Abstract
Purpose
Children with sensitization to aeroallergens have decreased lung function and nasal patency. Our purpose was to determine the association of sensitization to different aeroallergens with airway function and nasal patency.
Methods
Four hundred and eighty-six randomly selected 11 year-old children who lived in Seongnam City were examined. Serum specific immunoglobulin E (IgE) levels against 6 common allergens (Dermatophagoidesfarinae, birch, cat, dog, Japanese hop and Alternaria), impulse oscillometry (IOS) results for the evaluation of airway dysfunction, and acoustic rhinometry for the determination of nasal airway patency were obtained.
Results
IOS indicated that children sensitized to Alternaria (n = 38, 7.8%) and dog dander (n = 69, 14.2%) had decreased lung function, based on resistance at 10 Hz (Rrs10; aβ = 0.0072; 95% CI, 0.017, 0.127; P = 0.010) and 1 Hz (Rrs1; aβ = 0.038; 95% CI, 0.001, 0.074; P = 0.042). Children sensitized to D. farinae (n = 281, 57.8%) had decreased post-decongestant nasal volume at 0 to 5 cm (aβ = −0.605; 95% CI, −1.005, −0.205; P = 0.003), but normal IOS results at all measured frequencies (P > 0.05). Increased serum eosinophil level was associated with Rrs1 (P = 0.007) and Rrs2 (P = 0.018) and post-decongestant nasal volume at 0 to 5 cm (aβ = −0.885; 95% CI, −1.331, −0.439; P < 0.001).
Conclusions
Sensitivity to specific aeroallergens, serum eosinophil count and total IgE level had different associations with upper and lower airway dysfunction in urban children.
Keywords: Lung function tests, oscillometry, rhinometry, allergens, eosinophil count
INTRODUCTION
There are well-established associations of sensitization to aeroallergens with asthma and allergic rhinitis (AR) in children.1,2 Studies that documented increased bronchial hyper-responsiveness3 and decreased nasal volume4 in aeroallergen-sensitized individuals support this relationship. However, only a limited number of studies have suggested a relationship of specific allergen sensitization patterns with clinical outcomes.2,5,6 Because asthma and AR are heterogeneous conditions, the presence of aeroallergen sensitization in the upper or lower airways must be considered for the clinical management of these conditions. For example, sensitization to pollen is associated with AR,6,7 sensitization to fungi is associated with the severity8 and development of asthma,9,10 and sensitization to animal dander is associated with asthma5,7 and bronchial hyper-responsiveness.3 However, no comprehensive investigation that used quantitative examinations, such as impulse oscillometry (IOS) or acoustic rhinometry, has yet examined the relationships of sensitization to different aeroallergens with upper and lower airway diseases.
We hypothesized that the site-specific effect of sensitization to different aeroallergens impacts the clinical manifestations of asthma and AR. Therefore, we examined the sensitization to aeroallergens and their effects on upper and lower airways by measuring nasal volume using acoustic rhinometry and airway dysfunction using IOS. We also examined the association of serum eosinophil count and total immunoglobulin E (IgE) level with nasal volume change and small airway dysfunction.
MATERIALS AND METHODS
Subject enrollment
This prospective, general population-based and cross-sectional study was part of the Seongnam Atopy Project for Children's Happiness (SAP2017) program, which was sponsored by the Seongnam City Government for the prevention and education of allergic diseases in Korean children. The SAP2017 was performed at 11 elementary schools in Seongnam City (Gyeonggi Province, Republic of Korea) between March and August of 2017. The study subjects were 620 urban children who were 10 to 12 years-old.
Questionnaires
The Korean version of the International Study of Asthma and Allergies in Childhood study was used to determine the presence of allergic disease (asthma and AR), based on symptoms during the previous 12 months. Pet owners were those who answered “yes” to the question, “Do you have any pets in your household living with your child?” and were asked to identify the type of pet. Individuals who were exposed to ‘visual mold’ were those who answered “yes” to the question, “Do you see any wet moldy spots on the ceilings or walls in your household?” Individuals who were exposed to ‘smelling mold’ were those who answered “yes” to the question, “Do you smell mold in your household?”
Blood sampling
Serum total and allergen-specific IgE levels to 6 common aeroallergens in Korean children11 (D.farinae, dog dander, cat dander, birch, Japanese hop and Alternaria) were measured using the ImmunoCAP system (Phadia AB, Uppsala, Sweden). A serum allergen-specific IgE level of 0.35 kU/L or more was defined as positive. As some studies have suggested IgE level of 0.7 kU/L as positive, we have analyzed our data with both cutoff values and acquired similar results. Subjects with no positive results were considered non-sensitized, those with 1 positive result mono-sensitized, and those with 2 or more positive results poly-sensitized. The percentage of blood eosinophils was calculated, and serum total IgE was measured using a fluorescent enzyme-linked immunosorbent assay (ImmunoCAP system, Phadia AB).
Determination of airway dysfunction using IOS
Airway dysfunction was measured using the Jaeger MasterScreen IOS system (Jaeger Company, Wurtzburg, Germany).12 The signal duration was 30 seconds or longer, and measurements of 3 acceptable maneuvers were acquired at each time while monitoring flow curves. Airway resistance was measured at 1 Hz (Rrs1), Rrs2, Rrs3, Rrs5, Rrs10, Rrs15 and Rrs20. Rrs20-5 was calculated as the difference of Rrs5 and Rrs20.
Determination of nasal patency using acoustic rhinometry
Nasal patency was measured using the A1 Acoustic Rhinometer (GM Instruments Ltd, Kilwinning, UK), according to standardized international recommendations.13 A well-trained technician applied the probe to each subject's nostrils and asked the subject to hold his/her breath for approximately 5 seconds. Both nasal cavities were decongested by administering a single puff of intranasal xylometazoline (1 mg/mL), and rested for 15 minutes before volume measurement. The post-decongestant nasal patency characterized by measuring the volume (cm3) at 0 to 5 cm behind the nostril opening (vol 0–5 cm), the volume (cm3) at 2 to 5 cm behind the nostril opening (vol 2–5 cm), and the minimal cross-sectional area (MCA; cm2) of the nasal cavity. For quality control, measurements were repeated at least 3 times until the standard deviation was less than 15%.
Fractional exhaled nitric oxide (FeNO) measurements
FeNO (in parts per billion [ppb]) was measured using the Niox-mino (Aerocrine AB,Solna, Sweden) according to American Thoracic Society/European Respiratory Society recommendations.14 Each subject was seated in a comfortable position, took a full inhalation and then exhaled at a constant flow rate.
Statistical analysis
All analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA) and R Packages (version 3.5.0; R Foundation, Vienna, Austria). The primary outcome measures were the associations of small airway dysfunction (from IOS) and nasal patency (from acoustic rhinometry) with sensitization to 6 different aeroallergens. The secondary outcome measures were the associations of IOS and acoustic rhinometry data with total eosinophil count (TEC), total IgE level and FeNO level. To analyze the association of airway dysfunction with sensitization and of nasal volume with sensitization, Student's t-test was used for continuous variables and the χ2 test was used for categorical variables. All IOS results were adjusted for height, sex and body mass index (BMI) z-score and additional covariates that could affect small airway lung function based on previous studies. This analysis adjusted for all variables that affected the estimate by more than 10%. For IOS data, the odd ratios (ORs) for the relationship of resistance at 1, 2, 3, 5, 10, 15 and 20 Hz with categorical variables in the aero-allergen test, FeNO (20 ppb), TEC (4%) and total IgE (150 kU/L) were determined using generalized linear regression with a logit function.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of CHA Bundang Medical Center (IRB No. 2017-04-049). Written informed consent was obtained from the parents or guardians of all participants following a detailed explanation of the study.
RESULTS
Characteristics of the subjects
A total of 620 subjects completed the questionnaire, and serum specific-IgE data were available for 486 (78.4%) children (Table 1). The median age was 11.0 years, 50.4% of the subjects were male, 34.8% were not sensitized to any of the 6 aeroallergens, 34.2% were monosensitized, and 31.1% were polysensitized.
Table 1. Demographic and clinical characteristics of the children enrolled (n = 486).
| Variable | No.* (%) | |
|---|---|---|
| Sex | ||
| Boy | 245 (50.4) | |
| Girl | 241 (49.6) | |
| Age (yr) | 11.0 ± 0.89 | |
| Height (cm) | 149.5 ± 7.9 | |
| BMI (kg/m2) | ||
| Obesity | 31 (6.4) | |
| Overweight | 49 (10.1) | |
| Normal | 377 (77.6) | |
| Underweight | 29 (6.0) | |
| Asthma (n = 479) | ||
| No | 469 (97.9) | |
| Yes | 10 (2.1) | |
| AR (n = 482) | ||
| No | 206 (42.7) | |
| Yes | 276 (57.3) | |
| Aero-allergen sensitization (n = 486) | ||
| D. Farinae | 256 (53.1) | |
| Dog | 56 (11.5) | |
| Cat | 73 (15.0) | |
| Birch | 86 (17.7) | |
| Japanese hop | 32 (6.6) | |
| Alternaria | 35 (7.2) | |
| FeNO (n = 477) | ||
| High (35 ≥ ppb) | 34 (7.1) | |
| Low (35 < ppb) | 443 (92.9) | |
| Total IgE (N = 486) | ||
| High 150 kU/L ≤ | 190 (39.1) | |
| Low 150 kU/L > | 296 (60.9) | |
| Blood eosinophils (n = 472) | ||
| High 4% ≤ | 137 (29.0) | |
| Low 4% > | 335 (71.0) | |
| IOS (n = 469) | ||
| Rrs1 (hPa/L/s) | 7.19 (1.19)† | |
| Rrs2 (hPa/L/s) | 6.56 (1.20)† | |
| Rrs3(hPa/L/s) | 5.93 (1.21)† | |
| Rrs5 (hPa/L/s) | 4.94 (1.23)† | |
| Rrs10(hPa/L/s) | 3.88 (1.20)† | |
| Rrs15 (hPa/L/s) | 3.20 (1.23)† | |
| Rrs20 (hPa/L/s) | 3.16 (1.21)† | |
| Nasal decongestant test | ||
| Vol 0–5 cm | 11.0 (2.27) | |
| Vol 2–5 cm | 8.32 (1.93) | |
| MCA | 1.32 (0.59) | |
Values are reported as mean ± SD or number (%).
SD, standard deviation; BMI, body mass index; Obesity, BMI z-score more than 2 SDs above the mean; overweight, 1 to 2 SDs above the mean; underweight, more than 2 SDs below the mean; AR, allergic rhinitis; FeNO, fraction of exhaled nitric oxide; IgE, immunoglobulin E; IOS, impulse oscillometry system; Rr, resistance of respiratory system; vol 0–5 cm, nasal volume (cm3) at 0 to 5 cm behind the nostril opening; vol 2–5 cm, nasal volume (cm3) at 2 to 5 cm behind the nostril opening; MCA, minimal cross-sectional area (cm2) of the nasal cavity.
*Number with available data. Among the 480 children who performed acoustic rhinometry, 16 MCA and 32 decongestant test results were incomplete and excluded from analysis; †Geometric means with geometric SD.
Factors affecting small airway lung function and nasal patency
We obtained IOS data and nasal patency data for 469 (96.5%) children, and performed regression analysis to identify factors significantly associated with airway dysfunction and nasal patency. Age and height affected airway resistance at all frequencies tested, and BMI affected resistance at frequencies of 5 Hz and above. Moreover, airway resistance at 1 and 5 Hz was significantly greater for males than for females (Supplementary Tables S1 and S2). Nasal volume was also strongly associated with height and age, and MCA had a strong relationship with BMI z-score (Supplementary Table S3).
Prevalence of allergic diseases and allergen sensitization
A total of 281 (57.8%) children were sensitized to D. farinae, and fewer children were sensitized to birch (n = 110, 22.6%), cat dander (n = 80, 16.5%), dog dander (n = 69, 14.2%), Japanese hop and Alternaria (Table 2). The prevalence of asthma was low (n = 10, 2.1%) and was associated with sensitization to D. farinae (0.5% vs. 3.3%, P = 0.032), dog dander (0.7% vs. 10.4%, P < 0.001) and Alternaria (1.6% vs. 7.9%, P = 0.030). After adjustment for confounding factors, sensitization to dog dander (adjusted OR [aOR], 17.080) and Alternaria (aOR, 5.661) remained associated with asthma.
Table 2. Association of acoustic rhinometry results with aeroallergen sensitization status.
| Variables | MCA | Vol 0–5 cm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | aβ (95% CI) | P value | β (95% CI) | P value | Adjusted (95% CI) | P value | ||
| Sensitization | |||||||||
| Non | Ref | Ref | Ref | Ref | |||||
| Mono- | −0.119 (−0.203 ,−0.034) | 0.006 | −0.109 (−0.193, −0.024) | 0.012 | −0.047 (−0.091, −0.003) | 0.035 | −0.046 (−0.089, −0.004) | 0.034 | |
| Poly- | −0.137 (−0.228 ,−0.047) | 0.003 | −0.129 (−0.221, −0.038) | 0.006 | −0.059 (−0.105, −0.013) | 0.012 | −0.059 (−0.104, −0.013) | 0.011 | |
| Total IgE | |||||||||
| < 150 | Ref | Ref | Ref | Ref | |||||
| > 150 | 0.035 (−0.034, 0.104) | 0.320 | −0.121 (−0.234, −0.008) | 0.036 | −0.357 (−0.774, 0.060) | 0.093 | −0.432 (−0.840, −0.024) | 0.038 | |
| Eosinophil | |||||||||
| < 4% | Ref | Ref | Ref | Ref | |||||
| > 4% | −0.102 (−0.176, −0.028) | 0.007 | −0.202 (−0.325, −0.079) | 0.001 | −0.990 (−1.437, −0.544) | < 0.001 | −0.885 (−1.331, −0.439) | < 0.001 | |
| D. farinae | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | −0.100 (−0.173, −0.027) | 0.007 | −0.091 (−0.164, −0.018) | 0.015 | −0.044 (−0.082, −0.007) | 0.020 | −0.038 (−0.086, 0.010) | 0.117 | |
| Birch | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | −0.112 (−0.211, −0.014) | 0.025 | −0.100 (−0.200, 0.000) | 0.049 | −0.043 (−0.091, 0.006) | 0.088 | −0.038 (−0.086, 0.010) | 0.117 | |
| Cat | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | −0.024 (−0.128, −0.079) | 0.649 | −0.021 (−0.124, 0.082) | 0.688 | −0.013 (−0.065, 0.040) | 0.637 | −0.011 (−0.062, 0.040) | 0.675 | |
| Dog | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | 0.015 (−0.103, 0.133) | 0.801 | 0.011 (−0.105, 0.128) | 0.848 | −0.021 (−0.079, 0.037) | 0.480 | −0.016 (−0.072, 0.041) | 0.583 | |
| Japanese hop | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | - | - | −0.002 (−0.157, 0.154) | 0.983 | 0.044 (−0.031, 0.119) | 0.251 | 0.035 (−0.038, 0.107) | 0.352 | |
| Alternaria | |||||||||
| No | Ref | Ref | Ref | Ref | |||||
| Yes | −0.107 (−0.244, 0.030) | 0.126 | −0.092 (−0.228, 0.044) | 0.186 | −0.057 (−0.129, 0.015) | 0.120 | −0.056 (−0.125, 0.014) | 0.118 | |
Data were adjusted for height, sex and BMI z-score, and were analyzed using gamma-generalized linear regression. Independent variables: coefficients in regression analysis of sensitization status adjusted for sex, height and BMI; dependent variables: nasal decongestant test results.
IgE, immunoglobulin E; CI, confidence interval;vol 0–5 cm, nasal volume at 0 to 5 cm behind the nostril opening; MCA, minimal cross-sectional area of the nasal cavity.
The prevalence of AR was greater in children with sensitization to each of the aeroallergens tested except Japanese hop (Table 2). After adjustment for confounding factors, sensitization to D. farinae (aOR, 1.979; 95% CI, 1.340, 2.923; P = 0.001), cat dander (aOR, 1.751; 95% CI, 1.021, 3.005; P = 0.042), and birch (aOR, 1.729; 95% CI, 1.067, 2.801; P = 0.026) remained associated with AR.
Association of sensitization to aeroallergens with pet ownership and mold exposure
Among the 484 children for whom data were available, there were 81 pet-owners (16.7%); 44 (9.1%) children had dogs and 12 (2.5%) had cats. More dog owners than non-owners were sensitized to dog dander (47.7% vs. 10.7%, P < 0.001), but cat ownership was not associated with sensitization to cat dander (P = 0.410). There were no significant associations of Alternaria sensitization with visual exposure to mold (P = 0.577) or smelling of mold (P = 0.975).
Association of airway dysfunction with aeroallergen sensitization
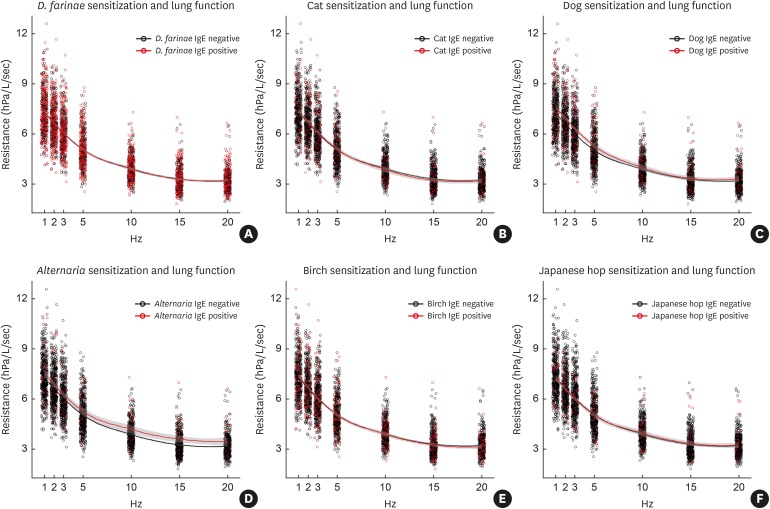
Airway dysfunction in children who were sensitized or not sensitized to 6 aeroallergens was determined by measuring resistance at 1, 2, 3, 5, 10 and 20 Hz, with adjustment for confounding factors (height, BMI z-score, gender, passive smoking and prematurity or low birth weight) (Figure). The Rrs values had no significant associations with sensitization to D. farinae (Figure A), cat (Figure B), birch (Figure D) or Japanese hop (Figure E). However, Rrs10 (aβ = 0.072; 95% CI, 0.017, 0.127; P = 0.010), Rrs15 (aβ = 0.101; 95% CI, 0.034, 0.168; P = 0.003) and Rrs20 (aβ = 0.084; 95% CI, 0.025, 0.144) were significantly lower in children sensitized to Alternaria (Figure D), although there was no significant association of Rrs 20-5 with Alternaria sensitization (aβ = −0.061; 95% CI, −0.183, 0.061; P = 0.328). Sensitization to dog dander was associated with Rrs1 (aβ = 0.038, 95% CI, 0.001, 0.074; P = 0.042) and Rrs2 (aβ = 0.037; 95% CI, 0.000, 0.074; P = 0.051), but not with resistance at any higher frequencies. The number of sensitized aeroallergens (mono- or poly-sensitization) was not associated with airway dysfunction at Rrs 1, 5 and 20 (P > 0.05 for Rrs1, 5 and 20).
Figure. Locally weighted scatterplot smoothing of impulse oscillometry resistance at 1, 2, 3, 5, 10, 15 and 20 Hz for 6 aeroallergens. Shaded areas indicate 80% confidence intervals. Sensitization to Alternaria (D) was significantly associated with Rrs10, Rrs15, and Rrs20 and sensitization to Dog (C) was significantly associated with Rrs1. Sensitization to D. farinae (A), Cat (B), Birch (E) and Japanese hop (F) were not significantly associated with small airway dysfunction at any tested frequency.
IgE, immunoglobulin E.
Association of nasal patency with aeroallergen sensitization
Analysis of the relationship of nasal patency (vol 0–5 cm, vol 2–5cm and MCA) with allergen sensitization indicated that MCA and vol 0–5 cm were significantly decreased in children who were mono- and poly-sensitized (Table 3). In addition, MCA and vol 0–5 cm were significantly decreased in children who were sensitized to D. farinae (MCA: aβ = −0.154; 95% CI, −0.264, −0.011; P = 0.006 and vol 0–5 cm: aβ = −0.605; 95% CI, −1.005, −0.205; P = 0.003), but not with any of the other aeroallergens tested. Vol 0–5 cm was also decreased in cat owners relative to non-owners
Table 3. Association of aeroallergen sensitization status with airway resistance at different frequencies.
| Hz | Dependent variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total IgE | TEC | Sensitization | |||||||
| Non- | Mono- | Poly- | |||||||
| aβ (95% CI) | P value | aβ (95% CI) | P value | Ref | aβ (95% CI) | P value | aβ (95% CI) | P value | |
| Rrs1 | 0.008 (−0.019, 0.034) | 0.578 | 0.040 (0.011, 0.069) | 0.007 | - | 0.013 (−0.017, 0.043) | 0.410 | 0.029 (−0.003, 0.061) | 0.075 |
| Rrs2 | 0.007 (−0.020, 0.034) | 0.592 | 0.036 (0.006, 0.066) | 0.018 | - | 0.017 (−0.013, 0.048) | 0.264 | 0.027 (−0.005, 0.060) | 0.097 |
| Rrs3 | 0.007 (−0.022, 0.036) | 0.636 | 0.031 (−0.001, 0.063) | 0.061 | - | 0.023 (−0.010, 0.057) | 0.174 | 0.026 (−0.010, 0.061) | 0.153 |
| Rrs5 | 0.005 (−0.028, 0.037) | 0.768 | 0.023 (−0.013, 0.059) | 0.209 | - | 0.025 (−0.012, 0.062) | 0.191 | 0.022 (−0.017, 0.062) | 0.269 |
| Rrs10 | 0.000 (−0.031, 0.031) | 0.983 | 0.010 (−0.024, 0.044) | 0.576 | - | 0.016 (−0.020, 0.052) | 0.383 | 0.016 (−0.022, 0.053) | 0.412 |
| Rrs15 | −0.002 (−0.039, 0.036) | 0.933 | −0.014 (−0.055, 0.028) | 0.523 | - | 0.018 (−0.025, 0.062) | 0.413 | 0.014 (−0.032, 0.060) | 0.556 |
| Rrs20 | −0.007 (−0.041, 0.027) | 0.680 | −0.009 (−0.046, 0.028) | 0.628 | - | 0.017 (−0.022, 0.055) | 0.399 | 0.014 (−0.026, 0.055) | 0.489 |
| Rrs20-5 | 0.029 (−0.040, 0.097) | 0.411 | 0.064 (−0.012, 0.140) | 0.100 | - | 0.066 (−0.012, 0.145) | 0.097 | 0.040 (−0.043, 0.122) | 0.347 |
Data were adjusted for sex, height and BMI, and were analyzed using gamma-generalized linear regression. Independent variables: immune status (total IgE, TEC and sensitization); dependent variables: lung function parameters. Positive serum total IgE: ≥150 kU/L; positive serum eosinophil: ≥4%; poly-sensitization: sensitive to 2 or more of the 6 tested aeroallergens. Risk factors were sex, height, BMI z-score, prematurity or low birth weight, and exposure to secondary smoke. P < 0.05 is in bold.
Rr, resistance of respiratory system; IgE, immunoglobulin E; CI, confidence interval; TEC, total eosinophil count.
Association of serum eosinophil count with total IgE level
Analysis of serum eosinophil level as a continuous variable indicated it had a significant association with Rrs1 (aβ = 0.040; 95% CI, 0.011, 0.069; P = 0.007). There was no association of total IgE level with sensitization status (non-, mono- or poly-sensitization) or airway resistance. Cat or dog ownership and visual mold or smelling mold had no significant associations with the IOS results. After adjusting for confounding factors, a serum eosinophil level above 4% was significantly associated with increased Rrs1 (aβ = 0.040; 95% CI, 0.011, 0.069; P = 0.007) and Rrs2 (aβ = 0.036; 95% CI, 0.006, 0.066; P = 0.018).
Total IgE level was associated with decreased MCA (aβ = −0.121; 95% CI, −0.234, −0.008; P = 0.036) and vol 0–5 cm (aβ = −0.432; 95% CI, −0.840, −0.024; P = 0.038). Moreover, MCA (aβ = −0.202; 95% CI, −0.325, −0.001) and vol 0–5 cm (aβ = −0.885; 95% CI, −1.331, −0.439; P < 0.001) were lower in subjects with serum eosinophil levels above 4% (Table 3).
DISCUSSION
We found that sensitization to Alternaria and dog dander was associated with airway dysfunction and current asthma status, although our IOS data indicated that these allergens affected slightly different frequencies of small airway function. On the other hand, sensitization to D. farinae and atopic status showed that strong associations with nasal volume, and current rhinitis increased the odds ratio for sensitization to D. farinae, cat dander, birch and any allergen, indicating that different aeroallergens vary in their effects on the upper and the lower respiratory tracts. Our results thus suggest that strategies for the management of respiratory allergic diseases depend on the aeroallergen and on the anatomical sites affected.
A major finding of this study is that sensitization to Alternaria and dog dander increased airway dysfunction in this general population of children. We focused on subjects in the general population who have not been diagnosed with asthma, but have decreased lung function and aeroallergen sensitization. Adults with asthma who are sensitive to mold have more hospital admissions overall and admissions to the intensive care unit for asthma exacerbations.8,10 In addition, sensitization to Alternaria is associated with airway hyper-responsiveness in non-asthmatic adults15 and in children with mild to moderate asthma.3 Interestingly, as in adult populations, our general pediatric population had an association of sensitization to Alternaria with airway dysfunction, as determined by resistance at 10 Hz, 15 Hz and 20 Hz, but not at Rrs20-5 (which represents small airway dysfunction).16 Similarly, children with dog dander sensitization had an increased risk of current asthma and increased airway resistance. This result is consistent with those of previous studies which reported that hypersensitivity to furry animals had a strong association with asthma, although sensitization to mites, grass pollen, and cockroach were not related to asthma.3,5,7,17 However, in contrast to our Alternaria results, dog dander sensitization was associated with Rrs1, implying small airway dysfunction. These findings indicate that sensitization to certain allergens plays an important role in modulating the bronchial hyper-responsiveness of children in the general population as well as in patients with asthma.
We found that sensitization to house dust mites was associated with decreased nasal volume and AR, and that sensitization to birch pollen and cat dander were associated with current AR. A genetic predisposition to allergic disease increases the risk of AR, and sensitization to pollen and/or dust mites are important determinants of AR.6,7,18 However, we found no significant decrease in nasal decongestant volume in children sensitized to tree and weed pollen, in contrast to those sensitized to house dust mites. This could be explained by the lower concentrations and shorter durations of exposure to pollen18 and by the unique allergenic properties of house dust mites.19 Thus, each aeroallergen appears to elicit a site-specific allergic response in the airway due to its unique properties.
We found that airway dysfunction was not related to the presence of atopy, mono-sensitization or poly-sensitization. Thus, multiple allergic diseases can occur in children who are poly-sensitized to seasonal and perennial allergens, and children may be sensitized to allergens that affect the upper and lower airways. We also found an association of elevated serum eosinophil level with upper and lower airway dysfunction, in agreement with the results of previous studies which showed that increased serum eosinophil level is associated with poor lung function.20 Serum and bronchial eosinophil levels are also related to airway obstruction20 and more frequent hospital admissions due to asthma exacerbations.8 However, the relationship between serum eosinophil level and upper airway function remains poorly elucidated. While there is paucity of investigation on the relationship between serum eosinophil level and upper airway function, it is noteworthy that our study demonstrated an association of elevated serum eosinophil level with upper airway dysfunction.
This study included a large number of subjects and examined the functional profiles of the upper and the lower airways. We measured the extent of site-specific airway dysfunction using the rigorous and quantitative methods of IOS and rhinometry. We did not independently examine each airway, but compared how allergens affect the upper and lower airways. Our study design had a few limitations. First of all, a selection bias may exist as we only included subjects whose parent or guardian signed an informed consent form, and it was more likely that those parent or guardian had concerns about allergic disease or their children already had allergic disease. Secondly, the nature of cross-sectional study design imposes limitations in the analysis and interpretation of the results. To establish causality, prospective and controlled studies are needed to verify the effects of different aeroallergens on the upper and lower airways.
In conclusion, we found that different aeroallergens had different effects on the upper and the lower airways of inner-city children in Korea. The presence of atopy had a strong association with nasal patency, and serum eosinophil count was associated with dysfunction of the upper and lower airways. Our results have implications for clinicians in analyzing the meaning of aeroallergen sensitization in relation to clinical presentation.
ACKNOWLEDGMENTS
This study was supported by a grant from the Seongnam Atopy Project of the Seongnam City Government, Republic of Korea. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. We are grateful to the children and families of the study for their support and dedication. We thank the Department of Environment Policy at Seongnam City Government for its assistance and cooperation.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Identification of continuous factors that could potentially confound analysis of small airway dysfunction
Identification of binary factors that could potentially confound analysis of small airway dysfunction
Prevalence (%) and aORs for asthma and AR in children with different aeroallergen sensitization status (n = 486)*
References
- 1.Roberts G, Zhang H, Karmaus W, Raza A, Scott M, Matthews S, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy. 2012;42:1501–1509. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Shin YH, Yum HY, Jee HM, Jang SJ, Yoon JW, et al. Patterns of sensitisation to common food and inhalant allergens and allergic symptoms in pre-school children. J Paediatr Child Health. 2013;49:272–277. doi: 10.1111/jpc.12150. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104:775–785. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 4.Chawes BL, Kreiner-Møller E, Bisgaard H. Objective assessments of allergic and nonallergic rhinitis in young children. Allergy. 2009;64:1547–1553. doi: 10.1111/j.1398-9995.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- 5.Schoos AM, Chawes BL, Melén E, Bergström A, Kull I, Wickman M, et al. Sensitization trajectories in childhood revealed by using a cluster analysis. J Allergy Clin Immunol. 2017;140:1693–1699. doi: 10.1016/j.jaci.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Krämer U, Oppermann H, Ranft U, Schäfer T, Ring J, Behrendt H. Differences in allergy trends between East and West Germany and possible explanations. Clin Exp Allergy. 2010;40:289–298. doi: 10.1111/j.1365-2222.2009.03435.x. [DOI] [PubMed] [Google Scholar]
- 7.Warm K, Hedman L, Lindberg A, Lötvall J, Lundbäck B, Rönmark E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol. 2015;136:1559–1565.e2. doi: 10.1016/j.jaci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.O'Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 10.Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 11.Ha EK, Baek JH, Lee SY, Park YM, Kim WK, Sheen YH, et al. Association of polysensitization, allergic multimorbidity, and allergy severity: a cross-sectional study of school children. Int Arch Allergy Immunol. 2016;171:251–260. doi: 10.1159/000453034. [DOI] [PubMed] [Google Scholar]
- 12.Sheen YH, Jee HM, Ha EK, Jang HM, Lee SJ, Lee S, et al. Impulse oscillometry and spirometry exhibit different features of lung function in bronchodilation. J Asthma. 2018;55:1343–1351. doi: 10.1080/02770903.2017.1418884. [DOI] [PubMed] [Google Scholar]
- 13.Clement PA, Gordts F Standardisation Committee on Objective Assessment of the Nasal Airway, IRS, and ERS. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–179. [PubMed] [Google Scholar]
- 14.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 15.Hiranrattana A, Stern DA, Guerra S, Halonen M, Wright AL, Daines M, et al. Alternaria sensitisation at age 6 years is associated with subsequent airway hyper-responsiveness in non-asthmatics. Thorax. 2018;73:1170–1173. doi: 10.1136/thoraxjnl-2017-210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karayama M, Inui N, Mori K, Kono M, Hozumi H, Suzuki Y, et al. Respiratory impedance is correlated with airway narrowing in asthma using three-dimensional computed tomography. Clin Exp Allergy. 2018;48:278–287. doi: 10.1111/cea.13083. [DOI] [PubMed] [Google Scholar]
- 17.Ingram JM, Sporik R, Rose G, Honsinger R, Chapman MD, Platts-Mills TA. Quantitative assessment of exposure to dog (Can f 1) and cat (Fel d 1) allergens: relation to sensitization and asthma among children living in Los Alamos, New Mexico. J Allergy Clin Immunol. 1995;96:449–456. doi: 10.1016/s0091-6749(95)70286-5. [DOI] [PubMed] [Google Scholar]
- 18.Katelaris CH, Lee BW, Potter PC, Maspero JF, Cingi C, Lopatin A, et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42:186–207. doi: 10.1111/j.1365-2222.2011.03891.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015;64:304–311. doi: 10.1016/j.alit.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51:1702536. doi: 10.1183/13993003.02536-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of continuous factors that could potentially confound analysis of small airway dysfunction
Identification of binary factors that could potentially confound analysis of small airway dysfunction
Prevalence (%) and aORs for asthma and AR in children with different aeroallergen sensitization status (n = 486)*