Abstract
Idiopathic membranous nephropathy is the most common cause of nephrotic syndrome in nondiabetic adults. The antibody most often implicated is the M-type phospholipase A2 receptor (PLA2R) antibody, found in >70% of primary membranous nephropathy cases. First-line therapy is immunosuppressive in nature, but for patients who are treatment-resistant there is a significant risk of end-stage renal disease (ESRD) and mortality. Hypercholesterolemia is not only a side effect of nephrotic syndrome, but its presence may worsen renal function. A recent single-arm observational study in Japan found that low-density lipoprotein apheresis (LDL-A) was able to ameliorate nephrotic syndrome in half of patients who were resistant to medication. We present a case of treatment resistant PLA2R negative membranous nephropathy who had significant improvement following two courses of LDL apheresis. To our knowledge, this is the first such reported case in the United States.
Keywords: membranous nephropathy, phospholipase A2, lipoprotein apheresis
Introduction
Idiopathic membranous nephropathy is the most common cause of nephrotic syndrome in nondiabetic adults.1 The etiology of this disease is best conceptualized as an autoimmune disorder. The majority (>70%) of cases of primary membranous nephropathy (MN) are associated with autoantibodies against the podocyte antigen M-type phospholipase A2 receptor (PLA2R) on the podocyte that activates complement.2 This leads to extracellular matrix accumulation of collagen in the subepithelial aspect of the glomerular basement membrane.3 The nonselective proteinuria found is due to complement stimulation of podocyte production of proteases, oxidants and cytokines that lead to disruption of the functional integrity of the slit diaphragm protein.4 These changes can be visualized on electron microscopy of a renal biopsy. A diffuse granular pattern of IgG deposition along the basement membrane sparing the mesangium is characteristic.
PLA2R antibodies are predominantly of the IgG4 class, and titers can be used to assess disease activity and monitor disease response.5 Other antibodies associated with this disorder include the thrombospondin type-1 domain-containing 7A (THSD7A) antibody which is found in 8–14% of patients and neutral endopeptidase (NEP) antibody which is found in neonates.6
The natural history of the disorder is highly variable. The long term outcome of patients who achieve partial or complete remission is excellent. With angiotensin-converting enzyme (ACE) inhibition or angiotensin II receptor blocker (ARB) treatment alone, one study found 70% of patients experienced a spontaneous partial remission.7 However, this may take several months to years to occur. If treatment is delayed, the patient is exposed to the complications of nephrotic syndrome such as increased infection risk, edema and thrombosis. In addition, those patients who are treatment-resistant and have persistence of nephrotic range proteinuria have an 11% mortality risk and a 50% risk of ESRD.
A recent single arm observational study (POLARIS) (N=44) found that LDL apheresis was able to ameliorate nephrotic syndrome in half of patients who were found to be drug resistant. Those patients who experienced disease remission continued to have a favorable outcome after 2 years of follow up.8 In this study, decreased urinary protein levels after treatment was most significantly correlated with a favorable two year outcome. Interestingly, the number of LDL apheresis procedures, age, sex, BMI and whether this was a primary versus recurrent case were not significantly correlated with remission.
Case report
A 49-year-old Caucasian female presented to an outside institution in 2015 with pedal edema (3+), approximately 7 kilograms above her baseline weight, with low serum albumin (2.3 g/dL) and proteinuria, with urine protein creatinine ratio (PCR) 9.043 mg/mg. At initial presentation to our institution, urine protein was 2084 mg/dL, and PCR was 8.27 mg/mg. Renal biopsy revealed membranous glomerulonephritis, early stage 2, associated with focal, minimal interstitial fibrosis and mild vascular sclerosis, negative for immune complex deposition; PLA2R antibodies were negative on immunofluorescence and in peripheral blood. Workup for secondary causes for membranous nephropathy revealed intermittently positive dsDNA (high titer) and ANA, but the patient did not meet criteria for systemic lupus erythematosus or dermatomyositis at the time. CT chest, abdomen and pelvis, colonoscopy, and mammogram with subsequent breast biopsy were negative for malignancy. There were no signs of infection. Additionally none of the patient’s medications had been implicated in nephrotic syndrome.
One of the established sequelae of nephrotic syndrome is increased risk of thromboembolism. The incidence of thromboembolism has been reported as affecting approximately a quarter of adults with nephrotic syndrome.9 Interestingly, this patient had a history of bilateral pulmonary emboli, and three previous miscarriages before manifestation of nephrotic syndrome symptoms. The comprehensive thrombophilia workup that followed was negative. The patient was subsequently placed on enoxaparin for anticoagulation and then transitioned to rivaroxaban as an outpatient.
Clinical Course
Initial medical management included induction and maintenance rituximab and cyclosporine, high dose dexamethasone, and subsequently a trial of cyclophosphamide and prednisone. Despite reaching maximal doses of immunosuppressive therapy after 8 months, significant proteinuria persisted (PCR 12.625 mg/mg) (Figure 1). Due to the patient’s limited response to treatment, and significant hyperlipidemia, the treating nephrology team requested a trial of LDL apheresis using the Kaneka (Liposorber) system. At the time of consultation, the patient’s total cholesterol was 771 mg/dL, triglycerides 1777 mg/dL; LDL levels exceeded the analytic capacity of the assay. LDL particles were predominantly small-sized 19.5 nm (normal 19.7–21.9 nm). PCR was 6.206 mg/mg prior to starting LDL apheresis. In the absence of consensus regarding the optimum timing of LDL-A in this clinical setting, we decided to initiate weekly LDL apheresis, with a plan to re-evaluate clinical and laboratory parameters of response after 6 weeks. For each procedure, 3000 mL of plasma (2 plasma volumes) were treated.
Figure 1.
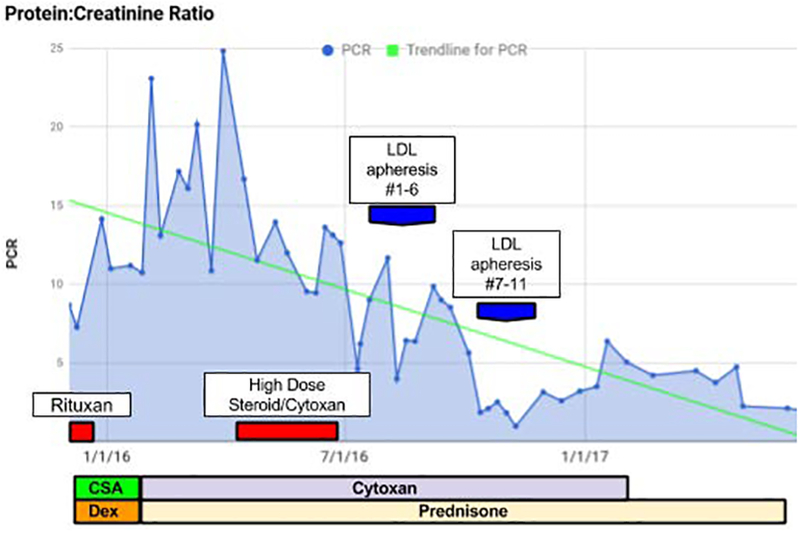
Trend of protein:creatinine ratio over the course of treatment regimes.
At the end of the first course of LDL apheresis, the patient reported symptomatic improvement; edema was reduced to +1 to trace in the peripheral extremities. Her physicians were able to lower her dose of cyclophosphamide and taper her dose of prednisone, leading to decreased treatment-related side effects. A second course of 5 weekly LDL-A procedures was requested 7 weeks later due to persistent hyperlipidemia. At the conclusion of the second course, her proteinuria had significantly decreased (PCR 0.442 mg/mg); 21 days post the last apheresis procedure, PCR rebounded to 3.028 mg/mg, but stabilized over the next several months on continued immunosuppression (Table I). She subsequently developed arthralgia and joint stiffness affecting her hands; on re-evaluation by rheumatology in December 2017 she was diagnosed with SLE, and started on Plaquenil with addition of Mycophenolate Mofetil in March 2018. At 21 months post her last LDL apheresis, proteinuria improved and stabilized (PCR 0.529 mg/mg), and renal function (eGFR 96 mL/min/1.73 m2) and serum albumin (4.3 g/dL) have returned to baseline.
Table I.
Serum and urine parameters before and after LDL-A treatment.
| Laboratory Parameters | Pre-Apheresis (first course) | Post-Apheresis (first course) | Pre-Apheresis (second course) | Post-Apheresis (second course) |
|---|---|---|---|---|
| Serum albumin (g/dL) | 3.1 | 3.3 | 3.0 | 3.7 |
| eGFR (mL/min/1.73 m2) | 79 | 77 | 81 | 83 |
| Total Cholesterol (mg/dL) | 553 | 244 | 409 | 153 |
| HDL (mg/dL) | 60 | 56 | 59 | 59 |
| Triglycerides (mg/dL) | 1063 | 990 | 481 | 234 |
| Albumin Creatinine Ratio (mg/g) | 4792 | 1927 | 6475 | 262.2 |
| Protein Creatinine Ratio (mg/mg) | 6.206 | 2.794 | 1.855 | 0.442 |
| Apolipoprotein B100 (mg/dL) | 291 | 100 | 212 | 63 |
Discussion
The proposed therapeutic mechanism of LDL apheresis in this setting is that it alleviates the tissue toxicity of persistent dyslipidemia in this disease while leaving plasma proteins intact. Elevated cholesterol is not only a side effect of nephrotic syndrome but its presence may worsen renal function.10 During hyperlipidemia, LDL accumulates in the matrix and becomes oxidized. Scavenger receptors on mesangial cells and monocyte-macrophages will take these up thus inducing localized inflammation and tissue injury.11 In a prospective cohort trial of initially healthy men, a high ratio of total cholesterol/HDL was significantly associated with an increased risk of renal dysfunction.12 In LDL-A using a dextran sulfate column (Liposorber, Kaneka) no HDL is absorbed. In addition, amelioration of the dyslipidemia may affect sensitivity to drug treatment. It is hypothesized that LDL-A improves immunosuppressive drug sensitivity by removing dyslipidemia- triggered Cyclosporine receptor downgrading.13
In Japan, national health insurance has covered LDL-A for nephrotic syndrome patients resistant to conventional therapy since 1993. An analysis of retrospective trials found that LDL-A has been effective in inducing remission in nearly 50% of patients with refractory nephrotic syndrome.13 Early results from the POLARIS study suggested that the time to treatment initiation may matter; treatment started within 8 weeks of nephrotic syndrome diagnosis was more effective than LDL-A treatment started after 8 weeks.14 The benefit of LDL-A in treatment of refractory nephrotic syndrome has been shown primarily in focal and segmental glomerulosclerosis (FSGS); there are few reports of response in patients with minimal change and membranous nephropathy (Table II). In view of these studies, the FDA recently expanded the indication for the Liposorber LA-15 system to include adult patients with nephrotic syndrome due to primary FSGS unresponsive to standard treatment.15 There is a paucity of literature describing the short and long-term efficacy of this treatment modality in patients with refractory membranous nephropathy. Anti-PLA2R is largely negative in patients with secondary causes of MN, including SLE, which was eventually diagnosed in our patient. To our knowledge, there are no specific reports on the use of LDL-A in anti-PLA2R positive idiopathic MN.
Table II.
Studies reporting clinical efficacy of LDL apheresis for treatment-resistant nephrotic syndrome.
| Group | Study Design | Method | Results |
|---|---|---|---|
| (Muso et al. 1994)16 | Retrospective (Japan) | 8 patients with steroid resistant nephrotic syndrome received a mean of 7 LDL-A treatments | 4 patients achieved full remission, one patient had partial remission |
| (Muso et al. 2001)17 | Prospective (Japan) | 17 patients with steroid resistant nephrotic syndrome due to FSGS underwent LDL-A, compared to 10 nephrotic patients treated with steroids only | The time needed to achieve less than nephrotic range proteinuria was significantly shorter in the LDL-A group |
| (Yoshizawa et al. 2003)18 | Case series (Japan) | 6 patients with steroid resistant nephrotic syndrome received an average of 11 LDL-A treatments | 4 patients achieved complete remission and one achieved a type one incomplete remission |
| (Muso et al. 2007)19 | Retrospective (Japan) | 41 patients with nephrotic syndrome whose short-term outcomes with LDL-A were reported from 1999–2004 | 29 patients with outcomes determined at 2 years were analyzed. 62% of them were classified into complete remission or type 1 incomplete remission |
| (Muso et al. 2015)14 | Multicenter prospective study (Japan) | 44 patients with drug resistant nephrotic syndrome were treated with LDL-A | More than half of the patients showed remission of NS based on the urinary protein level at the completion of LDL-A. Demonstrated LDL-A short efficacy for drug resistant nephrotic syndrome |
Conclusion
We report a case of treatment resistant PLA2R negative MN with persistent clinical improvement 21 months after LDL apheresis. We propose that LDL-A is a potential therapeutic option for refractory MN and contributes to the literature describing the long-term usefulness of LDL-A for this unusual indication.
Acknowledgement:
This work was supported by the Intramural Research Program of the NIH Clinical Center (project Z99 CL999999).
Footnotes
The views expressed are those of the authors and do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
The authors declared having no financial interests relevant to this article.
References
- 1.Bockenhauer D et al. Familial Membranous Nephropathy: An X-Linked Genetic Susceptibility? Nephron Clin. Pract 108, c10–c15 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Cattran DC & Brenchley PE Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int 91, 566–574 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Minto AW et al. Augmented expression of glomerular basement membrane specific type IV collagen isoforms (alpha3-alpha5) in experimental membranous nephropathy. Proc. Assoc. Am. Physicians 110, 207–217 (1998). [PubMed] [Google Scholar]
- 4.Nangaku M, Shankland SJ & Couser WG Cellular response to injury in membranous nephropathy. J. Am. Soc. Nephrol 16, 1195–1204 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Hofstra JM et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol 23, 1735–1743 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomas NM et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med 371, 2277–2287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuarrie EP, Stirling CM & Geddes CC Idiopathic membranous nephropathy and nephrotic syndrome: outcome in the era of evidence-based therapy. Nephrol. Dial. Transplant 27, 235–242 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Muso E et al. A Prospective Observational Survey on the Long-Term Effect of LDL Apheresis on Drug-Resistant Nephrotic Syndrome. Nephron Extra 5, 58–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerlin BA, Ayoob R & Smoyer WE Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin. J. Am. Soc. Nephrol 7, 513–520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorhead JF, Chan MK, El-Nahas M & Varghese Z Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2, 1309–1311 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Schlondorff D Cellular Mechanisms of Lipid Injury in the Glomerulus. Am. J. Kidney Dis 22, 72–82 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Schaeffner ES et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol 14, 2084–2091 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Muso E Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin. Exp. Nephrol 18, 286–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muso E et al. Immediate therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: evidence from the short-term results from the POLARIS Study. Clin. Exp. Nephrol 19, 379–386 (2015). [DOI] [PubMed] [Google Scholar]
- 15.FDA Liposorber® LA-15 System Summary of Safety And Probable Benefit Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf17/H170002B.pdf. (Accessed: 2nd August 2018)
- 16.Muso E et al. Does LDL-apheresis in steroid-resistant nephrotic syndrome affect prognosis? Nephrol. Dial. Transplant 9, 257–264 (1994). [PubMed] [Google Scholar]
- 17.Muso E et al. Significantly rapid relief from steroid-resistant nephrotic syndrome by LDL apheresis compared with steroid monotherapy. Nephron 89, 408–415 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa T, Suzuki T & Kanmatsuse K [Usefulness of LDL-apheresis for treatment of steroid-resistant nephrotic syndrome]. Nihon Jinzo Gakkai Shi 45, 25–31 (2003). [PubMed] [Google Scholar]
- 19.Muso E et al. Beneficial effect of low-density lipoprotein apheresis (LDL-A) on refractory nephrotic syndrome (NS) due to focal glomerulosclerosis (FGS). Clin. Nephrol 67, 341–344 (2007). [DOI] [PubMed] [Google Scholar]


