Abstract
Background
Sulfate availability is crucial for the sulfonation of brain extracellular matrix constituents, membrane phospholipids, neurosteroids, and neurotransmitters. Observations from humans and mouse models suggest dysregulated sulfate levels may be associated with neurodevelopmental disorders, such as autism. However, the cellular mechanisms governing sulfate homeostasis within the developing or adult brain are not fully understood.
Methods
We utilized a mouse model with a conditional allele for the sulfate transporter Slc13a4, and a battery of behavioral tests, to assess the effects of disrupted sulfate transport on maternal behaviors, social interactions, memory, olfaction, exploratory behavior, anxiety, stress, and metabolism. Immunohistochemistry examined neurogenesis within the stem cells niches.
Findings
The sulfate transporter Slc13a4 plays a critical role in postnatal brain development. Slc13a4 haploinsufficiency results in significant behavioral phenotypes in adult mice, notably impairments in social interaction and long-term memory, as well as increased neurogenesis in the subventricular stem cell niche. Conditional gene deletion shows these phenotypes have a developmental origin, and that full biallelic expression of Slc13a4 is required only in postnatal development. Furthermore, administration of N-acetylcysteine (NAC) within postnatal window P14-P30 prevents the onset of phenotypes in adult Slc13a4+/− mice.
Interpretation
Slc13a4 haploinsufficient mice highlight a requirement for adequate sulfate supply in postnatal development for the maturation of important social interaction and memory pathways. With evidence suggesting dysregulated sulfate biology may be a feature of some neurodevelopmental disorders, the utility of sulfate levels as a biomarker of disease and NAC administration as an early preventative measure should be further explored.
Keywords: Sulfate, Brain development, N-acetylcysteine, Slc13a4, Neurodevelopmental
Research in Context.
Evidence before this study
Sulfate conjugation regulates the biological activities of numerous substrates, and in the brain is an important metabolic biotransformation of neurotransmitters, extracellular matrix molecules and membrane phospholipids. Sulfate availability within the brain is maintained within a tight range, in keeping with an active transport process. While the sulfate transporter Slc13a4 is known to be expressed in brain, the precise cellular mechanisms governing brain sulfate homeostasis are not fully understood. Importantly, sulfate levels are highest during embryogenesis and infancy, reflecting an increased need for sulfate during the development of critical organ systems. The implications of altered sulfate availability for brain development and function remains an important and unanswered question.
Added value of this study
This study utilized transgenic mice with a conditional Slc13a4 allele to investigate the consequences of impaired sulfate transport within the brain for development and function. We found that Slc13a4 haploinsufficiency, which impairs sulfate uptake into the brain, results in significant behavioral and neurogenesis phenotypes in adult animals, including social interaction and long-term memory deficits. Importantly, conditional deletion of Slc13a4 using a tamoxifen-inducible Cre strategy demonstrated that full biallelic expression of Slc13a4 is only required in a narrow postnatal window, confirming the importance of sulfate availability for brain development. We also found that administration of the amino acid derivative N-acetylcysteine, which can be metabolized intracellularly into free sulfate, can prevent the onset of behavioral and neurogenesis defects, but only if given within the identified postnatal developmental window.
Implications of all the available evidence
Taken together, these data confirm the requirement for sulfate availability in the normal development and maturation of the brain. In the absence of adequate sulfate supply in times of high sulfate demand, the formation and maturation of pathways critical for social interaction and memory are disrupted, resulting in specific adult behavioral phenotypes and altered neurogenesis. Interestingly, dysregulated sulfate levels and handling has been reported in some autistic individuals, and Slc13a4 haploinsufficient mice have phenotypes which overlap with some dimensions of human and animal models of autism. This suggests that sulfate supply and availability, particularly during critical postnatal developmental periods, should be further investigated as a possible contributor to some of the social deficits seen in neurodevelopmental disorders such as autism.
Alt-text: Unlabelled Box
1. Introduction
Sulfate is an obligate nutrient required for numerous physiological processes during embryonic and postnatal development [14]. The conjugation of a sulfate group (sulfonation) is a metabolic biotransformation that alters the biological activity of numerous target molecules. For example, the degree and patterning of sulfonation on the glycosaminoglycan chains of extracellular matrix (ECM) molecules, such as heparan sulfate (HS) and chondroitin sulfate (CS), regulates growth factor binding within local environments and influences the steepness and shape of morphogen gradients throughout developing tissues [9]. Moreover, the sulfonation of drugs, steroids, peptide hormones and neurotransmitters alters their structure and function, often inactivating their biological activity [41]. These numerous and varied cellular sulfonation reactions are carried out by a wide array of substrate-specific sulfotransferase enzymes, although common to all these reactions is the use of a single sulfonate donor, 3′-phosphoadenosine-5′-phosphosulfate (PAPS). The bifunctional PAPS synthase enzyme generates PAPS from ATP and sulfate. To provide a sufficient supply of sulfate for these critical sulfonation reactions, cells either metabolize sulfur containing amino acids to release intracellular sulfate, or uptake inorganic sulfate across the plasma membrane via sulfate transporters [11].
Within the brain, sulfate availability is crucial for the sulfonation of ECM constituents, membrane phospholipids, neurosteroids, and neurotransmitters. ECM sulfonation is particularly important in brain development; the sulfonation of HS glycosaminoglycan (GAG) chains influences the binding of growth factors such as FGFs within the neural stem cell niche and can regulate neurogenesis [47], while the correct patterning of CS GAG chain sulfonation is critical for the assembly and function of perineuronal nets [27], which play a critical role in the stabilization and maturation of developmentally important synaptic connections and functions, and thus regulate windows of plasticity [37].
Interestingly, there is evidence to suggest that impaired sulfate metabolism is potentially associated with the neurodevelopmental condition autism spectrum disorder (ASD) [6,21,45]. Children with autism have been reported to have significantly lower free and total serum sulfate levels [1,6,19,20,46]. Significantly, Adams, et al. report lower serum sulfate levels to be among the most consistent differences observed in a study analyzing the broad metabolic status of children with autism, with 56% of ASD individuals within the study having serum sulfate levels below the neurotypical reference range. They also found a correlation between serum sulfate levels and autism severity [1]. In two studies by Geier DA et al., an approximate 50% reduction in serum sulfate levels was seen in all ASD subjects compared with age-matched controls ([20]; 2009a). To the best of our knowledge, few parameters measured in autistic individuals are affected within this high a percentage of study participants. While these studies are small, collectively analyzing just over 100 ASD individuals, together they show a consistent observation of reduced serum sulfate levels in ASD children. Moreover, a study of 232 ASD children, and 68 age-matched controls, found significantly higher (>50%) sulfate excretion in the urine of ASD children [45], while others have observed a reduced sulfonation capacity when compared to typically developing children [2,21,22,29,46]. Interestingly, a potential connection between altered heparan sulfate biology and autism has also been posited [33]. While no direct genetic associations between sulfate metabolism genes and ASD have yet been reported, altered sulfate availability and handling are known to be an indirect consequence of other factors such as drug metabolism or perturbations to oxidative stress pathways [11]. Impaired cellular uptake of sulfate is known to alter the sulfonation patterns of molecules, without directly altering sulfate metabolism genes [28], suggesting impaired sulfate availability in the developing brain could alter sulfonation reactions critical to important developmental processes such as axon migration, synaptogenesis and neurogenesis, and be a contributing factor to neurodevelopmental conditions such as intellectual disability and autism spectrum disorder.
While the cellular mechanisms governing sulfate homeostasis within the brain are not fully understood, the low ratio of cerebrospinal fluid/serum sulfate levels indicates a high barrier to, and selective transport of, sulfate to maintain brain levels within a tight range [12]. SLC13A4 is a sulfate transporter with expression largely restricted to the placenta and brain, and homozygous deletion of Slc13a4 leads to embryonic lethality [34]. A conditional genetic deletion strategy whereby Slc13a4 was deleted in embryos, but not extraembryonic tissues, resulted in normal embryonic development, revealing the essential role of placental SLC13A4 activity in embryonic development [34]. However, what role, if any, Slc13a4 plays in the brain is not known.
Since Slc13a4 deficient mice have a reported sulfate transport deficiency across the placenta, we hypothesized that impaired sulfate transport in the brain, due to a loss of Slc13a4, would result in behavioral and cellular phenotypes. In the current study we utilized Slc13a4 knockout mice to investigate the effects of impaired sulfate availability on brain development and function. Moreover, we used inducible Cre deleter mice combined with mice harboring a conditional Slc13a4 allele to test whether behavioral and cellular phenotypes had a developmental origin. Additionally, as the amino acid derivative N-acetylcysteine can be metabolized to free sulfate and has been shown to circumvent sulfate transporter deficiencies [28], we explored the possibility that administration of N-acetylcysteine could prevent or ameliorate resultant phenotypes in Slc13a4 deficient mice.
2. Materials and methods
2.1. Animals
All the experiments involving animals were approved by the University of Queensland Animal Ethics Committee (Ethics SBMS/147/17, SCMB/146/14, and SBMS/034/17/Breed), and were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All animals were housed under 12 h light/12 h dark conditions with free access to food and water. Slc13a4 strains [34] and UBC-CreERT2 mice [36] have been previously described. Wild-type C57BL/6 females were bred with Slc13a4+/− males (C57BL/6 background) to generate Slc13a4+/+ and Slc13a4+/− males and females for experiments. Offspring were weaned at postnatal day (P)21. For behavioral tests, all the animals were tested at 10–12 weeks of age, unless otherwise stated. Animals of each genotype and sex were taken from a minimum of four independent litters, and de-identified prior to behavioral experiments so that the experimenter was blind to the genotypes at the time of testing. The experimenter was also blinded to the treatment given to each animal in the case of NAC or sulfate administration. Sample sizes are in line with previous publications, and N values for individual experiments are listed in the figure legends. Animals were not randomly assigned to groups, as all animals of a particular sex from a given set of litters were used in experiments, and genotypes applied to the dataset after. The lighting in the behavioral testing rooms were 100 lx. BrdU (Sigma, USA) (50 mg/kg/injection) was administered to mice at 10–14 weeks of age via intraperitoneal (i.p.) injection. On the day of administration, animals were injected 5 times with BrdU, 2 h apart; 30 min after the final injection, mice were euthanized by cervical dislocation. Tamoxifen (Sigma, USA) was dissolved in 1% ethanol and sesame oil as a 10 mg/ml stock solution. For adult Slc13a4+/flx;UBC-CreERT2 and Slc13a4+/flx mice, 100 mg/kg tamoxifen was administered i.p. for 5 consecutive days. For neonatal animals, 50 mg/kg tamoxifen was injected i.p. for 4 consecutive days. Daily injections of 150 mg/kg NAC have previously been shown to rescue phenotypes in a glutamate transporter mutant mouse model after 4 weeks, showing this dose could effectively produce repletion of antioxidants in this model, and circumvent the loss of the glutamate transporter [10]. Moreover, NAC doses ranging from 50 to 200 mg/kg could provide protective effects against amyloid beta peptide-induced learning and memory deficits in mice [18]. Therefore, based on the efficacy of NAC these studies, we chose 150 mg/kg injected daily i.p. as the dose rate in our experiments, which were administered to mice i.p. once per day for 16 days, from P14-P30. The duration of the administration was based on our observations of the postnatal developmental window in which full Slc13a4 expression is required, plus the peak expression of sulfate transporters at P20 (see results section for details). For sodium sulfate (Na2SO4) administration, 250 mg/kg dissolved in PBS was administered to mice i.p. once per day for 16 days from P14-P30.
2.2. Genotyping
Genomic DNA (gDNA) was extracted from mouse toe samples using the KAPA mouse genotyping Kit (KAPA Biosystems, USA). 10 μl 10× KAPA lysis buffer, 2 μl KAPA enzyme and 88 μl RNase free H2O was added to each tissue sample and then lysed at 75 °C for 15 min, followed by a 5-min incubation at 95 °C. Primer sequences and PCR conditions for genotyping have been previously reported [34,36].
2.3. Pup retrieval test
The pup retrieval test was used to evaluate maternal behaviors and was performed as previously described [44]. Specifically, 12–14 week old females with their first litter were used to perform the test on postpartum day 2. Three pups were selected randomly and placed in the three corners in the home cage separately. After 30 min, mothers were re-introduced into the cage, the time the first pup was picked up was recorded, as well as the time to retrieve all the pups up to 5 min. If the female failed to retrieval all the pups within 5 min, the time of retrieval was recorded as 300 s.
2.4. Nest building test
Individual mice were placed in a fresh cage without environmental enrichment. 3 g of Nestlet material was placed in the cage before the dark phase. The nest was examined and scored (from 1 to 5) the next morning and the weight of the nest and the unshredded Nestlet material was recorded and compared between the groups, as previously described [16].
2.5. Resident-intruder test
Animals of age 10–12 weeks of age were housed in separate cages for 5 days to create resident cages. On the sixth day, a stranger mouse (intruder, non-littermate to the resident mouse) was introduced into the resident cage. The time the resident mouse spent in interacting with the intruder was recorded and compared between the groups [24].
2.6. Social memory test
The social memory test is divided into two phases, the familiar mouse phase and novel mouse phase. The familiar phase includes 4 trials, one per day for 4 days. For trial 1, a novel mouse is put into a dominant cage. The time that the dominant mouse spent sniffing the intruder within 1 min was recorded. For trials 2 to 4 the same mouse was introduced to the same cage for the same amount of time. Day 5 was the stranger phase, where a novel mouse was introduced into the dominant cage and time of sniffing/interaction from the dominant mouse was recorded [23].
2.7. Open field test
Mice were transferred to the test room in their home cages 1 h before the test. A standard apparatus constructed of grey acyclic measuring 40 cm × 40 cm with 30 cm walls was used. Lines on the floor divided the area into 16 squares (10 cm × 10 cm each). A camera was suspended over the apparatus to record the movement of the mice during the test sessions. Central square entries and duration, peripheral area entries and duration, corner area duration, rearing frequency, and self-grooming times were recorded. The freezing time in the center of the apparatus at the beginning of the test was excluded from the central duration.
2.8. Elevated plus maze test
The elevated plus maze was constructed of grey acyclic and consisted of four arms with a removable base. All the arms were of the same length (25 cm) and width (5 cm), although two of them were open without walls and the other two had walls of 10 cm height. The arms joined in the middle with a square of 5 cm × 5 cm. The removable base lifted the apparatus up 40 cm from the floor. A camera was suspended over the apparatus during the 10-min test.
Mice for the experiment were transferred to the test room in their home cages 1 h before the test to habituate to the environment. The mice were placed in the central square facing one of the open arms at the beginning of the test and allowed to explore the apparatus for 10 min. During the test, the following indexes were recorded: the entries/duration of the open arm, the entries/duration of the closed arm, the entries/duration of the central square, the distance travelled in the first 5 min and the total distance travelled during the entire test. Mice might exhibit freezing behavior in the open arm at the beginning of the test, which was excluded from the open arm duration.
2.9. Phenomaster home cage metabolic system
The Phenomaster is a metabolic home-cage system which was used to evaluate mouse movements and metabolism status during a 10-day experimental session. Body weight, food and water intake, movement (X and Y axis and fine movements) were recorded every hour during the test. Body fat composition was determined at the end of the experiment using a Buker Minispec NMR analyzer [35,42].
2.10. Novel object recognition (NOR)
The apparatus used was the same as the open field test. The assay was performed as previously described [4,43]. Discrimination index (DI) = (Tobject C - Tobject A)/ (Tobject C + Tobject A).
2.11. Odor habituation/dishabituation
Water, 1% vanilla abstract, 1% mint abstract, social scent 1, social scent 2 were used in this experiment. Social scents “1” and “2” were collected from two different cages by using cotton sticks to swab on the bottom of cages to collect scents of unfamiliar mice. On the day of the experiment, mice were carried to the test room and habituated in a new cage for 15 min. Cotton sticks soaked with water, 1% vanilla abstract, 1% mint abstract, social scent 1, social scent 2 were inserted into the cage through cage lids sequentially without touching the lid. The same odor was repeated three times and the time that mice sniffed the cotton sticks was recorded. The whole experiment included 15 sections with 3 min for each test with a 1 min break in-between [48].
2.12. Food bury test
The food bury test involves a 4-day protocol. On day 1 and day 2, a small piece of cookie was introduced into the cage. The mice were allowed to habituate the scent of the cookie. 24 h before the test, all of the food was removed from the cage including the leftover cookie. On the experimental day, mice were first placed in a clean cage for 5 min and then were transferred to another clean cage with a piece of cookie buried under the bedding. The time mice spent in finding the snack was recorded. The test duration was 3 min. If mice failed to find the snack within 3 min, it was recorded as a failure [48].
2.13. Forced swim test
Mice for the experiment were carried into the test room in their home cages 1 h before the test. A plastic beaker was filled with water of 25 °C to a depth of 15 cm. The mice were put into the beaker for 5 min. During the experiment, the time the mouse floated in the beaker versus swimming was recorded.
2.14. qPCR
RNA was extracted from tissue using Trizol (Life technologies, Australia) following the manufacturer's instructions, quantified using a Nano Drop and checked for integrity by electrophoresis on a 1.5% agarose gel. cDNA was synthesized from 1 μg of total RNA using the Quantitect cDNA transcription kit (Qiagen, USA) according to the manufacturer's instructions.
For each qPCR plate (MicroAmp Fast Optical plates, 0.1 ml, Applied Biosystems), RNase free H2O and a mixed cDNA “Bucket” was used as negative control and positive control, respectively. Each 10 μl qPCR reaction contained 50 ng (1 μl) cDNA, 5 mM primer mix (1 μl) (Rn18s: Forward: GTAACCCGTTGAACCCCATT and Reverse: CCATCCAATCGGTAGTAGCG; Slc13a4: Forward: GGAAGCTGCTATTGGTCATCTG; Reverse: GGTCACAAGCAACACGTAAGC), 5 μl SYBR Green Master Mix (Qiagen, USA/ Bio Rad) and 3 μl RNase free H2O. Each sample was run in triplicate (technical replicates), and at least 3 independent samples were used (biological replicates). qPCR was performed using a 384-well QuantiStudio7 PCR machine (Life Technologies and analyzed by the ΔΔCt method.
2.15. In situ hybridization (ISH)
ISH were performed as previously described [15]. Briefly, 7 μm paraffin sections mounted on SuperFrost Plus slides were dewaxed and rehydrated through xylene and an ethanol gradient to PBS. All solutions used up to the hybridization step were DEPC treated to ensure RNAse-free conditions. Slides were post-fixed with 4% PFA-PBS and then treated with 10 mg/ml Proteinase K (Roche, USA) for 20 min at room temperature. Before being incubated with the hybridization buffer, the slides were acetylated with 0.25% (v/v) acetic anhydride (Sigma, USA) in 0.1 M triethanolamine (Sigma, USA) buffer for 10 min. 200 μl of hybridization cocktail (Amresco, USA) containing either sense or antisense Digoxigenin-labeled RNA probes (diluted 1:2000 from the original probe synthesis reaction –made using DIG RNA labelling mix (Roche) according to the manufacturer's instructions) was added to each section (or 350 μL hyb/probe mixture for a whole slide). Slides were incubated at 65 °C overnight in a chamber humidified with 50% formamide/1× SSC. On the second day, slides were washed with post hybridization buffer (×2 for 30 min each), followed by MABT (×2 for 30 min each) and incubated with 20 μg/ml RNase A in 1 × RNA wash for 30 min at 37 °C. Slides were then washed in 1 × MABT for 5 min and incubated with blocking solution for 1 h at room temperature. Anti-DIG antibody was diluted 1:2500 in blocking solution and each slide was incubated with 300 μl antibody solution at 4 °C overnight. On the third day, slides were washed with 1 × MABT four times for 15 min each, followed by NTMT wash containing 0.05% (w/v) Levamisole (Sigma, USA) for 10 min at room temperature. 300 μl NBT/BCIP solution (Promega, USA) was put on each slide. Colour was developed in the dark at room temperature until purple stained was evident. The reaction was stopped by washing slides in PBS. Slides were counterstained with Nuclear fast red (Sigma, USA) before being dehydrated, cleared in xylene and mounted under DPX. Slides were scanned using an Aperio slide scanner and visualized using the Aperio ScanScope software.
2.16. Choroid plexus transfection
Choroid plexuses (ChP) were isolated from the lateral ventricle and 4th ventricle of adult brains and cultured in DMEM with 10% FBS (Thermo Fisher, Australia) under 5%CO2, at 37 °C overnight. The following day, ChPs were transferred gently to serum free DMEM medium and transfected using Lippofectamine™ 2000 following the manufacturer's instructions (Invitrogen, USA). 12 h after transfection, ChP was fixed with 4% PFA and embedded in OCT. 10 μm cryo sections were used for double immunofluorescence for GFP (DSHB, USA) and ATP1A1 (DSHB, USA).
2.17. Immunohistochemistry and immunofluorescence
For paraffin sections, deparaffinized slides were rehydrated to ddH2O, and placed in an antigen retriever (Antigen Retriever 2100; Electron Microscopy Sciences, Australia) with citric acid buffer (pH 6.0). Slides were then cooled to room temperature and washed with PBS. For cryosections, slides were brought to room temperature and washed with PBS 3 times. Both paraffin and cryosections were incubated with blocking buffer (10% donkey serum) for 1 h at room temperature. Primary antibodies (Table 2.4) were diluted in blocking buffer and incubated with the sections at 4 °C overnight (GFP, 1:50, DSHB; ATP1A1, 1:100, Novas; BrdU, 1:200, DSHB). On the second day, slides were washed with PBST, 5 min × 3 and incubated with secondary antibody, which was diluted in PBST, for 1 h at room temperature, avoiding light. Slides were counterstained with DAPI and mounted with 70% glycerol. Images were captured by Lecia DMi8 confocal microscopy or Olympus BX61 fluoresce microscope and analyzed by Image J. For SLC13A4, a TSA amplification kit (Perkin Elmer, USA) was used according to the manufacture's instructions (SLC13A4, 1:100, ProteinTech).
To quantify BrdU+ cell numbers, every 12th 8 μm sagittal serial section was collected from lateral 0.60 mm to lateral 1.80 mm (relative to Bregma). All the images were captured by Lecia DMi8 confocal microscopy or Olympus BX61 fluoresce microscope and analyzed by Image J.
2.18. Radioactive sulfate uptake assay
400 μCi of Na235SO4 was diluted with PBS to 200 μl and injected into adult Slc13a4+/+ and Slc13a4+/− mice through the tail vein. 5 min following the injection, mice were euthanized to collect brain tissues (olfactory bulb and cerebellum were removed). 1 ml solvable solution (Perkin Elmer) was added to every 100 mg wet brain tissue in glass centrifuge tubes and incubated overnight at 37 °C. The next day, the tubes were gently shaken to homogenize the tissue and 100 μl 30% H2O2 was added to the tube. This step was repeated once when no visible bubble can be observed. 200 μl of the mixture was added to 1.5 ml scintillation cocktail (Perkin Elmer, USA) and placed at room temperature for 30 min before being analyzed in the scintillation counter (Perkin Elmer, USA) [34].
2.19. Statistical analysis
Quantitative data is presented as mean ± standard error of the mean, analyzed and graphed using GraphPad Prism 6.0. Student's t-test was performed to analysis data between two groups. Welch's correction was performed if a significant difference was evident in F test. One-way ANOVA was used to analyze the data among multiple individual groups (more than two groups), followed by Bonferroni post hoc test. Repeated measurement two-way ANOVA was performed to analysis the data for the odor habitation experiment and social memory test, followed by Bonferroni's multiple comparisons test. P values and sample sizes are listed in the figure legends for each individual experiment.
3. Results
3.1. Slc13a4 expression and function within the brain
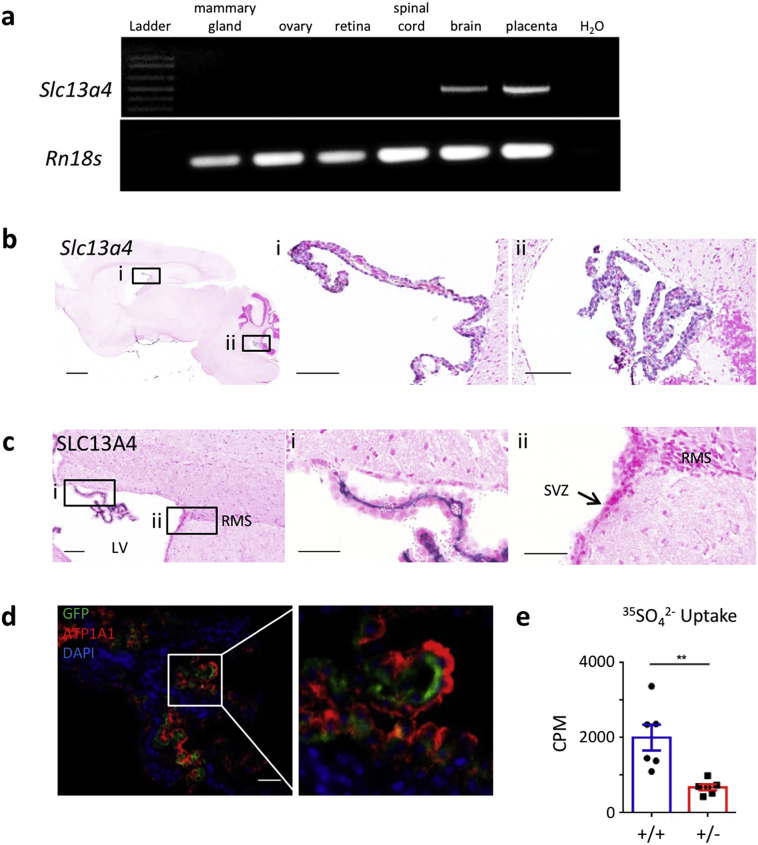
We confirmed previous observations that Slc13a4 mRNA expression is largely confined to the placenta and brain (Fig. 1a). Within the brain, SLC13A4 expression is found within all the four choroid plexuses and the pia mater (Fig. 1b–c). Immunostaining for the apically expressed ATP1A3 in GFP-tagged-SLC13A4 transfected choroid plexuses reveals a complementary expression pattern, confirming the basolateral membrane expression of SLC13A4 in choroid plexus epithelial cells seen using an SLC13A4 antibody (Fig. 1c–d). This cellular localization suggests SLC13A4 regulates uptake of sulfate from the blood into choroid plexus epithelium. To test this, we injected radio-labeled sulfate (35SO42−) into the tail vein and found that significantly less radio-tracer accumulated in the brains of Slc13a4+/− mice compared to their wildtype littermates (Fig. 1e). Therefore, SLC13A4 is likely to play an important role in brain sulfate uptake and availability.
Fig. 1.
SLC13A4 expression in the brain. (a) Slc13a4 is expressed in the placenta and brain. (b) Slc13a4 expression within all the four choroid plexuses (ChP) and the pia mater by ISH, choroid plexus of the lateral (i) and 4th ventricle (ii) shown in higher magnification. Sense probe negative control can be seen in Supplemental fig. S4d. (c) SLC13A4 protein expression in the basolateral membrane of ChP epithelium (i), but not in other cell types within the lateral ventricle (ii). (d) Isolated ChPs transfected with EGFP-SLC13A4 plasmid in vitro. Immunofluorescence for GFP (green), fused to SLC13A4, is complementary to ATP1A1 (red), a known marker for the apical membrane of ChP epithelium. (e) Five minutes following injection of 400 μCi 35SO42− into the tail vein, isolated Slc13a4+/− brains contained significantly lower radioactive counts than Slc13a4+/+ brains (each genotype n = 6, two-tailed t-test, Welch's correction, **P = .0022). Black scale bar = 100 μm, white scale bar = 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Slc13a4 haploinsufficiency results in the onset of atypical behavioral phenotypes
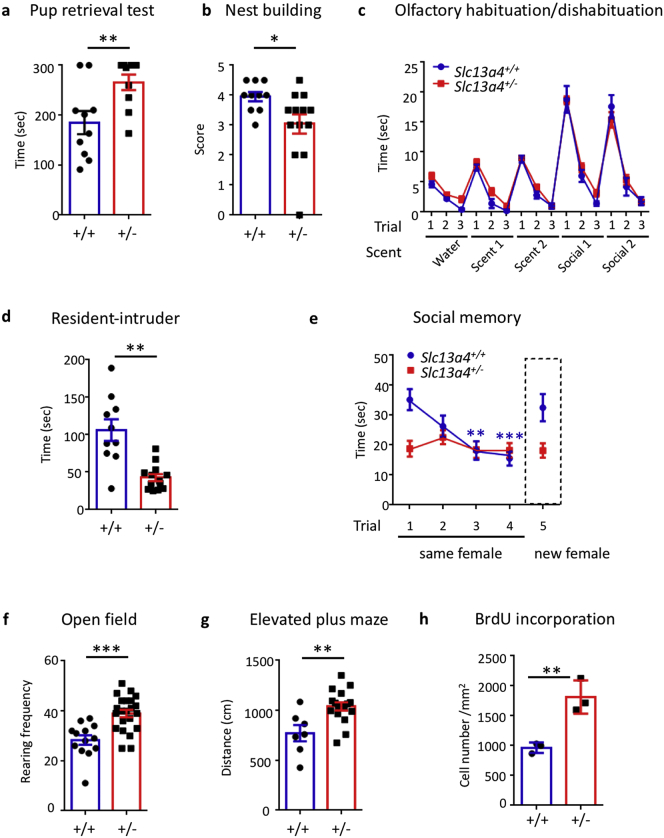
Our first indication of the functional relevance of SLC13A4 activity for animal behavior came from the observation that female Slc13a4+/− mice mated with a wildtype male had smaller litter sizes at postpartum day 2 (P2) compared to wildtype females mated with Slc13a4+/− males, despite these two crosses having the same genotypic makeup of pups, and normal litter sizes and pup weights at birth (Fig. S1a). This suggested that loss of pups between P0-P2 may be due to deficits in maternal behavior. Indeed, Slc13a4+/− females took longer to retrieve their pups in a pup retrieval test than wildtype littermate controls (Fig. 2a), and virgin Slc13a4+/− females show impaired nest building capability (Fig. 2b). We tested olfaction in Slc13a4+/− dams, as the olfactory system is essential for maternal behaviors such as nest building and pup retrieval [8]. However, we did not observe any gross differences in olfaction between Slc13a4+/− and wildtype controls (Fig. 2c, S1b). Since Slc13a4+/− dams also had normal gross mammary gland morphology (data not shown) we hypothesized that these aberrant maternal behaviors could reflect cognitive deficits in these mice.
Fig. 2.
Behavioral and adult neurogenic phenotypes of Slc13a4 haploinsufficient mice. (a) Slc13a4+/− females took longer to retrieve pups compared to Slc13a4+/+ females (Student's t-test, P = .0099, n = 10). (b) Slc13a4+/− females built poorer nests (Mann-Whitney test, P = .0191, n = 10 Slc13a4+/+, n = 13 Slc13a4+/−). (c) No differences in olfaction between genotypes (Two-way repeated ANOVA with Bonferroni post hoc test, n = 8 Slc13a4+/+, n = 17 Slc13a4+/−). (d) Slc13a4+/− mice spent significantly less time investigating the intruder in the resident-intruder test. Student's t-test, P = .0017 (n = 10 Slc13a4+/+, n = 13 Slc13a4+/−). (e) Slc13a4+/− females did not recognize the same intruder on sequential days, nor respond to a novel intruder on day 5 a social memory test. Asterisks represent significant changes over time (compared with trial 1) within the same genotype (Two-way repeated ANOVA with Bonferroni post hoc test, n = 10 Slc13a4+/+, n = 13 Slc13a4+/−). (f) There was a statistically significant increase in the rearing frequency between wildtype and Slc13a4+/− mice in the open field test (Student's test with Welch's correction, P = .0017, n = 10 Slc13a4+/+, n = 13 Slc13a4+/−). (g) Slc13a4+/− mice travelled further during the elevated plus maze test (Student's t-test, P = .003, n = 7 Slc13a4+/+, n = 17 Slc13a4+/−). (h) An increase in BrdU+ cells was observed in the V-SVZ of Slc13a4+/− brains (Student's t-test, P = .0072, n = 3 each genotype). All the data mean ± SEM. *P < .05, **P < .01, ***P < .001.
3.3. Slc13a4 haploinsufficiency results in the onset of atypical behavioral and neurogenic phenotypes
Most strikingly, Slc13a4 haploinsufficiency led to decreased social interaction with strangers (Fig. 2d) and an apparent impaired social memory (Fig. 2), although since Slc13a4+/− mice spent significantly less time interacting with another mouse, either familiar or new, in the social memory paradigm, it is unclear whether social memory per se is affected, or rather this represents simply a social interaction deficit. A significant impairment in long-term memory retention was evident in Slc13a4+/− mice however, as well as a trend towards short-term memory retention, as seen in the novel object recognition test (Fig. S2a). Slc13a4+/− mice also showed increased exploratory behavior in a novel environment, such as that provided by the Open Field or Elevated Plus Maze tests (Fig. 2f-g, see Fig. S2b-c), but this behavior was not observed long-term in a familiar environment, such as in a 10-day analysis within the Phenomaster system (Fig. S3a). No differences were observed in metabolic status, motor function, or depression levels (Fig. S3a-c). Therefore, the spectrum of behavioral deficits in Slc13a4+/− mice are relatively restricted to social interaction and long-term memory phenotypes, and haploinsufficiency for Slc13a4 does not affect the overall health of the animals.
As we observed a robust peak of Slc13a4 mRNA expression in the brains of pregnant wildtype mice around gestational day 8 (Fig. S4a), a time that coincides with a pregnancy-specific increase in adult neurogenesis [39], we investigated whether altered neurogenesis was also present in Slc13a4+/− mice. Increased proliferation was evident within the ventricular-subventricular zone (V-SVZ) in non-pregnant adult Slc13a4+/− mice (Fig. 2h, S5) compared to controls, although no gross differences in brain size or morphology were observed (data not shown). Interestingly, we observed decreased immunofluorescence for the sulfated-heparan sulfate (epitope 10E4) in the V-SVZ of Slc13a4+/− mice (Fig. S6), suggesting altered ECM sulfonation within the stem cell niche. While these phenotypes were originally observed in female mice, male Slc13a4+/− mice also displayed all the same phenotypes (Fig. S7).
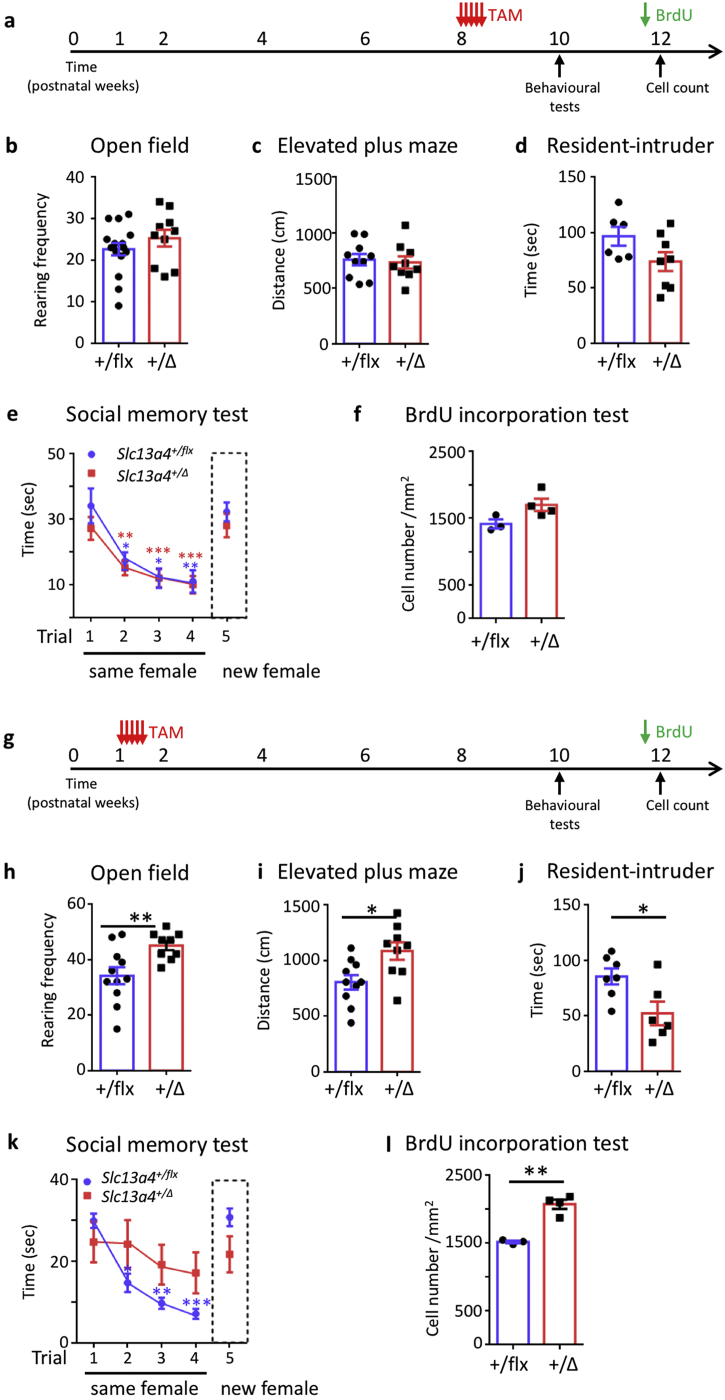
3.4. Slc13a4 activity is required during a postnatal developmental window only
As the expression of Slc13a4 gene expression is low in embryonic development and in non-pregnant adults, but peaks during postnatal development (Fig. S4b-c), we asked if the phenotypes observed in Slc13a4+/− mice arise from alterations in postnatal development. To address this question, we used a conditional strategy to delete Slc13a4 at different developmental time points. Slc13a4flx/flx females were crossed with a ubiquitous tamoxifen-inducible Cre recombinase deleter strain (UBC-CreERT2), and Cre excision of Slc13a4 following tamoxifen administration was confirmed by both genotyping PCR and qPCR (Fig. S8). Slc13a4 was first deleted in Slc13a4+/flx;UBC-CreERT2 adult mice at 8-weeks of age (Fig. 3a), however, no behavioral or V-SVZ proliferation phenotypes were observed at either 10–12 weeks (Fig. 3a-f), or later at 18–20 weeks (Fig. S9), suggesting a lack of Slc13a4 activity in adulthood does not contribute to the phenotypes we originally observed. Critically, when Slc13a4 was deleted earlier, in postnatal development prior to the peak of Slc13a4 mRNA expression at P20, the abnormal behaviors and increased cell proliferation in adulthood (P70) originally observed in Slc13a4+/− mice were recapitulated (Fig. 3g-l). Therefore, Slc13a4 activity is essential during a critical postnatal developmental window, and reduced Slc13a4 expression in this window results in the onset of atypical phenotypes in adulthood.
Fig. 3.
Behavioral phenotypes of tamoxifen injected Slc13a4+/flx;UBC-CreERT2 and Slc13a4+/flx mice. (a) 8-week old mice were injected with tamoxifen once per day for 5 days and behavioral tests were performed at 10 weeks of age. (b) No difference in rearing between groups in the open field test (P = .288, n = 16 Slc13a4+/flx, n = 10 Slc13a4+/Δ). (c) No difference in total distance travelled during the test phase of the elevated plus maze between genotypes (n = 10 Slc13a4+/flx, n = 9 Slc13a4+/Δ). (d) No significant defect in the resident-intruder test between genotypes (P = .121, n = 6 Slc13a4+/flx, n = 7 Slc13a4+/Δ). (e) In the social memory test, no differences in social interactions were observed between the genotypes. Asterisks represent significant changes over time (compared with trial 1) within the same genotype (n = 6 Slc13a4+/flx, n = 7 Slc13a4+/Δ). (f) No difference was found in BrdU+ cell numbers within V-SVZ between genotypes (P = .069, n = 3 Slc13a4+/flx, n = 4 Slc13a4+/Δ). (g) Tamoxifen was administered to P7 Slc13a4+/flx;UBC-CreERT2 and Slc13a4+/flx mice for 5 days. Behavioral tests were performed at 10 weeks (P70). (h) A significant increase in rearing frequency was detected in Slc13a4+/Δ mice compared with Slc13a4+/flx mice (P = .0081, n = 6 Slc13a4+/flx and n = 7 Slc13a4+/Δ). (i) Increased total distance travelled in elevated plus maze was observed in Slc13a4+/Δ mice (P = .0249, n = 6 Slc13a4+/flx and n = 7 Slc13a4+/Δ). (j) Slc13a4+/Δ mice showed decreased time in social interaction with intruders in the resident-intruder test (P = .0219, n = 7 Slc13a4+/flx and n = 6 Slc13a4+/Δ). (k) In the social memory test, Slc13a4+/Δ mice did not show the normal trend of decreasing interaction time with the same intruder over multiple days (Trials 1–4), or significantly more time when a new intruder was introduced in trial 5 (n = 7 Slc13a4+/flx and n = 6 Slc13a4+/Δ). (l) BrdU+ cell counts were significantly increased in the adult V-SVZ at P84 in Slc13a4+/Δ mice administered tamoxifen at P7 (P = .0011, n = 3 Slc13a4+/flx and n = 4 Slc13a4+/Δ). B, C, D, F, H, I, J, L - Student's t-test, E, K - Two-way repeated ANOVA with Bonferroni post hoc test. Data are mean±SEM. *P < .05, **P < .01.
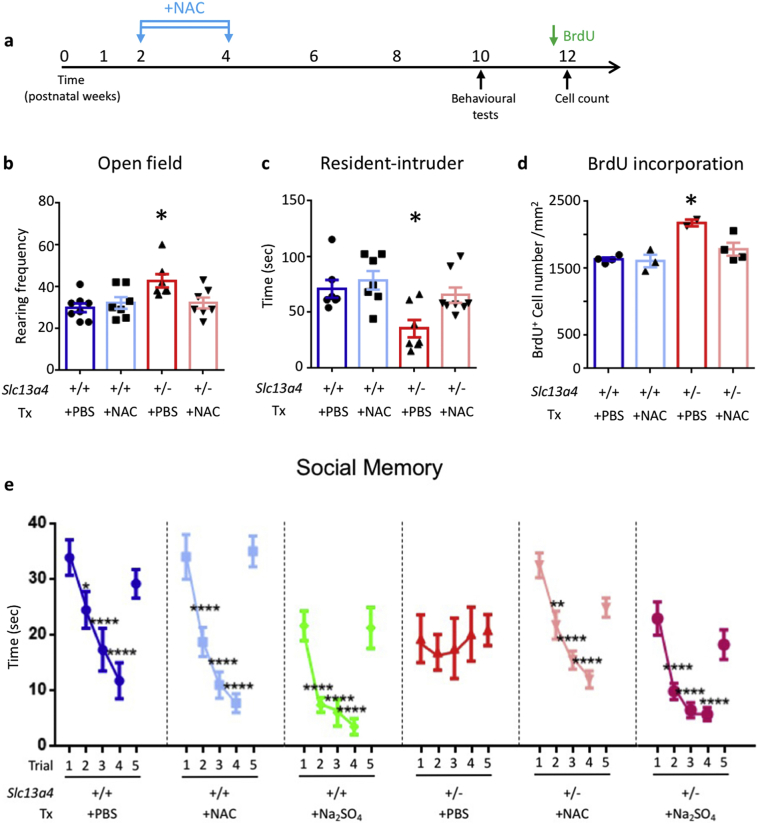
3.5. N-acetylcysteine administration during a postnatal window prevents the onset of phenotypes
N-acetylcysteine (NAC), a derivative of the amino acid cysteine, has previously been trialed for the treatment of various psychiatric and neurological disorders mainly due to its role as an antioxidant and the association of oxidative stress with these conditions (NAC is a precursor for the synthesis of glutathione) [26]. However, sulfur-containing amino acids and their derivatives, such as NAC, can also be metabolized to sulfate [11]. We therefore posited that administration of NAC during the critical developmental window may circumvent sulfate transporter deficiency and restore sulfate availability, thereby ameliorating the development of atypical behaviors in Slc13a4+/− mice.
To test this, we administered NAC (150 mg/kg NAC; i.p.) to Slc13a4+/+ and Slc13a4+/− mice for 16 days either during the critical postnatal period (P14-P30) or after the critical developmental window at 8–10 weeks of age, and then tested these cohorts of mice in our panel of behavioral and cellular assays. Adult mice treated with NAC did not show significant improvement in any of the phenotypes previously reported (Fig. S10). Remarkably, NAC administration to Slc13a4+/− mice from P14 to P30 prevented the onset of atypical phenotypes in 10–12 week-old adults (Fig. 4a-e), with the exception of the total distance travelled in the elevated plus maze (Fig. S11). In order to elucidate whether the effects of NAC reflect sulfate repletion, we also treated a cohort of mice with sodium sulfate (Na2SO4–250 mg/kg; i.p.). Na2SO4 administration from P14–30 also prevented the acquisition of observed behavioral phenotypes in adult haploinsufficient animals (Fig. 4 and Fig. S11).
Fig. 4.
Behavioral and cellular phenotypes of Slc13a4+/− adult mice administered NAC between P14-P30. (a) NAC (150 mg/kg) or PBS was administered to Slc13a4+/+ and Slc13a4+/− mice from P14-P30. Behavioral tests were performed from 10 weeks of age, followed by BrdU incorporation. (b) Slc13a4+/−mice administered PBS (+PBS) showed increased rearing frequency in the open field test compared with Slc13a4+/+ +PBS mice, which was prevented in Slc13a4+/−mice by administration of NAC (+NAC). Administration of NAC to Slc13a4+/+ mice had no effect. (c) Slc13a4+/− + PBS treated mice spent significantly less time exploring the intruder in the resident-intruder test, while no significant difference was evident among Slc13a4+/+ +PBS, Slc13a4+/+ +NAC and Slc13a4+/− + NAC groups. (d) Slc13a4+/− + PBS treated mice showed increased cell proliferation within V-SVZ compared to all other groups. (e) In the social memory test, Slc13a4+/− + PBS mice spent the same amount of time interacting with the same intruder, while mice from other groups spent less time interacting with the same intruder through trials 1–4. Slc13a4+/− + PBS mice spent the same amount of time interacting with a novel intruder in trial 5, while mice from other groups spent significantly more time interacting with the novel intruder. Adult mice administered sodium sulfate (Na2SO4–250 mg/kg) postnatally (P14–30) displayed the same pattern of social memory as wildtype mice or Slc13a4+/− mice administered NAC. Asterisks represent significant changes over time (compared with trial 1) within the same genotype. Two-way repeated ANOVA with Bonferroni post hoc test was performed for social memory test and one-way ANOVA was used for other tests. N = 7–9 per cohort for behavioral tests and n = 4 for BrdU analysis. *P < .05, **, P < .01, ***P < .001, ****P < .0001.
4. Discussion
In the current study, we describe a pivotal role for SLC13A4 activity in brain development. Specifically, haploinsufficiency of Slc13a4 leads to the acquisition of several atypical phenotypes in adulthood, in particular significant deficits in social interaction and long-term memory. Several observations suggest the phenotypes observed in Slc13a4+/− mice stem from altered sulfate availability at a time of high sulfate demand. Firstly, SLC13A4 is expressed within the choroid plexus and pia mater at the interface between the CSF and the blood supply. Secondly, adult Slc13a4+/− mice accumulate less radiolabeled sulfate within the brain than Slc13a4+/+ mice following injections into the peripheral circulation (via the tail vein). Finally, expression levels for Slc13a4, and other sulfate transporters (data not shown) are significantly elevated at P20, with much lower levels of expression in embryonic and adult brains. SLC13A4 therefore likely plays an important role in brain sulfate availability in postnatal development.
The elevated expression of sulfate transporters in postnatal development suggests Slc13a4 function might be most important during critical windows of brain formation. We therefore used a conditional deletion strategy to ascertain whether the phenotypes we see in Slc13a4+/− mice have a developmental origin. As expected, the deletion of one Slc13a4 allele in adulthood did not result in the acquisition of atypical phenotypes, although deletion of Slc13a4 in the first postnatal week recapitulated all the phenotypes in adult animals that we had previously observed. Therefore, full Slc13a4 activity is essential only during an early postnatal developmental window. This critical postnatal window overlaps with several described critical periods (CP), which are stages of increased neuroplasticity essential for the maturation of experience-dependent neuronal circuits. Some of the mechanisms thought to participate in closing these CPs, such as the establishment of perineuronal nets (PNNs), are dependent on sulfate chemistry [27].
Importantly, changes in sulfate transport that alter cellular sulfonation reactions can affect not only sulfonation of ECM constituents, but also the metabolism of neurotransmitters and neuroendocrine peptides [41]. Therefore, the molecular mechanisms that link impaired sulfate transport within the CP and impairments in social interactions and other atypical phenotypes seen in Slc13a4+/− mice may be wide-ranging and potentially multifactorial. Nevertheless, impairments in ECM sulfonation, such as required for the establishment of PNNs, is an attractive hypothesis deserving of further investigation. This is because PNN formation is dependent not only on the presence of chondroitin sulfate (CS), but specifically influenced by the degree/patterning of CS sulfonation [27]. Alterations in PNN formation as a cause of the behavioral phenotypes in Slc13a4+/− mice would align well with our data defining a postnatal requirement for SLC13A4 activity. These structural brakes on plasticity have been suggested to play a role in the etiology of autism, a prominent neurodevelopmental disorder, with the premature closing of critical periods a proposed feature of the autistic phenotype [5].
Of the neurodevelopmental disorders, the strongest potential link with abnormal sulfate chemistry is autism; children diagnosed with ASD have lower serum sulfate levels [1,6,19,46], higher sulfate excretion [45], and reduced sulfonation capacity [2,21,22,29,46]. Additionally, the BTBR T + tf/J mouse strain also exhibits significantly lower serum sulfate levels [13]. Moreover, a potential connection between heparan sulfate biology and autism has been posited [33], with decreased heparan sulfate expression within the V-SVZ observed in both autistic children [31], and BTBR T + tf/J mice [25]. Increased neurogenesis within the V-SVZ has also been observed in the brains of ASD mouse models [3,30] and young human autistic individuals [31]. Both of these phenotypes were observed in Slc13a4+/− mice. Consequently, the Slc13a4+/− mouse model may be of significance to ASD, as it shares some commonalities with several ASD mouse models, the most obvious being the profound impairments in social interactions. However, it should be noted that Slc13a4+/− phenotypes do not overlap with all dimensions of human autism or mouse models of ASD, as we did not observe repetitive grooming behaviors, nor did we test for impaired vocal communications. The phenotypes of Slc13a4+/− mice may be specific to social and memory pathways, which are commonly found in several neurodevelopmental disorders. For example, the deficits in object recognition seen in Slc13a4+/− mice are found not only in mouse models of ASD, but also models of intellectual disability and schizophrenia that present with social interaction deficits, such as En2 [7] and the 16.p11.2 deletion [40] mice. Additionally, it is important to note that genetic associations between sulfate metabolism genes and ASD have yet to be reported. However, serum sulfate levels, and thus sulfate availability, can be impacted by numerous pathways and physiological conditions independent of changes to genes directly responsible for handling sulfate metabolism, such as transporters or sulfotransferases. As a consequence of drug metabolism or alterations in the oxidative stress pathways for example, organismal sulfate levels can vary greatly, affecting downstream sulfonation reactions without changes to the sulfonation and transport machinery itself. Therefore, it is possible that consistent changes in sulfate availability could be a downstream consequence of numerous ASD-linked pathways. In this light, Slc13a4 mice can be viewed not as a model of genetic ASD per se, but rather one that demonstrates how altered sulfate availability affects brain development and function, resulting in some overlapping phenotypes relevant to ASD and other neurodevelopmental disorders.
NAC has previously been trialed for the treatment of several psychiatric disorders, including autism, mainly due to its role as an antioxidant and regulator of neurotransmitter pathways [17,26]. However, much less appreciated is the role of NAC as a potential source of intracellular sulfate; sulfur-containing amino acids and their derivatives can be metabolized to inorganic sulfate as well as the antioxidant glutathione [11]. When extracellular sulfate levels are low, or cellular uptake is impaired, sulfur-containing amino acids can act as sources of intracellular sulfate for sulfonation reactions. Indeed, Luca and colleagues demonstrated that NAC administration could rescue the decrease in cartilage ECM sulfonation that underlies chondrodysplasia phenotypes in mice null for the sulfate transporter Slc26a2 [28,32]. Therefore, we hypothesized that NAC, if administered during the postnatal developmental window we identified, could prevent the onset of cellular and behavioral phenotypes in adults by circumventing impaired sulfate transport and providing a source of sulfate for critical sulfonation reactions. Excitingly, when NAC was administered to Slc13a4+/− mice from P14-P30, they did not develop any of the previously observed phenotypes in adulthood. Not surprisingly, when NAC was administered to Slc13a4+/− adults, no significant improvement in behavioral deficits or neurogenesis was observed. This result supports the notion that full Slc13a4 expression is required for brain development during a critical postnatal window (P14–30), and that NAC, administered within this window, can prevent the onset of social impairments and perturbations to neurogenesis. Previous trials of NAC administration for the treatment of autism in human children have met with disappointing results, with only modest improvements in irritability and self-harm and no significant improvements in the core symptom of social impairment [26]. However, the children trialed in these studies ranged in age from 3 to 12. Our data reveal a critical developmental window in Slc13a4+/− mice ~P20, which may be more analogous to ~1–4 years of age in humans in terms of ongoing developmental processes [38]. Therefore, if impaired sulfate availability underlies some forms of social interaction phenotypes in ASD, NAC may have been administered too late in human trials to have an appreciable effect. Furthermore, we treated animals with sodium sulfate from P14–30, and the impairments in behavior were also prevented in adult Slc13a4+/− mice, suggesting that NAC is likely to be an important source of sulfate, rather than as a source of antioxidants.
5. Conclusions
Our study identifies SLC13A4 as a critical regulator of early postnatal brain development. Haploinsufficiency of Slc13a4 leads to atypical phenotypes in mice that display a developmental origin similar to the onset of neurodevelopmental disorders in humans such as ASD. Our study supports the view that sulfate levels as a biomarker for neurodevelopmental disorders that include social impairments should be further explored. Our research highlights the importance of neurodevelopmental windows, which should be more fully considered when designing interventions in the clinic. Excitingly, administration of NAC, a commonly used, safe and well-tolerated amino acid derivative, within a defined developmental window, prevented the onset of atypical behaviors in adult Slc13a4+/− animals. It is tempting therefore to speculate that better outcomes for the amelioration of social impairments in humans with neurodevelopmental disorders, such as ASD, might be gained through NAC administration as early as 1–2 years-old, should sulfate deficiencies underlie the etiology of these phenotypes.
Acknowledgments
Acknowledgements
The authors would like to thank the animal house staff at the University of Queensland for excellent technical assistance. Thank you also to A/Prof Paul Baldock and Jackson labs for access to the UBC-ERT2Cre mice. The authors acknowledge Dr. Sean Millard for helpful discussions and contributions to the manuscript, Dr. Lachlan Harris for technical assistance and intellectual input for the V-SVZ analysis.
Data and materials availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding sources
The authors thank the following institutions for funding: NHMRC to D. Simmons (APP1130255) and ARC support to A/Prof Piper (DP160100368; DP180100017).
Declaration of interests
Dr. Simmons has nothing to disclose.
Dr. Zhang has nothing to disclose.
Dr. Dawson has nothing to disclose.
Dr. Piper has nothing to disclose.
Author contributions
Z.Z. conceptualized and conducted the experiments, performed formal data analysis and co-wrote the original manuscript; D.G.S. conceptualized the experiments, lead project supervision and wrote the manuscript; M.P. had a supervisory role and contributed to the initial draft, review and editing of the manuscript; P.D. had a supervisory role and reviewed and edited the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.081.
Appendix A. Supplementary data
Supplementary material
References
- 1.Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond) 2011;8:34. doi: 10.1186/1743-7075-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti A., Pirrone P., Elia M., Waring R.H., Romano C. Sulphation deficit in “low-functioning” autistic children: a pilot study. Biol Psychiatry. 1999;46:420–424. doi: 10.1016/s0006-3223(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 3.Amiri A., Cho W., Zhou J., Birnbaum S.G., Sinton C.M., McKay R.M. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci. 2012;32:5880–5890. doi: 10.1523/JNEUROSCI.5462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger J.M., Rohn T.T., Oxford J.T. Autism as the early closure of a neuroplastic critical period normally seen in adolescence. Biol Syst Open Access. 2013;1:1–7. doi: 10.4172/2329-6577.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowling F.G., Heussler H.S., McWhinney A., Dawson P.A. Plasma and urinary sulfate determination in a cohort with autism. Biochem Genet. 2013;51:147–153. doi: 10.1007/s10528-012-9550-0. [DOI] [PubMed] [Google Scholar]
- 7.Brielmaier J., Matteson P.G., Silverman J.L., Senerth J.M., Kelly S., Genestine M. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunjes P.C. Lessons from lesions: the effects of olfactory bulbectomy. Chem Senses. 1992;17:729–763. [Google Scholar]
- 9.Bülow H.E., Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- 10.Cao L., Li L., Zuo Z. N-acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience. 2012;220:85–89. doi: 10.1016/j.neuroscience.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole D.E., Evrovski J. The clinical chemistry of inorganic sulfate. Crit Rev Clin Lab Sci. 2000;37:299–344. doi: 10.1080/10408360091174231. [DOI] [PubMed] [Google Scholar]
- 12.Cole D.E., Shafai J., Scriver C.R. Inorganic sulfate in cerebrospinal fluid from infants and children. Clin Chim Acta. 1982;120:153–159. doi: 10.1016/0009-8981(82)90086-9. [DOI] [PubMed] [Google Scholar]
- 13.Corley M.J., Meyza K.Z., Blanchard D.C., Blanchard R.J. Reduced sulfate plasma concentrations in the BTBR T+tf/J mouse model of autism. Physiol Behav. 2012;107:663–665. doi: 10.1016/j.physbeh.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson P.A. Role of sulphate in development. Reproduction. 2013;146:R81–R89. doi: 10.1530/REP-13-0056. [DOI] [PubMed] [Google Scholar]
- 15.Dawson P.A., Rakoczy J., Simmons D.G. Placental, renal, and ileal sulfate transporter gene expression in mouse gestation. Biol Reprod. 2012;87:43. doi: 10.1095/biolreprod.111.098749. [DOI] [PubMed] [Google Scholar]
- 16.Deacon R.M.J. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 17.Dean O., Giorlando F., Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu A.-L., Dong Z.-H., Sun M.-J. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Geier D.A., Kern J.K., Garver C.R., Adams J.B., Audhya T., Geier M.R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009;34:386–393. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- 20.Geier D.A., Kern J.K., Garver C.R., Adams J.B., Audhya T., Nataf R. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Hartzell S., Seneff S. Impaired sulfate metabolism and epigenetics: is there a link in autism? Entropy. 2012;14:1953–1977. [Google Scholar]
- 22.Horvath K., Perman J.A. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14:583–587. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Jin D., Liu H.-X., Hirai H., Torashima T., Nagai T., Lopatina O. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 24.Koolhaas J.M., Coppens C.M., de Boer S.F., Buwalda B., Meerlo P., Timmermans P.J.A. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyza K.Z., Blanchard D.C., Pearson B.L., Pobbe R.L.H., Blanchard R.J. Fractone-associated N-sulfated heparan sulfate shows reduced quantity in BTBR T+tf/J mice: a strong model of autism. Behav Brain Res. 2012;228:247–253. doi: 10.1016/j.bbr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minarini A., Ferrari S., Galletti M., Giambalvo N., Perrone D., Rioli G. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13:279–292. doi: 10.1080/17425255.2017.1251580. [DOI] [PubMed] [Google Scholar]
- 27.Miyata S., Komatsu Y., Yoshimura Y., Taya C., Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15 doi: 10.1038/nn.3023. 414–22– S1–2. [DOI] [PubMed] [Google Scholar]
- 28.Monti L., Paganini C., Lecci S., De Leonardis F., Hay E., Cohen-Solal M. N-acetylcysteine treatment ameliorates the skeletal phenotype of a mouse model of diastrophic dysplasia. Hum Mol Genet. 2015;24:5570–5580. doi: 10.1093/hmg/ddv289. [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly B.A., Waring R.H. Enzyme and sulphur oxidation deficiencies in autistic children with known food/chemical intolerances. J Orthomolecular Med. 1993;8:198–200. [Google Scholar]
- 30.Packer A. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci Biobehav Rev. 2016;64:185–195. doi: 10.1016/j.neubiorev.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Pearson B.L., Corley M.J., Vasconcellos A., Blanchard D.C., Blanchard R.J. Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav Brain Res. 2013;243:138–145. doi: 10.1016/j.bbr.2012.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pecora F., Gualeni B., Forlino A., Superti-Furga A., Tenni R., Cetta G. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochem J. 2006;398:509–514. doi: 10.1042/BJ20060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez C., Sawmiller D., Tan J. The role of heparan sulfate deficiency in autistic phenotype: potential involvement of slit/Robo/srGAPs-mediated dendritic spine formation. Neural Dev. 2016;11 doi: 10.1186/s13064-016-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakoczy J., Zhang Z., Bowling F.G., Dawson P.A., Simmons D.G. Loss of the sulfate transporter Slc13a4 in placenta causes severe fetal abnormalities and death in mice. Cell Res. 2015;25:1273–1276. doi: 10.1038/cr.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson L., Plano A., Cobb S., Riedel G. Long-term home cage activity scans reveal lowered exploratory behaviour in symptomatic female Rett mice. Behav Brain Res. 2013;250:148–156. doi: 10.1016/j.bbr.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz N.B., Domowicz M.S. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018;9:89. doi: 10.1002/1873-3468.13026. [DOI] [PubMed] [Google Scholar]
- 38.Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 40.Stoppel L.J., Kazdoba T.M., Schaffler M.D., Preza A.R., Heynen A., Crawley J.N. R-baclofen reverses cognitive deficits and improves social interactions in two lines of 16p11.2 deletion mice. Neuropsychopharmacology. 2018;43:513–524. doi: 10.1038/npp.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strott C.A. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 42.Tschöp M.H., Speakman J.R., Arch J.R.S., Auwerx J., Brüning J.C., Chan L. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel-Ciernia A., Wood M.A. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014;69 doi: 10.1002/0471142301.ns0831s69. 8.31.1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z., Storm D.R. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36:772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waring R.H., Klovrza L.V. Sulphur metabolism in autism. J Nutr Environ Med. 2000;10:25–32. [Google Scholar]
- 46.Waring R.H., Ngong J.M., Klovrza L., Green S., Sharp H. Biochemical parameters in autistic children. Dev Brain Dsyfunct. 1997;10:40–43. [Google Scholar]
- 47.Yamaguchi Y. Heparan sulfate proteoglycans in the nervous system: their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin Cell Dev Biol. 2001;12:99–106. doi: 10.1006/scdb.2000.0238. [DOI] [PubMed] [Google Scholar]
- 48.Yang M., Crawley J.N. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0824s48. Chapter 8, Unit 8.24–8.24.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material