Abstract
Background
Hypersensitivity to one's own sex hormones is not a new concept but it is an underappreciated one. Such a phenomenon may explain a large proportion of symptoms related to the menstrual cycle (such as premenstrual syndrome, PMS), cyclic pain syndromes or unexplained infertility. In this study we propose a novel diagnostic tool of hormonal skin testing which reveals sex hormones sensitivity with high clinical correlation, and a subsequent successful desensitization technique.
Methods
A group of 35 women with confirmed diagnosis of PMS were enrolled in the study in which they underwent a hormonal skin diagnostic skin testing procedure by intradermal injections of Progesterone (P), Estradiol (E2), Estrone (E1) and Estriol (E3). Skin reaction was monitored, and according to the reaction the patients were treated by serial desensitization by similar serial injections or placebo solvent. Response to treatment was monitored by assessing the change in the severity of PMS related symptoms.
Results
We show a positive correlation between PMS severity and skin sensitivity to sex hormones. Subsequent desensitization procedure led to a significant improvement in the severity of PMS related symptoms.
Conclusions
The testing and desensitization procedure is safe, sensitive and bares a high therapeutic potential in approach to resistant hormonal cycle related syndromes.
ClinicalTrials.gov Identifier
NCT00873262: Evaluation of Safety/Efficacy of Diagnostic Skin Test Panel and Desensitization Hormone Kit for Treatment of Premenstrual Syndrome (PMS) 2009.
Keywords: Sex hormones skin test, Hormonal desensitization therapy, Premenstrual syndrome, Mastalgia
Introduction
PMS (Premenstrual Syndrome) is a group of more than 100 physical and emotional symptoms occurring before the onset of menstruation, and disappearing at or during menses. The symptoms happen repetitively and in a cyclic pattern, and are associated with the luteal phase of the menstrual cycle (two weeks before the onset of menstruation). The symptoms are absent between two luteal phases.
According to the American College of Obstetricians and Gynecologists, 3–8% of all women suffer from a severe form of PMS, called premenstrual dysphoric disorder (PMDD). PMDD is a debilitating condition that renders those affected virtually paralyzed by their symptoms.
Sensitivity to their own hormones was initially reported in 1921 by Geber which demonstrated a flare in the cyclic urticaria following administration of the patient's own pre-menstrual serum.1 Further Zondec and Bromberg and Shelley et al.2, 3, 4, 5 demonstrated that a case of severe generalized erythema multiform was caused by progesterone sensitivity and estrogen related dermatitis.
The increased sensitivity to autoimmunity that is abundant in post pubertal women was speculated to be secondary to hormone related mast cell activation, probably through mast-cell degranulation by estrogens. Moreover, in human dendritic cells, in vitro, estrogen and progesterone upregulated IL-10 production.6, 7, 8
Therefore, the well-established effect of perimenstrual fluctuations of sex hormones in women is probably at least partially responsible for different perimenstrual syndromes especially in autoimmune and pain related conditions, such as headache and pelvic pain.
In this study we investigated an effect of desensitization treatment with hormones on women with severe PMS.
Study objectives
-
1.
To evaluate the safety and efficacy of Diagnostic Hormonal Skin Test Panel in subjects with severe PMS symptoms
-
2.
To evaluate the safety of desensitization treatment in subjects with severe PMS symptoms sensitive to one or more female reproductive hormones
-
3.
To evaluate the efficacy of desensitization treatment in subjects with severe PMS symptoms sensitive to one or more female reproductive hormones as compared to Placebo treatment
-
4.
To evaluate a prevalence of intolerance to sex hormones in subjects with severe breast swelling and tenderness (mastalgia), as a dominant PMS symptom
-
5.
To evaluate the efficacy of desensitization treatment to reduce mastalgia in subjects with severe PMS symptoms sensitive to one or more female reproductive hormones as compared to placebo treatment
Study design
A Phase II, prospective, single-blind, randomized, two-arms, cross controlled clinical study.
Population and study groups
Inclusion criteria: a group of 35 women aged 20–45 and premenopausal women aged 45–50 with severe PMS and mastalgia (breast swelling and tenderness) as a dominant symptom were enrolled in the study in accordance with acceptable PMS criteria (Appendix 1). “Severe symptom” is defined as scored at level 7–10 according to the standard (0- no symptom to 10- severe symptom) scale of PMS symptoms (Appendix 2).
Exclusion criteria: Pregnant or lactating women, women with serious health problems and abnormal laboratory tests, women using contraceptives during last three months.
A skin test for hormone sensitivity was performed in all enrolled participants. Following a daily follow-up after symptoms and skin reactions for one month the subjects were allocated randomly to two groups in a blinded to participant fashion:
-
a.
During the period starting on April 14, 2009 to April 13, 2011, 35 subjects have been enrolled in the study, based on outpatient clinic of Clinical Immunology and Allergy of Tel Aviv University in Tel Aviv Medical Center.
-
b.Following skin testing the patients had been randomly allocated to 2 treatment groups:
-
1.PLACEBO group: following skin testing - treated only with a solvent solution.
-
2.HORMONE group: treated with one or more hormone solutions in accordance with positive skin test results, following an initial skin test.
-
1.
-
c.
The trial was approved by the Institutional Ethics Committee
ClinicalTrials.gov Identifier: NCT00873262: Evaluation of Safety/Efficacy of Diagnostic Skin Test Panel and Desensitization Hormone Kit for Treatment of Premenstrual Syndrome (PMS) 2009.
Study structure
Description of the Diagnostic Skin Test Panel.
-
a.
Diagnostic Skin Test Panel is designed to detect hormones to which subjects might be sensitive.
-
b.
The Diagnostic Skin Test Panel includes four hormones -- Progesterone (P), Estradiol (E2), Estrone (E1), Estriol (E3) -- dissolved in Ethyl Oleate with 10% Benzyl Alcohol and a solvent, as a negative control.
Screening period
All recruited subjects with PMS and mastalgia (breast pain), as a dominant PMS symptom, underwent a baseline demographic and medical evaluation after signature on an Informed Consent Form. The evaluation included also a daily PMS Questionnaire, relating to PMS symptoms and their severity. Enrollment of study participants was performed on the 16th-21st day (luteal phase) of the menstrual cycle. Allocation to both groups was performed by a randomization procedure using a computer generated random rule.
Detection period
Skin testing to sex hormones was performed during the luteal phase of the patient's menstrual cycle 1 to 2 days after the screening.
All solutions were injected intradermally into the volar aspect of the forearm. Four hormones and one control (EO) solutions were administrated in a volume of 0.02 ml each. Positive control histamine (prick test), with negative control (Saline) were administrated in parallel to the hormone administration to confirm a potency of the immune system to react. Skin reactions were evaluated after 20 minutes and were defined as Immediate Response.
Second skin response was assessed by the study investigators after 48 hours, defined as Late Response, and then self-assessed by the subject daily, using ruler.
Daily examination and recording of skin reaction size and filling of the PMS Severity Questionnaire were performed by the subjects every evening (8–9pm) during the following month until the scheduled visit in the next luteal phase.
Diameter of intradermal wheal (not erythema) was considered for evaluation as a skin cellular reaction.
Treatment period
At the end of the first month of skin response observation, in the luteal phase of the next menstrual cycle, desensitization treatment was designed based on the positive skin responses to hormones.
Three sessions of desensitization were planned to be performed once a month. Each session consisted of separate hormone intradermal injections according to the positive skin test reaction. Each month the volume of intradermal injection was doubled, beginning with 0.04 ml in the first month, 0.08 ml in the second month, and 0.16 ml in the third month.
Upon completion of the placebo treatment and data collection the subjects from the PLACEBO group were proposed to undergo desensitization treatment according to the skin test results.
During the treatment period the participants continued daily recording of the PMS symptoms and skin sensitivity if it continued to exist.
Repeated skin test was not performed as part of the study protocol.
Intradermal injection specifications and technique
Hormone concentrations in skin testing
-
1.
Progesterone 1 mmol/L; Estradiol 1 mmol/L; Estrone 3 mmol/L; Estriol 3 mmol/L
Vehicle/solvent (in PLACEBO group)
-
1.
Saline (NaCl) 0.9%; Ethyl Oleate with 10% Benzyl Alcohol; Histamine phosphate 1 mg/ml (epicutaneous-prick test)
Hormone injection protocol in HORMONE group
Subjects with positive skin test to at least one sex hormone were injected with an increasing volume of the appropriate hormone at the same concentration as it was used in the skin test as follows:
-
1.
Week 4 of the study (luteal phase of 2nd menstrual cycle) - 0.04 ml
-
2.
Week 8 of the study (luteal phase of 3rd menstrual cycle) - 0.08 ml
-
3.
Week 12 of the study (luteal phase of 4th menstrual cycle) - 0.16 ml
Hormone injection technique
-
1.
Hormone solutions were heated to body temperature (36.6C) in the water bath and were injected according to the standard technique (10°) accompanied by visual control of the formation of the intradermal papule. The procedure was performed by a single qualified professional.
Data analysis
The data from the diaries and questionnaires, as well as physiological data of the subjects in the HORMONE group were compared with the similar data of the subjects in the PLACEBO group. Demographic and baseline variables were summarized and analyzed to access the comparability of the PLACEBO and HORMONE groups. For treatment efficacy the following method was employed. Mean score for each symptom was calculated during the luteal phase of the first and last month. The comparison was performed between means of PLACEBO and HORMONE groups by a Student's t-test, between final and initial scores of each symptom in each patient.
Study flow chart
Results
Patient enrollment
During the 2 year period starting on April 14, 2009 to April 13, 2011, 35 subjects have been enrolled in the study (Table 1). Twenty20 subjects (57%) dropped out of the study due to various personal reasons and screening failures such as hepatitis, willingness to use contraceptives, a long trip, operation etc., but more interestingly due to resolution of symptoms during the skin test phase and lack of incentive to continue their participation.
Table 1.
Enrollment of participants to the study, Average age of the subjects in HORMONE group was 37.5 ± 6.4 and in PLACEBO group was 35.9 ± 5.4 (Standard error).
| No of subjects | |
|---|---|
| 20 | Dropped out |
| 7 | PLACEBO |
| 8 | HORMONE |
Of 15 subjects that finally participated in the study 8 have been randomly allocated to HORMONE group and underwent at least 2 treatment sessions. 7 have been randomly allocated to PLACEBO group.
Sensitivity to hormones
Sensitivity to one or more female sex hormones has been detected at different levels in 91% (20 out of 22) of the patients with PMS. The results of the skin tests in both groups are shown in Table 2A, Table 2BA and 2B (for the drop out group) (see Table 2C).
Table 2A.
Results of the skin test in HORMONE (A) and PLACEBO (B) groups. Note the relatively high response rate to Estrogen derivatives.
| HORMONE group |
PLACEBO group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient No. | P |
E2 |
E1 |
E3 |
Patient No. | P |
E2 |
E1 |
E3 |
| Progesterone | Estradiol | Estrone | Estriol | Progesterone | Estradiol | Estrone | Estriol | ||
| 1 | V | 3 | V | V | V | V | |||
| 2 | V | V | 4 | ||||||
| 6 | V | V | 11 | V | V | V | |||
| 13 | V | V | 17 | V | V | V | |||
| 14 | V | V | 20 | V | V | V | V | ||
| 21 | 30 | V | V | ||||||
| 26 | V | 33 | V | ||||||
| 28 | V | V | V | ||||||
| Positive response to hormone (%) | 38% | 50% | 38% | 50% | Positive response to hormone (%) | 57% | 71% | 57% | 71% |
Table 2B.
Results of the skin test in the drop out group. Bolded numbers represent patients that stopped participation due to improvement of symptoms. (ND – skin test not done; neg-negative test).
| Patient No. | P |
E2 |
E1 |
E3 |
|---|---|---|---|---|
| Progesterone | Estradiol | Estrone | Estriol | |
| 5 | V | V | ||
| 7 | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND |
| 9 | V | V | V | V |
| 10 -HEPATITIS C | ND | ND | ND | ND |
| 12 | ND | ND | ND | ND |
| 15 | V | V | ||
| 16 | ND | ND | ND | ND |
| 18 | V | V | V | |
| 19 | ND | ND | ND | ND |
| 22 | Lost on follow-up | |||
| 23 | ND | ND | ND | ND |
| 24 | Lost on follow-up | |||
| 25 | V | V | ||
| 27 | neg | neg | neg | neg |
| 29 | neg | neg | neg | neg |
| 31 | V | V | ||
| 32 | ND | ND | ND | ND |
| 34 | V | V | V | |
| 35 | V | V | V | V |
Table 2C.
Summary of skin test positivity in 23 tested patients with cyclic mastalgia (Placebo, Hormone and Dropout groups), as % of positive skin tests (4%= one patient). Note that the majority of patients had more than a single positivity, with predominant double positivity to Estrone and Estradiol, followed by Progestrone and Estrone double positivity.
| Progesterone | Estradiol | Estrone | Estriol | |
|---|---|---|---|---|
| Progesterone | 4 | 35 | 39 | 17 |
| Estradiol | 35 | 0 | 48 | 39 |
| Estrone | 39 | 48 | 4 | 35 |
| Estriol | 17 | 39 | 35 | 4 |
Diary of PMS symptoms
Representative data from individual PMS questionnaires of subjects responded and not responded to the treatment is depicted in Fig. 1. Red point indicates a menstruation onset. The graph is presented as a mean severity score of the PMS-associated symptoms measured daily during the study. The differences in time duration of treatments are due to technical differences of show-up schedules of individual patients.
Fig. 1.
Representative symptom severity during the treatment course. Red point indicates a menstruation onset. The graph is presented as a mean severity score of the PMS-associated symptoms measured daily during the studyThe differences in time duration of treatments are due to technical differences of show-up schedules of individual patients. (A-responders to desensitization therapy. B – non responders to desensitization therapy. Treatment 1–3 – treatment periods – days post trial enrollment).
Responsivity to treatment
Means of initial and final scores are presented in Table 3. This mean score, in contrast to the mean score in the next section, represents a mean of individual daily scores in luteal phase assessed for each symptom separately. Blank spaces indicate lack of symptom appearance in the patient.
Table 3.
Mean initial and final scores for each PMS symptom in each patient in HORMONE (A) and PLACEBO (B) groups Blank spaces indicate lack of symptom appearance in the patient. (I = Initial, F = Final).
| HORMONE group | Pt No |
1 |
2 |
6 |
13 |
14 |
21 |
26 |
28 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean score Initial/Final | I | F | I | F | I | F | I | F | I | F | I | F | I | F | I | F | |
| Symptoms | |||||||||||||||||
| Headache | 3.3 | 0.0 | 2.4 | 1.4 | 5.1 | 1.8 | 6.1 | 6.9 | 4.4 | 3.3 | 2.0 | 0.0 | 8.7 | 1.3 | |||
| Breast tenderness or swelling | 6.4 | 0.0 | 3.5 | 3.3 | 5.3 | 7.4 | 8.4 | 8.4 | 2.0 | 0.0 | 10.0 | 1.3 | 5.8 | 4.3 | |||
| Menstrual cramps/Joint or muscle pain | 8.6 | 7.2 | 3.5 | 3.8 | 5.2 | 5.5 | 2.6 | 5.8 | 5.2 | 6.2 | 5.4 | 0.0 | 8.0 | 1.3 | 5.8 | 4.3 | |
| Bloatedness | 9.3 | 7.8 | 5.5 | 0.3 | 6.0 | 5.9 | 10.0 | 10.0 | 2.1 | 0.0 | 10.0 | 1.3 | |||||
| Hot flushes | 7.7 | 2.0 | 1.3 | 4.1 | 4.4 | 0.0 | 9.2 | 0.0 | 2.1 | 1.8 | |||||||
| Nausea/diarrhea/constipation | 8.9 | 8.0 | 1.4 | 6.5 | 5.7 | 10.0 | 9.8 | 1.3 | |||||||||
| Palpitations | 6.8 | 0.0 | 2.0 | 8.0 | 2.3 | 0.0 | 7.6 | 0.0 | |||||||||
| Weight gain | 7.2 | 0.2 | 3.9 | 5.6 | 3.5 | 2.4 | 10.0 | 9.8 | 10.0 | 0.0 | |||||||
| Confusion/lack of concentration/feeling out of control | 6.7 | 0.0 | 2.8 | 0.3 | 4.1 | 7.6 | 5.6 | 4.4 | 10.0 | 0.7 | |||||||
| Tearful | 1.8 | 0.0 | 3.6 | 7.9 | 8.1 | 0.7 | |||||||||||
| Dizziness | 4.8 | 0.0 | 2.6 | 0.0 | 0.9 | 5.6 | 9.7 | 0.0 | |||||||||
| Acne | 2.7 | 1.2 | 7.3 | 0.7 | 7.3 | 9.3 | 7.6 | 0.0 | 1.7 | 1.4 | |||||||
| Food cravings | 7.8 | 6.5 | 4.0 | 3.9 | 7.7 | 4.4 | 7.0 | 5.4 | 10.0 | 10.0 | 4.6 | 0.0 | 10.0 | 0.7 | 3.2 | 0.0 | |
| Forgetfulness | 3.2 | 0.0 | 2.8 | 3.1 | 4.6 | 0.0 | 6.7 | 0.0 | |||||||||
| Anger/Irritability | 9.0 | 6.0 | 3.8 | 3.5 | 2.7 | 0.0 | 5.9 | 9.0 | 6.2 | 6.1 | 1.3 | 0.0 | 9.9 | 0.7 | 1.9 | 0.0 | |
| Depression | 9.1 | 2.0 | 4.0 | 9.1 | 5.3 | 1.6 | 8.7 | 0.0 | |||||||||
| Arguments/violent tendencies | 8.0 | 7.3 | 2.5 | 0.9 | 6.3 | 0.7 | 7.8 | 3.3 | |||||||||
| Anxiety/tension | 4.2 | 3.0 | 5.8 | 4.6 | 5.8 | 9.5 | 6.4 | 1.0 | 10.0 | 2.0 | |||||||
| Mood swings | 8.4 | 7.1 | 3.7 | 1.9 | 2.2 | 0.0 | 3.3 | 8.9 | 5.4 | 0.0 | 10.0 | 2.0 | 1.9 | 0.0 | |||
| Increased appetite | 7.4 | 7.2 | 6.3 | 6.1 | 6.1 | 3.5 | 5.9 | 4.3 | 10.0 | 10.0 | 4.9 | 0.0 | 10.0 | 1.3 | 3.7 | 0.0 | |
| Over-sensitivity | 7.7 | 5.9 | 3.0 | 1.1 | 2.0 | 0.0 | 6.4 | 8.8 | 7.8 | 4.4 | 10.0 | 0.0 | 1.9 | 0.0 | |||
| Isolation/less interest in daily activities (work, school, Friends, Hobbies) | 9.0 | 1.5 | 5.2 | 1.0 | 5.5 | 0.0 | 4.0 | 6.0 | 5.6 | 2.0 | 5.0 | 0.0 | 10.0 | 4.3 | 1.5 | 0.0 | |
| Fatigue, lack of energy | 10.0 | 7.4 | 6.7 | 5.6 | 8.4 | 4.6 | 7.1 | 8.3 | 9.8 | 1.2 | 3.9 | 0.0 | 10.0 | 4.3 | 2.7 | 0.0 | |
| Slept more/difficulty to get up/trouble falling asleep or staying asleep | 6.4 | 3.3 | 4.8 | 0.3 | 7.7 | 4.6 | 6.4 | 5.9 | 9.8 | 4.2 | 2.8 | 0.0 | 10.0 | 4.3 | 2.8 | 0.0 | |
| Reduced productivity/inefficiency at work/school/home/daily routine, as a result of any of the above | 4.5 | 2.0 | 2.8 | 1.9 | 2.5 | 4.4 | 8.6 | 2.0 | 3.0 | 0.0 | 10.0 | 4.3 | |||||
| Avoidance/less participation in hobbies/social activities, as a result of any of the above | 3.6 | 1.5 | 2.2 | 0.0 | 10.0 | 4.3 | |||||||||||
| B. PLACEBO group | Pt No |
3 |
4 |
11 |
17 |
20 |
30 |
33 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score Initial/Final | I | F | I | F | I | F | I | F | I | F | I | F | I | F | |
| Symptoms | |||||||||||||||
| Headache | 4.0 | 2.0 | 2.4 | 3.1 | 5.1 | 3.7 | |||||||||
| Breast tenderness or swelling | 3.3 | 2.3 | 6.1 | 9.4 | 6.0 | 3.2 | 5.2 | 3.0 | 7.0 | 4.0 | 8.5 | 0.0 | |||
| Menstrual cramps/Joint or muscle pain | 4.7 | 4.6 | 9.1 | 9.4 | 6.4 | 3.2 | 5.8 | 5.7 | 9.0 | 6.5 | 6.4 | 0.7 | |||
| Bloatedness | 4.0 | 3.7 | 2.4 | 1.7 | 6.9 | 0.0 | 4.5 | 2.8 | 9.8 | 6.5 | 6.1 | 0.4 | |||
| Hot flushes | 2.3 | 4.3 | 5.4 | 8.0 | 3.9 | 0.0 | 3.0 | 0.0 | 7.7 | 0.0 | |||||
| Nausea/diarrhea/constipation | 2.3 | 4.1 | 6.7 | 1.7 | |||||||||||
| Palpitations | |||||||||||||||
| Weight gain | 7.7 | 2.9 | 1.9 | 1.5 | 2.7 | 0.0 | 4.7 | 0.0 | 5.8 | 0.4 | |||||
| Confusion/lack of concentration/feeling out of control | 8.3 | 6.6 | 5.5 | 0.0 | 7.2 | 0.0 | 5.4 | 0.0 | 2.3 | 0.9 | |||||
| Tearful | 1.7 | 0.0 | 1.1 | 7.1 | 4.6 | 0.0 | 9.3 | 0.0 | 6.6 | 0.7 | |||||
| Dizziness | |||||||||||||||
| Acne | 0.9 | 0.0 | 1.9 | 1.6 | 3.5 | 0.7 | 2.0 | 6.5 | |||||||
| Food cravings | 10.0 | 10.0 | 7.4 | 6.9 | 3.8 | 3.5 | 10.0 | 0.0 | 6.7 | 0.7 | |||||
| Forgetfulness | 2.1 | 2.9 | 6.3 | 0.0 | 3.2 | 0.0 | 1.0 | 0.4 | |||||||
| Anger/Irritability | 1.9 | 1.9 | 5.0 | 7.4 | 8.3 | 0.0 | 4.5 | 2.2 | 10.0 | 5.7 | 7.4 | 0.7 | |||
| Depression | 3.7 | 0.0 | 6.7 | 0.7 | 2.9 | 2.0 | |||||||||
| Arguments/violent tendencies | 1.9 | 1.0 | 3.4 | 5.7 | 6.8 | 0.0 | 2.5 | 1.0 | 8.0 | 4.0 | 6.7 | 0.0 | |||
| Anxiety/tension | 2.9 | 4.3 | 3.9 | 0.0 | 7.4 | 0.7 | 1.8 | 0.7 | |||||||
| Mood swings | 5.0 | 4.3 | 7.3 | 0.0 | 2.7 | 0.3 | 8.7 | 0.0 | 7.4 | 0.7 | |||||
| Increased appetite | 2.9 | 3.7 | 10.0 | 6.6 | 6.8 | 0.8 | 2.3 | 4.0 | 4.8 | 0.0 | 6.9 | 1.6 | |||
| Over-sensitivity | 6.0 | 8.0 | 8.1 | 3.5 | 2.7 | 0.0 | 4.7 | 0.0 | 6.1 | 1.8 | |||||
| Isolation/less interest in daily activities (work, school, Friends, Hobbies) | 3.3 | 1.7 | 8.6 | 7.4 | 9.2 | 0.0 | 6.1 | 1.8 | |||||||
| Fatigue, lack of energy | 3.9 | 4.6 | 10.0 | 8.9 | 9.8 | 0.0 | 1.7 | 0.0 | 7.8 | 7.3 | 8.1 | 1.8 | 5.0 | 2.9 | |
| Slept more/difficulty to get up/trouble falling asleep or staying asleep | 4.0 | 3.7 | 7.9 | 5.7 | 10.0 | 0.0 | 4.3 | 3.7 | 5.9 | 0.7 | |||||
| Reduced productivity/inefficiency at work/school/home/daily routine, as a result of any of the above | 2.3 | 0.7 | 9.4 | 7.1 | 8.1 | 0.0 | 8.3 | 0.0 | 5.4 | 0.7 | |||||
| Avoidance/less participation in hobbies/social activities, as a result of any of the above | 9.7 | 5.0 | 8.6 | 0.0 | 6.7 | 0.0 | 5.4 | 0.7 | |||||||
Relative symptom severity improvement (RI) of each of the PMS symptoms was calculated according to the below formula (depicted in Fig. 2) - RI = (average of initial scores - average of final scores)/average of initial scores. No difference between PLACEBO and HORMONE groups was observed.
Fig. 2.
Graphic presentation of the relative symptom severity reduction (condition improvement) in PLACEBO and HORMONE groups (red bar = median). Each symbol represents an average Relative Improvement (RI) of the group for one of the 26 symptoms assessed.
It is worth mentioning that the PLACEBO group was subjected to the skin test which actually represents a first non-specific treatment by a low hormone concentration. Thus, an excessive efficacy of the PLACEBO group might be due to the high responsiveness of certain patients to the single low-dose treatment.
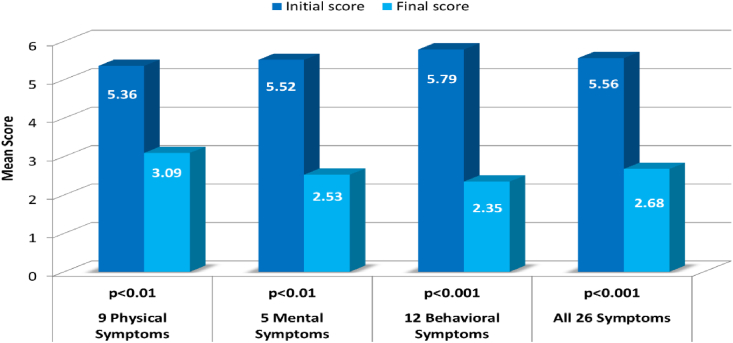
Further, both HORMONE and PLACEBO groups have been combined to one group including different number of treatments. The efficacy of the treatment (specific and non-specific) was examined through comparison of initial and final scores in 15 patients. Changes in severity of clusters of physical (9 symptoms), mental (5 symptoms) and behavioral (12 symptoms) symptoms (Appendix 2) as well as total of 26 symptoms have been examined were found to be highly significant (t-test p values between 0.01 and 0.001, Fig. 3).
Fig. 3.
Mean scores of 15 patients at the beginning and at the end of treatment. Each bar represents a Mean Severity Score of 15 PMS patients. The treatment was found to be effective in all clusters of PMS symptoms. (p values according to t-test analysis).
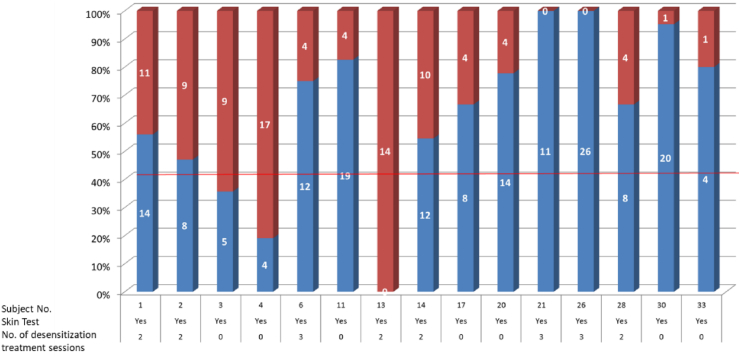
Fig. 4 demonstrates a responder rate of patients exposed to skin test alone (in PLACEBO group) or to skin test and 2–3 specific hormone treatment (HORMONE group). The level of response was calculated as an >40% improvement in PMS-associated symptoms according to the following scheme:
-
1.
R = Responder - improvement in ≥40% of the PMS-associated symptoms
-
2.
NR = Non-responder - improvement in <40% of the PMS-associated symptoms
Fig. 4.
Responder rate of 15 patients following one to four sessions of hormone administration. Each bar represents no. of PMS symptoms in each patient. (Red bars – represent Non-Responders (i.e. - improvement in <40% of the PMS-associated symptoms); Blue bars – represent Responders (i.e. - improvement in ≥40% of the PMS-associated symptoms)).
Among the HORMONE group 7 out of 8 patients (88%) responded to the treatment. In the PLACEBO group 5 out of 7 patients (71%) responded to the skin test and placebo treatment. Total responder rate for 15 patients was 80%.
Despite the inclusion criteria of severe mastalgia, based on the patient inquiry, only 13 out of 15 patients reported mastalgia as a severe symptom during the first month. More than 40% improvement in the symptom was observed in 7 out of 13 patients (54%). Of those 4 out of 6 (75%) patients in the PLACEBO group, which received only one injection (skin test), demonstrated 40% improvement in the symptom severity. As mentioned in the Methods section, repeated skin test was not performed as part of the study protocol.
Adverse events
No serious adverse events (AE) have been reported. Most AE included local skin response (itching, redness, swelling) that disappeared during the first month.
Discussion
Modern classification of menstrual related diseases can be found in the fields of gynecology, psychiatry and endocrinology in modern medicine terminology: Psycho-Neuro-Endocrine-Immunology (P.N.E.I.). It is a scientific field of study that focuses on the link between the nervous system, the endocrine system, and the immune system. With respect to the therapeutic concept proposed in this study, the Low Dose Medicine is a promising approach that will be able to allow design of innovative therapeutic strategies for the treatment of menstrual cycle diseases based on the rebalance of the immune response.9
Due to an absence of reliable biological marker of cyclic menstrual pains we established a skin test (allergologically based) directed to provoke an allergic reaction by intradermal administration of small concentrations of sex hormones. The skin test to sex hormones and desensitization treatment were first described by Mandatory Palestinian scientists.2 This study hypothesized that an immune-endocrine-allergic mechanism does exist, that the mechanism is reflected by provoked skin sensitivity and that such a mechanism is responsible for cyclic pain syndromes. We therefore continued the development of this theory (and clinical practice) over the last 20 years.15, 16, 17, 18, 19
We postulate that the mechanism responsible for intolerance to sex hormones is in fact an adaptive reaction of several bodily systems to stress, enhanced during ovulation, thus leading to temporary inbalance, manifested as a well-known and well-described entity of premenstrual syndrome array (see Appendix 2 for the list of PMS symptoms). Moreover, the known mechanism of allergic skin response is probably responsible for skin sensitivity to dermal injections.10 Thus, it is possible that sex hormone intolerance plays a pivotal role in the pathophysiology of various autoimmune diseases.11 Additionally, these clinical syndromes may transform themselves into proliferative inflammatory or even neoplastic processes. For instance, in a prospective French study12 a 5- fold increased breast cancer risk was found in women with cyclic mastalgia.
Possible markers for pain syndromes, dysmenorrhea, low back pain and menstrual headache are tissue or even plasma Ca/Mg and vitamin D reduced levels often found in these conditions. The intradermal application of sex hormones in optimal tissue Ca/Mg and vit D microenvironment and optimally standardized technique that induces effective Dendritic/Ts cells inhibiting inflammatory response become an attractive therapeutic method for different clinical situations.13
Japanese scientists14 found a cyclic pattern of autonomic nervous system functioning shifts in women suffering from PMS, which may be attributed to the serum biochemical cyclic shifts symptoms in these women.
It is safe to suggest that the reproducibility of these symptoms, especially the metabolic stress and electrolyte inbalance, may influence the gene expression array responsible for prolonged pain sensitization. Many genes of the stress response system are similar in plants and humans (NFkB, Bax, Map kinase, etc). Even a precursor gene exists for management of stress in yeast and plants of the p53 gene, which is a hallmark gene in mammals and humans for cell repair and is also involved in human stress response. The DNA repair gene, p53, is regulated in humans by the androgens (DHEA and testosterone) metabolite androstenediol and rostanediol.
A reduction of androgens and their metabolites over a longer time, impairs therefore cell repair and neurogenesis, and they are also involved in the modulating of immunity of the mother during pregnancy.
Thus prolonged pain syndromes such as PMS, recurrent or chronic pain resulting from traumatic brain injury, especially in recurrent trauma, probably lead to central sensitization of various systems – a concept well-accepted by immunologists, endocrinologists and pain specialists.
Our experience indicates that an immunological reaction of sex hormone intolerance correction, brought about a stable remission of pain syndromes including reduced Visual Assessment Score pain levels in our patients (non-published data).
We want to mention a design flaw of our experiment such as a lack of a placebo group in the skin test phase and a lack or Ca/Mg or Vitamin D levels, which probably explains the similar positive effect in the PLACEBO and HORMONE groups. Moreover, a high proportion of dropout was partially due to resolution of symptoms in the skin test phase, probably due to such an early positive effect. Therefore, in a future study we suggest to optimize our design taking into consideration the aforementioned points.
Therefore, induction of tolerance via intradermal application with naive or bioidentical sex hormones could be used to inhibit cell-mediated immune responses, as we found in PMS, and animal model of recurrent abortions,15, 16, 17, 18, 19 or even in a broader spectrum of autoinflammatory diseases.20
It is important to note that sex hormone intolerance is critically dependent on Ca/Mg and Vitamin D levels. This fact may explain an unexpected immediate effect to initial skin test prior to de-sensitization procedure in many cases (in which the Mg or Vitamin D group were not measured, which may explain such a prompt response).
To conclude, we feel that it is safe to suggest that the efficacy of this novel approach might be of high value in treatment of PMS and additional pathologies, especially in the field of idiopathic autoimmune disorders such as small fiber neuropathy and fibromyalgia.
Conclusions
-
1.
Severe PMS is correlated with a high predominance of sensitivity (>90%) to one's own female hormones (Progesterone, Estradiol, Estrone, Estriol).
-
2.
A high safety profile of the Diagnostic Skin Test Panel and desensitization treatment is observed.
-
3.
A high treatment response rate (80%) in patients suffering from PMS symptoms was shown.
-
4.
Even a single exposure of the patient to the smallest dose of the hormones during the skin test, might have a therapeutic effect, expelling the PLACEBO group positive effect.
-
5.
Equal effect of the treatment on physical, mental and behavioral symptoms was demonstrated.
-
6.
In a future optimized study, a skin test placebo group is needed to be included.
-
7.
Sex hormone desensitization therapy to be considered in various related pathologies such as infertility, gynecology-oncology and breast cancer, endocrine and idiopathic autoimmune disorders.
Author Contributions
Author IA was responsible for the design of the study including treatment and diagnostic protocol, as well as for the diagnostic skin tests and desensitization procedures and further clinical follow-up; Author YB was responsible for the design of the study and statistical anaylisis; Author ZIH was responsible for manuscript design, statistical anaylisis, revision of data and submission process; Author MZ was responsible for diagnostic selection of patients and clinical follow-up; Authro SK was responsible for the design of the study, patient selection, treatment and diagnostic protocol revision and manuscript draft.
Funding
The study was funded by Israel Ministry of Health special budget for biomedical startups.
Conflicts of interest
None.
Footnotes
The study was approved by Tel Aviv Sourasky Medical Center Institutional Ethics Committee.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100041.
Contributor Information
Alek M. Itsekson, Email: itsekson@netvision.net.il.
Bomstein Yonit, Email: ybomstein@gmail.com.
Itsekson-Hayosh Ze'ev, Email: zeevits@gmail.com.
Zolti Matitiyahu, Email: mati.zolti@gmail.com.
Kivity Shmuel, Email: allergy@tlvmc.gov.il.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Geber H. Einege Daten zur Pathologie der Urticaria menstrationalis. Dermat Z. 1921;32:143. [Google Scholar]; Geber H: Einege Daten zur Pathologie der Urticaria menstrationalis. Dermat Z 1921; 32:143.
- 2.Zondek B., Bromberg Y.M. Endocrine allergy: I. Allergic sensitivity to endogenous hormones. J Allergy. 1945;16(1):1–16. [Google Scholar]; Zondek B, Bromberg YM. Endocrine allergy: I. Allergic sensitivity to endogenous hormones. J Allergy. 1945;16(1):1-16.
- 3.Zondek B., Bromberg Y.M. Endocrine allergy; clinical reactions to allergy to endogenous hormones and their treatment. J Obstet Gynaecol Br Emp. 1947;54(1):1–19. doi: 10.1111/j.1471-0528.1947.tb05383.x. [DOI] [PubMed] [Google Scholar]; Zondek B, Bromberg YM. Endocrine allergy; clinical reactions to allergy to endogenous hormones and their treatment. J Obstet Gynaecol Br Emp. 1947;54(1):1-19. [DOI] [PubMed]
- 4.Shelley W.B., Preucel R.W., Spoont S.S. Autoimmune progesterone dermatitis. Cure by oophorectomy. JAMA. 1964;190:35–38. [PubMed] [Google Scholar]; Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis. Cure by oophorectomy. JAMA. 1964;190:35-38. [PubMed]
- 5.Shelley W.B., Shelley E.D., Talanin N.Y., Santoso-Pham J. Estrogen dermatitis. J Am Acad Dermatol. 1995;32(1):25–31. doi: 10.1016/0190-9622(95)90179-5. [DOI] [PubMed] [Google Scholar]; Shelley WB, Shelley ED, Talanin NY, Santoso-Pham J. Estrogen dermatitis. J Am Acad Dermatol. 1995; 32(1):25-31. [DOI] [PubMed]
- 6.Huck B., Steck T., Habersack M., Dietl J., Kammerer U. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):85–94. doi: 10.1016/j.ejogrb.2005.02.017. [DOI] [PubMed] [Google Scholar]; Huck B, Steck T, Habersack M, Dietl J, Kammerer U. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol 2005; 122(1): 85-94. [DOI] [PubMed]
- 7.Kyurkchiev D., Ivanova-Todorova E., Hayrabedyan S., Altankova I., Kyurkchiev S. Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am J Reprod Immunol. 2007;58(5):425–433. doi: 10.1111/j.1600-0897.2007.00526.x. [DOI] [PubMed] [Google Scholar]; Kyurkchiev D, Ivanova-Todorova E, Hayrabedyan S, Altankova I, Kyurkchiev S. Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am J Reprod Immunol 2007; 58(5): 425-433. [DOI] [PubMed]
- 8.Hughes G.C., Thomas S., Li C., Kaja M.K., Clark E.A. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]; Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol 2008; 180(4): 2029-2033. [DOI] [PubMed]
- 9.Bottaccioli Francesco, Bottaccioli Anna Giulia. Psycho-neuro-endocrino-immunology paradigm and cardiovascular diseases. Integrative Cardiology. 2017:139–151. [Google Scholar]; Francesco Bottaccioli, Anna Giulia Bottaccioli. Psycho-neuro-endocrino-immunology Paradigm and Cardiovascular Diseases. Integrative Cardiology, 2017 pp 139-151.
- 10.Gittler Julia K., Krueger James G., Guttman-Yassky Emma. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis. J Allergy Clin Immunol. 2013;131:300–313. doi: 10.1016/j.jaci.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; Julia K. Gittler, James G. Krueger, and Emma Guttman-Yassky. Atopic dermatitis results in intrinsic barrier and immune abnormalities: Implications for contact dermatitis, J Allergy Clin Immunol 2013;131:300-313. [DOI] [PMC free article] [PubMed]
- 11.Rogers Mary A.M., Levine Deborah A., Blumberg Neil, Fisher Gwenith G., Kabeto Mohammed, Langa Kenneth M. Antigenic challenge in the etiology of autoimmune disease in women. J Autoimmun. 2012 May;38(2-3):J97–J102. doi: 10.1016/j.jaut.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mary A.M. Rogers, Deborah A. Levine, Neil Blumberg, Gwenith G. Fisher, Mohammed Kabeto, and Kenneth M. Langa, Antigenic Challenge in the Etiology of Autoimmune Disease in Women. J Autoimmun. 2012 May; 38(2-3): J97-J102. [DOI] [PMC free article] [PubMed]
- 12.Plu-Bureau G., Lê M.G., Sitruk-Ware R., Thalabard J.C. Cyclical mastalgia and breast cancer risk: results of a French cohort study. Cancer Epidemiol Biomark Prev. 2006 Jun;15(6):1229–1231. doi: 10.1158/1055-9965.EPI-05-0745. [DOI] [PubMed] [Google Scholar]; Plu-Bureau G, Le MG, Sitruk-Ware R, Thalabard JC. Cyclical mastalgia and breast cancer risk: results of a French cohort study. Cancer Epidemiol Biomarkers Prev. 2006 Jun;15(6):1229-1231. [DOI] [PubMed]
- 13.Hufnagl K., Jensen-Jarolim E. Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease. Allergo J Int. 2018;27(3):72–78. doi: 10.1007/s40629-018-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hufnagl K, Jensen-Jarolim E. Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease. Allergo J Int. 2018;27(3):72-78. [DOI] [PMC free article] [PubMed]
- 14.Matsumoto T., Ushiroyama T., Kimura T., Hayashi T., Moritani T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc Med. 2007 Dec 20;1:24. doi: 10.1186/1751-0759-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]; Matsumoto T, Ushiroyama T, Kimura T, Hayashi T, Moritani T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc. Med. 2007 Dec 20;1:24. [DOI] [PMC free article] [PubMed]
- 15.Itsekson A., Lazarov A., Cordoba M., Zeitune M., Abraham D., Seidman D.S. Premenstrual syndrome and associated skin diseases related to hypersensitivity to female sex hormones. J Reprod Med. 2004 Mar;49(3):195–199. [PubMed] [Google Scholar]; Itsekson A, Lazarov A, Cordoba M, Zeitune M, Abraham D, Seidman DS. Premenstrual syndrome and associated skin diseases related to hypersensitivity to female sex hormones. J Reprod Med. 2004 Mar;49(3):195-199. [PubMed]
- 16.Itsekson A.M., Seidman D.S., Zolti M., Lazarov A., Carp H.J. Recurrent pregnancy loss and inappropriate local immune response to sex hormones. Am J Reprod Immunol. 2007 Feb;57(2):160–165. doi: 10.1111/j.1600-0897.2006.00461.x. [DOI] [PubMed] [Google Scholar]; Itsekson AM, Seidman DS, Zolti M, Lazarov A, Carp HJ. Recurrent pregnancy loss and inappropriate local immune response to sex hormones. Am J Reprod Immunol. 2007 Feb;57(2):160-165. [DOI] [PubMed]
- 17.Seidman D.S., Itsekson A., Alesker M., Zolti M., Carp H., Wolman I. Estradiol valerate as a possible endocrine reproductive disruptor: evidence from an in vivo rat model. Fertil Steril. 2009 Apr;91(4 Suppl):1510–1512. doi: 10.1016/j.fertnstert.2008.08.018. [DOI] [PubMed] [Google Scholar]; Seidman DS, Itsekson A, Alesker M, Zolti M, Carp H, Wolman I. Estradiol valerate as a possible endocrine reproductive disruptor: evidence from an in vivo rat model. Fertil Steril. 2009 Apr;91(4 Suppl):1510-1512. [DOI] [PubMed]
- 18.Itsekson A.M., Seidman D.S., Zolti M., Alesker M., Carp H.J. Steroid hormone hypersensitivity: clinical presentation and management. Fertil Steril. 2011 Jun 30;95(8):2571–2573. doi: 10.1016/j.fertnstert.2011.05.025. [DOI] [PubMed] [Google Scholar]; Itsekson AM, Seidman DS, Zolti M, Alesker M, Carp HJ. Steroid hormone hypersensitivity: clinical presentation and management. Fertil Steril. 2011 Jun 30;95(8):2571-2573. [DOI] [PubMed]
- 19.Itsekson A.M., Soriano D., Zolti M., Seidman D.S., Carp H.J. Intradermal sex hormone desensitization for relief of premenstrual symptoms may improve the obstetric outcome of women with recurrent pregnancy loss. Gynecol Endocrinol. 2013 Feb;29(2):169–172. doi: 10.3109/09513590.2012.730582. [DOI] [PubMed] [Google Scholar]; Itsekson AM, Soriano D, Zolti M, Seidman DS, Carp HJ. Intradermal sex hormone desensitization for relief of premenstrual symptoms may improve the obstetric outcome of women with recurrent pregnancy loss. Gynecol Endocrinol. 2013 Feb;29(2):169-172. [DOI] [PubMed]
- 20.Untersmayr E., Jensen A.N., Walch K. Sex hormone allergy: clinical aspects, causes and therapeutic strategies - update and secondary publication. World Allergy Organ J. 2017 Dec 27;10(1):45. doi: 10.1186/s40413-017-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Untersmayr E, Jensen AN, Walch K. Sex hormone allergy: clinical aspects, causes and therapeutic strategies - Update and secondary publication. World Allergy Organ J. 2017 Dec 27;10(1):45. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.