Abstract
Background
Despite the success of antiretroviral therapy (ART), latent HIV-1 continues to persist in a long-lived population of resting memory CD4+ T cells within those who are infected. Finding a safe and effective means to induce latency reversal (LR) during ART to specifically expose this latent HIV-1 cellular reservoir for immune elimination has been a major barrier to a functional cure.
Methods
In this study, we test the use of antigen-presenting type 1-polarized, monocyte-derived dendritic cells (MDC1) generated from chronic HIV-1-infected individuals on ART as a means to induce HIV-1 latency reversal in autologous CD4+ T cells harboring replication-competent provirus. We use the same MDC1 for ex-vivo generation of autologous HIV-1 antigen-specific CD8+ cytotoxic T cells (CTL) and test their effector responses against the MDC1-exposed HIV-1- infected CD4+ T cell targets.
Findings
MDC1 presentation of either HIV-1 or cytomegalovirus (CMV) antigens to CD4+ T cells facilitated HIV-1 LR. This antigen-driven MDC1-mediated LR was sharply diminished with blockade of the CD40L/CD40 ‘helper’ signaling pathway. Importantly, these antigen-presenting MDC1 also activated the expansion of CTL capable of killing the exposed HIV-1-infected targets.
Interpretation
Inclusion of virus-associated MHC class II ‘helper’ antigens in MDC1-based HIV-1 immunotherapies could serve both as a targeted means to safely unmask antigen-specific CD4+ T cells harboring HIV-1, and to support CTL responses that can effectively target the MDC1-exposed HIV-1 cellular reservoir as a functional cure strategy.
Fund
This study was supported by the NIH-NAID grants R21-AI131763, U01-AI35041, UM1-AI126603, and T32-AI065380.
Keywords: HIV-1 latency reversal, Dendritic cells, Cytomegalovirus, T cells, CD40 ligand, Immunotherapy
Research in context.
Evidence before this study
Finding a nontoxic, effective means to purge the latent HIV-1 reservoir remains a major obstacle to a functional cure. The ‘kick and kill’ approach to controlling HIV-1 involves inducing HIV-1 latency reversal (LR) during antiretroviral therapy (ART) to expose infected cells, while creating an arsenal of immune effector cells, such as cytotoxic T lymphocytes (CTL), capable of eliminating these targets. While pharmacological latency reversal agents (LRAs) have achieved limited success in ex vivo studies, they have yet to be found effective at reducing the latent reservoir in HIV-1-infected individuals. In addition, some LRAs have been shown to negatively impact antigen-specific CD8+ T cell effector responses in vitro. Besides latency itself, major hurdles for effective CTL elimination of HIV-1 infected cells include issues related to CD8+ T cell exhaustion, alterations in CTL epitopes, antigen processing, and antigen presentation associated with immune escape; the establishment of epitope variants that act as partial agonists to induce dysfunctional noncytolytic cross-reactive memory CTL responses, and presentation of target antigen decoys by cells harboring defective virus. Thus, an optimal cure strategy must address not only induction of proviral gene expression but also clearance of reactivated cells presenting HIV-1-associated peptide epitopes by either highly functional CTL, or through incorporation of other immune-based strategies, including vaccines and adjuvants, broadly neutralizing antibodies, or compounds modulating pro-apoptotic pathways.
Added value of this study
Conventional dendritic cells (DC) have been safely and widely used in both cancer and HIV-1 clinical trials for their capacity to induce antigen-specific T cell responses, but their HIV-1 LRA potential has been underexplored. Though not designed to specifically address their function as a therapeutic LRA, a recent study suggested a link between administration of a DC-based HIV-1 vaccine and increased residual viremia in ART-suppressed individuals prior to analytic treatment interruption. Here we report that antigen-presenting pro-inflammatory type 1-polarized monocyte-derived dendritic cells (MDC1) generated from chronic HIV-1-infected individuals on ART can induce HIV-1 LR in autologous CD4+ T cells. Importantly, this effect of driving the virus out of latency was facilitated through the strategic use both CMV- and HIV-associated antigen to specifically promote MHC-class II antigen restricted interactions between CD4+ T cells responsive to these pathogens and MDC1. Furthermore, we demonstrate the potential of this single MDC1-based therapeutic tool to promote both the antigen-specific exposure (the ‘kick’) and CTL killing (the ‘kill’) of CD4+ T cells harboring replication-competent provirus.
Implications of all the available evidence
To date, clinical trials of pharmacological LRAs have demonstrated minimal or no reduction in the latent reservoir in vivo. Deliberately programmed to release high amounts of the critical CTL-inducing cytokine IL-12p70 upon interaction with the CD4+ T helper cell factor CD40L, clinically applicable MDC1 are uniquely capable of both activating HIV-1 transcription in latently infected CD4+ T cells and inducing broad HIV-1-specific CTL responses. Strategic inclusion of virus-associated MHC class II ‘helper’ antigens in MDC1-based HIV-1 immunotherapies could serve both as a targeted means to safely unmask virus antigen-specific CD4+ T cells harboring HIV-1, and to support CTL responses that can effectively target the MDC1-exposed HIV-1 reservoir as a functional cure strategy.
Alt-text: Unlabelled Box
1. Introduction
Despite the success of antiretroviral therapy (ART), HIV-1 is managed as a chronic disease due to its persistence in a long-lived population of resting memory CD4+ T cells [1]. This latent reservoir of inducible, replication-competent HIV-1 in ART-suppressed individuals is considered a critical barrier to a cure [2], since the lack of viral protein expression in latently infected cells allows the reservoir to escape immune surveillance. The ‘kick and kill’ (or ‘shock and kill’) approach to controlling HIV-1 involves inducing HIV-1 latency reversal (LR) during ART to expose infected cells, while creating an arsenal of immune effector cells, such as cytotoxic T lymphocytes (CTL), capable of eliminating these targets [3]. Successful CTL targeting of the latent HIV-1 reservoir will require recognition of HIV-1-associated peptide epitopes presented on infected cells after LR. Besides latency itself, major hurdles for effective CTL elimination of HIV-1 infected cells include issues related to CD8+ T cell exhaustion [4], alterations in CTL epitopes, antigen processing, and antigen presentation associated with immune escape [5,6]; the establishment of epitope variants that act as partial agonists to induce dysfunctional noncytolytic cross-reactive memory CTL responses [7,8], and presentation of target antigen decoys by cells harboring defective virus [9]. Together, these points highlight the need to generate highly functional CTL either through induction of de novo CD8+ T cell responses, or subdominant memory responses targeting relevant conserved epitopes of the reservoir-associated virus.
Finding an effective means to expose and purge the latent reservoir in a nontoxic manner has been elusive and remains a major hurdle to this cure approach. While pharmacological latency reversal agents (LRAs) have achieved limited success in ex vivo studies, none have been shown to reduce the latent reservoir in HIV-1-infected individuals [10]. Although the protein kinase C (PKC) agonist bryostatin-1 was shown to achieve T cell activation comparable to that induced by PHA/Il-2 stimulation in vitro and ex vivo [10,11], its use in cancer clinical trials resulted in serious adverse events [[12], [13], [14], [15]]. Furthermore, in a phase I HIV-1 clinical trial, tolerable but conservative drug dosing of bryostatin-1 prevented it from reaching detectable systemic concentrations associated with PKC activation and from reactivating latent HIV-1 reservoirs [16].
Some LRAs, specifically histone deacetylase inhibitors and PKC modulators, can negatively impacting antigen-specific CD8+ T cell effector responses in vitro [11,17,18]. However, it is unclear if this negative effect on HIV-1-specific immunity occurs with their use in vivo. More promising data, have emerged from studies utilizing TLR agonists as LRAs, including those targeting TLR7 [[19], [20], [21], [22]]. This strategy to target such germline-encoded innate immune activation receptors effectively contributes to impacting the latent reservoir and delaying viral rebound In nonhuman primate models, particularly when combined either with therapeutic vaccination using Ad26/MVA (recombinant adenovirus serotype 26 prime/modified vaccinia Ankara boost) expressing gag, pol and env [20], or with HIV-broadly neutralizing antibody (bNAb) therapy [21]. While promising, the potential for toxicity associated with TLR-induced broad activation of innate immune cells exists. These findings support the need to continue exploring eradication strategies to reverse HIV latency without inducing nonspecific global immune activation [23], while enhancing HIV antigen-specific adaptive immunity.
Although conventional dendritic cells (DC) have been safely used in HIV-1 clinical trials for their capacity to induce antigen-specific T cell responses [[24], [25], [26], [27]], their HIV-1 LRA potential has been underexplored. Interestingly, a study from our group suggested a link between administration of a DC-based HIV-1 therapeutic and increased residual viremia in ART-suppressed individuals prior to analytic treatment interruption [28]. However, that study was not designed to specifically address the use of the DC therapeutic as an LRA. Thus a number of important questions remain, including the roles that DC polarization status and antigen presentation could have in the noted phenomenon, and the underlying mechanisms involved.
We hypothesize that under optimal conditions, a DC-based therapeutic strategy can be designed to safely facilitate both the ‘kick’ and ‘kill’ of the latent HIV-1 reservoir. Here, we show that clinically applicable, type 1-polarized, monocyte-derived DC (MDC1) are uniquely capable of both activating HIV-1 transcription in latently infected CD4+ T cells harboring replication-competent virus and inducing broad HIV-1-specific CTL responses that can effectively target the exposed infected cells. To promote strong HIV-1-specific CTL responses, these antigen-presenting MDC1 are deliberately programmed to subsequently release high amounts of the critical CTL-inducing cytokine IL-12p70 upon interaction with the CD4+ T helper (TH) cell factor CD40L [29]. We found that the strategic inclusion of heterologous cytomegalovirus (CMV)- associated antigen designed to encourage such CD4+ T cell ‘helper’ activity through MHC class II presentation also facilitated MDC1-mediated LR. The demonstrated antigen-dependent LRA activity of CMV and HIV-1 antigen-presenting MDC1 suggests that CD4+ T cells having antigen specificity to these viruses contribute to the latent reservoir, thus offering a safe and directed means to immunologically expose and target this compartment as part of a functional cure strategy for HIV-1 infection.
2. Materials and methods
2.1. Study participants
HIV-1-infected ART-treated participants of the Pittsburgh clinical site of the Multicenter AIDS Cohort Study (MACS) were selected for this research. These participants were documented as having begun ART with a median virally controlled treatment duration of 12.3 years (range 1.7–20.8 years; Table S1). Whole blood products from HIV-1-negative blood donors were purchased from the Central Blood Bank of Pittsburgh. Written informed consent was obtained from participants prior to inclusion in the study. The University of Pittsburgh Institutional Review Board approved this study.
2.2. Isolation of monocytes and peripheral blood lymphocytes
Peripheral blood mononuclear cells (PBMC) obtained from buffy coat or whole blood were isolated by standard density gradient separation using Lymphocyte Separation Medium (Corning Cat# 25–072-CV). PBMC were further separated into monocytes and peripheral blood lymphocytes (PBL) using a positive selection human CD14 microbeads kit (Miltenyi Biotec Cat# 130–05-201; RRID: AB_2665482) according to manufacturer's specifications, and the differentially isolated cells were cryopreserved until use.
2.3. Generation of monocyte-derived DC
Immature DC were generated from monocytes isolated and cultured for 7 days in Iscove's Modified Dulbecco's Media (IMDM; Gibco Cat# 12440-053) containing 10% fetal bovine serum Atlanta biologicals Cat# S12450H) and 0.5% gentamicin (Gibco Cat# 15710-064) in the presence of granulocyte-monocyte colony-stimulating factor (GM-CSF; 1000 IU/mL; Sanofi-aventis Cat# NAC2004–5843-01) and interleukin-4 (IL-4; 1000 IU/mL; R&D Systems Cat# 204-1 L). As previously described [29], mature, high IL-12p70-producing MDC1 and IL-12p70 deficient, prostaglandin E2-treated DC (PGE2-DC) were generated as previously described [29] by exposure of immature DC cultures at day 5 for 48 h to a cocktail of maturation factors containing either interferon (IFN)-α (1000 U/mL; Schering Corporation Cat# NDC:0085–1110-01), IFN-γ (1000 U/mL; R&D Systems Cat# 285-1F), IL-1β (10 ng/mL; R&D Systems #201-LB), tumor necrosis factor (TNF)-α (25 ng/mL; R&D Systems Cat# 210-TA), and polyinosinic:polycytidylic acid (20 ng/mL; Sigma-Aldrich Cat# P9582-5MG), or IL-1β (10 ng/mL), TNF-α (25 ng/mL), IL-6 (1000 U/mL; R&D Systems Cat# 206–1 L), and PGE2 (2 μM; Sigma-Aldrich Cat# P6532-1MG), respectively.
2.4. Flow cytometry
Phenotypic characterization of DC was determined by flow cytometry using cells stained with the following antibodies: CD14-PE (clone TÜK4; Miltenyi Biotec Cat# 130-098-067; RRID: AB_2660171), CD83-PE (clone HB15A; Beckman Coulter Cat# IM2218U), CD86-PE (clone HA5.2B7; Beckman Coulter Cat# IM2729U), CCR7-FITC (clone 150503; R&D Systems Cat# FAB197F; RRID: AB_2259847), OX40L-PE (clone ik-1; BD Biosciences Cat# 558164; RRID: AB_647195), Siglec-1/CD169-Alexa Fluor® 488 (clone 7-239; Bio-Rad Cat# MCA2517A488T; RRID: 2286027), CD209-APC (clone DCN46; BD Biosciences Cat# 551545; RRID: AB_647161), and HLA-DR-APC-Cy7 (clone L243; Biolegend Cat# 307618). For surface staining, cells were preincubated with 1× PBS labeling buffer containing 2% BSA, 0.1% NaN3, and unfractionated murine IgG (1.0 μg/mL; Sigma-Aldrich Cat# 15381-1MG) to block Fc-receptor binding. CD4+ T cells cocultured with MDC1 were tested weekly for the presence of HIV-1 p24 by surface staining for CD3 (APC-H7, clone SK7; BD Biosciences Cat# 641397; RRID: AB_1645731) and CD4 (Pacific Blue, clone RPA-T4; BD Biosciences Cat# 558116; RRID: AB_397037), and intracellular staining with KC57-FITC antibody (clone FH190–1-1, Beckman Coulter Cat# 6604665). Antigen-specific CTL responses were assessed by exposing CTL to HIV-1 Gag 9-mer peptides (1 μg/mL) or media alone, and incubating with CD107a-FITC (clone H4A3; BD Biosciences Cat# 555800; RRID: AB_396134) stain mix containing 0.1% monensin (BD Golgi Stop™, BD Biosciences Cat# 554724) for six hours at 37 °C. Cells were then stained for viability (LIVE/DEAD™ Fixable Aqua Dead Cell Stain, Life Technologies Cat# L34957), surface expression of CD3 (APC-H7, clone SK7; BD Biosciences) and CD8 (PerCP-Cy5.5, clone SK1; BD Biosciences Cat# 341051; RRID: AB_400298), and intracellular expression of IFN-γ (IFN-γ-AlexaFluor® 700, clone B27; BD Biosciences Cat# 557995; RRID: AB_396977).
2.5. Functional characterization of differentially matured DC
DC production of IL-12p70 in response to CD40L-transfected J558 cells (J558-CD40L; a gift from Dr. P. Lane, Birmingham, UK) stimulation was determined as previously described [7]. Briefly, DC were plated (2.5 × 104 cells/well) in a 96-well flat-bottom plate and stimulated with J558-CD40L (5 × 104 cells/well) for 24 h. Culture supernatants were collected and tested by IL-12p70 ELISA using the following reagents: Recombinant Human IL-12 Standard (R&D Systems Cat# 219-IL-005), Primary Human IL-12 mAb (Thermo Scientific Cat#M122), Secondary Human IL-12 mAb, Biotin-labeled (Thermo Scientific Cat# M121B), HRP-conjugated Streptavidin (Thermo Scientific Cat# N100), TMB Substrate Solution (Thermo Scientific Cat# N301), Stop Solution (Thermo Scientific Cat# N600).
2.6. Induction of HIV-1 LR in CD4+ T cells
MDC1 were tested for their ability to induce HIV-1 LR by coculture with autologous CD4+ T cells in the absence or presence of SEB (Sigma-Aldrich Cat #S4481), CMV pp65 (CMVpp65 Recombinant Protein, Miltenyi Biotec Cat# 130-091-824, or PepTivator® CMV pp65, Miltenyi Biotec Cat# 130-093-435), HIV-1 Gag (HIV-1 IIIB PR55 Gag Recombinant Protein, NIH AIDS Reagent Program Cat# 3276; HIV-1 Gag Recombinant Protein, Sigma-Aldrich Cat# H 0160; or HIV-1 Consensus 15-mer Peptides (Sigma-Aldrich), or influenza M1 antigen (Influenza M1 Protein (A/California/04/2009) (H1N1), eEnzyme Cat# IA-M1-023P, or PepTivator® Influenza A (H1N1) MP1, Miltenyi Biotec Cat# 130-097-285). Briefly, total CD4+ T cells were isolated from cryopreserved PBL derived from HIV-1-infected MACS participants by negative magnetic bead separation using an EasySep™ Human CD4+ T Cell Enrichment Kit (STEMCELL Technologies Cat# 19052). CD4+ T cells were cocultured with DC in complete IMDM at a ratio of either 1:7 (100,000 DC: 750,000 CD4+ T cells) or 1:10 (100,000:1 × 106) for seven days in 48-well plates. Total CD4+ T cells from HIV-1-infected MACS participants were treated with Dynabeads® Human T-Activator CD3/CD28 (Life Technologies Cat# 11131D) and implemented as a positive control in LR experiments. The cytokines rhIL-2 (Proleukin®, 100 U/mL; Prometheus Laboratories, Inc. Cat# NDC65483-116-07) and rhIL-7 (1 μg/mL; Miltenyi Biotec Cat# 130-095-361) were added to the cultures on day 4, and culture supernatants were harvested on day 7 for quantitation of HIV-1 RNA. Where stated, cocultures were maintained and the T cells tested for intracellular expression of p24 on days 14-20 by flow cytometry. CD40L blocking antibody (clone MK13A4, 10 μg/mL; Enzo Life Sciences Cat# ALX-805-037-C100; RRID: AB_2076315) or Leaf™ Purified Mouse IgG1,k isotype antibody (clone MG1-45, 10 μg/mL; Biolegend Cat# 401404) was used where shown.
2.7. Relative quantification of HIV-1 gag RNA
Culture supernatants were ultra-centrifuged (Sorvall Stratos Biofuge) at 45,000 x g for 1 h at 4 °C to obtain viral pellets from which total RNA was isolated by the RNA-Bee™ method (TEL-TEST, Inc. Cat# CS-105B). Five microliters of RNA were used for detection of reverse transcription using TaqMan® Reverse Transcription Reagents (Life Technologies Cat# N8080234) according to the manufacture's protocol. A 20 μL TaqMan® PCR was performed by mixing 5 μL cDNA with TaqMan® Universal PCR Master Mix (Thermo Fisher Cat# 4364340), 500 nM each of forward (5′-CCCATGTTTTCAGCATTATCAGAA-3′, Integrated DNA Technologies) and reverse primers (5′-CCACTGTGTTTAGCATGGTGTTTAA-3′, Integrated DNA Technologies), and 250 nM FAM/MGB-labeled probe (5′-FAM-AGCCACCCCACAAGA-MGB-3′; Thermo Fisher Cat# 4316033, TMgagP2). Real-time PCR was performed using the ViiA 7 A&B Applied Biosystems instrument (Life Technologies) and the following cycling conditions: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Real-time PCR primers and probes were based on the HIV-1 pNL4.3 sequence encoding the gag region. Serially diluted pNL4.3 plasmid DNA ranging from 101 to 106 copies applied to each PCR assay served as the HIV-1 standard curve. A no template control was included in each assay to control for PCR cross-contamination, and each sample was assayed in triplicate. QuantStudio™ Real-time PCR Software (Applied Biosystems, Foster City, CA) was used for PCR data analysis.
2.8. Generation and characterization of HIV-1-infected CD4+ T cell targets
Total CD4+ T cells cocultured with antigen-presenting MDC1 and were tested weekly for the presence of HIV-1 p24 antigen by intracellular flow cytometry staining with KC57-FITC antibody (clone FH190–1-1, Beckman Coulter Cat# 6604665). Target cells were pre-screened for p24 expression, and cryopreserved for later use as targets when they reached at least 10% positivity.
2.9. Induction and expansion of autologous CTL
Total CD8+ T cells were isolated from cryopreserved PBL by negative magnetic bead separation using an EasySep™ Human CD8+ T Cell Enrichment Kit (STEMCELL Technologies Cat# 19053). To induce CTL responses as previously described [7], CD8+ T cells were cocultured with autologous differentially matured DC loaded with either HLA-A2-restricted Gag p24 Gag151–159 9-mer peptide epitopes when using HIV-1-negative blood donors, or Gag p17/p24 overlapping 15-mer peptides (1 μg/mL, Sigma-Aldrich) when using HIV-1-infected MACS participants. The cocultures (75,000 DC: 750,000 CD8+ T cells) were treated with or without the addition of either 25,000 gamma-irradiated (5000 rad) CD40L-transfected J588 cells [30] or MEGACD40L® Protein (0.25 μg/mL; Enzo Life Sciences Cat# ALX-522-110-C010) where stated. On day 5, rhIL-2 (250 U/mL) and rhIL-7 (10 ng/mL) were added to the cultures and every three days thereafter. On day 12, T cell cultures were restimulated with either gamma-irradiated HLA-A2+ T2 cells (for induction of primary CTL responses in HLA-A2+ HIV-1-negative donors) or differentially matured autologous DC loaded with autologous 9-mer peptides (1 μg/mL) corresponding to the viral antigens and DC type used in the initial stimulation. Antigen-specific readout assays were performed between days 20–24 to assess CTL activity.
2.10. IFN-γ ELISPOT assays
Autologous CTL (3 × 104/well) were tested for reactivity to individual and pooled Gag 9-mer peptide antigens (1–10 μg/mL) by ELISPOT assay using anti-human IFN-γ and biotin monoclonal antibodies (clones 1-D1K and 7-B6–1; Mabtech Cat# 3420–6-1000) as previously described [7,8]. Recorded values were net responses compared to control wells consisting of CTL exposed to assay medium alone.
2.11. HIV-1 antigen-expressing cell killing assays
CTL effector function was assessed as described previously, with modifications [8]. Briefly, MDC1-stimulated total CD8+ T cells were cocultured with autologous MDC1-induced CD4+ target cells at various effector:target (E:T) ratios for 18 h at 37 °C. Harvested cocultures were stained for surface expression of CD8 (PerCP-Cy5.5, clone SK1; BD Biosciences Cat# 341051; RRID: AB_400298) and intracellular expression of HIV-1 p24 (KC57-FITC, clone FH190-1-1; Beckman Coulter Cat# 6604665). Effector CD8+ cells were excluded from analysis gating, and the percent reduction in infected CD4+ T cells was determined at each E:T ratio. For the colorimetric cytolytic assays described in the supplemental material, autologous CD4 cells were stained with either CFSE (eBioscience Cat# 65–0850) or CellTrace™ Violet (Thermo Fisher, Cat# C34557) dyes following the manufacturer's protocols. Target cells (CFSE) were then loaded with individual peptides at 100 ng/mL in PBS for 60 min at room temperature (RT); excess unbound peptide was removed by washing. The CFSE and CellTrace Violet dye-labeled cells were mixed in equal numbers and coincubated for 18 h with autologous CTL at various E:T ratios. The antigen-specific killing of HIV-1 peptide-loaded CD4+ T cells (green) was calculated based on relative changes in percentages of the differentially stained target cells remaining, using by flow cytometry analysis.
2.12. Viral outgrowth assays
Total CD8+ T cells were cocultured with autologous p24-expressing CD4+ target cells at various E:T ratios as described for the CTL kill assay. Cultures were maintained for eight days, after which culture supernatants were harvested and tested by p24 ELISA (Frederick National Laboratory for Cancer Research, Frederick, MD) for CTL-induced viral suppression [7].
2.13. Quantification of replication-competent HIV-1
Culture supernatants harvested from LR and viral outgrowth assays were spinoculated onto TZM-bl cell (NIH AIDS Reagent Program Cat# 8129-422; RRID: CVCL_B478) monolayers (30,000 cells/well) for four hours at 300 g and cocultured for 48 h. Beta-Glo® reagent (Promega Cat# E4740) was added to PBS-washed TZM-bl cell monolayers and incubated for 1 h at room temperature. Control supernatants from cultured CD4+ T cells of an uninfected donor were treated in parallel. Chemiluminescence from the TZM-bl cells was detected by luminometer as previously described [31]. Sample wells were considered positive for the presence of replication-competent virus if the chemiluminescent signal exceeded the mean + 2 S.D. of a control sample.
2.14. Statistical analyses
Statistical analyses for ELISA and ELISPOT data (Fig. 1b and c) were calculated using Wilcoxon matched-pairs signed-ranks test and a linear mixed model with 95% confidence intervals, respectively. Differences between MDC1-mediated LR were determined by multilevel mixed-effects tobit regression analyses (Fig. 2, Fig. 4a) and Wilcoxon matched-pairs signed-ranks test (Fig. 3, Fig. 4c–d).
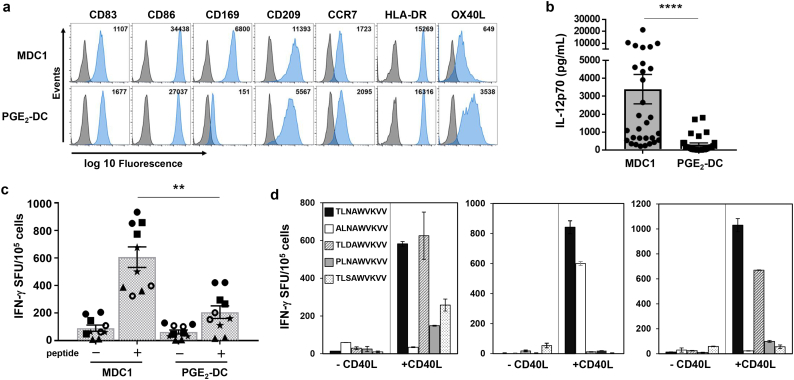
Fig. 1.
MDC1 are superior inducers of HIV-1-specific CTL responses. a) Differentially polarized mature DC were analyzed for surface phenotype. Gray histogram peaks of flow cytometry plots indicate unstained control samples; peaks shaded in blue represent positive staining for the phenotypic markers indicated. Inset numbers refers to MFI. b) Mature DC were tested for their net IL-12p70-producing capacity above background in response to CD40L stimulation. P values were determined by Wilcoxon matched-pairs signed-ranks test. Error bars indicate mean ± SEM; n = 30. ****P<0.0001. c) MDC1 and PGE2-DC loaded with HIV-1 Gag151–159 peptide (TLNAWVKVV) were cocultured with autologous CD8+ T cells from HLA-A2+ HIV-1-naïve individuals. The in vitro expanded antigen-specific CTLs were quantified by IFN-γ ELISPOT. Shown are values from unstimulated (−) and peptide stimulated (+) CTLs generated using antigen presenting MDC1 or PGE2-DC. P values were calculated using a linear mixed model with 95% confidence intervals. Error bars indicate mean ± S.D. **P<0.01. d) IFN-γ ELISPOT results of CD8+ T cell responses to Gag151–159 peptide variants induced in 3 different HIV-uninfected donors by autologous antigen-presenting MDC1 in the absence or presence of CD40L. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
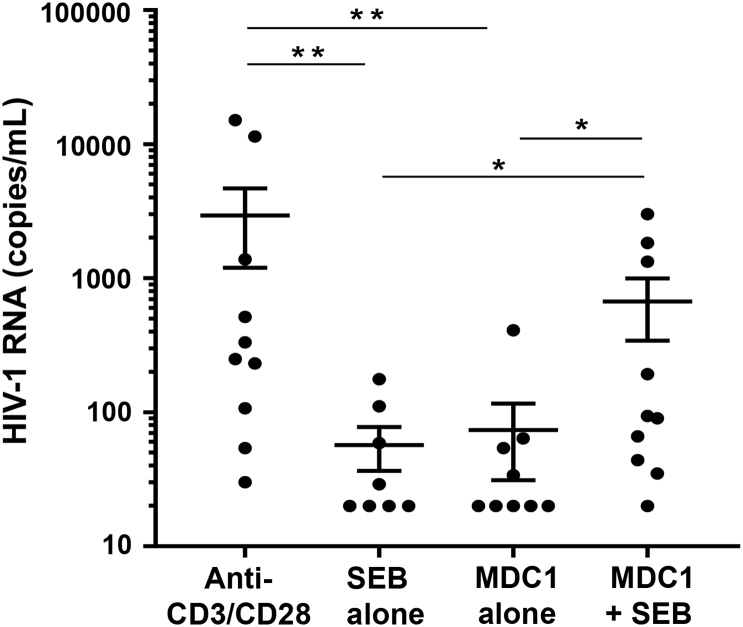
Fig. 2.
Influence of antigen presentation on MDC1-mediated HIV-1 latency reversal in CD4+ T cells. MDC1 were cocultured with autologous CD4+ T cells in the presence or absence of SEB antigen. Cell culture supernatants were analyzed by qRT-PCR for HIV-1 RNA at day 7. P values comparing viral RNA levels were determined by multilevel mixed-effects tobit regression analyses. Error bars indicate mean ± SEM. *P<0.05 and **P<0.01.
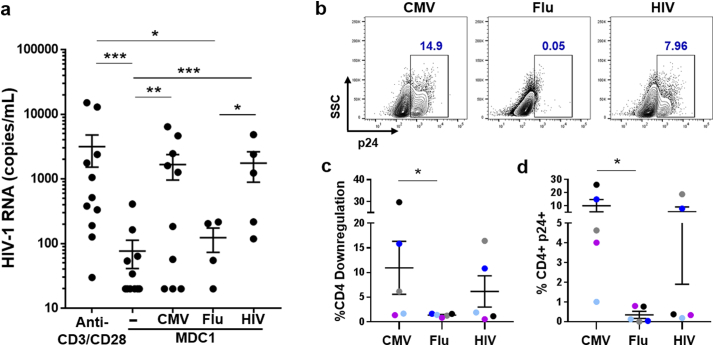
Fig. 4.
CMV and HIV antigen presentation drives MDC1-mediated HIV-1 latency reversal. a) MDC1 were loaded with either CMV pp65, HIV-1 Gag, or influenza M1 antigen and tested for their ability to induce LR in autologous CD4+ T cells. Culture supernatants were assayed by qRT-PCR for detection of HIV-1 RNA at day 7. b) Representative flow cytometry plots of p24 expression of day 20 cocultures, gated on total CD4+ T cells. c) Graphical representation of MDC1/antigen-induced CD4 downregulation in cocultures described in (b), as measured by flow cytometry. d) Expression of p24 expression in cell populations detailed in (b). Each colour in (c) and (d) represents an individual study participant. P values comparing viral RNA levels were determined by multilevel mixed-effects tobit regression analyses. Error bars indicate mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
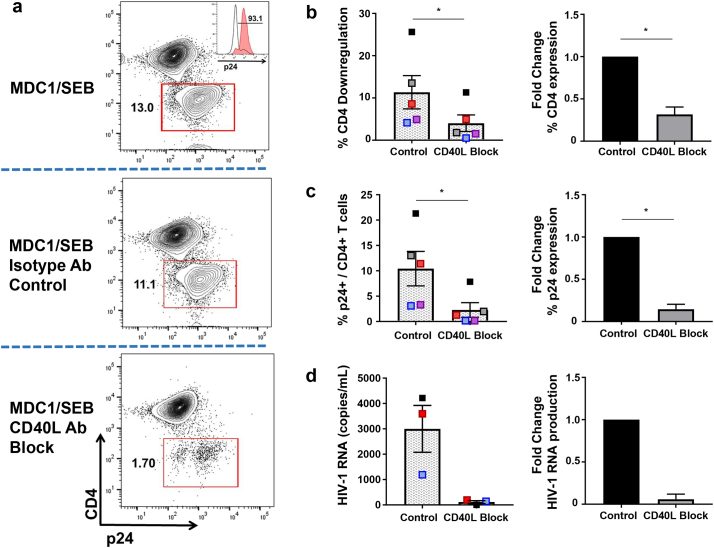
Fig. 3.
Role of CD40/CD40L interaction in the MDC1-mediated ‘kick’ of latent HIV-1. MDC1 were cocultured with autologous CD4+ T cells and SEB to induce HIV-1 LR, in the presence or absence of CD40L blocking antibody. a) Representative flow cytometry plot of day 15 cultures. Downregulation of CD4 expression (red gate) corresponds with increased expression of p24 in the absence of CD40L blockade. Red histogram peak corresponds with p24 expression of CD4 downregulated population. b, c) Graphical representation of CD4 downregulation (b) and p24 expression (c) of populations described in (A); n = 5. D) Day 7 cell coculture supernatants were analyzed by qRT-PCR for HIV-1 RNA; n = 3. Differences between MDC1/SEB-mediated LR in the absence or presence of CD40L blocking antibody were compared by Wilcoxon matched-pairs signed-ranks test. Error bars indicate mean ± SEM. *P<0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. MDC1 effectively induce primary HIV-1-specific CTL responses with CD40L ‘help’
We initially compared the use of two clinically applicable, differentially activated DC preparations using blood products from HLA-A2+ HIV-1-naïve blood bank donors to test their capacity to induce primary HIV-1-specific CTL responses. MDC1 were characterized and defined by their mature phenotypic status, expressing high levels of CD86 and CD83, and their high capacity to produce IL-12p70 upon subsequent stimulation with the CD4+ T cell ‘helper’ signal CD40L, while PGE2-DC were IL-12p70-deficient and less responsive to CD40L signaling (Fig. 1a and b, and Fig. S1) [29,32]. These DC types were loaded with HLA-A2-restricted HIV-1 peptide antigen and used as in vitro stimulators of autologous CD8+ T cells, in the presence of gamma-irradiated (5000 rad) CD40L-expressing J558 cells (J558-CD40L), which served as a CD40L+ TH cell surrogate. In doing so, we found that the MDC1 had a higher CTL priming capacity compared to PGE2-DC (Fig. 1c). Importantly, the effective in vitro induction of long-term CTL responses by MDC1 required the presence of CD40L ‘help’ during the initiation of the priming cocultures (Fig. 1d).
3.2. Antigen presentation by autologous MDC1 drives HIV-1 LR in CD4+ T cells
Recent evidence linked a DC therapeutic with an increase in residual HIV-1 viremia while the study participants were on ART, suggesting that the DC therapeutic acted in some way as an LRA [28]. However, the mechanisms involved in this phenomenon are not yet clear, including the role that antigen presentation may have played. Because MDC1 were shown to be strong inducers of primary CTL responses (Fig. 1), we next tested their capacity to reactivate or ‘kick’ latent HIV-1 from latency to expose the infected cells for subsequent CTL targeting.
MDC1 were generated from ART virally suppressed HIV-1-infected MACS participants and cocultured with autologous peripheral blood CD4+ T cells in the presence or absence of SEB antigen. SEB was used because as a superantigen, it can effectively facilitate immune cross-talk between the antigen-presenting cells and a large percentage (~30%) of SEB responsive T cells [29]. CD4+ T cells treated with anti-CD3/CD28 mAb-coated beads were used as a positive LRA control [33]. qRT-PCR analysis of HIV-1 RNA presence in day 7 coculture supernatants revealed that MDC1 indeed acted as a strong LRA in an SEB antigen-dependent manner (Fig. 2, MDC1 alone vs MDC1 + SEB, P < 0.05).
3.3. Role of CD40/CD40L interaction in MDC1-mediated ‘kick’ of latent HIV-1
DC crosstalk with CD40L+ TH cells plays a critical role in the induction and survival of long-term CTL responses [[34], [35], [36], [37]]. Because we previously showed that MDC1 are particularly sensitive to CD40L signaling [29], and that this CD4+ T cell-derived ‘helper’ factor is required for effective MDC1-mediated in vitro priming of de novo CTL responses (Fig. 1d), we wanted to determine if CD40/CD40L cross-talk between the MDC1 and CD4+ T cells was playing a role in the MDC1-mediated HIV-1 LR. Indeed, we found that blocking CD40/CD40L interaction strongly decreased the effectiveness of MDC1-mediated LR. The impact of this CD40L signaling inhibition on MDC1-mediated LR was clearly evident when analyzing the activated CD4+ T cells by flow cytometry, where the addition of an anti-CD40L blocking antibody resulted in a marked inhibition of CD4 downregulation (87.7% ± 3.1%, P < 0.05; Fig. 3a and b), a phenomenon associated with HIV-1 protein translation [38]. As expected, this inhibition of CD4 downregulation by addition of the CD40L blocking antibody was associated with abrogation of intracellular p24 expression (90.8% ± 7.0%) (Fig. 3a, c; P < 0.05) induced in autologous CD4+ T cells, and with the reduction in HIV-1 RNA content in the day 7 coculture supernatants measured by qRT-PCR (Fig. 3d, 94.1% ± 6.1% inhibition). Importantly, the addition of an isotype control antibody to the MDC1:T cell cocultures had no significant impact on the induced changes in CD4 expression or HIV-1 expression resulting from LR (Fig. 3a-d). Taken together, these data support the required involvement of cognate antigen-driven bidirectional signaling events between MDC1 and antigen-responsive CD4+ T cells in HIV-1 LR.
3.4. CMV and HIV-1 antigen-driven reactivation of latent HIV-1 by MDC1
We have shown that MDC1-mediated transcription of HIV-1 DNA (Fig. 2) and subsequent translation of p24 (Fig. 3a, c) are both dependent on the presence of SEB superantigen and on CD40/CD40L signaling. However, to simulate a clinically relevant method of HIV-1 LR, we posited that the inclusion of common viral MHC class II antigens as part of our MDC1-based therapeutic could promote interaction with CD40L-expressing CD4+ TH cells, to both provide immune ‘help’ for MDC1-mediated induction of HIV-1-specific CTL responses and facilitate MDC1-mediated exposure of viral antigen-specific CD4+ T cells harboring latent HIV-1.
In choosing which viral antigens to incorporate in our model of MDC1-mediated LR, we considered previous findings that a significant pool of latently infected CD4+ T cells are HIV-1-specific [39,40]. As such, an HIV-1-based vaccine or LRA construct could potentially reactivate this population while also facilitating the ‘kill’ through CTL priming. We also considered the fact that approximately 95% of HIV-1-infected individuals are coinfected with CMV [41], in whom CMV-specific CD4+ T cell memory inflation occurs [42], with some having greater than 25% of their T cells specific to CMV [43]. Therefore, we hypothesized that inclusion of heterologous CMV antigen would effectively promote MDC1 interaction with CD4+ TH cells to facilitate ‘help’ for HIV-1 specific CTL induction and to induce CMV antigen-specific MDC1-mediated LR.
We tested MDC1 alone or loaded with CMV pp65, HIV-1 Gag, or influenza A virus M1 protein (representing a common, non-persistent virus antigen) for their ability to induce latency reversal in autologous CD4+ T cells (Fig. 4). We found that MDC1-mediated LR was antigen-dependent, with CMV and HIV-1 antigen having notable LR activity, while the influenza virus antigen did not (Fig. 4a–d). MDC1/CMV- and MDC1/HIV-1-mediated increases in extracellular virion-associated HIV-1 RNA were significantly greater than those induced by MDC1 alone (Fig. 4A; P < 0.01 and P < 0.001, respectively). Importantly, MDC1 presenting either CMV or HIV-1 antigen exposed latent HIV-1 cellular reservoir targets, identified by a marked downregulation in CD4 expression (Fig. 4c) that corresponded to increases in intracellular p24 (Fig. 4b, d).
3.5. MDC1-induced CTL effectively kill MDC1-exposed CD4+ T cell targets harboring replication-competent HIV-1
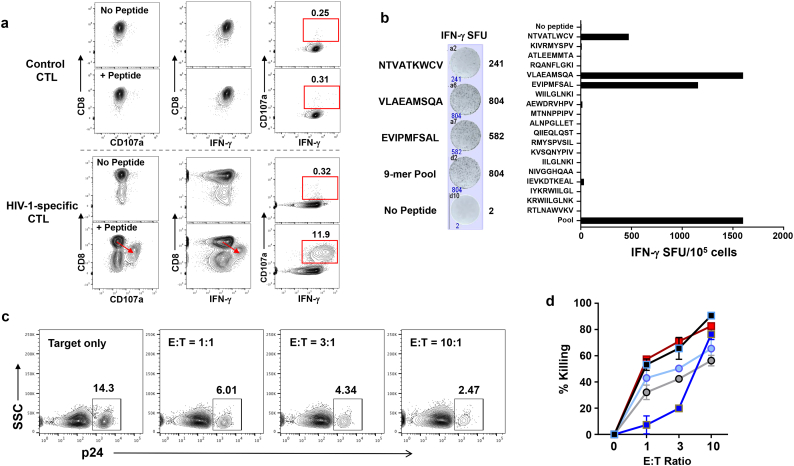
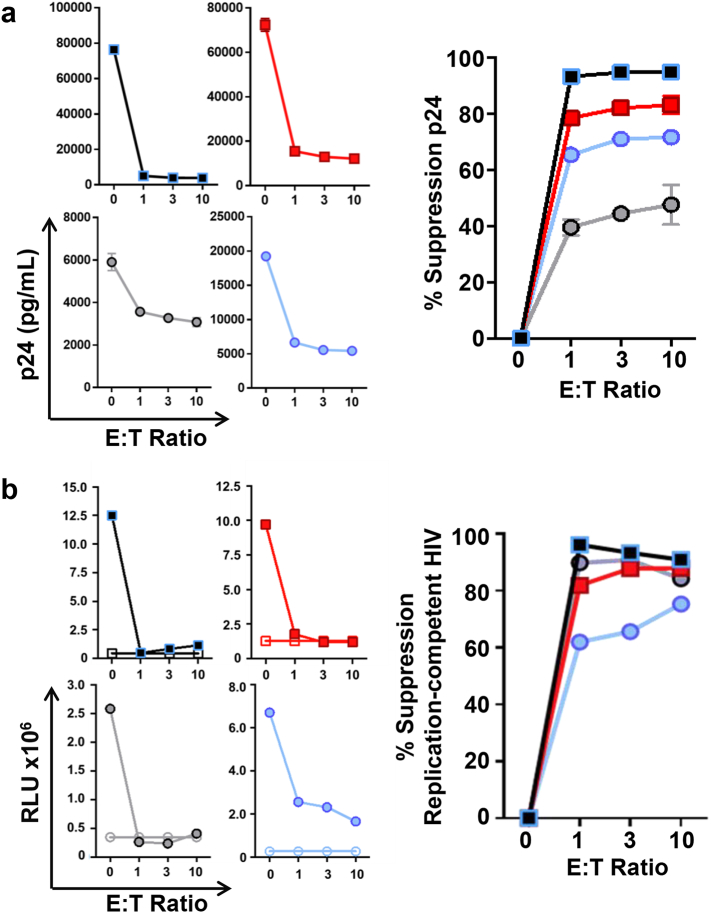
MDC1 loaded with autologous HIV-1 Gag peptides were used to induce antigen-specific CTL, as determined by flow cytometry analysis for antigen-induced expression of CD107a and interferon (IFN)-γ (Fig. 5a) and by IFN-γ ELISPOT (Fig. 5b). Antigen-induced downregulation of CD8 expression, a characteristic previously shown to be associated with enhanced cytolytic capacity [7,44], was evident along with high expression of CD107a and IFN-γ in the CTL generated ex vivo using the HIV-1 antigen-presenting MDC1 (Fig. 5a). Also, the CTL responses induced by MDC1 were broadly reactive to a range of individual Gag 9-mer epitopes by IFN-γ ELISPOT (Fig. 5b). The antigen-specific killing capacity of these CTL was initially tested by coculturing them overnight with differentially labeled Gag 9-mer peptide antigen-loaded or antigen negative (control) autologous CD4+ T cell targets, which clearly showed the selective elimination of the antigen-loaded target cells as determined by flow cytometry analysis (Fig. S2a, b). More importantly, the LR activity of CMV and HIV-1 antigen-presenting MDC1 resulted in the effective exposure of HIV-1-infected antigen-expressing target cells that were also recognized and efficiently controlled by the MDC1/Ag-induced HIV-1-specific CTL in short-term cytotoxicity assays (75.4% ± 14.3% killing), indicated by a CTL dose-dependent decrease of p24-expressing CD4+ T cells (Fig. 5c, d; S2c, d). This pattern was consistent regardless of the type of antigen used to induce MDC1-mediated LR. Furthermore, the CTL suppressed viral outgrowth from the infected cells in long-term cocultures by 74.4% ± 20.2%, as measured by p24 ELISA (Fig. 6a, Right).
Fig. 5.
MDC1-induced broadly reactive HIV-1-specific CTL effectively kill HIV-1 infected CD4+ T cells exposed by MDC1. a) MDC1 generated from HIV-1-infected, ART-suppressed individuals were loaded with HIV-1 Gag peptides and used to induce broadly reactive antigen-specific autologous CTL as determined by flow cytometry staining for CD107a and IFN-γ. b) Polyclonal IFN-γ responses to individual Gag 9-mer epitopes by MDC1-induced CTL described in (a). c) HIV-1 latency reversal was induced by MDC1 and SEB or antigen in CD4+ T cells. Target cells (T) were coincubated with autologous MDC1-induced effector CTL (E) at various E:T ratios for 18 h. CTL-induced target killing was measured by loss of HIV-1 Gag p24-expressing target cells using flow cytometry. d) Summary of 5 independent flow cytometry cytotoxicity experiments. Square symbols represent MDC1/SEB-induced target cells; circles indicate MDC1/viral antigen-induced targets. Error bars indicate mean ± S.D.
Fig. 6.
HIV-1-specific CTL control MDC1-exposed targets harboring replication-competent HIV-1. a) HIV-1 latency reversal was induced in CD4+ T cells by MDC1 presenting SEB, CMV pp65, HIV-1 Gag, or influenza M1 antigen. Target cells (T) were cocultured with autologous MDC1-induced effector CTL (E) at various E:T ratios for 8 days. Left panels: Culture supernatants were tested by p24 ELISA to measure CTL-induced viral suppression. Right panel: Graphical compilation of individual experiments. Error bars indicate mean ± S.D. b) Culture supernatants collected from viral outgrowth assays and cultured on TZM-bl cell monolayers were tested for chemiluminescence. Left: Graphical compilation of individual experiments (RLU, relative light units). Solid symbols indicate HIV-1-infected participant samples; open symbols represent HIV-1-negative samples assayed in parallel. Right: CTL-induced suppression of replication-competent HIV-1. Square symbols represent MDC1/SEB-induced target cells; circles indicate MDC1/viral peptide antigen-induced targets.
Recent studies have posited that CTL preferentially target cells containing defective HIV-1 proviruses, which in effect act as decoy targets to prevent elimination of the true latent reservoir [9,45,46]. Therefore, we sought to determine whether MDC1-mediated LR activity unmasked cells harboring replication-competent virus that could be subsequently recognized and killed by the CTL. To do so, culture supernatants collected from viral outgrowth assays at various effector-to-target ratios (Fig. 6a) were subsequently cultured on TZM-bl reporter cells [31] for quantification of infectious HIV-1 (Fig. 6b). Importantly, we found that MDC1 LR activity exposed those targets harboring replication-competent virus, whose dose-dependent elimination (Fig. 6b, Left) resulted in 84.5% ± 6.7% suppression of replication-competent HIV-1 (Fig. 6b, Right). Thus, MDC1-primed HIV-1-specific autologous CTL were capable of eliminating HIV-1-infected cells harboring replication-competent virus following their subsequent unveiling through the LR activity of MHC-class II antigen-presenting MDC1.
4. Discussion
We have shown that antigen-presenting MDC1 are capable of inducing both HIV-1 latency reversal in infected CD4+ T cells isolated from ART-treated HIV-1+ MACS participants, and HIV-1 antigen-specific CTL responses that can effectively kill the MDC1-exposed HIV-1-infected targets. This MDC1-mediated ‘kick’ was found to be antigen-dependent, and bidirectional signaling events between the MDC1 and CD4+ T cells involving the CD40/CD40L signaling pathway contributed to this process. Other studies have explored the LRA potential of immature DC using in vitro models of HIV-1 latency with infected immortalized cell lines [47,48], through in vitro establishment of HIV-1 latency in primary CD4+ T cells of uninfected donors [49], or by addressing their nonspecific impact on in vitro pre-expanded polyclonal-activated T cells [50]. However, we demonstrate, in a natural setting of chronic HIV-1 infection, the use of an effective, clinically relevant autologous mature DC type specifically programmed to both mediate LR in freshly isolated CD4+ T cells derived from individuals undergoing successful ART, and to induce effector cells capable of recognizing and eliminating the infected cells. Our current data imply that a component of the HIV-1 reservoir is contained within the pool of both CMV- and HIV-1-specific CD4+ T cells. Importantly, our study was limited to a small number of viral antigen sources, and to one target protein antigen for each of the respective viruses tested. Therefore, the levels of LR induced most likely underrepresent the magnitude of HIV-1 reactivation possible if the number and selection of antigens had been optimized. This is especially true when considering antigen-specific CMV immunity, where CD4+ T cell responses to pp65 and IE-1 protein antigens comprise less than 12% of total CD4+ T cell responses to CMV in coinfected individuals [43]. As CMV is one of the largest and most complex viruses, with a genome encoding over 200 open reading frames [51], our study has room for optimization through incorporation of other CMV antigens that could enhance the effectiveness of our MDC1-based LR strategy. Nevertheless, our study supports the concept that HIV-1 LR can be achieved to expose cells infected with replication-competent virus for CTL elimination, in both a safe and directed antigen-specific fashion.
Previous studies in HIV-1/CMV-coinfected individuals indicate that HIV-1-specific CD4+ T cells are preferentially infected and depleted by HIV-1 [40,52], and that a portion of latently infected cells that remain during ART are indeed HIV-1-specific [39]. Interstingly, in contrast to our findings, it has been reported that CMV-specific CD4+ T cells are less susceptible to HIV-1 infection in vivo [53]. In spite of this, a large body of data exists to support the notion that CMV antigen-specific CD4+ T cells, in particular, likely contribute to a sizeable portion of the latent HIV-1 cellular reservoir. For example, CMV infection is frequent in HIV-1-infected individuals, with a seroprevalence of approximately 95% [41]. Furthermore, CMV occupies an inflated proportion, on average 10%, of memory T cell responses in healthy individuals, and CMV-specific CD4+ T cells persist at high levels in HIV-1 and CMV coinfected individuals [42,51,54,55], with greater than 1 out of 4 of the total number of CD4+ T cells in peripheral blood being CMV-specific in some individuals [43]. Subclinical CMV replication often occurs in the mucosal and peripheral tissues of HIV-1-infected individuals, contributing to T cell dysfunction, impaired immune recovery, and chronic immune activation during ART [[56], [57], [58], [59]]. Consequently, recruitment of target cells to sites of inflammation that are also major sites of HIV-1 persistence, such as gut and other lymphoid tissues, creates a favorable environment for reservoir seeding [[60], [61], [62], [63]]. Recent evidence supporting this scenario was provided by a study of ART-suppressed individuals in whom CMV replication in the gut was associated with inflammation, mucosal barrier damage, and microbial translocation [60]. Manipulation of HIV-1 coreceptor expression by CMV could also serve as a mechanism for establishment of the latent reservoir in susceptible target cells. For example, CMV upregulates CCR5 expression on newborn umbilical cord blood central memory CD4+ T cells that could facilitate in utero transmission of HIV-1 [64]. In addition, in vitro studies demonstrate the ability of CMV to manipulate AP-1 and NF-κB signaling for induction of HIV-1 gene expression in infected bystander cells through direct transactivation of the HIV-1 LTR [[65], [66], [67], [68]]. Each of these mechanisms could potentially lead to enhanced HIV-1 infection of target cells in CMV-coinfected individuals. Once established, the latent HIV-1 cellular reservoir could also be subject to CMV-mediated proliferation or clonal expansion. In support of this theory, cross-sectional studies have shown a correlation between CMV replication in blood and semen and higher levels of HIV-1 DNA in both ART-naïve and ART-suppressed individuals [69,70]. In a related longitudinal study, CMV replication in peripheral blood mononuclear cells (PBMC) of men initiating early ART was associated with delayed decay of HIV-1 DNA reservoirs [63]. Furthermore, proviral and integration site analyses in ART-suppressed individuals have implicated clonal expansion of latently infected CD4+ T cells as a major mechanism of HIV-1 persistence [[71], [72], [73], [74], [75]], and recent findings estimate that these expanded clones comprise 50–60% of the latent HIV-1 reservoir [[76], [77], [78]]. Of note, in a study of 15 HIV-1-infected patients who underwent myeloablative chemotherapy for CMV- and Epstein-Barr virus (EBV)-associated malignancies, increases in HIV-1 DNA were preferentially found in CMV- and EBV-specific CD4+ T cells after immune reconstitution [79]. It is possible that self-renewal of stem cell memory T cells (TSCM) contributes to homeostatic proliferation of the latent HIV reservoir [80]. This subset of memory T cells plays a significant role in the maintenance of long-term immunological memory and contains the most copies of integrated provirus per cell in HIV-1-infected individuals [80]. Finally, CMV-infected individuals possess functional CMV-specific TSCM cells that could promote expansion of the HIV-1 reservoir in CMV/HIV-1-coinfected persons through homeostatic proliferation, even during ART [81].
Recent studies in vivo studies utilizing TLR agonists to promote HIV-1 LR have shown promise [[19], [20], [21], [22]], and are currently being studied in human trials (NIH Clinical Trials NCT02858401 (GS-9620; NCT03060447; NCT03837756). However, the specifics of how these innate immune receptor activators are leading to reactivation of the latent reservoir have yet to be fully elucidated. Evidence from both human and non-human primate studies points toward the IFN-α-producing plasmacytoid DC (pDC) as being an important cellular component in this process [[19], [20], [21], [22]]. It is worth noting that the combination of factors used in the maturation and generation process of the specialized antigen-presenting type-1 programmed MDC1 used in our study, which includes IFN-α, IFN-γ, and a TLR3 agonist, was designed to mimic maturation events expected to occur as a result of DC crosstalk with responding IFN-α-producing pDC and IFN-γ-producing NK cells during the early stages of a successful antiviral immune response [32,82]. The factors produced by these early immune responders programs the maturing DC for being hyper-responsive to subsequent signaling factors they receive during antigen cognate interactions with CD4+ TH cells. While the underlying mechanisms of the MDC1-mediated LR observed in our study have not been fully identified, our findings do indicate that bidirectional antigen-driven cross-talk between MDC1 and CD4+ T cells involving the CD40/CD40L signaling pathway contributed to the noted LR activity. Based on gene chip analysis of CD40L-activated MDC1 that revealed upregulations in galectin-9, TNF-α, and IL-15 mRNA expression (data not shown), all of which have been implicated as inducers of LR [50,83,84], the potential contribution of these factors warrants further investigation.
There are a number of caveats and limitations to our study that we acknowledge. Although our results point to CMV- and HIV-1-specific CD4+ T cells as harboring latent provirus, it is likely that CD4+ T cells specific to other viruses that manifest as chronic infections, including EBV and herpes simplex virus, and others contribute to the latent reservoir as well. Moreover, while we did not observe influenza antigen-mediated LR ex vivo, others have documented increases in cell-associated HIV-1 RNA expression in HIV-1-infected individuals receiving influenza vaccination during suppressive ART [85,86]. However, deep sequencing studies pre- and post-vaccination suggested nonselective induction of proviral expression from a broad pool of HIV-1-infected bystander cells [86]. While the LR we show in our study was antigen-driven, we did not truly identify the antigen specificity of the infected CD4+ T cells, and therefore we do not rule out the possibility that reactivation of the virus could reflect nonspecific bystander effects of a potent MDC1-mediated antigen-specific response, rather than a direct impact on an antigen-specific reservoir. Furthermore, targeting the latent reservoir poses numerous challenges with regard to antigen delivery by a DC vaccine to certain sites that are anatomical sanctuaries of HIV-1, such as lymph node B cell follicles [87]. Because of this, the LRA potential of antigen-presenting B cells and antigen-presenting DC-derived exosomes should be explored for their ability to drive HIV-1 LR and to target these anatomical sites. Alternatively, developing strategies for direct antigen delivery to the antigen presenting cells residing in these areas in vivo should be considered. Nonetheless, our results provide strong rationale for the incorporation of MDC1 and ‘helper’ antigen derived from heterologous viral sources (such as CMV) into the design of a dual therapeutic approach to address both the ‘kick’ and the ‘kill’ of latent HIV-1.
Acknowledgments
Acknowledgements
The authors thank Ming Ding, Anwesha Sanyal, and Holly Bilben for technical assistance, and John W. Mellors, Simon Barratt-Boyes, Jennifer M. Zerbato, and Paolo Piazza for helpful discussions.
Funding sources
This work was supported by NIH-NAID grants R21-AI131763, U01-AI35041, UM1-AI126603, and T32-AI065380. The funding sources had no role in the study design, data collection and analysis, interpretation of results, preparation of the manuscript, or decision to have it published.
Declaration of interests
The authors have nothing to disclose.
Author contributions
J Kristoff performed experiments, analyses, and co-wrote the manuscript, ML Palma and TM Garcia-Bates provided technical assistance and contributed to experimental data, C Shen performed statistical analysis, N Sluis-Cremer provided intellectual input and edited the manuscript, P Gupta provided intellectual input and data interpretation, CR Rinaldo provided intellectual input, supervision, and edited the manuscript, and RB Mailliard designed the study, provided supervision, technical assistance, and co-wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.077.
Appendix A. Supplementary data
Fig. S1. MDC1 are programmed to produce enhanced levels of IL-12p70 upon stimulation with CD40L. DC were plated (2.5 x 104 cells/well) in a 96-well flat-bottom plate and cultured in presence or absence of J558-CD40L (5 x 104 cells/well) for 24 hours. Culture supernatants were collected and tested by IL-12p70 ELISA. bd = below detection limit of the assay (37pg/ml).
Fig. S2. Selective killing of HIV-1 antigen-expressing CD4+ T cells by autologous MDC1-induced HIV-specific CTL. MDC1 generated from HIV-1-infected, ART-suppressed individuals were cultured with or without HIV-1 Gag peptides or with irrelevant control peptides (Influenza M1) and used to induce autologous CTL. a) CD4+ T cells were labeled with CellTrace™ dye (violet) or CFSE (green) for use as target cells. The CFSE-labeled cells were additionally loaded with relevant HIV-1 Gag 9-mer peptides. The cells were mixed in equal numbers and coincubated for 18 hours with MDC1-induced autologous CTL at various E:T ratios. b) The antigen-specific killing of HIV-1 peptide-loaded CD4+ T cells (green) was calculated based on relative changes in the percentages of the viable differentially colored target cells. c) p24-expressing HIV-1 infected target cells (T) were coincubated with autologous MDC1-induced effector CTL at various E:T ratios for 18 hours. CTL-induced target killing was measured by loss of HIV-1 Gag p24-expressing target cells using flow cytometry. d) Comparison of cytotoxic activity of HIV-specific CTL with CTL cultured in the absence of HIV-1 Gag peptides (Control CTL) in a representative donor (shown in a). Error bars indicate mean ± S.D.
Supplementary material
References
- 1.Ruelas D.S., Greene W.C. An integrated overview of HIV-1 latency. Cell. 2013;155(3):519–529. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruner K.M., Hosmane N.N., Siliciano R.F. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015;23(4):192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks S.G. HIV: Shock and kill. Nature. 2012;487(7408):439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 4.Jones R.B., Walker B.D. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest. 2016;126(2):455–463. doi: 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulder P.J., Watkins D.I. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4(8):630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 6.Boucau J., Le Gall S. Antigen processing and presentation in HIV infection. Mol Immunol. 2018 doi: 10.1016/j.molimm.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailliard R.B., Smith K.N., Fecek R.J., Rappocciolo G., Nascimento E.J., Marques E.T. Selective induction of CTL helper rather than killer activity by natural epitope variants promotes dendritic cell-mediated HIV-1 dissemination. J Immunol. 2013;191(5):2570–2580. doi: 10.4049/jimmunol.1300373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith K.N., Mailliard R.B., Piazza P.A., Fischer W., Korber B.T., Fecek R.J. Effective cytotoxic T lymphocyte targeting of persistent HIV-1 during antiretroviral therapy requires priming of naive CD8+ T cells. mBio. 2016;7(3) doi: 10.1128/mBio.00473-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack R.A., Jones R.B., Pertea M., Bruner K.M., Martin A.R., Thomas A.S. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe. 2017;21(4):494–506.e4. doi: 10.1016/j.chom.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullen C.K., Laird G.M., Durand C.M., Siliciano J.D., Siliciano R.F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20(4):425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clutton G., Xu Y., Baldoni P.L., Mollan K.R., Kirchherr J., Newhard W. The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function. Sci Rep. 2016;6:30749. doi: 10.1038/srep30749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam A.P., Sparano J.A., Vinciguerra V., Ocean A.J., Christos P., Hochster H. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am J Clin Oncol. 2010;33(2):121–124. doi: 10.1097/COC.0b013e3181a31920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith B.D., Jones R.J., Cho E., Kowalski J., Karp J.E., Gore S.D. Differentiation therapy in poor risk myeloid malignancies: results of a dose finding study of the combination bryostatin-1 and GM-CSF. Leuk Res. 2011;35(1):87–94. doi: 10.1016/j.leukres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan R.J., Jr., Leong L., Chow W., Gandara D., Frankel P., Garcia A. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: a California cancer consortium study. Invest New Drugs. 2012;30(2):723–728. doi: 10.1007/s10637-010-9557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajani J.A., Jiang Y., Faust J., Chang B.B., Ho L., Yao J.C. A multi-center phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Invest New Drugs. 2006;24(4):353–357. doi: 10.1007/s10637-006-6452-1. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez C., Serrano-Villar S., Madrid-Elena N., Perez-Elias M.J., Martin M.E., Barbas C. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS. 2016;30(9):1385–1392. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 17.Jones R.B., O'Connor R., Mueller S., Foley M., Szeto G.L., Karel D. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014;10(8):e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M., De Crignis E., Rokx C., Verbon A., van Gelder T., Mahmoudi T. T cell toxicity of HIV latency reversing agents. Pharmacol Res. 2019;139:524–534. doi: 10.1016/j.phrs.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Tsai A., Irrinki A., Kaur J., Cihlar T., Kukolj G., Sloan D.D. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. 2017;91(8) doi: 10.1128/JVI.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borducchi E.N., Cabral C., Stephenson K.E., Liu J., Abbink P., Ng'ang'a D. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–287. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borducchi E.N., Liu J., Nkolola J.P., Cadena A.M., Yu W.H., Fischinger S. Publisher correction: antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;564(7734):E8. doi: 10.1038/s41586-018-0721-y. [DOI] [PubMed] [Google Scholar]
- 22.Vibholm L., Schleimann M.H., Hojen J.F., Benfield T., Offersen R., Rasmussen K. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis. 2017;64(12):1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman D.D., Margolis D.M., Delaney M., Greene W.C., Hazuda D., Pomerantz R.J. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 24.Connolly N.C., Whiteside T.L., Wilson C., Kondragunta V., Rinaldo C.R., Riddler S.A. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15(2):284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia F., Climent N., Guardo A.C., Gil C., Leon A., Autran B. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5(166):166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 26.Brezar V., Ruffin N., Richert L., Surenaud M., Lacabaratz C., Palucka K. Decreased HIV-specific T-regulatory responses are associated with effective DC-vaccine induced immunity. PLoS Pathog. 2015;11(3) doi: 10.1371/journal.ppat.1004752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy Y., Thiebaut R., Montes M., Lacabaratz C., Sloan L., King B. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol. 2014;44(9):2802–2810. doi: 10.1002/eji.201344433. [DOI] [PubMed] [Google Scholar]
- 28.Macatangay B.J., Riddler S.A., Wheeler N.D., Spindler J., Lawani M., Hong F. Therapeutic vaccination with dendritic cells loaded with autologous HIV type 1-infected apoptotic cells. J Infect Dis. 2016;213(9):1400–1409. doi: 10.1093/infdis/jiv582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaccard C.R., Watkins S.C., Kalinski P., Fecek R.J., Yates A.L., Salter R.D. CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J Immunol. 2015;194(3):1047–1056. doi: 10.4049/jimmunol.1401832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanyal A., Mailliard R.B., Rinaldo C.R., Ratner D., Ding M., Chen Y. Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4(+) T cells. Nat Med. 2017;23(7):885–889. doi: 10.1038/nm.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mailliard R.B., Wankowicz-Kalinska A., Cai Q., Wesa A., Hilkens C.M., Kapsenberg M.L. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64(17):5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 33.Spina C.A., Anderson J., Archin N.M., Bosque A., Chan J., Famiglietti M. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9(12) doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feau S., Garcia Z., Arens R., Yagita H., Borst J., Schoenberger S.P. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nat Commun. 2012;3:948. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett S.R., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F., Heath W.R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 36.Ridge J.P., Di Rosa F., Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 37.Schoenberger S.P., Toes R.E., van der Voort E.I., Offringa R., Melief C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 38.Chen B.K., Gandhi R.T., Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol. 1996;70(9):6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demoustier A., Gubler B., Lambotte O., de Goer M.G., Wallon C., Goujard C. In patients on prolonged HAART, a significant pool of HIV infected CD4 T cells are HIV-specific. AIDS. 2002;16(13):1749–1754. doi: 10.1097/00002030-200209060-00006. [DOI] [PubMed] [Google Scholar]
- 40.Douek D.C., Brenchley J.M., Betts M.R., Ambrozak D.R., Hill B.J., Okamoto Y. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 41.Stone S.F., Price P., French M.A. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother. 2006;57(4):585–588. doi: 10.1093/jac/dkl049. [DOI] [PubMed] [Google Scholar]
- 42.Abana C.O., Pilkinton M.A., Gaudieri S., Chopra A., McDonnell W.J., Wanjalla C. Cytomegalovirus (CMV) epitope-specific CD4(+) T cells are inflated in HIV(+) CMV(+) subjects. J Immunol. 2017;199(9):3187–3201. doi: 10.4049/jimmunol.1700851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Margolick J.B., Bream J.H., Nilles T.L., Langan S., Bui H.T. Heterogeneity of CD4+ and CD8+ T-cell responses to cytomegalovirus in HIV-infected and HIV-uninfected men who have sex with men. J Infect Dis. 2014;210(3):400–404. doi: 10.1093/infdis/jiu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maile R., Siler C.A., Kerry S.E., Midkiff K.E., Collins E.J., Frelinger J.A. Peripheral "CD8 tuning" dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174(2):619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 45.Bruner K.M., Murray A.J., Pollack R.A., Soliman M.G., Laskey S.B., Capoferri A.A. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. 2016;22(9):1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S.H., Ren Y., Thomas A.S., Chan D., Mueller S., Ward A.R. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest. 2018;128(2):876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren X.X., Ma L., Sun W.W., Kuang W.D., Li T.S., Jin X. Dendritic cells maturated by co-culturing with HIV-1 latently infected Jurkat T cells or stimulating with AIDS-associated pathogens secrete TNF-alpha to reactivate HIV-1 from latency. Virulence. 2017;8(8):1732–1743. doi: 10.1080/21505594.2017.1356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norton T.D., Miller E.A., Bhardwaj N., Landau N.R. Vpx-containing dendritic cell vaccine induces CTLs and reactivates latent HIV-1 in vitro. Gene Ther. 2015;22(3):227–236. doi: 10.1038/gt.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marini A., Harper J.M., Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol. 2008;181(11):7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 50.van der Sluis R.M., van Montfort T., Pollakis G., Sanders R.W., Speijer D., Berkhout B. Dendritic cell-induced activation of latent HIV-1 provirus in actively proliferating primary T lymphocytes. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sylwester A.W., Mitchell B.L., Edgar J.B., Taormina C., Pelte C., Ruchti F. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenchley J.M., Ruff L.E., Casazza J.P., Koup R.A., Price D.A., Douek D.C. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J Virol. 2006;80(14):6801–6809. doi: 10.1128/JVI.00070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casazza J.P., Brenchley J.M., Hill B.J., Ayana R., Ambrozak D., Roederer M. Autocrine production of beta-chemokines protects CMV-specific CD4 T cells from HIV infection. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komanduri K.V., Donahoe S.M., Moretto W.J., Schmidt D.K., Gillespie G., Ogg G.S. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279(2):459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 55.Naeger D.M., Martin J.N., Sinclair E., Hunt P.W., Bangsberg D.R., Hecht F. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman M.L., Lederman M.M., Gianella S. Partners in crime: the role of CMV in immune Dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep. 2016;13(1):10–19. doi: 10.1007/s11904-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith D.M., Nakazawa M., Freeman M.L., Anderson C.M., Oliveira M.F., Little S.J. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis. 2016;63(11):1517–1524. doi: 10.1093/cid/ciw612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poizot-Martin I., Allavena C., Duvivier C., Cano C.E., Guillouet de Salvador F., Rey D. CMV+ Serostatus associates negatively with CD4:CD8 ratio normalization in controlled HIV-infected patients on cART. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dan J.M., Massanella M., Smith D.M., Spina C.A., Schrier R., Daar E.S. Brief report: effect of CMV and HIV transcription on CD57 and PD-1 T-cell expression during suppressive ART. J Acquir Immune Defic Syndr. 2016;72(2):133–137. doi: 10.1097/QAI.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maidji E., Somsouk M., Rivera J.M., Hunt P.W., Stoddart C.A. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gianella S., Chaillon A., Mutlu E.A., Engen P.A., Voigt R.M., Keshavarzian A. Effect of cytomegalovirus and Epstein-Barr virus replication on intestinal mucosal gene expression and microbiome composition of HIV-infected and uninfected individuals. AIDS. 2017;31(15):2059–2067. doi: 10.1097/QAD.0000000000001579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christensen-Quick A., Vanpouille C., Lisco A., Gianella S. Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res Hum Retroviruses. 2017;33:S23–S30. doi: 10.1089/aid.2017.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gianella S., Anderson C.M., Var S.R., Oliveira M.F., Lada S.M., Vargas M.V. Replication of human Herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol. 2016;90(8):3944–3952. doi: 10.1128/JVI.02638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson E.L., Howard C.L., Thurman J., Pontiff K., Johnson E.S., Chakraborty R. Cytomegalovirus upregulates expression of CCR5 in central memory cord blood mononuclear cells, which may facilitate in utero HIV type 1 transmission. J Infect Dis. 2015;211(2):187–196. doi: 10.1093/infdis/jiu424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barry P.A., Pratt-Lowe E., Peterlin B.M., Luciw P.A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarthy M., Auger D., He J., Wood C. Cytomegalovirus and human herpesvirus-6 trans-activate the HIV-1 long terminal repeat via multiple response regions in human fetal astrocytes. J Neurovirol. 1998;4(5):495–511. doi: 10.3109/13550289809113494. [DOI] [PubMed] [Google Scholar]
- 67.Murayama T., Ohara Y., Obuchi M., Khabar K.S., Higashi H., Mukaida N. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol. 1997;71(7):5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saleh S., Lu H.K., Evans V., Harisson D., Zhou J., Jaworowski A. HIV integration and the establishment of latency in CCL19-treated resting CD4(+) T cells require activation of NF-kappaB. Retrovirology. 2016;13(1):49. doi: 10.1186/s12977-016-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gianella S., Anderson C.M., Vargas M.V., Richman D.D., Little S.J., Morris S.R. Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis. 2013;207(6):898–902. doi: 10.1093/infdis/jis777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gianella S., Massanella M., Richman D.D., Little S.J., Spina C.A., Vargas M.V. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol. 2014;88(14):7818–7827. doi: 10.1128/JVI.00831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Stockenstrom S., Odevall L., Lee E., Sinclair E., Bacchetti P., Killian M. Longitudinal genetic characterization reveals that cell proliferation maintains a persistent HIV type 1 DNA Pool during effective HIV therapy. J Infect Dis. 2015;212(4):596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner T.A., McKernan J.L., Tobin N.H., Tapia K.A., Mullins J.I., Frenkel L.M. An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. J Virol. 2013;87(3):1770–1778. doi: 10.1128/JVI.01985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner T.A., McLaughlin S., Garg K., Cheung C.Y., Larsen B.B., Styrchak S. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345(6196):570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maldarelli F., Wu X., Su L., Simonetti F.R., Shao W., Hill S. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345(6193):179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohn L.B., Silva I.T., Oliveira T.Y., Rosales R.A., Parrish E.H., Learn G.H. HIV-1 integration landscape during latent and active infection. Cell. 2015;160(3):420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bui J.K., Sobolewski M.D., Keele B.F., Spindler J., Musick A., Wiegand A. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017;13(3) doi: 10.1371/journal.ppat.1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosmane N.N., Kwon K.J., Bruner K.M., Capoferri A.A., Beg S., Rosenbloom D.I. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med. 2017;214(4):959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lorenzi J.C., Cohen Y.Z., Cohn L.B., Kreider E.F., Barton J.P., Learn G.H. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc Natl Acad Sci U S A. 2016;113(49) doi: 10.1073/pnas.1617789113. (E7908-E16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henrich T.J., Hobbs K.S., Hanhauser E., Scully E., Hogan L.E., Robles Y.P. Human immunodeficiency virus type 1 Persistence following systemic chemotherapy for malignancy. J Infect Dis. 2017;216(2):254–262. doi: 10.1093/infdis/jix265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzon M.J., Sun H., Li C., Shaw A., Seiss K., Ouyang Z. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20(2):139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmueck-Henneresse M., Sharaf R., Vogt K., Weist B.J., Landwehr-Kenzel S., Fuehrer H. Peripheral blood-derived virus-specific memory stem T cells mature to functional effector memory subsets with self-renewal potency. J Immunol. 2015;194(11):5559–5567. doi: 10.4049/jimmunol.1402090. [DOI] [PubMed] [Google Scholar]
- 82.Mailliard R.B., Son Y.I., Redlinger R., Coates P.T., Giermasz A., Morel P.A. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171(5):2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 83.Abdel-Mohsen M., Chavez L., Tandon R., Chew G.M., Deng X., Danesh A. Human Galectin-9 is a potent mediator of HIV transcription and reactivation. PLoS Pathog. 2016;12(6) doi: 10.1371/journal.ppat.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones R.B., Mueller S., O'Connor R., Rimpel K., Sloan D.D., Karel D. A subset of latency-reversing agents expose HIV-infected resting CD4+ T-cells to recognition by cytotoxic T-lymphocytes. PLoS Pathog. 2016;12(4) doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yek C., Gianella S., Plana M., Castro P., Scheffler K., Garcia F. Standard vaccines increase HIV-1 transcription during antiretroviral therapy. AIDS. 2016;30(15):2289–2298. doi: 10.1097/QAD.0000000000001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christensen-Quick A., Chaillon A., Yek C., Zanini F., Jordan P., Ignacio C. Influenza vaccination can broadly activate the HIV reservoir during antiretroviral therapy. J Acquir Immune Defic Syndr. 2018;79(3) doi: 10.1097/QAI.0000000000001829. (e104-e7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perreau M., Savoye A.L., De Crignis E., Corpataux J.M., Cubas R., Haddad E.K. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210(1):143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. MDC1 are programmed to produce enhanced levels of IL-12p70 upon stimulation with CD40L. DC were plated (2.5 x 104 cells/well) in a 96-well flat-bottom plate and cultured in presence or absence of J558-CD40L (5 x 104 cells/well) for 24 hours. Culture supernatants were collected and tested by IL-12p70 ELISA. bd = below detection limit of the assay (37pg/ml).
Fig. S2. Selective killing of HIV-1 antigen-expressing CD4+ T cells by autologous MDC1-induced HIV-specific CTL. MDC1 generated from HIV-1-infected, ART-suppressed individuals were cultured with or without HIV-1 Gag peptides or with irrelevant control peptides (Influenza M1) and used to induce autologous CTL. a) CD4+ T cells were labeled with CellTrace™ dye (violet) or CFSE (green) for use as target cells. The CFSE-labeled cells were additionally loaded with relevant HIV-1 Gag 9-mer peptides. The cells were mixed in equal numbers and coincubated for 18 hours with MDC1-induced autologous CTL at various E:T ratios. b) The antigen-specific killing of HIV-1 peptide-loaded CD4+ T cells (green) was calculated based on relative changes in the percentages of the viable differentially colored target cells. c) p24-expressing HIV-1 infected target cells (T) were coincubated with autologous MDC1-induced effector CTL at various E:T ratios for 18 hours. CTL-induced target killing was measured by loss of HIV-1 Gag p24-expressing target cells using flow cytometry. d) Comparison of cytotoxic activity of HIV-specific CTL with CTL cultured in the absence of HIV-1 Gag peptides (Control CTL) in a representative donor (shown in a). Error bars indicate mean ± S.D.
Supplementary material