection Increases Postoperative Esophagectomy Complications. Ann Thorac Surg 97:1959–1965. [DOI] [PubMed] [Google Scholar]
- 10.Kent MS, Pennathur A, Fabian T, McKelvey A, Shuchert MJ, Luketich JD, Landreneau RJ (2007) A pilot study of botulinum toxin injection for the treatment of delayed gastric emptying following esophagectomy. Surg Endosc 21:754–757. [DOI] [PubMed] [Google Scholar]
- 11.Cerfolio RJ, Bryant AS, Canon CL, Dhawan R, Eloubeidi MA (2009) Is botulinum toxin injection of the pylorus during Ivor–Lewis esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 137:565–572. [DOI] [PubMed] [Google Scholar]
- 12.Mungo B, Lido O, Stem M, Molena D (2016) Early experience and lessons learned in a new minimally invasive esophagectomy program. Surg Endosc 30: 1692–1698. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deldycke A, Van Daele E, Ceelen W, Van Nieuwenhove Y, Pattyn P (2016) Functional outcome after Ivor Lewis esopahgectomy for cancer. J Surg Oncol 113:24–28. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay TK, Gupta S, Padhy AK, Kapoor VK (1991) Is pyloroplasty necessary following intrathoracic transposition of stomach? Results of a prospective clinical study. Aust N Z J Surg 61:366–369. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rice D, EL-Zein R, Huang JL, Vaporciyan A, Goodyear A, Mehta A, Correa A, Walsh G, Roth J, Swisher S, Hofstetter W (2011) Minimally invasive esophagectomy versus open esophagectomy, a symptom assessment study. Dis Esophagus 24:147–152. [DOI] [PubMed] [Google Scholar]
- 17.Martin JT, Federico JA, McKelvey AA, Kent MS, Fabian T (2009) Prevention of delayed gastric emptying after esophagectomy: a single center’s experience with botulinum toxin. Ann Thorac Surg 87:1708–1713. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano DN, Berger AC, Meidl H, Pucci MJ, Rosato EL, Keith SQ, Evans NR, Palazzo F (2017) Do intraoperative pyloric interventions predict the need for postoperative endoscopic interventions after minimally invasive esophagectomy? Dis Esophagus 30:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NT, Dholakia C, Nguyen X-MT, Reavis K (2010) Outcomes of minimally invasive esophagectomy without pyloroplasty: analysis of 109 cases. Am Surg 2010;76:1135–1138. [PubMed] [Google Scholar]
- 20.Kukar M, Roswell Park Cancer Institute (2018) Botulinum Toxin Type A in Preventing Complications After Surgery in Patients With Esophageal Cancer. https://clinicaltrials.gov/ct2/show/NCT02965976. Accessed July 10, 2018.