Abstract
Fluoropyrimidines (FLs) have been widely used for more than 60 years against a range of solid tumors and still remains the cornerstone for the treatment of colorectal, gastric, and breast cancer. Here, we performed an economic analysis to estimate the cost of DPYD-guided toxicity management and the clinical benefit expressed as quality adjusted life years (QALYs) in a large group of 571 individuals of Italian origin suffering from cancer and treated with a fluoropyrimidines-based chemotherapy. Individuals suffering from cancer with a histologically confirmed diagnosis of cancer, who received a fluoropyrimidines-based treatment, were retrospectively genotyped in the DPYD gene. Effectiveness was measured as survival of individuals from chemotherapy, while study data on safety and efficacy as well as on resource utilization associated with each adverse drug reaction were used to measure costs to treat these adverse drug reactions. A generalized linear regression model was used to estimate cost differences for both study groups. DPYD extensive metabolizers (528 individuals) had greater effectiveness and lesser cost, representing a cost-saving option over DPYD intermediate and poor metabolizers (43 individuals) with mean QALYs of 4.18 (95%CI: 3.16–5.55) versus 3.02 (95%CI: 1.94–4.25), respectively. Our economic analysis showed that there are some indications for differences in survival between the two groups (p > 0.05), while the cost of DPYD extensive metabolizers was significantly lower (p < 0.01) compared with those belonging to the group of intermediate/poor metabolizers. These findings suggest that DPYD-guided fluoropyrimidines treatment represent a cost-saving choice for individuals suffering from cancer in the Italian healthcare setting.
Keywords: economic evaluation, antimetabolite fluoropyrimidines, FL, DPYD genotyping, cost-effectiveness
Introduction
Fluoropyrimidines (FLs) are historically among the most widely used anticancer drugs. FLs include 5-fluorouracil (5-FU), capecitabine, and tegafur. Both capecitabine and tegafur are inactive prodrugs that are metabolized to 5-FU. Although most individuals can be safely treated with FLs, 20%–30% are likely to develop severe (grade ≥ 3) to life-threatening toxicities.1 The rate-limiting step of 5-FU catabolism is dihydropyrimidine dehydrogenase (DPD)-mediated conversion of 5-FU to dihydrofluorouracil. Several germline genomic variants in the DPD-encoding gene (DPYD [MIM: 274270]) result in deficient DPD activity and increased drug half-life that can translate into severe or even lethal FL-related FU toxicity (MIM: 274270).
Even though more than 160 single-nucleotide polymorphisms (SNPs) have been identified in the DPYD gene, only 4 of them are classified to date as being clinically relevant and listed within the international pharmacogenomic guidelines for drug dose adjustments in the Pharmacogenomics Knowledgebase (PharmGKB) that have been recently revised by the Clinical Pharmacogenomics Implementation Consortium (CPIC). The DPYD∗2A (rs3918290) and DPYD∗13 (rs55886062) loss-of-function variants abolish DPD activity almost completely, while the c.2846A>T (rs67376798) and c.1236G>A-HapB3 (rs56038477) variant alleles have been correlated to a moderate loss of protein function.2
The association between DPYD∗2A, DPYD∗13, c.2846A>T, and c.1236G>A-HapB3 variants and the increased risk of severe FL-related toxicity is widely reported in the literature, thus providing increasing support for the translation of DPYD pre-emptive genotyping in the clinical practice.3, 4, 5 However pre-emptive DPYD genotyping is poorly implemented in the clinical practice, and the recent publication of ESMO guidelines for colorectal cancer management, not including any recommendation for pre-emptive DPYD genotyping, rekindled the debate about the validity and utility of this test in the clinical practice. Besides, while the administration of 5-FU and capecitabine in individuals with low or absent DPD activity is contraindicated by FDA, no dose adjustment is recommended in the drug label based on the DPYD genotype (see Xeloda in Web Resources).
One of the limiting factors for the clinical uptake of the DPYD pharmacogenomic test is the lack of information about the cost effectiveness of a pre-treatment DPYD genotyping, raising the question whether DPYD genotype-guided FL dosing can save healthcare resources. Only one prospective clinical study has evaluated so far, from an economical point of view, the effect of an upfront FL dose reduction in DPYD∗2A carriers, reporting a financial advantage of the genotyping approach.6 We previously reported that the differential costs required to manage chemotherapy-related toxicity can be predicted by the individuals’ genotype for both the UGT1A1 (MIM: 191740)-irinotecan and DPYD-FL gene-drug interactions in a large group of individuals suffering from colorectal cancer from a real world clinical practice.7, 8
Here, we aimed to estimate the effectiveness within the study period of DPYD genotyping based on the cost of toxicity management and the clinical benefit expressed as quality adjusted life years (QALYs) per genotype group, namely extensive (group A) and intermediate and poor metabolizers (group B) in a large group of individuals, treated with a FL-based chemotherapy, who suffered from various types of cancer within the Italian healthcare setting.
Material and Methods
Analysis Perspective
The perspective of the present economic analysis was that of third-party payers (sickness funds) in Italy. In this light, only direct health care costs were considered.9 Direct costs are those associated directly with the medical care of individuals and are reimbursed by the payers. The remaining indirect costs (such as productivity loss, traveling costs, etc.), which attempt to quantify the non-medical financial impact of the disease, were not considered here.
Data Collection and Genotyping
Individuals of Italian descent, suffering from cancer with a histologically confirmed diagnosis of cancer, who received a FL-based treatment, were recruited with consent. The study was previously approved by the local ethical committee. Information about chemotherapy schedule, drug dose reduction, delays, or early treatment interruption and the complete list of treatment-related toxic events at each chemotherapy cycle were recorded and retrieved by an existing database.5, 10, 11 The causality of the toxic events was assessed by the physician at the time of occurrence, and only the chemotherapy-related events were recorded. All the toxic events were graded according to NCI Common Terminology Criteria for Adverse Events v.3.0.
Subjects were subsequently retrospectively genotyped for the DPYD∗2A, DPYD∗13, DPYD c.2846A>T, and DPYD-HapB3 variants, after the individuals completed their treatments, according to previously reported methods.5, 10 Primer sequences and genotyping details are available upon request. Actionability rate (i.e., the number of carriers of an actionable genotype over the total number of individuals in the population study) in this study was 7.53%
Methods for Economic Analysis
In the present analysis, the effectiveness of treatments was measured in terms of mean survival per treatment group. Frequently, in biostatistics the difference between alternative interventions is determined by the median survival, but in economic analysis mean value is used instead because it is the most suitable measure for calculating the actual economic effects in society.12 Survival was calculated as the time from chemotherapy start to death from any cause or loss to follow-up or to the end of study period. We estimated effectiveness in two ways: within the study period but without correction for censoring, as well as with the use of Kaplan-Meier curve as a restricted mean survival. We also included information concerning the quality of life of individuals in order to estimate quality adjusted life years (QALYs) per arm.
Concerning the costing methodology of ADRs, we collected data on safety and efficacy as well as on resource utilization associated with each ADR. Patient-level resource utilization data were combined with unit cost data and they were then aggregated to calculate the total treatment cost per person. Thus, the total cost of individuals reflects the cost of any ADR multiplied by the probability of experiencing this event during the course of the study. Variation in resource utilization reflects the fact that some individuals experienced more severe ADRs than others. Reimbursement tariffs used were obtained from the official Government Gazette and are common to all public hospitals and public payers in Italy. All price data and economic estimates refer to the economic year 2018. All values presented here were undiscounted. Since the scope of the analysis was to compare the cost which is related with the ADRs, person-specific data on chemotherapy doses delivered in each cycle were not taken into consideration. On the other hand, all resources used for the treatment of ADRs were taken into consideration. The cost per ADR also includes other medications, such as antivomiting medication, growth factors, and antibiotics that were given during the treatment of these ADRs. The cost of each hospitalization admission was based on Italian Diagnosis Related Groups (DRGs) CRO-Aviano case mix, since we do not have available the number of in-hospital days for all the individuals.
Total cost of events incorporates the cost of visits to oncologists and nurses. The cost of medical examinations in each cycle includes diagnostic imaging (e.g., computed tomography scans and X-rays) and for laboratory tests such as full blood cell count with differential and platelet count as well as full biochemistry tests (alanine aminotransferase, aspartate aminotransferase, c-glutamyltransferase, albumin, bilirubin, sodium, potassium, lactate dehydrogenase, alkaline phosphatase, uric acid, serum creatinine, etc.). The following ADRs were considered for cost evaluation: hepatic events, acute pancreatitis, hand and foot syndrome, hematochezia, neurological events, cardiovascular events, nausea and vomiting, infection, thrombocytopenia, leukopenia, febrile neutropenia, and stomatitis. Based on raw data, it was determined that only grades IV and V ADRs had a significant resource and cost impact, so grades I, II, and III were not included in this assessment, a common practice in similar economic studies for cancer patients.13 Total cost also incorporated the cost of the genotyping test for those belonging to group A. DPYD genetic test is currently not included in Italian National Tariff, so the cost of the test has been estimated from internal hospital data in the amount of 120€. This is consistent with the costs reported in other European healthcare contexts.3, 14
Statistical Analysis and Cost Evaluation in the Presence of Censoring
All analyses were undertaken using Excel sheets with the use of VBA (Visual Basic for Applications) and SPSS V.22 software. A key issue in economic evaluation study is the estimation of the mean population healthcare costs, as they do not follow the normal distribution and vary significantly from zero to extremely high.
In addition to that, a methodological issue concerns the handling of the censored data due to either loss of follow-up or the fact that some individuals are still alive at the time of study completion. “Censoring” occurs when the complete costs of some individuals are not available and hence hypothesis testing for the distribution of cost is invalid if done with conventional approaches (i.e., Kaplan Maier cost estimator, etc.).15 The problem arises because if we included only uncensored subjects in the analysis, we might have lost a substantial amount of information, as individuals who survive a long time are likely to be censored and not included in analysis, while subjects who died early are likely to be uncensored and included in analysis. Individuals were stratified into group A (non-carriers for DPYD variants) and group B (carriers of any of the DPYD c.2846A>T, DPYD∗2A, DPYD∗13, and DPYD-HapB3 variants). In our case, the survival time data (and the corresponding cost data) used in this study were censored by 66.7% in group A and 62.8% in group B, respectively. To deal with such cases for cost estimation, one may choose among a wide range of approaches, each of which has both strengths and limitations.16 We used the Bang-Tsiatis estimator which provides a consistent approach for cost estimation without covariates.
The Bang-Tsiatis estimator belongs to a class of weighted estimators that account appropriately for censoring and have been shown to be consistent and asymptotically normal with easily estimated variances. The efficiency of these estimators is also confirmed, while this method is the most prominent given the fact that the cost history (pattern of cost accumulation) throughout the study is not available and only the final cost has been estimated.
In short, the Bang-Tsiatis estimator employs the weighted cost for each uncensored individual, for each group, based on the inverse probability of being censored at the time of failure.17 The same method was used for the estimation of effectiveness as well.
In a further step, we attempted to estimate the cost within a regression analysis framework. In such a case, it is more appropriate to use a generalized linear model18 for the mean of the total cost since this type of model provides greater flexibility than linear models in formulating the effects of covariates (e.g., age, BMI, gender, number of chemotherapy cycles) on various aspects of cost accumulation. We used a gamma distribution for cost and a Tweedie distribution as a link function since it represents a useful form of variables that are a mixture of zeros and positive values.19 As proposed by Lin,20 we included only the non-censored individuals weighted by the Kaplan-Meier inverse probability of not being censored at this point in time.
To deal with uncertainty, we used nonparametric bootstrapping. In particular, based on the initial dataset, 5,000 new datasets with the same number of observations were drawn at random with replacement. Mean values of the parameters of interest were obtained from each dataset and were used to construct a new matrix with the same observations. Their variability measures were used to estimate confidence intervals (CIs) using the straightforward percentile method.21
Quality of Life
Due to the lack of available information for this specific Italian study population, utility weights were extracted from related literature.22, 23, 24, 25, 26, 27 In particular, the “well” state was set at 0.84, while for those experiencing any major event, utility decrements were presented in Table 1.
Table 1.
Calculation of Utility Decrements
| Duration (Days) | Utility | Calculation | Explanation | |
|---|---|---|---|---|
| Neutropenia G5 | 30 | 0.72 | 0.84−0.12 | 0.12 utility decrements from baseline22, 23 |
| Neutropenia G4 | 30 | 0.74 | 0.84−0.12+0.02 | 0.02 utility increment due to improved condition23 |
| Leukopenia G5 | 30 | 0.72 | 0.84−0.12 | assuming the same utility valuesa as neutropenia22, 23 |
| Leukopenia G4 | 30 | 0.74 | 0.84−0.12+0.02 | assuming the same utility valuesa as neutropenia22, 23 |
| Stomatitis G5 | 10 | 0.70 | – | literature |
| Stomatitis G4 | 10 | 0.72 | 0.70+0.02 | 0.02 utility increment due to improved condition23 |
| Infection G5 | 10 | 0.724 | 0.84−0.13+0.014 | utility of diarrhea G5 +0.01422, 23, 24 |
| Infection G4 | 10 | 0.744 | 0.84−0.13+0.014+0.02 | 0.02 utility increment due to improved condition |
| Diarrhea G5 | 20 | 0.71 | 0.84−0.13 | 0.13 utility decrements from baseline22, 23 |
| Diarrhea G4 | 20 | 0.73 | 0.84−0.13+0.02 | 0.02 utility increment due to improved condition22, 23 |
| Cardiovascular G5 | 90 | 0.64 | – | literature25 |
| Cardiovascular G4 | 90 | 0.66 | 0.64+0.02 | 0.02 utility increment due to improved condition23 |
| Thrombocytopenia G5 | 45 | 0.70 | – | assuming the same utility valuesa as neutropenia |
| Thrombocytopenia G4 | 45 | 0.72 | – | 0.02 utility increment due to improved condition23 |
| Hepatic G4 | 30 | 0.73 | – | literature24 |
| Acute_pancreatitis G4 | 30 | 0.73 | – | assuming the same utility valuesa as hepatic G4 |
| Hand_and_ foot_syndrome G4 | 30 | 0.69 | – | literature26 |
| Febrile neutropenia G4 | 30 | 0.59 | 0.84−0.27+0.02 | 0.27 utility decrements from baseline22, 23 |
| Hematochezia G4 | 30 | 0.79 | – | literature27 |
| Neurological G4 | 45 | 0.71 | 0.84−0.15+0.02 | 0.15 utility decrements from baseline22, 23 |
| Nausea & Vomiting G4 | 10 | 0.72 | 0.84−0.14+0.02 | 0.14 utility decrements from baseline22, 23 |
G indicates grade; duration of decrements was based on expert opinions
Based on clinical experts
Results
Subjects and Treatments
Subject characteristics and demographics are reported in Table 2, stratified by DPYD metabolizer status. Based on the inclusion criteria, 571 individuals were considered eligible for the downstream economic analysis. Price data used for the determination of cost of interventions are listed in Table 3. Table 4 presents the outcomes concerning the total cost and the effectiveness (life years) by treatment arm with and without taking into account censoring of variables. In short, the total cost of ADRs in group A (without censoring) was €1,150 (95%CI: €928–€1,391) and in group B was €3,712 (95%CI: €1,875–€6,005). Thus, the cost in the latter group was much higher than the cost difference being estimated at €2,562 (95%CI: €720–€4,872, p < 0.0001). Group B had provided slightly fewer life years compared to group A; in particular the mean effectiveness (survival) was estimated at 2.91 (95%CI: 2.73–3.10) for group B, as compared to 2.97 (95%CI: 2.43–3.55) for group A, indicating a difference of 0.06 (95%CI: −0.52–0.67). Similar results were taken for the differences in QALYs. When censoring was taken into consideration, group A had greater effectiveness and lesser cost, representing a dominant option over the comparator, namely group B. The mean QALYs was 4.18 (95%CI: 3.16–5.55) in group A and 3.02 (95%CI: 1.94–4.25) in group B, indicating a difference at 1.16 (95%CI: −2.90–0.46) in favor of group A.
Table 2.
Patient Characteristics Used in the Analysis
| Group A | Group B | |
|---|---|---|
| Gender (%) | ||
| All | 528 (100%) | 43 (100%) |
| Male | 314 (59.5%) | 25 (58.14%) |
| Female | 214 (40.5%) | 18 (41.86%) |
| Age (SD) | ||
| All | 61.54 (11.84) | 58.04 (12.51) |
| Male | 63.35 (11.14) | 60.86 (10.86) |
| Female | 58.88 (12.35) | 54.12 (13.94) |
| BMI | ||
| All | 25.05 (3.45) | 25.67 (3.54) |
| Male | 25.48 (3.36) | 26.48 (2.49) |
| Female | 24.42 (3.47) | 24.53 (4.45) |
| Number of Cycles (SD) | ||
| All | 8.47 (3.66) | 7.95 (4.55) |
| Male | 8.60 (3.63) | 8.08 (4.41) |
| Female | 8.16 (3.74) | 7.77 (4.85) |
| Interruption (%) | ||
| All | 115 (21.78%) | 17 (39.53%) |
| Male | 64 (12.12%) | 9 (20.93%) |
| Female | 51 (9.66%) | 8 (18.6%) |
| Tumor response (%) | ||
| Complete Response | 18 (3.41%) | 1 (2.33%) |
| Partial Response | 77 (14.58%) | 8 (18.60%) |
| Steady Disease | 65 (12.31%) | 5 (11.63%) |
| Progress Disease | 73 (13.64%) | 1 (2.33%) |
| Non-Evaluated | 295 (55.87%) | 26 (60.47%) |
Group A (DPYD variants non-carriers), group B (carriers of any DPYD variants among DPYD c.2846A>T, DPYD∗2A, and DPYD∗13 and DPYD-HapB3). SD, standard deviation.
Table 3.
Cost per Item Used in the Economic Analysis Described in This Paper
| Medical Intervention | Cost (Euro) | Source |
|---|---|---|
| Hospitalization | ||
| Hospital access (ordinary recovery) | 5,807 | local economic dataa |
| Hospital access (ordinary recovery) with inpatient death | 6,806 | local economic dataa |
| Day-hospital access | 412 | local economic dataa |
| Blood/platelet transfusion (per die) | 39 | FVG Regional Health System website |
| Supportive Therapy Costs | ||
| GCSF standard treatment for 2 days | 22 | Health Agency of FVG Region |
| Mucositis supportive therapy for 1 week | 17 | Health Agency of FVG Region |
| Instrumental Examination Costs | ||
| Ultrasound abdomen | 80 | FVG Regional Health System website |
| Colonoscopy | 120 | FVG Regional Health System website |
| Echocardiogram | 66 | FVG Regional Health System website |
| Blood analysis (haemachrome) | 11.80 | local economic dataa |
| Health Practitioner Hourly Rate | ||
| Physician (minimum hourly rate) | 27 | FVG Regional Health System website |
| Nurse (minimum hourly rate) | 12 | FVG Regional Health System website |
| Cost of the genetic test | 120 | local economic dataa |
Abbreviations: GCSF, growth colony stimulating factor; FVG, Friuli Venezia Giulia; DRG, diagnosis-related groups.
Based on the definition of Italian DRGs CRO-Aviano case mix.
Table 4.
Main Results of the Economic Analysis
|
Group A |
Group B |
Differences |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (€) | Survival (LY) | QALYs | Cost (€) | Survival (LY) | QALYs | Cost (€) | Survival (LY) | QALYs | |
| Without Correction for Censoring | |||||||||
| Mean | 1,008 | 2.91 | 2.40 | 3,697 | 3.16 | 2.65 | 2,689 | 0.25 | 0.25 |
| SD | 118 | 0.10 | 0.08 | 1,077 | 0.29 | 0.25 | 1,083 | 0.31 | 0.26 |
| UCI | 1,246 | 3.10 | 2.56 | 6,007 | 3.76 | 3.14 | 4,998 | 0.89 | 0.79 |
| LCI | 786 | 2.72 | 2.24 | 1,811 | 2.60 | 2.18 | 784 | −0.34 | −0.26 |
| Min | 659 | 2.55 | 2.10 | 789 | 2.10 | 1.76 | −356 | −0.81 | −0.66 |
| Max | 1,587 | 3.26 | 2.70 | 8,278 | 4.35 | 3.64 | 7,413 | 1.47 | 1.25 |
| With Correction for Censoring | |||||||||
| Mean | 1,383 | 4.99 | 4.18 | 4,661 | 3.62 | 3.02 | 3,278 | −1.37 | −1.16 |
| SD | 334 | 0.73 | 0.61 | 2,268 | 0.70 | 0.59 | 2,297 | 1.02 | 0.86 |
| UCI | 2,077 | 6.62 | 5.55 | 9,596 | 5.07 | 4.25 | 8,277 | 0.55 | 0.46 |
| LCI | 771 | 3.77 | 3.16 | 1,052 | 2.31 | 1.94 | −442 | −3.46 | −2.90 |
| Min | 459 | 3.20 | 2.68 | 95 | 1.14 | 0.96 | −1,594 | −5.34 | −4.48 |
| Max | 2,774 | 8.62 | 7.24 | 17,382 | 6.39 | 5.35 | 15,864 | 4.16 | 3.49 |
Abbreviations: LY, life years; SD, standard deviation; LCI, lower confidence interval; UCI, upper confidence interval. Results were based on 5,000 non-parametric bootstrap experiments; cost measured in EUR; effectiveness evaluated at life years; survival analysis with correction for censoring was based on Kaplan-Meier estimator via inverse probability weights for uncensored subjects.
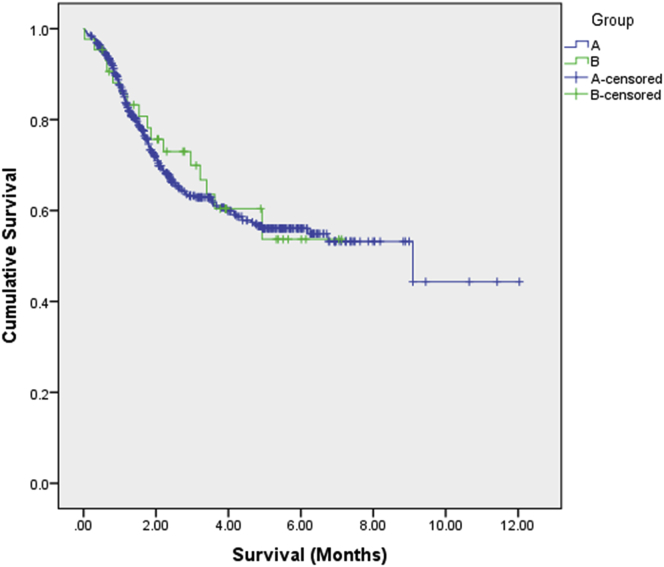
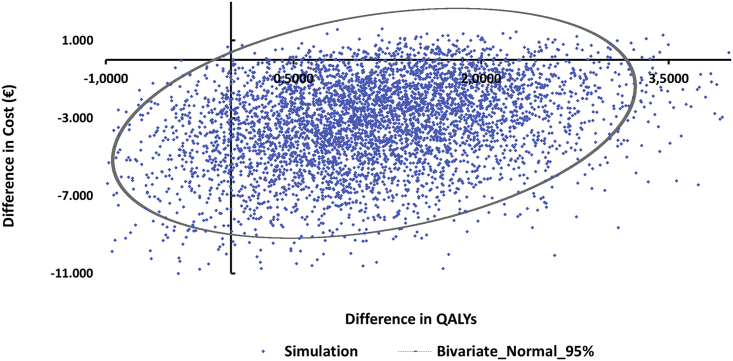
We also estimated survival with Kaplan-Mayer and the mean was 4.86 (95%CI: 4.01–5.73) in group B and 7.13 (95%CI: 6.43–7.82) in group A, p < 0.001 in favor of group A. The test for equality of survival distributions with non-parametric log rank (Mantel-Cox) test (test for medians) indicated that these results do not reach statistically significant differences (x2 = 0.03, df = 1, p = 0.855). Figure 1 depicts the survival curves for the two groups and Figure 2 the distribution of the difference of statistical experiments for cost and QALYs corrected from censoring. The latter graph shows 5,000 simulations, for which the parameter values were changed each time at random based on the empirical distributions of raw data. Each dot represents the result of one individual model simulation run. In accordance with these probabilistic results, the majority of experiments fall into the southeast quadrant (Figure 2), indicating that individuals in group A are a dominant group over the intermediate and poor metabolizer individuals in group B.
Figure 1.
Survival Curves
Survival curves for group A (DPYD variants non-carriers) and group B (carriers of any DPYD variants among DPYD c.2846A>T, DPYD∗2A, and DPYD∗13 and DPYD-HapB3).
Figure 2.
Scatterplot of Distribution of Cost and Effectiveness for the Two Study Groups
Results were based on 5,000 non-parametric bootstrap experiments. Ellipse represents the 95% confidence intervals based on bivariate normal distribution.
Table 5 shows the results of the generalized linear model. Statistically significant variables were the group and the number of chemotherapy cycles. BMI, age, and survival does not provide any additional predicted value to the model and were excluded from the analysis. The mean cost per individual was higher for those belonging to group B (regression coefficient = 0.186 [95%CI: 0.11–0.315], p < 0.001). The estimated cost per individual is described by the following equation:
For instance, the average cost of a female with 8 chemotherapy cycles belonging to group A is 728€, while for group B, the corresponding cost is €3,914.
Table 5.
Association of the Cost of ADRs with Group and Study Participants’ Characteristics
| B | 95% LCI | 95% UCI | Exp (B) | 95% LCI | 95% UCI | p Value | |
|---|---|---|---|---|---|---|---|
| (Intercept) | 9,113 | 8,509 | 9,716 | 9,070 | 4,961 | 16,583 | 0.000 |
| [Group = A]a | −1.682 | −2.207 | −1.157 | 0.186 | 0.11 | 0.315 | 0.000 |
| [Female]a | −0.345 | −0.78 | 0.089 | 0.708 | 0.458 | 1.093 | 0.119 |
| Cycles | −0.062 | −0.112 | −0.012 | 0.94 | 0.894 | 0.988 | 0.015 |
Dependent variable, cost; generalized linear model; Tweedie distribution with log-link function; adjusted by: (intercept), group, gender, chemotherapy cycles; LCI, lower confidence interval; UCI, upper confidence interval.
Group B and male was set at 1, as reference case.
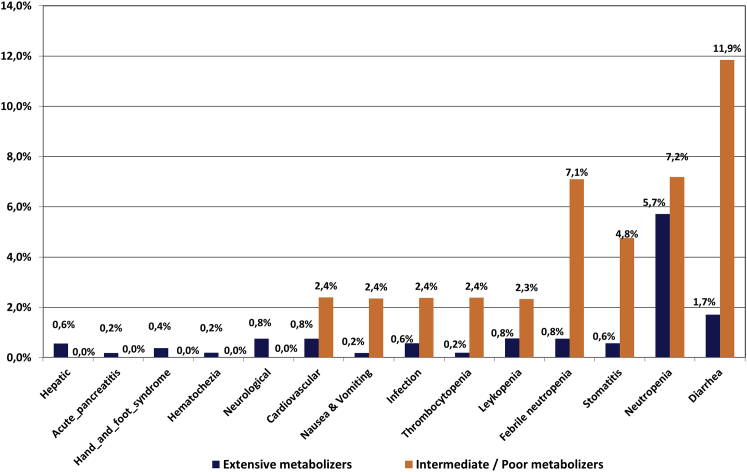
Table 6 and Figure 3 show the percentages of serious ADRs (grades IV and V). The probability of an individual belonging to group A to experience any of these events was limited to 13.49%, as compared to 41.64% for those belonging to group B, a difference of 28.15% in favor of group A. The most frequent ADRs occurring in group A were grade IV neutropenia at 5.71% (95%CI: 3.78%–7.81%) followed by febrile neutropenia at 0.94% (95%CI: 0.19%–1.89%). The rest of serious ADRs were limited to a level well below 1%. For group B, the percentage of those experiencing a grade IV neutropenia was estimated at 7.35% (95%CI: 4.14%–17.07%), with a difference of 1.63% (95%CI: 4.26%–10.85%) compared to the corresponding percentage of group A. Febrile neutropenia was 4.92% (95%CI: 3.37%–12.20%) in group B, a difference of 3.97% (95%CI: 3.40%–11.44%) compared to group A.
Table 6.
Percentage of Serious Adverse Drug Reactions per Study Group
| Mean | SD | UCI | LCI | Min | Max | |
|---|---|---|---|---|---|---|
| Group A (Extensive Metabolizers) | ||||||
| Neutropenia G4 | 5.72% | 1.08% | 7.98% | 3.63% | 2.47% | 9.73% |
| Neutropenia G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Leukopenia G4 | 0.57% | 0.33% | 1.33% | 0.00% | 0.00% | 2.08% |
| Leukopenia G5 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.52% |
| Stomatitis G4 | 0.57% | 0.32% | 1.33% | 0.00% | 0.00% | 1.89% |
| Stomatitis G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Infection G4 | 0.57% | 0.33% | 1.33% | 0.00% | 0.00% | 1.89% |
| Infection G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Diarrhea G4 | 1.70% | 0.56% | 2.84% | 0.76% | 0.00% | 4.36% |
| Diarrhea G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Cardiovascular G4 | 0.57% | 0.33% | 1.33% | 0.00% | 0.00% | 2.27% |
| Cardiovascular G5 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.14% |
| Thrombocytopenia G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Thrombocytopenia G5 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.52% |
| Hepatic G4 | 0.56% | 0.42% | 1.52% | 0.00% | 0.00% | 3.03% |
| Acute_pancreatitis G4 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.14% |
| Hand_and_foot_syndrome G4 | 0.38% | 0.26% | 0.95% | 0.00% | 0.00% | 1.52% |
| Febrile neutropenia G4 | 0.75% | 0.38% | 1.52% | 0.19% | 0.00% | 2.46% |
| Hematochezia G4 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.14% |
| Neurological G4 | 0.76% | 0.38% | 1.52% | 0.19% | 0.00% | 2.27% |
| Nausea & Vomiting G4 | 0.19% | 0.19% | 0.57% | 0.00% | 0.00% | 1.14% |
| Group B (Intermediate and Poor Metabolizers) | ||||||
| Neutropenia G4 | 7.18% | 4.02% | 16.67% | 0.00% | 0.00% | 26.19% |
| Neutropenia G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Leukopenia G4 | 2.33% | 2.36% | 7.14% | 0.00% | 0.00% | 14.29% |
| Leukopenia G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Stomatitis G4 | 2.39% | 2.42% | 7.50% | 0.00% | 0.00% | 14.29% |
| Stomatitis G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Infection G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Infection G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Diarrhea G4 | 9.47% | 4.53% | 19.05% | 2.38% | 0.00% | 28.57% |
| Diarrhea G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Cardiovascular G4 | 2.40% | 2.31% | 7.14% | 0.00% | 0.00% | 16.67% |
| Cardiovascular G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Thrombocytopenia G4 | 2.38% | 2.37% | 7.14% | 0.00% | 0.00% | 14.29% |
| Thrombocytopenia G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Hepatic G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Acute_pancreatitis G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Hand_and_foot_syndrome G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Febrile neutropenia G4 | 7.10% | 4.01% | 16.67% | 0.00% | 0.00% | 21.43% |
| Hematochezia G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Neurological G4 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Nausea & Vomiting G4 | 2.35% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Differences (Group B – Group A) | ||||||
| Neutropenia G4 | 1.46% | 4.17% | 10.48% | −5.68% | −9.51% | 21.45% |
| Neutropenia G5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Leukopenia G4 | 1.76% | 2.39% | 6.95% | −1.14% | −2.08% | 13.72% |
| Leukopenia G5 | −0.19% | 0.19% | 0.00% | −0.57% | −1.52% | 0.00% |
| Stomatitis G4 | 1.82% | 2.45% | 7.13% | −1.14% | −1.89% | 13.91% |
| Stomatitis G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Infection G4 | −0.57% | 0.33% | 0.00% | −1.33% | −1.89% | 0.00% |
| Infection G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Diarrhea G4 | 7.76% | 4.56% | 17.72% | 0.11% | −3.22% | 27.62% |
| Diarrhea G5 | 2.38% | 2.33% | 7.14% | 0.00% | 0.00% | 14.29% |
| Cardiovascular G4 | 1.83% | 2.34% | 6.95% | −1.14% | −2.27% | 16.10% |
| Cardiovascular G5 | −0.19% | 0.19% | 0.00% | −0.57% | −1.14% | 0.00% |
| Thrombocytopenia G4 | 2.38% | 2.37% | 7.14% | 0.00% | 0.00% | 14.29% |
| Thrombocytopenia G5 | −0.19% | 0.19% | 0.00% | −0.57% | −1.52% | 0.00% |
| Hepatic G4 | −0.56% | 0.42% | 0.00% | −1.52% | −3.03% | 0.00% |
| Acute_pancreatitis G4 | −0.19% | 0.19% | 0.00% | −0.57% | −1.14% | 0.00% |
| Hand_and_foot_syndrome G4 | −0.38% | 0.26% | 0.00% | −0.95% | −1.52% | 0.00% |
| Febrile neutropenia G4 | 6.34% | 4.03% | 15.53% | −0.76% | −2.08% | 21.24% |
| Hematochezia G4 | −0.19% | 0.19% | 0.00% | −0.57% | −1.14% | 0.00% |
| Neurological G4 | −0.76% | 0.38% | −0.19% | −1.52% | −2.27% | 0.00% |
| Nausea & Vomiting G4 | 2.17% | 2.33% | 7.14% | −0.57% | −1.14% | 14.29% |
Abbreviations: SD, standard deviation; LCI, lower confidence interval; UCI, upper confidence interval; G, grade. Results were based on 5,000 non-parametric bootstrap experiments.
Figure 3.
Frequency of the Serious ADRs (Grade IV and V) per Study Group
Shown are group A (DPYD variants non-carriers, depicted in dark blue) and group B (carriers of any DPYD variants among DPYD c.2846A>T, DPYD∗2A, and DPYD∗13 and DPYD-HapB3, depicted in red). y axis depicts the percentage of the individuals, presenting with various adverse drug reactions, which as summarized in the x axis.
Discussion
Cancer is one of the leading causes of mortality and morbidity in both men and women in Italy and represents also a severe burden in economic terms. Most of the available information on cancer management-related costs refers to the direct medical expenditures for cancer treatment, rather than costs associated to treatment-related toxicity.28 In providing cancer care, chemotherapy-related toxicities affect cancer individuals’ quality of life, producing treatment delays and discomfort and resulting in large expenditures of medical care for treatment.
The present economic analysis performed in the Italian healthcare setting indicates that individuals without any of the DPYD c.2846A>T, DPYD∗2A, and DPYD∗13 and DPYD-HapB3 risk variant alleles (group A) accrued more QALYs and had a better clinical outcome, in terms of survival months from the beginning of chemotherapy, compared to those individuals that carry any one of the DPYD c.2846A>T, DPYD∗2A, and DPYD∗13 and DPYD-HapB3 risk variant alleles (group B), if treated with a FL-containing chemotherapy regimen. Specifically, the mean FL-related toxicity management cost associated with group A was estimated at €1,010, well below the average cost of group B (€3,712). These incremental costs were mainly driven by higher percentage of severe ADRs in group B compared to group A, notably G4 diarrhea and febrile neutropenia (see above).
These results are consistent with the limited published data on the cost effectiveness of genotyping the four DPYD variants listed in the guidelines (DPYD∗2A, DPYD∗13, DPYD c.2846A>T, and DPYD-HapB3). Such analyses regarding DPYD screening are hindered by the ethical and logistic issues to perform randomized clinical trials comparing the outcomes in DPYD-genotyped individuals compared to the outcomes for those treated with the standard of care. In addition, the required sample size to achieve a proper statistical power for these low-frequency risk alleles needs to be very large.
Among the parameters used for the evaluation of the benefit of pharmacogenomic screening is the number needed to genotype (NNG), defined as the number of individuals that must be screened for a certain genomic variant, to identify one individual that will develop a toxic event.29 The minor allele frequency of the variant of interest greatly impacts the NNG. When the frequency is low, NNG becomes high (100–1,000), negatively affecting the cost effectiveness of the genetic screening approach.30 However, there is not a fixed NNG cut-off able to define the cost effectiveness of a test. The severity of the impacted clinical events and the attendant costs relative to the cost of testing would also determine whether high NNG’s prohibited cost effectiveness, as in the case of HLA-related Steven-Johnsons reactions and toxic epidermal necrolysis.31 Among the four DPYD variants considered, three are indeed rare (0.2%–1.4% in white Europeans) while DPYD-HapB3 has a higher minor allele frequency (4.8%). As the low minor allele frequency of DPYD variant alleles makes forbiddingly expensive the screening of each DPYD gene variant individually, a multi-SNPs approach, combining all the four DPYD gene variants, conducted in the present study, raises the chances to bypass all the above-mentioned issues, resulting in a combined frequency of the variants of about 7%, similar to the frequency reported in other studies on Europeans.14
Two prospective clinical trials have been published applying an upfront FL dose adjustment based on the individuals’ DPYD genotype.3, 6 Genotype-guided dose-individualization significantly reduced the incidence of severe toxicity in the genotype-guided treatment cohort for both studies, demonstrating that the pre-treatment genotyping approach is feasible in the clinical practice and can spare the occurrence of severe ADRs. An economic analysis has been performed within the pharmacogenomic study by Deenen et al.,6 which referred, though, only to the DPYD∗2A variant. The average treatment cost per individual was demonstrated to be significantly lower in the individuals screened prior to drug prescription (€2,772) versus those individuals that received a standard of care treatment (€2,817), proven to be slightly cost effective.
In a simulation study, Cortejoso et al. provided evidence that screening for the three rarer DPYD variants (DPYD c.2846A>T, DPYD∗2A, and DPYD∗13) could be cost effective, provided that at least 2.21 case subjects of severe FL-induced neutropenia are avoided per 1,000 treated subjects.32 More recently, the pharmacoeconomic analysis of the prospective clinical trial NCT02324452 demonstrated a net cost saving of 51 Euros per individual if comparing a pre-treatment genotyping strategy for the four genetic variants currently included in the pharmacogenomic guidelines with the standard of treatment.33
This group previously reported the results of a cost survey demonstrating that the toxicity management costs are related to the individual’s genotype for specific pharmacogenomic variants related to toxicity risk, in a large group of individuals suffering from cancer treated according to the standard clinical practice.7 A recent retrospective study was also published in the Italian population providing proof of evidence that carriers of the four DPYD variants mentioned in this study have higher toxicity management costs than non-carriers. By including the UGT1A1∗28 genotype in individuals treated with an association of FL and irinotecan, the incremental cost between carriers and non-carriers further increased.8
Despite these very recent studies trying to address the issue of the FL-related toxicity costs according to the individual’s DPYD genotype, the effect of the genotype on the individual’s quality of life and survival was never investigated before. The present study demonstrated that the QALYs for wild-type DPYD individuals (group A) was estimated at 4.18 years, as opposed to 3.03 years for those carrying a heterozygous genotype for any DPYD genetic variants studied (group B). Thus, a difference of 1.16 years was observed between these two groups in favor of group A, but this difference was not statistically significant.
In this light, the clinical outcomes, the quality of life, and the costs associated with these two study groups are quite different from a health economic point of view.
The present analysis may suffer from certain limitations. At first, this analysis does not represent a head-to-head comparison but rather an observational study that compares two reasonably well-balanced study groups of individuals suffering from cancer. Since the results represent a combinational effect of several type of health technologies used, the analysis may incorporate a bias factor and thus we have to interpret the results with caution. In addition to that, individuals in group B are fewer compared to group A and this may further increase the probability of a potential estimating error.
Concerning utility weights, it is worth mentioning that they do not necessarily fully represent the actual population. Given the lack of data for this specific population, utility decrement for all ADRs was used based on the literature and expert opinions. A general comment that partly affects the present study is that in the related literature a considerable variation in reported utilities associated with measurement instrument still exist, while similar individuals frequently report different levels of quality of life.34
Finally, the analysis was conducted from a sickness fund perspective and not the society overall and thus only specific type of costs were extracted from the analysis. We also need to indicate that the cost of chemotherapy drug is not considered here, but only the cost of ADRs. Since policy makers are primarily interested in the value-for-money for specific health technologies, the identification and validation of appropriate predictive genomic biomarkers and genomic technologies must be a priority and potentially would help to enrich the responding population and increase cost effectiveness in the future.
The results must also be considered in the Italian setting only and on the basis of current resources and drug prices, but other similar studies have to be conducted to produce more decisive conclusions and to establish better treatment options for the individuals and other healthcare systems worldwide.
Conclusions
According to the analysis performed in the Italian healthcare setting, there are strong indications for survival differences between individuals that do not bear any of the high risk DPYD variants analyzed in this study (group A) and individuals that carry at least one of the same DPYD variants (group B) in favor of the former group taking also into consideration the quality of life of individuals. Additionally, the economic evaluation reported in this study provides further insights based on cost considerations. In particular, group A has significantly less total cost compared with group B, and thus represents a cost-saving option against its comparator. Further studies are needed in order to evaluate the cost, effectiveness, and quality of life for these individuals, since this evidence will further aid decision making to focus primarily on important cost drivers, such as DPYD genotype.
Declaration of Interests
The authors declare no conflict of interests.
Acknowledgments
This work was partly funded by a European Commission grant (H2020-668353; U-PGx) to C.M. and encouraged and coordinated by the Genomic Medicine Alliance Health Economics Working Group. Nothing contained in this paper is intended to guarantee the appropriateness of any medical treatment or to be used for therapeutic purposes or as a substitute for a health professional’s advice.
Published: May 30, 2019
Contributor Information
George P. Patrinos, Email: gpatrinos@upatras.gr.
Christina Mitropoulou, Email: c.mitropoulou@goldenhelix.org.
Web Resources
Clinical Pharmacogenetics Implementation Consortium, https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/
Italian Government Gazette, https://egas.sanita.fvg.it
OMIM, http://www.omim.org/
Pharmacogenomics Knowledgebase (PharmGKB), https://www.pharmgkb.org
Xeloda prescribing information (accessed at 17/11/2018), https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
References
- 1.Johnson M.R., Diasio R.B. Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with 5-fluorouracil. Adv. Enzyme Regul. 2001;41:151–157. doi: 10.1016/s0065-2571(00)00011-x. [DOI] [PubMed] [Google Scholar]; Johnson, M.R., and Diasio, R.B. (2001). Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with 5-fluorouracil. Adv. Enzyme Regul. 41, 151-157. [DOI] [PubMed]
- 2.Offer S.M., Diasio R.B. Is It Finally Time for a personalized medicine approach for Fluorouracil-based therapies? J. Clin. Oncol. 2016;34:205–207. doi: 10.1200/JCO.2015.64.2546. [DOI] [PubMed] [Google Scholar]; Offer, S.M., and Diasio, R.B. (2016). Is It Finally Time for a personalized medicine approach for Fluorouracil-based therapies? J. Clin. Oncol. 34, 205-207. [DOI] [PubMed]
- 3.Henricks L.M., Lunenburg C.A.T.C., de Man F.M., Meulendijks D., Frederix G.W.J., Kienhuis E., Creemers G.J., Baars A., Dezentjé V.O., Imholz A.L.T. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19:1459–1467. doi: 10.1016/S1470-2045(18)30686-7. [DOI] [PubMed] [Google Scholar]; Henricks, L.M., Lunenburg, C.A.T.C., de Man, F.M., Meulendijks, D., Frederix, G.W.J., Kienhuis, E., Creemers, G.J., Baars, A., Dezentje, V.O., Imholz, A.L.T., et al. (2018). DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 19, 1459-1467. [DOI] [PubMed]
- 4.Lee A.M., Shi Q., Pavey E., Alberts S.R., Sargent D.J., Sinicrope F.A., Berenberg J.L., Goldberg R.M., Diasio R.B. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147) J. Natl. Cancer Inst. 2014;106:dju298. doi: 10.1093/jnci/dju298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, A.M., Shi, Q., Pavey, E., Alberts, S.R., Sargent, D.J., Sinicrope, F.A., Berenberg, J.L., Goldberg, R.M., and Diasio, R.B. (2014). DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147). J. Natl. Cancer Inst. 106, dju298. [DOI] [PMC free article] [PubMed]
- 5.Toffoli G., Giodini L., Buonadonna A., Berretta M., De Paoli A., Scalone S., Miolo G., Mini E., Nobili S., Lonardi S. Clinical validity of a DPYD-based pharmacogenetic test to predict severe toxicity to fluoropyrimidines. Int. J. Cancer. 2015;137:2971–2980. doi: 10.1002/ijc.29654. [DOI] [PubMed] [Google Scholar]; Toffoli, G., Giodini, L., Buonadonna, A., Berretta, M., De Paoli, A., Scalone, S., Miolo, G., Mini, E., Nobili, S., Lonardi, S., et al. (2015). Clinical validity of a DPYD-based pharmacogenetic test to predict severe toxicity to fluoropyrimidines. Int. J. Cancer 137, 2971-2980. [DOI] [PubMed]
- 6.Deenen M.J., Meulendijks D., Cats A., Sechterberger M.K., Severens J.L., Boot H., Smits P.H., Rosing H., Mandigers C.M., Soesan M. Upfront genotyping of DPYD∗2A to individualize fluoropyrimidine therapy: A safety and cost analysis. J. Clin. Oncol. 2016;34:227–234. doi: 10.1200/JCO.2015.63.1325. [DOI] [PubMed] [Google Scholar]; Deenen, M.J., Meulendijks, D., Cats, A., Sechterberger, M.K., Severens, J.L., Boot, H., Smits, P.H., Rosing, H., Mandigers, C.M., Soesan, M., et al. (2016). Upfront genotyping of DPYD∗2A to individualize fluoropyrimidine therapy: A safety and cost analysis. J. Clin. Oncol. 34, 227-234. [DOI] [PubMed]
- 7.Roncato R., Cecchin E., Montico M., De Mattia E., Giodini L., Buonadonna A., Solfrini V., Innocenti F., Toffoli G. Cost Evaluation of Irinotecan-Related Toxicities Associated With the UGT1A1∗28 Patient Genotype. Clin. Pharmacol. Ther. 2017;102:123–130. doi: 10.1002/cpt.615. [DOI] [PubMed] [Google Scholar]; Roncato, R., Cecchin, E., Montico, M., De Mattia, E., Giodini, L., Buonadonna, A., Solfrini, V., Innocenti, F., and Toffoli, G. (2017). Cost Evaluation of Irinotecan-Related Toxicities Associated With the UGT1A1∗28 Patient Genotype. Clin. Pharmacol. Ther. 102, 123-130. [DOI] [PubMed]
- 8.Toffoli, G., Innocenti, F., Polesel, J., et al. The genotype for DPYD risk variants in patients with colorectal cancer and the related toxicity management costs in clinical practice. Clin. Pharmacol. Ther. 105, 994–1002. [DOI] [PubMed]
- 9.Byford S., Raftery J. Perspectives in economic evaluation. BMJ. 1998;316:1529–1530. doi: 10.1136/bmj.316.7143.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]; Byford, S., and Raftery, J. (1998). Perspectives in economic evaluation. BMJ 316, 1529-1530. [DOI] [PMC free article] [PubMed]
- 10.Toffoli G., Cecchin E., Corona G., Russo A., Buonadonna A., D’Andrea M., Pasetto L.M., Pessa S., Errante D., De Pangher V. The role of UGT1A1∗28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]; Toffoli, G., Cecchin, E., Corona, G., Russo, A., Buonadonna, A., D’Andrea, M., Pasetto, L.M., Pessa, S., Errante, D., De Pangher, V., et al. (2006). The role of UGT1A1∗28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 24, 3061-3068. [DOI] [PubMed]
- 11.Cecchin E., D’Andrea M., Lonardi S., Zanusso C., Pella N., Errante D., De Mattia E., Polesel J., Innocenti F., Toffoli G. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 2013;13:403–409. doi: 10.1038/tpj.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cecchin, E., D’Andrea, M., Lonardi, S., Zanusso, C., Pella, N., Errante, D., De Mattia, E., Polesel, J., Innocenti, F., and Toffoli, G. (2013). A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 13, 403-409. [DOI] [PMC free article] [PubMed]
- 12.Lousdal M.L., Kristiansen I.S., Møller B., Støvring H. Predicting mean survival time from reported median survival time for cancer patients. Med. Decis. Making. 2017;37:391–402. doi: 10.1177/0272989X16655341. [DOI] [PubMed] [Google Scholar]; Lousdal, M.L., Kristiansen, I.S., Moller, B., and Stovring, H. (2017). Predicting mean survival time from reported median survival time for cancer patients. Med. Decis. Making 37, 391-402. [DOI] [PubMed]
- 13.Wong W., Yim Y.M., Kim A., Cloutier M., Gauthier-Loiselle M., Gagnon-Sanschagrin P., Guerin A. Assessment of costs associated with adverse events in patients with cancer. PLoS ONE. 2018;13:e0196007. doi: 10.1371/journal.pone.0196007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wong, W., Yim, Y.M., Kim, A., Cloutier, M., Gauthier-Loiselle, M., Gagnon-Sanschagrin, P., and Guerin, A. (2018). Assessment of costs associated with adverse events in patients with cancer. PLoS ONE 13, e0196007. [DOI] [PMC free article] [PubMed]
- 14.Murphy C., Byrne S., Ahmed G., Kenny A., Gallagher J., Harvey H., O’Farrell E., Bird B. Cost implications of reactive versus prospective testing for dihydropyrimidine dehydrogenase deficiency in patients with colorectal cancer: A single-institution experience. Dose Resp. 2018;16 doi: 10.1177/1559325818803042. [DOI] [PMC free article] [PubMed] [Google Scholar]; Murphy, C., Byrne, S., Ahmed, G., Kenny, A., Gallagher, J., Harvey, H., O’Farrell, E., and Bird, B. (2018). Cost implications of reactive versus prospective testing for dihydropyrimidine dehydrogenase deficiency in patients with colorectal cancer: A single-institution experience. Dose Resp. 16, [DOI] [PMC free article] [PubMed]
- 15.Huang Y. Cost analysis with censored data. Med. Care. 2009;47(7, Suppl 1):S115–S119. doi: 10.1097/MLR.0b013e31819bc08a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huang, Y. (2009). Cost analysis with censored data. Med. Care 47(7, Suppl 1) S115-S119. [DOI] [PMC free article] [PubMed]
- 16.Wijeysundera H.C., Wang X., Tomlinson G., Ko D.T., Krahn M.D. Techniques for estimating health care costs with censored data: an overview for the health services researcher. Clinicoecon. Outcomes Res. 2012;4:145–155. doi: 10.2147/CEOR.S31552. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wijeysundera, H.C., Wang, X., Tomlinson, G., Ko, D.T., and Krahn, M.D. (2012). Techniques for estimating health care costs with censored data: an overview for the health services researcher. Clinicoecon. Outcomes Res. 4, 145-155. [DOI] [PMC free article] [PubMed]
- 17.Bang H., Tsiatis A.A. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]; Bang, H., and Tsiatis, A.A. (2000). Estimating medical costs with censored data. Biometrika 87, 329-343.
- 18.Barber J., Thompson S. Multiple regression of cost data: use of generalised linear models. J. Health Serv. Res. Policy. 2004;9:197–204. doi: 10.1258/1355819042250249. [DOI] [PubMed] [Google Scholar]; Barber, J., and Thompson, S. (2004). Multiple regression of cost data: use of generalised linear models. J. Health Serv. Res. Policy 9, 197-204. [DOI] [PubMed]
- 19.Kurz C.F. Tweedie distributions for fitting semicontinuous health care utilization cost data. BMC Med. Res. Methodol. 2017;17:171. doi: 10.1186/s12874-017-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kurz, C.F. (2017). Tweedie distributions for fitting semicontinuous health care utilization cost data. BMC Med. Res. Methodol. 17, 171. [DOI] [PMC free article] [PubMed]
- 20.Lin D.Y. Regression analysis of incomplete medical cost data. Stat. Med. 2003;22:1181–1200. doi: 10.1002/sim.1377. [DOI] [PubMed] [Google Scholar]; Lin, D.Y. (2003). Regression analysis of incomplete medical cost data. Stat. Med. 22, 1181-1200. [DOI] [PubMed]
- 21.Campbell M.K., Torgerson D.J. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM. 1999;92:177–182. doi: 10.1093/qjmed/92.3.177. [DOI] [PubMed] [Google Scholar]; Campbell, M.K., and Torgerson, D.J. (1999). Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM 92, 177-182. [DOI] [PubMed]
- 22.Ayvaci M.U., Shi J., Alagoz O., Lubner S.J. Cost-effectiveness of adjuvant FOLFOX and 5FU/LV chemotherapy for patients with stage II colon cancer. Med. Decis. Making. 2013;33:521–532. doi: 10.1177/0272989X12470755. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ayvaci, M.U., Shi, J., Alagoz, O., and Lubner, S.J. (2013). Cost-effectiveness of adjuvant FOLFOX and 5FU/LV chemotherapy for patients with stage II colon cancer. Med. Decis. Making 33, 521-532. [DOI] [PMC free article] [PubMed]
- 23.Paracha N., Abdulla A., MacGilchrist K.S. Systematic review of health state utility values in metastatic non-small cell lung cancer with a focus on previously treated patients. Health Qual. Life Outcomes. 2018;16:179. doi: 10.1186/s12955-018-0994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paracha, N., Abdulla, A., and MacGilchrist, K.S. (2018). Systematic review of health state utility values in metastatic non-small cell lung cancer with a focus on previously treated patients. Health Qual. Life Outcomes 16, 179. [DOI] [PMC free article] [PubMed]
- 24.Stahmeyer J.T., Rossol S., Liersch S., Guerra I., Krauth C. Cost-Effectiveness of Treating Hepatitis C with Sofosbuvir/Ledipasvir in Germany. PLoS ONE. 2017;12:e0169401. doi: 10.1371/journal.pone.0169401. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stahmeyer, J.T., Rossol, S., Liersch, S., Guerra, I., and Krauth, C. (2017). Cost-Effectiveness of Treating Hepatitis C with Sofosbuvir/Ledipasvir in Germany. PLoS ONE 12, e0169401. [DOI] [PMC free article] [PubMed]
- 25.Stein J.D., Brown G.C., Brown M.M., Sharma S., Hollands H., Stein H.D. The quality of life of patients with hypertension. J. Clin. Hypertens. (Greenwich) 2002;4:181–188. doi: 10.1111/j.1524-6175.2002.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stein, J.D., Brown, G.C., Brown, M.M., Sharma, S., Hollands, H., and Stein, H.D. (2002). The quality of life of patients with hypertension. J. Clin. Hypertens. (Greenwich) 4, 181-188. [DOI] [PMC free article] [PubMed]
- 26.Lloyd A., Nafees B., Narewska J., Dewilde S., Watkins J. Health state utilities for metastatic breast cancer. Br. J. Cancer. 2006;95:683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lloyd, A., Nafees, B., Narewska, J., Dewilde, S., and Watkins, J. (2006). Health state utilities for metastatic breast cancer. Br. J. Cancer 95, 683-690. [DOI] [PMC free article] [PubMed]
- 27.Malinowski K.P., Kawalec P. Health utility of patients with Crohn’s disease and ulcerative colitis: a systematic review and meta-analysis. Expert Rev. Pharmacoecon. Outcomes Res. 2016;16:441–453. doi: 10.1080/14737167.2016.1190644. [DOI] [PubMed] [Google Scholar]; Malinowski, K.P., and Kawalec, P. (2016). Health utility of patients with Crohn’s disease and ulcerative colitis: a systematic review and meta-analysis. Expert Rev. Pharmacoecon. Outcomes Res. 16, 441-453. [DOI] [PubMed]
- 28.Calhoun E.A., Chang C.H., Welshman E.E., Fishman D.A., Lurain J.R., Bennett C.L. Evaluating the total costs of chemotherapy-induced toxicity: results from a pilot study with ovarian cancer patients. Oncologist. 2001;6:441–445. doi: 10.1634/theoncologist.6-5-441. [DOI] [PubMed] [Google Scholar]; Calhoun, E.A., Chang, C.H., Welshman, E.E., Fishman, D.A., Lurain, J.R., and Bennett, C.L. (2001). Evaluating the total costs of chemotherapy-induced toxicity: results from a pilot study with ovarian cancer patients. Oncologist 6, 441-445. [DOI] [PubMed]
- 29.Tonk E.C.M., Gurwitz D., Maitland-van der Zee A.H., Janssens A.C.J.W. Assessment of pharmacogenetic tests: presenting measures of clinical validity and potential population impact in association studies. Pharmacogenomics J. 2017;17:386–392. doi: 10.1038/tpj.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tonk, E.C.M., Gurwitz, D., Maitland-van der Zee, A.H., and Janssens, A.C.J.W. (2017). Assessment of pharmacogenetic tests: presenting measures of clinical validity and potential population impact in association studies. Pharmacogenomics J. 17, 386-392. [DOI] [PMC free article] [PubMed]
- 30.Swen J.J., Nijenhuis M., van Rhenen M., de Boer-Veger N.J., Buunk A.M., Houwink E.J.F., Mulder H., Rongen G.A., van Schaik R.H.N., van der Weide J., Dutch Pharmacogenetics Working Group (DPWG) of the Royal Dutch Pharmacists Association (KNMP) Pharmacogenetic Information in Clinical Guidelines: The European Perspective. Clin. Pharmacol. Ther. 2018;103:795–801. doi: 10.1002/cpt.1049. [DOI] [PubMed] [Google Scholar]; Swen, J.J., Nijenhuis, M., van Rhenen, M., de Boer-Veger, N.J., Buunk, A.M., Houwink, E.J.F., Mulder, H., Rongen, G.A., van Schaik, R.H.N., van der Weide, J., et al.; Dutch Pharmacogenetics Working Group (DPWG) of the Royal Dutch Pharmacists Association (KNMP) (2018). Pharmacogenetic Information in Clinical Guidelines: The European Perspective. Clin. Pharmacol. Ther. 103, 795-801. [DOI] [PubMed]
- 31.Hughes D.A., Plumpton C.O. Rare disease prevention and treatment: the need for a level playing field. Pharmacogenomics. 2018;19:243–247. doi: 10.2217/pgs-2017-0300. [DOI] [PubMed] [Google Scholar]; Hughes, D.A., and Plumpton, C.O. (2018). Rare disease prevention and treatment: the need for a level playing field. Pharmacogenomics 19, 243-247. [DOI] [PubMed]
- 32.Cortejoso L., García-González X., García M.I., García-Alfonso P., Sanjurjo M., López-Fernández L.A. Cost-effectiveness of screening for DPYD polymorphisms to prevent neutropenia in cancer patients treated with fluoropyrimidines. Pharmacogenomics. 2016;17:979–984. doi: 10.2217/pgs-2016-0006. [DOI] [PubMed] [Google Scholar]; Cortejoso, L., Garcia-Gonzalez, X., Garcia, M.I., Garcia-Alfonso, P., Sanjurjo, M., and Lopez-Fernandez, L.A. (2016). Cost-effectiveness of screening for DPYD polymorphisms to prevent neutropenia in cancer patients treated with fluoropyrimidines. Pharmacogenomics 17, 979-984. [DOI] [PubMed]
- 33.Henricks L.M., Lunenburg C.A.T.C., de Man F.M., Meulendijks D., Frederix G.W.J., Kienhuis E., Creemers G.J., Baars A., Dezentjé V.O., Imholz A.L.T. A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur. J. Cancer. 2019;107:60–67. doi: 10.1016/j.ejca.2018.11.010. [DOI] [PubMed] [Google Scholar]; Henricks, L.M., Lunenburg, C.A.T.C., de Man, F.M., Meulendijks, D., Frederix, G.W.J., Kienhuis, E., Creemers, G.J., Baars, A., Dezentje, V.O., Imholz, A.L.T., et al. (2019). A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur. J. Cancer 107, 60-67. [DOI] [PubMed]
- 34.Djalalov S., Rabeneck L., Tomlinson G., Bremner K.E., Hilsden R., Hoch J.S. A Review and Meta-analysis of Colorectal Cancer Utilities. Med. Decis. Making. 2014;34:809–818. doi: 10.1177/0272989X14536779. [DOI] [PubMed] [Google Scholar]; Djalalov, S., Rabeneck, L., Tomlinson, G., Bremner, K.E., Hilsden, R., and Hoch, J.S. (2014). A Review and Meta-analysis of Colorectal Cancer Utilities. Med. Decis. Making 34, 809-818. [DOI] [PubMed]