Abstract
The three trefoil factor family peptides (TFF1, TFF2, TFF3) with their lectin activities play important roles in mucosal protection and repair. Major gaps in understanding their molecular function have however hampered therapeutic development for gastrointestinal disorders. Here, we provide a critical overview of the status quo.
Keywords: cell migration, gastrointestinal wound healing, lectin, mucosal protection, mucins, mucositis
The trefoil factor family: key players in mucosal protection and repair
The three members of the trefoil factor family (TFF) of peptides (TFF1, TFF2, TFF3; Box 1) are typical constituents of mucous epithelia such as the alimentary, respiratory, and urogenitary tracts, the conjunctiva, and the inner ear, where they are typically co-secreted with mucins [1, 2]. The major expression site for TFF1 and TFF2 is the stomach and for TFF3 the intestine [1]. TFF peptides appear also in the saliva, gastric juice, urine, blood, and breast milk [1, 3]. Pathologically, TFF peptides are ectopically expressed after wounding, in inflammatory diseases, and in various tumours [1]. They are also secreted in an endocrine manner, for example, in the immune and central nervous systems [1, 4]. Numerous in vivo and in vitro studies point to TFF peptides as key players in mucosal protection and repair processes. Accordingly, TFF peptides are constituents of the mucus barrier, where they display lectin-like behaviour, enhance mucosal restitution (cell migration), whilst modulating cell junctions, apoptosis, angiogenesis, and mucosal differentiation processes [1]. Furthermore, transgenic TFF-deficient mice display abnormalities in the gastrointestinal (GI) tract and the immune system [1].
Therapeutic Potential
Based on a diverse range of GI injury models [1], TFF holds particular therapeutic promise for GI inflammatory disorders, but also for oral mucositis [5, 6]. However, translation of this GI therapeutic potential has been limited to a single Phase I/II trial (16 patients) where recombinant TFF3 in enemas was given in combination with orally-delivered mesalazine for the treatment of ulcerative colitis [1]. In this study, no additional improvement was observed when compared with mesalazine treatment alone, however, the small trial size combined with the limited understanding of TFF3’s mode of action limits the interpretation of these outcomes. More successful was TFF's therapeutic response in oral mucositis, a side effect of radio- and chemotherapy. In a Phase I trial, AG013, a mouth rinse formulation of Lactococcus lactis secreting TFF1, reduced oral mucositis in cancer patients receiving chemotherapy [6]. AG013 has been granted Orphan Drug status in the European Union and Fast Track designation by the U.S. Food and Drug Administration and is currently in Phase II. Additionally, homodimeric TFF3 was also well-tolerated in a Phase II study and reduced the incidence and severity of chemotherapy-induced oral mucositis in cancer patients [5]. Both trials support TFF’s therapeutic potential and highlight different delivery strategies.
Mechanisms of Action: Potential Interaction Partners
Currently, we still know very little about the molecular mechanisms of TFF peptides, which is a recognised problem for clinical translation. TFF's three-loop trefoil domain hosts multiple secondary structural elements, suggesting multiple pharmacophores, which corresponds with the variety of observed functions [1].
For a long time, no TFF binding molecule with characteristics of a classical receptor could be identified [4]. Only recently, chemokine receptor types 4 and 7 (CXCR4 and CXCR7) have been reported to mediate TFF2- and TFF3-induced chemotaxis [1, 7]. Other signal transduction data concerning TFF remain elusive and mainly result from migration and apoptotic assays, without providing target receptors nor mechanistic details. Typical activated cascades include ERK1/2, JNK, Akt, and NFκB [1, 8]. The following interactions of TFF peptides with proteins and carbohydrate moieties have been documented (see also Figure 1, Table 1).
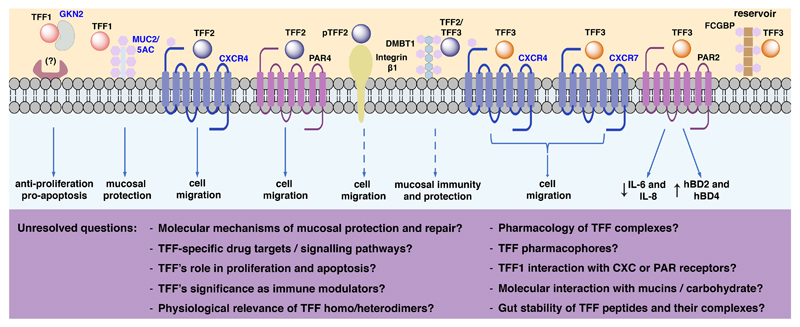
Figure 1. Interactions of TFF peptides.
The figure includes validated and hypothetical interactions, potential associated physiological functions, and unresolved questions. Targets highlighted in blue are supported by independent research groups. Dashed arrows and ? denotes functions that have not been experimentally validated. The TFF1-GKN2 heterodimer displays synergistic anti-proliferative and pro-apoptotic effects, yet its mechanism of action remains unknown. TFF1-MUC2/5AC interaction might enhance mucosal protection. TFF2 interaction with CXCR4 or PAR4 accelerates cell migration. TFF2 binds specifically to the GlcNAcα1→4Galβ1→R moiety of MUC6. Interaction of TFF2 and β integrin is supposed to have motogenic effects. TFF2/3 interaction with DMBT1 might play a role in mucosal immunity and protection. TFF3 interaction with PAR2 downregulates the levels of IL-6 and IL-8 and upregulates hBD2 and hBD4. The TFF3-FCGBP complex is considered to act as a reservoir for continuous TFF3 release. CXCR: C-X-C chemokine receptor; DMBT1: deleted in malignant brain tumour 1; FCGBP: IgG Fc binding protein; GKN2, gastrokine 2; PAR: protease-activated receptor; MUC: mucin; hBD human beta defensin.
Table 1. Overview of TFF interactions.
| Interaction | Target location | Experimental approach | Note | Ref. | |
|---|---|---|---|---|---|
| TFF1 | GKN2 | AGS, HT-29, HGT-1 and NS-1 cells | Immunoprecipitation | Synergistic anti-proliferative and pro-apoptotic effects | [1, 10] |
| MUC2 MUC5AC |
Stomach and duodenum | Yeast two-hybrid system | Interacts with VWFC1/VWFC2 domains. Demonstrated in vitro | [1, 7] | |
| RF-LPS | Helicobacter pylori | SPR, coimmunofluoresence and incubation with tissue sections | Explains the bacteria tropism for colonising gastric tissue. Interacts with the carbohydrate moiety. | [15] | |
| TFF2 | CXCR4 | Jurkat and AGS expressing CXCR4 cell lines | Ca2+mobilization | Activity at 500 nM | [4] |
| PAR4 | HT-29 cells | Receptor knockdown by siRNA | Induced cell migration at 200 nM | [9] | |
| DMBT1 | Porcine stomach membranes | Affinity chromatography | Putative receptor. No signalling upon binding was shown | [7] | |
| β-integrin | Porcine stomach membranes | Affinity chromatography | Non-covalent interaction; potential/role in cell migration | [7] | |
| MUC6 | Gastric mucus | MS identification & binding assay | Binds to GlcNAcα1→4Galβ1→R | [13, 14] | |
| TFF3 | CXCR4 CXCR7 |
Ocular surface tissue | Computational docking and functional assays | Induced cell migration at 760 nM | [8] |
| PAR2 | HT-29 cells | Immunoprecipitation, siRNA and functional assays | Downregulates IL-6 and IL-8 | [3] | |
| FCGBP | Human colonic tissue | Purification of heteromer and identification by MS | TFF3-FCGBP considered reservoir. Lacks motogenic activity | [12] | |
| DMBT1 | Bronchoalveolar lavage and colon | ELISA assay | Ca2+ dependent manner (typical of lectins) | [11] |
GKN2: gastrokine 2; MUC: mucin; VWFC: von Willebrand factor; RF-LPS: oligosaccharide portion of lipopolysaccharide; SPR: surface plasmon resonance; CXCR: C-X-C chemokine receptor; PAR: protease-activated receptors; DMBT1: deleted in malignant brain tumours 1; siRNA: small interfering RNA; FCGBP: IgG Fc binding protein; MS mass spectrometry.
Interaction with CXCR4/7
Promising data support TFF interaction with CXCR4 and CXCR7 in the low affinity range [8]. TFF2 triggers ERK1/2 signalling via CXCR4 [4], which might be linked to the recruitment of immune cells [8]. TFF3 also interacts with CXCR4 and CXCR7 expressed on ocular surface tissues, where cell migration is induced via an ERK1/2-independent signalling pathway [8].
Interaction with Protease-Activated Receptors (PARs)
TFF2 and TFF3 have been reported to activate PAR4 and PAR2, respectively [3, 9]. PAR4 knockdown abolishes the mucosal healing effect of TFF2 [9]. TFF3 activates PAR2, but not PAR1, as shown in cytosolic Ca2+ activity measurements in HT-29 cells, causing downregulation of proinflammatory cytokines and upregulation of defensin expression [3].
Interaction with Integrins
Porcine TFF2 binds non-covalently to integrin β1, as determined by affinity chromatography [2, 7]. This is of interest considering that integrins play an important role in cell migration that is enhanced by TFF peptides.
Interaction with Gastrokine 2 (GKN2)
Large amounts of gastric TFF1 are linked to GKN2 (formerly: GDDR, trefoil factor interactions 1 / TFIZ1) via a disulfide bridge [7, 10]. The TFF1-GKN2 heterodimer has anti-proliferative and pro-apoptotic effects [10].
Interaction with Deleted in Malignant Brain Tumours 1 (DMBT1)
Porcine TFF2 binds non-covalently to the cysteine-rich repetitive glycoprotein (MW > 340 kDa) DMBT1 (formerly: hensin, muclin) [2, 11]. DMBT1 is an extracellular matrix-associated multifunctional protein playing a role in mucosal innate immunity and protection. Dimeric human TFF3 also binds to human DMBT1 in a Ca2+ dependent manner [11]. The high expression of DMBT1 and TFF3 in inflammatory bowel diseases underpins its role in mucosal protection [9].
Interaction with IgG Fc Binding Protein (FCGBP)
The majority of colonic TFF3 is bound to the mucus-associated cysteine-rich repetitive glycoprotein FCGBP (MW > 500 kDa) [12]. Gaseous hydrogen sulfide (H2S) liberates TFF3 from the TFF3-FCGBP complex in vitro. H2S is present in relatively high amounts in the colon promoting injury repair and resolution of inflammation [12]. It is speculated that this is a mechanism to control monomeric TFF3 levels at the site of action [12].
Interaction with Mucins MUC2 and MUC5AC
TFF peptides can crosslink secreted mucins such as MUC5AC in the stomach and MUC2 in the small intestine and colon resulting in increased viscosity conveying protective effects for the gut [7, 13]. TFF1 has been reported to interact with the cysteine-rich von Willebrand factor C1 and C2 domains of the mucins MUC2 and MUC5AC using the yeast 2-hybrid system [1, 7]. However, the majority of gastric TFF1 is not associated with MUC5AC or MUC2 in vivo [1].
Interactions with Carbohydrate Moieties
TFF2 can be considered a lectin, stabilising the gastric mucus barrier thereby affecting its viscoelastic properties [13, 14]. In addition, pH-dependent lectin activities have been reported for TFF1 and TFF3 [13, 15]. Furthermore, homodimeric TFF1 (and to a lesser extend homodimeric TFF3, but not TFF2) interacts with the core oligosaccharide of Helicobacter pylori lipopolysaccharide during colonisation of the gastric mucus [13, 15]. In contrast, TFF2 binds highly specifically to the GlcNAcα1→4Galβ1→R moiety of MUC6; this epitope is evolutionarily conserved and the terminal α-GlcNAc has antimicrobial activity against Helicobacter pylori [2, 13, 14]. Of note, Helicobacter pylori via LabA might also adhere to the LacdiNAc oligosaccharide of TFF2, suggesting another colonization mechanism [13].
Conclusions and Future Perspectives
TFF peptides regulate mucosal and epithelial protection and repair. Their therapeutic potential is promising despite substantial gaps in mechanistic knowledge. Additional work at the molecular level is required to define TFF-related signalling pathways and drug targets to improve and accelerate therapeutic translation. Advances in peptide synthesis and peptidomimetic design could provide novel tools to overcome these challenges and deliver enhanced drug candidates. TFF interactions with carbohydrate moieties is another promising avenue that warrants further research. Systematic characterisation of these lectin interactions along with TFF activation of glycosylated receptors (e.g. CXCR4/7, integrins) would be of great interest, as this might explain the low binding affinities and weak biological effects observed. Taken together, the TFF peptides remain an intriguing class of molecules that will keep the field engaged for time to come.
Acknowledgements
M.M. is supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no 714366) and by the Australian Research Council Discovery Early Career Researcher Awards (DE150100784). S.M.B is a National Health and Medical Research Council of Australia (NHMRC) R.D Wright Biomedical Research Fellow (APP1126378) and is funded by NHMRC Australia Project Grants #1083480, #1139366 and #1140297. We apologise to all authors whose work could not be cited due to space limitations.
References
- 1.Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350–1369. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int J Oncol. 2015;47:806–816. doi: 10.3892/ijo.2015.3090. [DOI] [PubMed] [Google Scholar]
- 3.Roa B, Tortolero S. Trefoil factor 3 (TFF3) from human breast milk activates PAR-2 receptors, of the intestinal epithelial cells HT-29, regulating cytokines and defensins. Bratisl Med J. 2016;117:332–339. doi: 10.4149/bll_2016_066. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann W. Trefoil Factor Family (TFF) peptides and chemokine receptors: a promising relationship. J Med Chem. 2009;52:6505–6510. doi: 10.1021/jm9008136. [DOI] [PubMed] [Google Scholar]
- 5.Peterso DE, et al. Phase II, randomized, double-blind, placebo-controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil-based chemotherapy. J Clin Oncol. 2009;27:4333–4338. doi: 10.1200/JCO.2008.21.2381. [DOI] [PubMed] [Google Scholar]
- 6.Limaye SA, et al. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer. 2013;119:4268–4276. doi: 10.1002/cncr.28365. [DOI] [PubMed] [Google Scholar]
- 7.Otto W, Thim L. Trefoil factor family-interacting proteins. Cell Mol Life Sci. 2005;62:2939–2946. doi: 10.1007/s00018-005-5482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieckow J, et al. CXCR4 and CXCR7 mediate TFF3-induced cell migration independently from the ERK1/2 signaling pathway. Invest Ophthalmol Vis Sci. 2016;57:56–65. doi: 10.1167/iovs.15-18129. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cell Mol Life Sci. 2011;68:3771–3780. doi: 10.1007/s00018-011-0678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim O, et al. Heterodimeric interaction between GKN2 and TFF1 entails synergistic antiproliferative and pro-apoptotic effects on gastric cancer cells. Gastric Cancer. 2017;20:772–783. doi: 10.1007/s10120-017-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen J, et al. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3) Plos One. 2013;8:11. doi: 10.1371/journal.pone.0064441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert TK, et al. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res. 2010;9:3108–3117. doi: 10.1021/pr100020c. [DOI] [PubMed] [Google Scholar]
- 13.Sturmer R, et al. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: in vitro binding studies. Chembiochem. 2018;19:2598–2608. doi: 10.1002/cbic.201800622. [DOI] [PubMed] [Google Scholar]
- 14.Hanisch FG, et al. Human trefoil factor 2 is a lectin that binds alpha-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J Biol Chem. 2014;289:27363–27375. doi: 10.1074/jbc.M114.597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves EP, et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology. 2008;135:2043–2054. doi: 10.1053/j.gastro.2008.08.049. 2054 e2041-2042. [DOI] [PubMed] [Google Scholar]