Abstract
Purpose
In our previous study, we demonstrated that both titrated extract of Centella asiatica (TECA) and astaxanthin (AST) have anti-inflammatory effects in a 5% phthalic anhydride (PA) mouse model of atopic dermatitis (AD). The increasing prevalence of AD demands new therapeutic approaches for treating the disease. We investigated the therapeutic efficacy of the ointment form of TECA, AST and a TECA + AST combination in a mouse model of AD to see whether a combination of the reduced doses of 2 compounds could have a synergistic effect.
Methods
An AD-like lesion was induced by the topical application of 5% PA to the dorsal ear and back skin of an Hos:HR-1 mouse. After AD induction, TECA (0.5%), AST (0.5%) and the TECA (0.25%) + AST (0.25%) combination ointment (20 μg/cm2) were spread on the dorsum of the ear or back skin 3 times a week for 4 weeks. We evaluated dermatitis severity, histopathological changes and changes in protein expression by Western blotting for inducible nitric oxide synthase (iNOS), cyclocxygenase (COX)-2, and nuclear factor (NF)-κB activity. We also measured the concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and immunoglobulin E (IgE) in the blood of AD mice by enzyme-linked immunosorbent assay (ELISA).
Results
PA-induced skin morphological changes and ear thickness were significantly reduced by TECA, AST and TECA + AST treatments, but these inhibiting effects were more pronounced in the TECA + AST treatment. TECA, AST and the TECA+AST reatments inhibited the expression of iNOS and COX-2; NF-κB activity; and the release of TNF-α, IL-6 and IgE. However, the TECA+AST treatment showed additive or synergistic effects on AD.
Conclusions
Our results demonstrate that the combination of TECA and AST could be a promising therapeutic agent for AD by inhibiting NF-κB signaling.
Keywords: Astaxanthin, atopic dermatitis, inflammation, titrated extract of Centella asiatica, NF-κB
INTRODUCTION
Atopic dermatitis (AD) is a complex chronic inflammatory skin disease which is characterized by hypersensitivity against various types of antigens1 as well as poorly defined erythema with edema, eczematous lesions and lichenification, along with elevated plasma levels of immunoglobulin E (IgE), interleukin (IL)-4, IL-13, eosinophils and mast cells.2 Currently, anti-inflammatory drugs, such as corticosteroids, immunosuppressants and calmodulin inhibitors, are considered the first line of treatment, due to their established effectiveness.3 These drugs control the development of AD by inhibiting several immune responses, but the risk of prolonged use of these drugs has been demonstrated in several studies.4,5 Local and systemic side effects, such as itching and development of skin infections, occur with topical corticosteroids.4
Nuclear factor (NF)-κB is a key transcription factor that regulates the expression of genes in immune and inflammatory responses inducing the transcription of various pro-inflammatory genes.5 It promotes the transcription of the Ig kappa chain and cytokines such as IL-1, IL-2, IL-6, IL-8 and interferon gamma.6,7 NF-κB is often activated in tissues in autoimmune diseases including AD.8 Facilitated translocation of NF-κB may exacerbate allergic inflammation by increasing inflammatory cytokines and chemokines.9 Therefore, a compound that suppresses NF-κB activation could act as a therapeutic agent for allergic disorders. Topical application of dehydroxymethylepoxyquinomicin (DHMEQ) ointment has been found to suppress allergic inflammation via its NF-κB inhibition on AD-like lesions in NC/Nga mice.10 Tanaka et al.11 demonstrated that the NF-κB inhibitor IMD-0354 suppressed the abnormal proliferation of mast cells and reduced the allergic responses in AD mice. Thus, inhibition of NF-κB could be a good strategy for AD treatment.
We previously demonstrated that topical treatments of titrated extract of Centella asiatica (TECA), a traditional herbal medicine, and astaxanthin (AST; 3,3′-dihydroxy-β, β-carotene-4,4′-dione), a type of xanthophylls, both have an anti-inflammatory effect on AD through inhibiting NF-κB.9,12 Oxidative stress is critical for inflammatory skin damage and NF-κB activation.9 Because AST has strong antioxidant effects,9,12 a combination of TECA and AST could synergistically be effective even at lower doses are administered. In this study, we investigated the combined effect of lower doses of TECA and AST ointment against AD as well as the NF-κB-involved mechanism in a phthalic anhydride (PA)-induced AD mouse model. The TECA + AST treatment more effectively inhibited NF-κB signals, pro-inflammatory cytokine production, and the expression of inducible nitric oxide synthase (iNOS) and cyclocxygenase (COX)-2 in a PA-induced AD animal model compared to the TECA or AST treatment alone. Therefore, we suggest that the TECA + AST treatment could be considered candidate combination agents for AD therapy.
MATERIALS AND METHODS
Ethical approval
The experimental protocols were carried out according to the guidelines for animal experiments of the Institutional Animal Care and Use Committee (IACUC) of Laboratory Animal Research Center at Chungbuk National University, Korea (CBNUA-929-16-01). All efforts were made to minimize animal suffering and to reduce the number of animals used. All mice were housed in 3 mice per cage with automatic temperature control (21°C–25°C), relative humidity (45%–65%) and 12-hour light-dark cycle illuminating from 08:00 a.m. to 08:00 p.m. Food and water were available ad libitum. They were fed pellet diet consisting of crude protein 20.5%, crude fat 3.5%, crude fiber 8.0%, crude ash 8.0%, calcium 0.5%, phosphorus 0.5% per 100 g of the diet (collected from Daehan Biolink, Eumseong-gun, Korea). During the study, all mice were specially observed for body posture, piloerection, ataxia, urination, etc 2 times per day.
Animal treatment
The protocols for the animal experiment used in this study were carefully reviewed for ethical and scientific care procedures and approved by the Chungbuk National University-IACUC (approval No. CBNUA-1073-17-01). Hos:HR-1 mice (8-week-old, n = 40) were randomly divided into 1 of the 4 groups. In the first group (PA, n = 10), 100 μL (10 μL/cm2) of 5% PA solution was spread on the dorsal ear and back skin 3 times a week for 4 weeks. The second group (TECA, n = 10), the third group (AST, n = 10) and the forth group (TECA + AST, n = 10) were applied with PA, and 3 hours after TECA (0.5%), AST (0.5%) and the TECA (0.25%) + AST (0.25%) combination ointment (20 μg/cm2) were applied, respectively. Age-matched Hos:HR-1 mice were used as the control group (Con, n = 10).
Scratching behaviors and histological techniques
The number of scratching in 10 minutes was blindly counted by the investigators. Generally, the mice scratched several times with their hind paws for 1 second, and these movements were counted as 1 scratching. The dorsal ear and back skin tissues were removed from mice, fixed with 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 5-μm thick slices. The skin sections were then stained with hematoxylin and eosin (H&E). The thickness of the epidermis and dermis were also measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
Mast cells were detected by staining with toluidine blue. After deparaffinization and dehydration, ear and back skin sections were stained with 0.25% solution of toluidine blue (Sigma-Aldrich, St. Louis, MO, USA) and examined by light microscopy for the presence of mast cells. The number of mast cell per specific area was measured with Leica Application Suite (Leica Microsystems).
Enzyme-linked immunosorbent assay (ELISA) for the detection of serum IgE concentration
The serum IgE concentration was measured using an ELISA kit (Shibayagi Inc., Gunma, Japan) according to the manufacturer's instructions. Briefly, capture antibodies were plated on the Nunc C bottom immunoplate supplied in the kit. Next, wells were washed 3 times with washing solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0). Then, serum samples and standards diluted with buffer solution were added to the wells, and the plate was incubated for 2 hours. The wells were washed again with washing solution, 50 µL of biotin-conjugated anti-IgE antibodies (1,000-fold dilution) were added to each well and incubated further for 2 hours to bind with captured IgE. The wells were washed again with washing solution, after which horseradish peroxidase-conjugated detection antibodies (2,000-fold dilution) were added to each well and incubated for 1 hour. After that, an enzyme reaction was initiated by adding tetramethylbenzidine substrate solution (100 mM sodium acetate buffer pH 6.0, 0.006% H2O2) and incubating the plate at room temperature in the dark for 20 minutes. Finally, the reaction was terminated by adding acidic solution (reaction stopper, 1 M H2SO4), and absorbance of yellow product is measured spectrophotometrically at 450 nm. The final concentration of IgE was calculated using the standard curve.
Cytokine assay
By the end of the study period, blood specimens were collected. Serum levels of mouse tumor necrosis factor (TNF)-α and IL-6 were measured by ELISA kits provided by Thermo Fisher scientific Inc. (Rockford, IL, USA) according to the manufacturer's protocol.
Western blot analysis
The dorsal ear and back skin tissues (100 mg) or about 1 × 106 cells were harvested and homogenized with lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.2% sodium dodecyl sulfate [SDS], 1 mM phenyl methylsulfonyl fluoride [PMSF], 10 μL/mL aprotinin, 1% igapel 630 [Sigma Chem Co. St. Louis, MO, USA], 10 mM NaF, 0.5 mM EDTA, 0.1 mM EGTA and 0.5% sodium deoxycholate). The extracts were centrifuged at 23,000 g for 1 hour. An equal amount of protein (20 μg) was separated on a SDS/10%-polyacrylamide gel, and then transferred to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). Blots were blocked for 2 hours at room temperature with 5% (w/v) non-fat dried milk in tris-buffered saline (10 mM Tris [pH 8.0] and 150 mM NaCl) solution containing 0.05% tween-20. The membrane was incubated for 4 hours at room temperature with specific antibodies: Mouse monoclonal antibodies directed against c-Jun N-terminal kinase (JNK), phosphor-JNK, p38, phosphor-p38, extracellular signal-regulated kinase (ERK), phosphor-ERK (1:1,000) (Cell Signaling Technology, Beverly, MA, USA), and against p50 (1:500) (Santa Cruz Biotechnology Inc.), and rabbit polyclonal antibodies against iNOS, COX-2, p65 and IκB-α (1:500) (Santa Cruz Biotechnology Inc.) were used in the study. The blot was then incubated with the corresponding conjugated anti-rabbit IgG-horseradish peroxidase (Santa Cruz Biotechnology Inc.). Immunoreactive proteins were detected with the enhanced chemiluminescent western blotting detection system.
Cell culture
The RAW 264.7 murine macrophage cell line was obtained from the Korea Cell Line Bank (Seoul, Korea). These cells were grown at 37°C in Dulbecco's Modified Eagle's medium supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin sulfate (100 μg/mL) in humidified atmosphere of 5% CO2. Cells were incubated with TECA (5 μg/mL), AST (10 μM), and TECA (2.5 μg/mL) + AST (5 μM) or positive chemicals and then stimulated with lipopolysaccharides (LPS) 1 μg/mL for the indicated time in figure legends. The final concentration of ethanol used was less than 0.05%. Cells were treated with 0.05% ethanol as the vehicle control.
Reporter gene assay
RAW 264.7 cells were plated at 1 × 105 cells/well on 24-well culture plate and transiently transfected with NF-κB-luciferase reporter (Affymetrix Inc., Santa Clara, CA, USA) or pNF-κB-luciferase reporter (Stratagene California, La Jolla, CA, USA) using Lipofectamine® LTX & PLUS in OPTI-MEM media (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instruction. The transfected cells were treated with LPS (1 μg/mL) in the absence or presence of various concentrations of TECA, AST and TECA + AST for 24 hours. Reporter gene activity was assayed using the luciferase assay kit (Promega Co., Madison, WI, USA), measured by a luminescence counter (Wallac Victor2 1420; PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
The experiments were conducted in triplicate, and all experiments were repeated at least 3 times with similar results. All statistical analyses were performed using the GraphPad Prism 5 software (version 5.03; GraphPad software, Inc., San Diego, CA, USA). Group differences were analyzed by 1-way analysis of variance followed by Tukey's multiple comparison test. All values are presented as mean ± standard deviation. Significance was set at P < 0.05 for all tests.
RESULTS
Effects of the TECA + AST treatment on dorsal ear skin thickness and morphology
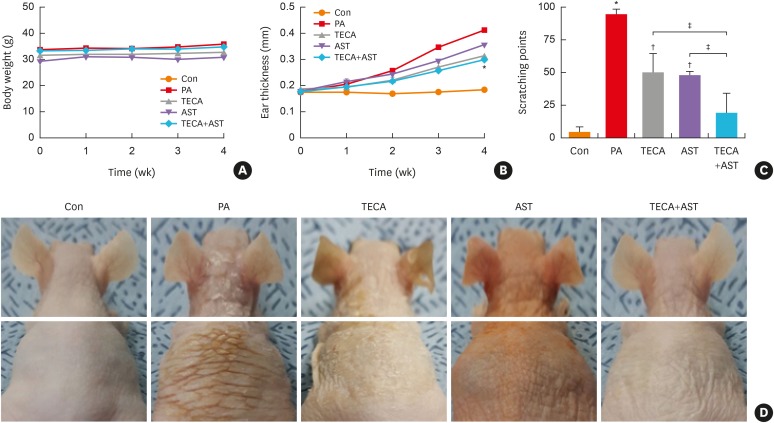
Changes in body weight were measured during the experimental period. No significant difference in body weight was detected after any treatment (Fig. 1A). To investigate whether the TECA + AST treatment suppressed changes in ear phenotype induced by PA treatment, the thickness and morphology of the dorsal ear skin were observed. The increase in dorsal ear skin thickness was more rapid in PA-treated mice than in control mice. However, it only slowly increased in TECA-, AST-, and TECA + AST-treated mice (Fig. 1B). Following the sequential application of PA to the mice, they vigorously scratched their lesional skins with their hind paws. Scratching behaviors in the PA group were markedly exacerbated by up to 100 behavioral points at the end of the experiment, but it was reduced by the TECA or AST treatment alone and more significantly reduced by the TECA + AST treatment (Fig.1C). Furthermore, unlike control mice, PA-treated mice showed erythema, edema and erosion. These changes in the dorsal ear and back skin morphologies and dorsal ear skin thickness were dramatically inhibited upon TECA, AST and TECA + AST treatment, but the TECA + AST treatment was more effective than the TECA or AST treatment alone (Fig. 1D).
Fig. 1. Differences in body weight, dorsal ear skin thickness and phenotypes, as well as back skin phenotypes. Body weights of mice in the 5 groups were measured using a chemical balance (A). PA solution was repeatedly applied to the dorsal ear and back skin during the topical application of Centella asiatica phytosome. After 4 weeks, dorsal ear skin thickness (B), the number of times scratching at the end of the application experiment (C) and phenotypes (D) were observed following the procedure described in Materials and Methods.
Data shown are the mean ± standard deviation (n = 10).
Con, control; PA, phthalic anhydride; TECA, titrated extract of Centella asiatica; AST, astaxanthin.
*P < 0.05 is the significance level compared to the control group. †P < 0.05 is the significance level compared to the PA treatment group. ‡P < 0.05 is the significance level compared to the TECA or AST treatment alone group.
Effect of the TECA + AST treatment on inflammatory responses in dorsal ear and back skin
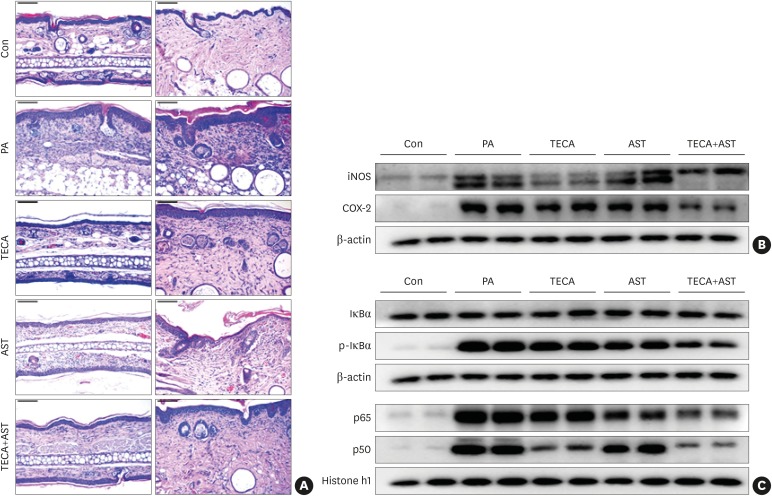
To investigate the suppressive effect of the TECA + AST treatment, histological analysis of the dorsal ear and back skin was performed (Fig. 2A). There was no significant difference in the thickness of the epidermis and dermis, but immune cell infiltration was observed in the PA-treated group compared with the control group. These changes in the dorsal ear and back skin histology (immune cell infiltration) were reversed upon TECA, AST and TECA + AST treatments, but the TECA + AST treatment was more effective than the TECA or AST treatment alone.
Fig. 2. Histopathological analysis of dorsal ear and back skin tissues and the anti-inflammatory effects of the TECA + AST treatment through inhibiting NF-κB in the back skin. Histopathology of dorsal ear and back skin tissues (A). PA solution was repeatedly applied to the dorsal ear and back skin during the topical application of TECA, AST and the TECA+AST combination. Histopathological changes were examined in the slide sections of dorsal ear and back skin tissues by staining with H&E followed by observation at 200× magnification (scale bars, 100 μm). Alterations in the expression of iNOS and COX-2 proteins of the back skin were measured by Western blotting (B). The effect of TECA, AST and the TECA + AST combination on NF-κB (p50 and p65) subunit translocation into the nucleus and IκBα phosphorylation in back skin cytosol (C). Equal amounts of nuclear proteins (20 μg/lane) or total proteins (20 μg/lane) were subjected to 10% SDS-PAGE, and the expressions of p50, p65, IκBα and p-IκBα proteins were detected by Western blotting using specific antibodies. Histone h1 protein and β-actin protein were used here as internal controls. Data shown are the mean ± standard deviation (n = 10).
Con, control; TECA, titrated extract of Centella asiatica; AST, astaxanthin; NF-κB, nuclear factor-κB; PA, phthalic anhydride; H&E, hematoxylin and eosin; iNOS, inducible nitric oxide synthase; COX, cyclooxygenase; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
In addition, the protein expression of iNOS and COX-2 was significantly up-regulated in PA-treated mice. The expression was significantly suppressed in TECA- and AST-treated mice, but the suppressive effects were greater in the TECA+AST-treated mice (Fig. 2B).
Effect of the TECA + AST treatment on p50, p65 and p-IκB expression in PA-induced AD mice
NF-κB is implicated in inflammatory responses in AD. To investigate whether the TECA+AST treatment could inhibit NF-κB activation more than the TECA or AST treatment alone, a nuclear extract from back skin tissue was prepared and assayed for a western blot (Fig. 2C). As shown in Fig. 2C, PA-treated mice showed significant IκBα degradation in the cytosolic fraction when compared to control group mice in the back skin. However, IκBα degradation was reduced significantly more in TECA, AST and TECA + AST-treated mice than in PA-treated mice. PA-treated mice also showed increased nuclear expression of p65 and p50. While TECA, AST and the TECA + AST treatments inhibited the translocation of p65 and p50 into the nucleus, the TECA and AST treatment more clearly inhibited p65 and p50 as well as p-IκB expression (Fig. 2C).
Effect of the TECA + AST treatment on IgE concentration and the release of inflammatory cytokines
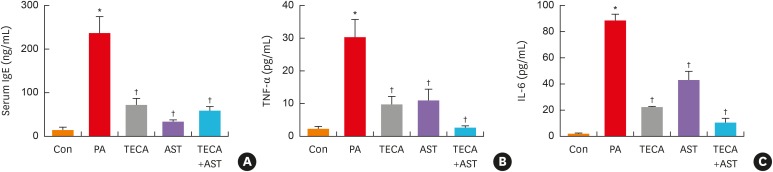
To determine if the TECA + AST treatment could alter IgE concentration and inflammatory cytokine release in PA-induced skin inflammation, the levels of IgE, TNF-α and IL-6 were measured in mouse sera of the control, PA, TECA, AST and TECA + AST treatment groups. The levels of IgE, TNF-α and IL-6 were generally higher in the PA treatment group than in the control group. However, these levels in the TECA, AST and TECA + AST treatment groups were dramatically decreased to those in the control group (Fig. 3). These decreasing effects were the most significant in the TECA + AST-treated group.
Fig. 3. Changes in serum cytokine concentration. After the final treatment, mice from each group were euthanized under anesthesia. The serum used to measure the cytokine concentration was prepared from blood samples collected from the abdominal veins of mice. Serum IgE (A), TNF-α and IL-6 (B) concentrations were quantified by ELISA. Data shown are the mean ± standard deviation (n = 10).
Con, control; PA, phthalic anhydride; TECA, titrated extract of Centella asiatica; AST, astaxanthin; IgE, immunoglobulin E; TNF, tumor necrosis factor; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
*P < 0.05 is the significance level compared to the control group. †P < 0.05 is the significance level compared to the PA treatment group.
Effect of the TECA + AST treatment on mast cell infiltration
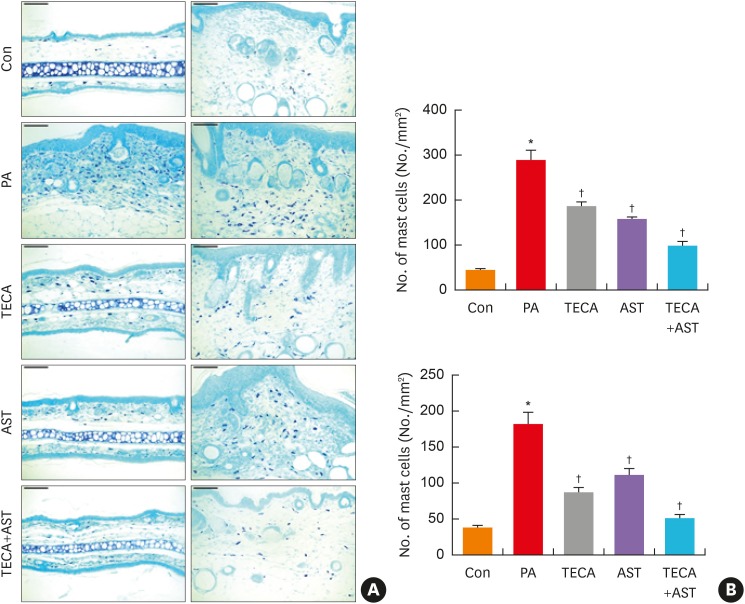
Mast cells play important roles in AD. To investigate the suppressive effect of the TECA + AST treatment on mast cell infiltration, both ear and back skin were stained with toluidine blue, and the alteration of mast cells was observed under a microscope. Mast cells stained blue were significantly greater in number in the PA-treated group compared to the control group for both dorsal ear and back skin (Fig. 4A). However, their numbers were significantly reduced in the TECA, AST and TECA + AST treatment groups for both dorsal ear and back skin (Fig. 4B). These decreasing effects were the most significant in the TECA + AST-treated group. These data demonstrate that the TECA + AST treatment may contribute to the suppression of mast cell infiltration in the skin of PA-induced AD more than the TECA or AST treatment alone.
Fig. 4. The inhibition of mast cell infiltration by topical application of the TECA + AST combination in dorsal ear and back skin. Mast cell infiltrations in dorsal ear and back skin (A). Slide sections of dorsal ear and back tissues were examined by staining with 0.25% toluidine blue followed by observation at 200× magnification (scale bars, 100 μm). The number of infiltrated mast cells per specific area was measured as described in Materials and Methods (B). Data shown are obtained from the same mice treated as shown in Fig. 1. Data shown are the mean ± standard deviation (n=10). *P < 0.05 is the significance level compared to the control group.
Con, control; PA, phthalic anhydride; TECA, titrated extract of Centella asiatica; AST, astaxanthin.
*P < 0.05 is the significance level compared to the control group. †P < 0.05 is the significance level compared to the PA treatment group.
Effect of the TECA+AST treatment on LPS-induced NO production as well as iNOS and COX-2 expression in RAW 264.7 cells
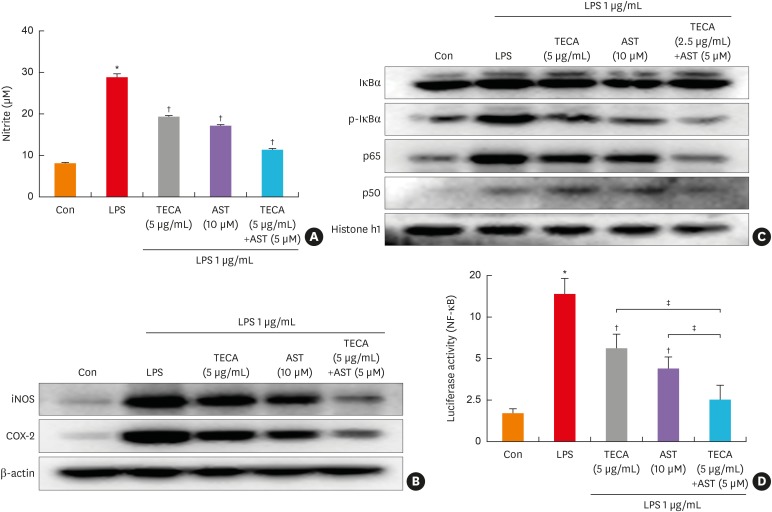
The effect of AST on LPS-induced NO production in RAW 264.7 cells was also investigated by measuring nitrate released in the culture medium by Griess reaction after co-treatment with LPS and TECA (5 μg/mL), AST (10 μM), and the TECA (2.5 μg/mL) + AST (5 μM) combination for 24 hours. LPS-induced nitrite concentration in the medium was decreased remarkably by both TECA and AST, but the inhibiting effect of TECA+AST was more significant (Fig. 5A). Additionally, to investigate whether the TECA+AST combination treatment is more effective compared to the TECA or AST treatment alone on corresponding gene expression, we determined iNOS expression by Western blot analysis. We also determined COX-2 expression since iNOS can be modulated by COX-2. As shown in Fig. 5B, the cells expressed extremely low levels of iNOS and COX-2 protein in an unstimulated condition. However, iNOS and COX-2 protein expressions were markedly increased in response to LPS (1 μg/ml) after 24 hours. Treatment with the TECA (2.5 μg/mL) + AST (5 μM) treatment caused more significant decreases in LPS-induced iNOS and COX-2 expression than the TECA (5 μg/mL) or AST (10 μM) treatment alone in RAW 264.7 cells (Fig. 5B).
Fig. 5. Effects of the TECA + AST treatment on NO production and the expression of iNOS and COX-2 in LPS-treated RAW 264.7 macrophages. The cells were treated with 1 μg/mL LPS alone or LPS with TECA (5 μg/mL), AST (10 μM) and the combination of TECA (2.5 μg/mL) + AST (5 μM) for 24 hours. At the end of incubation, 50 μL of the medium was removed to measure NO production (A). Control values were obtained in the absence of LPS. In the culture medium, NO production was measured by the Griess reaction as described in Materials and Methods. Equal amounts of total proteins (20 μg/lane) were subjected to 10% SDS-PAGE, and alterations in the expression of iNOS and COX-2 proteins were detected by Western blotting using specific antibodies (B). The β-actin protein was used here as an internal control. The effect of the TECA + AST treatment on the LPS-induced translocation of the NF-κB subunits (p50 and p65) into the nucleus and the phosphorylation of IκBα in cytosol (C). Equal amounts of nuclear proteins (20 μg/lane) or total proteins (20 μg/lane) were subjected to 10% SDS-PAGE, and the expressions of p50, p65, IκBα and p-IκBα proteins were detected by Western blotting using specific antibodies. Histone h1 protein and β-actin protein were used here as internal controls. The effect of the TECA + AST treatment on the LPS-induced NF-κB transcriptional activity (D). Data shown are the mean ± standard deviation from 3 experiments in duplicate.
Con, control; LPS, lipopolysaccharides; TECA, titrated extract of Centella asiatica; AST, astaxanthin; NF-κB, nuclear factor-κB; iNOS, inducible nitric oxide synthase; COX, cyclooxygenase; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
*P < 0.05 is the significance level compared to the control group. †P < 0.05 is the significance level compared to the PA treatment group. ‡P < 0.05 is the significance level compared to the TECA or AST treatment alone group.
Effect of the TECA + AST treatment on NF-κB activity in RAW 264.7 cells
Because the activation of NF-κB is critical for the induction of both iNOS and COX-2 by LPS or other inflammatory cytokines, we determined whether the TECA + AST treatment could suppress NF-κB activation more in LPS-activated RAW 264.7 cells. RAW 264.7 cells were co-treated with LPS and TECA, AST, and the TECA + AST combination for 1 hour, which is the time it takes to maximally activate NF-κB from LPS treatment (data not shown). We investigated the inhibitory effect of the TECA, AST and TECA + AST treatments on the translocation of the NF-κB subunit and IκB phosphorylation. The nuclear translocation of p65 and p50 was inhibited, and the LPS-induced phosphorylation of IκBα was also inhibited by TECA or AST alone treatment, and more significantly by the TECA + AST treatment (Fig. 5C). Moreover, the TECA + AST treatment also synergistically inhibited NF-κB transcriptional activity (Fig. 5D).
DISCUSSION
The skin barrier limits the clinical efficacy of herbal extracts despite several functional components in the extracts.13 PA-induced inflammatory responses on the dorsal ear skin and the clinical effects of TECA ointment, AST ointment and the TECA + AST combination ointment were first determined by changes in dorsal ear skin thickness and the phenotype of the back skin. Although the TECA or AST treatment alone slightly decreased PA-induced dermatitis, the TECA + AST treatment further enhanced the therapeutic effect of TECA or AST, strongly inhibiting PA-mediated AD phenotypes. The effect of combination treatment with TECA and AST was further confirmed by a histological analysis of the skin tissues. The increased number of dermal immune cells and increased thickness of the epidermis are the major characteristics of AD skin lesions, which were induced by repeated PA treatment.14 This atopic skin damage was significantly inhibited by the TECA + AST treatment, and the combination's inhibitory effects were greater than those of the TECA or AST treatment alone.
Many kinds of transcription factors have been reported to play key roles in the development of AD, and NF-κB is one of the major targets of AD treatment. Several anti-inflammatory drugs, including glucocorticoids and FK-506, have been reported as significant therapeutic agents for AD, and their effects are associated with inhibitory effects on NF-κB activity.11 Our previous studies also demonstrated that TECA and AST inhibited the activity of NF-κB in a PA-induced AD mouse model.9,12 As shown in Fig. 2, treatment of TECA ointment or AST ointment alone does affect PA-mediated NF-κB (p50 and p65) translocalization, but a combination of lower concentrations of TECA and AST showed successful inhibition of NF-κB (p50 and p65) from the nucleus. Moreover, the TECA + AST treatment also synergistically inhibited NF-κB transcriptional activity followed by further inhibition of NO generation and the expression of iNOS and COX-2. Several studies have demonstrated the importance of NF-κB on B-cell-mediated IgE production and the activation of mast cells.15,16 Hyperproduction of IgE in skin lesions is a typical clinical characteristic of AD.17 Chronic IgE-dominated immune responses contribute to skin barrier damage, causing the release of inflammatory mediators that correlate with the severity of AD.18 Therefore, to diagnose AD, assessment of the total IgE level is required as a significant therapeutic target. Excessive immune responses accompanied by increases in IgE and T helper 2 (Th2) cytokine production impair barrier function and lead to skin hyperplasia.19 Mast cells are considered to be key effector cells in IgE-mediated immediate hypersensitivity and allergic disorders as well as in the protection of immune responses to parasites and bacteria.20 In our present study, the number of mast cells that infiltrated into the dermis was significantly increased in the PA-treated mice compared to the control mice. While the number of mast cells was decreased in the TECA-, AST- and TECA + AST-treated mice, the TECA + AST treatment was more effective than the TECA or AST treatment alone.
Our results suggest that the anti-inflammatory effect of the TECA + AST treatment might be related to the inhibition of NF-κB. Activation of NF-κB has also been observed after IgE-induced TNF-α and IL-6 production in mast cells.21 Direct evidence relating NF-κB to the pathogenesis of AD is limited; however, some reports have suggested the role of NF-κB in the immunological disturbance that is observed in AD. NF-κB is also activated in B cells and T cells when they are stimulated via CD40 or T-cell receptors, respectively, indicating the critical roles of NF-κB for both Ig production and mast cell activation in AD.22 Moreover, many studies have shown that compounds suppressing NF-κB activation could act as therapeutic agents for AD.23,24 Cyclosporine and tacrolimus (i.e.,FK506), potent immunosuppressants, have been used to treat AD.18 Tanaka et al.25 reported that the NF-κB inhibitor IMD-0354 suppressed the abnormal proliferation of mast cells and reduced allergic responses. In our present study, the data indicated that the TECA + AST treatment could ameliorate PA-induced cytokine production, mast cell activation and IgE production through inactivating NF-κB and that the TECA + AST treatment was more effective than the TECA or AST treatment alone. The anti-oxidant effect of AST could potentiate the anti-inflammatory effect of the TECA + AST treatment, even though the doses used were half of those used for TECA or AST treatment alone. Therefore, our results suggest that the TECA + AST treatment could be considered a candidate agent for AD therapy.
ACKNOWEDGMENTS
This work is financially supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (No. MRC, 2017R1A5A2015541) and by the Ministry of Trade, Industry & Energy (MOTIE, 1415139249) through the fostering project of Osong Academy-Industry Convergence (BAIO).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goindi S, Kumar G, Kumar N, Kaur A. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013;14:1284–1293. doi: 10.1208/s12249-013-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10:207–215. doi: 10.4168/aair.2018.10.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Hachem M, Gesualdo F, Ricci G, Diociaiuti A, Giraldi L, Ametrano O, et al. Topical corticosteroid phobia in parents of pediatric patients with atopic dermatitis: a multicentre survey. Ital J Pediatr. 2017;43:22. doi: 10.1186/s13052-017-0330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CS, Lee SA, Kim YJ, Seo SJ, Lee MW. 3,4,5-tricaffeoylquinic acid inhibits tumor necrosis factor-α-stimulated production of inflammatory mediators in keratinocytes via suppression of Akt- and NF-κB-pathways. Int Immunopharmacol. 2011;11:1715–1723. doi: 10.1016/j.intimp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Umezawa K, Chaicharoenpong C. Molecular design and biological activities of NF-kappaB inhibitors. Mol Cells. 2002;14:163–167. [PubMed] [Google Scholar]
- 7.Park CS, Kim TB, Moon KA, Bae YJ, Lee HR, Jang MK, et al. Chlamydophila pneumoniae enhances secretion of VEGF, TGF-beta and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment. Allergy Asthma Immunol Res. 2010;2:41–47. doi: 10.4168/aair.2010.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karuppagounder V, Arumugam S, Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, et al. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp Dermatol. 2015;24:418–423. doi: 10.1111/exd.12685. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Yeo IJ, Han JH, Suh JW, Lee HP, Hong JT. Anti-inflammatory effect of Astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp Dermatol. 2018;27:378–385. doi: 10.1111/exd.13437. [DOI] [PubMed] [Google Scholar]
- 10.Hamasaka A, Yoshioka N, Abe R, Kishino S, Umezawa K, Ozaki M, et al. Topical application of dehydroxymethylepoxyquinomicin improves allergic inflammation via NF-kappaB inhibition. J Allergy Clin Immunol. 2010;126:400–403. doi: 10.1016/j.jaci.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka A, Muto S, Jung K, Itai A, Matsuda H. Topical application with a new NF-kappaB inhibitor improves atopic dermatitis in NC/NgaTnd mice. J Invest Dermatol. 2007;127:855–863. doi: 10.1038/sj.jid.5700603. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Choi JY, Son DJ, Park EK, Song MJ, Hellström M, et al. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci. 2017;18:E738. doi: 10.3390/ijms18040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi VK, Jain N, Valli KS. Importance of novel drug delivery systems in herbal medicines. Pharmacogn Rev. 2010;4:27–31. doi: 10.4103/0973-7847.65322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011;66:351–359. doi: 10.1111/j.1398-9995.2010.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinnell SB, Jacobs-Helber SM, Sterneck E, Sawyer ST, Conrad DH. STAT6, NF-kappaB and C/EBP in CD23 expression and IgE production. Int Immunol. 1998;10:1529–1538. doi: 10.1093/intimm/10.10.1529. [DOI] [PubMed] [Google Scholar]
- 16.Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-kappaB activity. J Allergy Clin Immunol. 2000;105:500–505. doi: 10.1067/mai.2000.104942. [DOI] [PubMed] [Google Scholar]
- 17.Yoshihisa Y, Andoh T, Matsunaga K, Rehman MU, Maoka T, Shimizu T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS One. 2016;11:e0152288. doi: 10.1371/journal.pone.0152288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–876. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 19.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong HJ, Koo HN, Na HJ, Kim MS, Hong SH, Eom JW, et al. Inhibition of TNF-alpha and IL-6 production by aucubin through blockade of NF-kappaB activation RBL-2H3 mast cells. Cytokine. 2002;18:252–259. doi: 10.1006/cyto.2002.0894. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Yamada T, Yoshinaga SK, Boone T, Horan T, Fujita S, et al. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002;169:1151–1158. doi: 10.4049/jimmunol.169.3.1151. [DOI] [PubMed] [Google Scholar]
- 23.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Kim MS, Jeong GS, Yoon J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J Ethnopharmacol. 2015;171:85–93. doi: 10.1016/j.jep.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, et al. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]