Abstract
Background
Bromodomain and extra-terminal inhibitors (BETi) have shown efficacy for the treatment of aggressive triple negative breast cancer (TNBC). However, BETi are plagued by a narrow therapeutic window as manifested by severe toxicities at effective doses. Therefore, it is a limitation to their clinical implementation in patient care.
Methods
The impact of vitamin C on the efficacy of small compounds including BETi was assessed by high-throughput screening. Co-treatment of TNBC by BETi especially JQ1 and vitamin C was evaluated in vitro and in vivo.
Findings
High-throughput screening revealed that vitamin C improves the efficacy of a number of structurally-unrelated BETi including JQ1, I-BET762, I-BET151, and CPI-203 in treating TNBC cells. The synergy between BETi and vitamin C is due to suppressed histone acetylation (H3ac and H4ac), which is in turn caused by upregulated histone deacetylase 1 (HDAC1) expression upon vitamin C addition. Treatment with JQ1 at lower doses together with vitamin C induces apoptosis and inhibits the clonogenic ability of cultured TNBC cells. Oral vitamin C supplementation renders a sub-therapeutic dose of JQ1 able to inhibit human TNBC xenograft growth and metastasis in mice.
Interpretation
Vitamin C expands the therapeutic window of BETi by sensitizing TNBC to BETi. Using vitamin C as a co-treatment, lower doses of BETi could be used to achieve an increased therapeutic index in patients, which will translate to a reduced side effect profile.
Fund
University of Miami Sylvester Comprehensive Cancer Center, Bankhead Coley Cancer Research program (7BC10), Flight Attendant Medical Research Institute, and NIH R21CA191668 (to GW) and 1R56AG061911 (to CW and CHV).
Keywords: Triple negative breast cancer, Vitamin C, BET inhibitor, Combination therapy, Histone acetylation, HDAC1
Research in context.
Evidence before this study
Preclinical studies have shown that BETi are effective in treating breast cancer especially TNBC. Multiple BETi are under evaluation in ongoing clinical trials. However, severe side effects of BETi at effective doses have been reported in patients. Our earlier work shows that vitamin C sensitizes melanoma to BETi treatment. Hence, it is plausible that vitamin C may also improve the response of TNBC to BETi.
Added value of the study
In this study, we present that vitamin C at 100 μM, achievable in the plasma in vivo by oral delivery, markedly increases the efficacy of BETi such as JQ1. Oral vitamin C supplementation renders a sub-therapeutic dose of JQ1 able to inhibit human TNBC xenograft growth and metastasis in mice. The molecular mechanisms for vitamin C to sensitize TNBC and melanoma to BETi are different. In TNBC, vitamin C inhibits H3ac and H4ac mainly by upregulation of HDAC1. In melanoma, vitamin C suppresses H4ac specifically H4K5ac and H4K12ac by downregulation of HAT1.
Implication of all the available evidence
This study provides evidence that vitamin C can help expand the therapeutic window of BETi for TNBC treatment. Future clinical trials may consider testing co-treatment of TNBC with BETi and vitamin C.
Alt-text: Unlabelled Box
1. Introduction
Bromodomain and extra-terminal domain (BET) proteins, such as BRD4, play key roles in cancer by binding to acetylated histones to promote the expression of oncogenes such as c-Myc [1]. Compounds have been developed to block BET proteins from binding to acetylated lysines in histones, primarily by mimicking the structure of an acetylated lysine to impair BET protein action. These compounds are collectively termed BET inhibitors (BETi). It has been shown that BETi reduce the expression of c-Myc, a key target for cancer treatment [2]. As anti-cancer agents, BETi are now being evaluated in clinical trials for the treatment of various cancers.
Triple negative breast cancer (TNBC) is an aggressive malignancy that currently lacks targeted therapy. Preclinical studies have shown that BETi alone are effective in treating TNBC. For instance, JQ1 (thieno-triazolo-1,4-diazepine), the first published BETi, inhibits TNBC tumor growth in rodents, likely through multiple molecular mechanisms [[3], [4], [5], [6], [7], [8]]. In addition to inhibiting tumor growth, JQ1 also impairs angiogenesis and TNBC response to hypoxia [9]. As a corroboration of these studies, BET protein degraders have also shown strong pre-clinical antitumor activity in treating TNBC [10]. Furthermore, BETi in combination with other cancer drugs have been used to treat TNBC in model systems. For example, JQ1 in combination with a PI3K inhibitor inhibits the growth of TNBC that harbors PI3K mutations [11]. Co-treatment with JQ1 and histone deacetylase (HDAC) inhibitors reduces TNBC cell viability [12]. Treatment with the BETi, OTX015, and the mTOR inhibitor, everolimus, is more effective than OTX015 alone in treating TNBC, even though everolimus alone has no effect [13]. Overall, preclinical studies indicate that BETi alone, or in combination with other drugs, may prove effective for TNBC treatment.
While BETi are promising cancer drugs, at effective doses, severe BETi side effects have been reported in multiple phase-I clinical trials, which include gastrointestinal toxicity, anemia, thrombocytopenia, neutropenia, diarrhea, fatigue, and nausea [[14], [15], [16]]. Most of these side effects are dose-dependent and compromise the potential for BETi to be used in clinical care. If TNBC cells could be sensitized to BETi by use of a sensitizing agent, to permit effective use of lower BETi doses and achieve an increased therapeutic index, this might translate to a reduced side effect profile in patients.
Genetic and epigenetic variations underlie inter-individual differences in cancer drug response. Unlike genetic variation, epigenetic variation is reversible and therefore provides an opportunity to improve the response of cancer to therapeutic drugs by targeting epigenetic regulators [17]. We and others have shown that vitamin C, which exists mainly as ascorbate anion under physiological pH conditions, promotes active DNA demethylation catalyzed by ten-eleven translocation (TET) methylcytosine dioxygenases [[18], [19], [20], [21], [22]]. Thus, the bioavailability of vitamin C affects DNA demethylation in the cell. Emerging evidence suggests that cancer cells may be effectively vitamin C deficient. Vitamin C enters and accumulates in breast epithelial cells mainly through sodium-dependent vitamin C transporter 2 (SVCT2) [23]. Our earlier work discovered that SVCT2 expression was decreased in human breast cancers compared to normal breast tissues from the same patients [24]. Thus, breast cancer cells may need to compensate for a potential intracellular vitamin C deficiency. Conventional drug discovery and development often involves cell-based in vitro screening. However, vitamin C is absent from the media used to culture TNBC cells [25]. Thus, the function of vitamin C in regulating DNA demethylation and the potential role of vitamin C in modulating cellular functions and drug responses in cancer has been largely overlooked. Previously, we reported that vitamin C sensitizes melanoma to BETi treatment [26]. Thus, it is plausible that vitamin C may also improve the response of TNBC to BETi.
Here, we show that vitamin C at 100 μM, which is achievable in the plasma in vivo by oral supplementation, markedly decreases the EC50 of BETi by upregulating the expression of HDAC1 and reducing H3 and H4 acetylation. Vitamin C synergistically improves the response of TNBC cells to BETi in culture conditions and in modeled mice. Since vitamin C is a safe and well-tolerated micronutrient, this work supports translation to clinical trials that would test if vitamin C supplementation can permit therapeutic efficacy of BETi used at lower, better tolerated, doses with a reduced side effect profile in patients.
2. Materials and methods
2.1. Cell culture and treatment
TNBC cell lines including MDA-MB-231, BT-549, and HCC1937 were purchased from ATCC (Manassas, VA) in a period between the year of 2013 to 2016 without further authentication. Frozen cells were newly thawed from low (3−10) passages. Testing for Mycoplasma was performed using PlasmoTest Mycoplasma detection kits (Thermo Fisher, Waltham, MA) and only negative cells were included in all experiments. All cells were maintained under a 5% CO2 atmosphere in RPMI medium (Life technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 Units/mL penicillin, and 100 μg/mL of streptomycin. DT-28 cells, which were maintained in IMEM with 10% FBS as described previously [27], were a kind gift from Dr. Dorraya El-Ashry (University of Minnesota). TNBC cells were seeded for 24 h and subsequently treated with vitamin C (sodium ascorbate, Sigma-Aldrich, St. Louis, MO). Media was changed daily to ensure the presence of fresh vitamin C.
2.2. Compound screening
For primary screen, MDA-MB-231 cells were pretreated with or without 50 μM vitamin C for 72 h in flasks. Subsequently, cells were seeded in 384-well plates. Compounds were added in duplicates to the testing plates at a final concentration of 1 μM. After plates were incubated for 72 h, CellTiter-Glo reagents (Promega, Madison, WI) were added to each well. Cell viability was measured on an Envision Multi-Label Reader (Perkin Elmer, Waltham, MA) and reported as a percentage of response relative to both cells treated with DMSO alone (0% response) and cells treated with 10 μM Velcade (100% response). The Z-factor for all the plates were between 0.5 and 1, ensuring the robustness of the assay.
2.3. EC50 measurements
TNBC cells including MDA-MB-231, BT-549, HCC1937, and DT-28 were pretreated with or without vitamin C at various concentrations. On 384-well plates, a 3-fold serial dilution of BETi such as I-BET762, CPI-203, I-BET151 (Selleck chemicals, Houston, TX) or JQ1 (kindly provided by the Bradner lab, Dana-Farber Cancer Institute, Harvard University) were prepared with a starting concentration of 10 μM. On the day of treatment, BETi were simultaneously transferred to the cell plates with a 384-pipettor head using a FLIPR tetra instrument (Molecular Devices, Sunnyvale, CA). Cell viability was measured after 72 h by CellTiter-GLO assay. Each concentration of BETi was plotted against percent cell survival. The half maximal effective concentration (EC50) values were calculated from 4-parameter fitted curves by solving for the X-intercept value at the 50% inhibition level of the Y-intercept value.
2.4. Gene silencing
Individual Accell siRNA against human TET1, TET2, and TET3 were designed and synthesized by Dharmacon (Lafayettte, CO) and transfected following the manufacturer's instruction. Human BRD4 siRNA and non-targeting scrambled siRNA were purchased from Thermo Fisher. MDA-MB-231 cells were plated and grown until achieving 30–50% confluence. Transfection of siRNA was performed using Lipofectamine 2000 (Thermo Fisher). Media was changed after 6 h of transfection to eliminate the possible toxicity of transfecting reagents. Cells were then used for BETi EC50 measurement or harvested after 5 days for RNA and protein extraction.
2.5. Gene expression analysis
Total RNA was extracted from cells using RNeasy mini kit (Qiagen). cDNA was synthesized from 1 μg of total RNA with the Supercript III First-Strand Synthesis System (Thermo Fisher). Real-time quantitative PCR was performed with 100 ng of cDNA on a Quantstudio 12 K Flex real-time PCR system (Applied Biosystems, Foster City, CA) using PowerUp Sybr Green Master Mix (Applied Biosystems, Foster City, CA). Primers were designed to span introns with the following sequences; HDAC1 (forward primer: 5′-GGAAATCTATCGCCCTCACA -3′, reverse primer: 5′-AACAGGCCATCGAATACTGG -3′); HDAC6 (forward primer: 5′-AAGAAGACCTAATCGTGGGACT-3′, reverse primer: 5′-GCTGTGAACCAACATCAGCTC-3′); GAPDH (forward primer: 5′-TGGACCTGACCTGCCGTCTA-3′, reverse primer: 5′- CCCTGTTGCTGTAGCCAAATTC-3′) and Relative gene expression was determined using the 2−ΔΔCT method.
2.6. Immunofluorescence and image analysis
TNBC cells were seeded on coverslips for 24 h followed by treatment with vitamin C (50 μM). Cells were then washed with cold PBS and fixed for 10 min at room temperature with 4% paraformaldehyde, permeabilized for 5 min with 0.2% Triton X-100 PBS, and blocked for 30 min with 5% BSA. This was followed by incubation with the primary antibodies (1:500) overnight at 4 °C, PBS wash, and then by the secondary antibodies at 1:1000 dilution for another hour at room temperature. Primary antibodies used in this study include anti-PanH3ac, anti-PanH4ac, anti-H3 total, anti-H4 total (Active motif, Carlsbad, CA); anti-HDAC6 (Cell signaling, Denver, MA); anti-GAPDH (Santa Cruz Biotechnology, Dallas, TX) and anti-BRD4 (Abcam, Cambridge, UK). To stain the nucleus, cells were incubated with 40 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) for 20 min at room temperature. The coverslips were then mounted on glass slides and imaged. All fluorescence images were acquired using a Zeiss LSM 710 confocal microscope and captured into a 512 × 512 frame size by averaging 4 times at a bit depth of 16. Fluorescence intensities from the images were quantified using Fiji (ImageJ). Average intensity values were measured from every cell within the image field from a minimum of three 20× images per condition. The intensity values from individual cells were plotted and statistically analyzed by one-way ANOVA with Tukey post hoc test using GraphPad Prism 7 from GraphPad Software (San Diego, CA).
2.7. Co-immunoprecipitation
TNBC cells pretreated with or without vitamin C (50 μM) were subjected to co-immunoprecipitation (co-IP). Briefly, after crosslinking with 1% formaldehyde, cells were sonicated in a Covaris ultrasonicator. Proteins were subsequently extracted and quantified. 100 μg of protein from each sample were incubated with either 5 μg of IgG or 3 μg of mouse monoclonal anti-H3ac or anti-H4ac primary antibody overnight by rotating at 14 rpm at 4 °C. Captured immunocomplexes were boiled at 95 °C for 5 min with 1 × laemmli buffer (BioRad, Hercules, CA), electrophoresed in SDS-PAGE gels, and subjected to Western blot.
2.8. Western blot
Total proteins were extracted using RIPA buffer (Fisher Scientific, Hampton, NH) in combination with protease/phosphatase inhibitor cocktail and phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich). Protein samples were quantified by BCA protein assay following the manufacturer's instruction. Samples were then diluted in SDS sample buffer (60 mM Tris–HCl, 1% SDS, 10% glycerol, 0.05% bromophenol blue, pH 6.8, with 2% β-mercaptoethanol), separated on 10% SDS-PAGE (Bio-Rad, Hercules, CA), and transferred to PVDF membranes (Bio-Rad). Transfer efficiency was determined by Ponceau S staining (Sigma-Aldrich). PVDF membranes were incubated with blocking solution (TBS containing 0.1% Tween 20 and 5% BSA) and probed with different primary antibodies (anti-Pan H4ac, anti-Pan H3ac, anti-H3 total, Active Motif, Carlsbad, CA; anti-GAPDH: Santa Cruz Biotechnology, Dallas, TX; anti-BRD4: Abcam Cambridge UK). Protein bands were detected using chemiluminescence and ImageJ was used to quantify Western blot band densities.
2.9. Apoptosis and clonogenic assays
TNBC cells were seeded in 24-well plates and pretreated with or without vitamin C (50, 100 μM) for 72 h. One group with and another group without vitamin C were then further treated with 0.5 μM JQ1 for an additional 48 h. All treatments were conducted in triplicate. Apoptotic cells were determined by fluorescein-based TUNEL (Sigma-Aldrich) assay following the manufacturer's protocol. Clonogenic survival was defined by the ability of the cells to form colonies. Each colony contained at least 50 cells. Briefly, 500 cells were seeded into 6-well dishes in 2 mL medium. Two days later, different wells were treated with 50 μM or 100 μM vitamin C alone or in conjunction with either 0.25 μM or 0.5 μM of JQ1. Plates were maintained for additional 12 days with a daily treatment regimen of vitamin C and JQ1 treatment every 3 days. Cells were fixed with methanol-acetic acid (3:1) solution followed by crystal violet staining. Colonies were imaged and percentage colony area was determined using ImageJ and ‘colony area’ plugin. Each experiment was repeated in triplicate.
2.10. Animal studies
All procedures were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee at the University of Miami. Female NSG mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MBA-MD-231 (106) cells were engrafted on the 4th mammary fat pad of each animal. In the pilot experiment, two groups of 10 mice each received vitamin C (3.3 g/L) supplementation in the water supply for one month before tumor cell implantation. Five days post implantation, one group of animals with or without vitamin C supplementation received JQ1 (50 mg/ kg body weight/day) intraperitoneal injection (IP) for 2 weeks. For the following mouse experiment, three weeks post implantation all animals obtained tumors around 100–150 mm3 volume measured by caliper. Mice were then randomly distributed into four groups of 10 animals each. One group received vitamin C (3.3 g/L) supplementation in the water supply, one group received JQ1 (30 mg/kg body weight/day, IP) every day, another group received both vitamin C in the water supply and JQ1 IP. For both experiments the control group received vehicle only. Tumor volume and body weight were measured by caliper and scale. After extraction, tumors were washed in PBS, weighed, and then fixed for further experiments. Organs (liver and lung) were collected and fixed in Bouin's solution for analyzing metastasis. Visible metastatic nodules were counted and enumerated on lungs and livers post fixation under a dissecting microscope.
2.11. Statistical analysis
All observations in this study were analyzed in triplicate or more and each experiment was repeated three times. GraphPad Prism was also used to generate and analyze data. Dose response data were analyzed by ANOVA followed by Tukey Post hoc comparison. Values represent the mean ± SEM of three independent experiments. To compare two groups, student's t-test was used and P < .05 was considered as statistically significant.
3. Results
3.1. Vitamin C improves BETi efficacy in treating TNBC cells
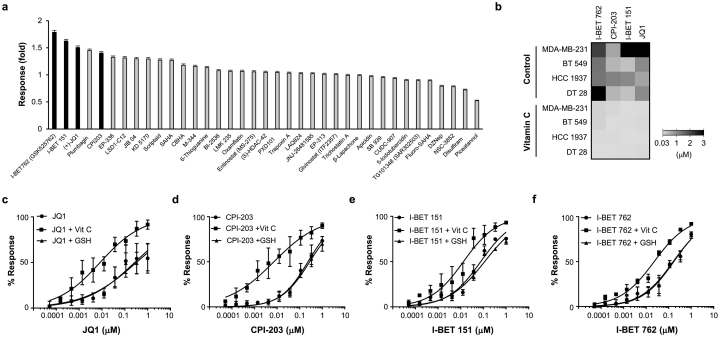
Cell-based in vitro screening is critical to cancer drug discovery and development. Vitamin C is not only an essential micronutrient, but also has been proven to be a regulator of DNA demethylation [[18], [19], [20], [21], [22]]. However, vitamin C is often not included in the formulation of media used for culturing cancer cells. We reasoned that vitamin C might change the response of TNBC cells to certain drugs by altering the epigenome and transcriptome. To test this hypothesis, we screened a panel of small molecules (n = 164) (Supplementary Table S1) against MDA-MB-231 cells, with or without pretreatment with vitamin C (50 μM, the median plasma level in healthy humans) for 72 h. All compounds were tested at 1 μM dose and cell viability was measured at the end of treatment for the primary screen. A dose of velcade (Bortezomib, 10 μM), which completely inhibited MDA-MB-231 cell proliferation, was considered 100% drug response. Overall, vitamin C treatment increased the activity of 72.5% and suppressed the activity of 15.6% of the tested compounds, while the rest remained essentially unchanged. Strikingly, the top hit compounds, whose inhibitory effect on TNBC cell survival was enhanced by vitamin C, were a number of structurally different BETi including JQ1, I-BET762, I-BET151, and CPI-203 (Fig. 1a).
Fig. 1.
Vitamin C enhances the efficacy of BETi. (a) Representative data of the compound screen (n = 164) targeting epigenetic modulators in MDA-MB-231 cells. Of the top 5 hits with increased responses after vitamin C (Vit C, 50 μM) treatment, 4 are BETi including JQ1, CPI-203, I-BET151, and I-BET 762 (black bars). (b) Heat map showing the reduction of BETi EC50 in 4 different TNBC cell lines after treatment with vitamin C (50 μM). (c-f) Dose response curves show that vitamin C (50 μM) increases the response of MDA-MB-231 cells to JQ1, CPI-203, I-BET151, and I-BET 762 while GSH (50 μM), a potent antioxidant, had no effect (n = 3). All data are presented as mean ± SEM (t-test).
BETi block the binding of BET proteins to acetylated lysine residues in histones, an effect that can be mimicked by decreasing BET expression. To verify the findings of our high-throughput screening, we used siRNA to knock down the expression of BRD4, a major BET protein that plays an important role in cancers, including TNBC [28]. The loss of BRD4 expression by siRNA transduction was confirmed by Western blot (Supplementary Fig. S1a). BRD4 siRNA caused a modest decrease in MDA-MB-231 cell survival compared to scrambled siRNA. In combination with vitamin C, BRD4 siRNA further inhibited cell growth, while vitamin C (50 μM) alone had no obvious effect (Supplementary Fig. S1b).
To further confirm the effect of vitamin C on BETi, we tested different TNBC cell lines including MDA-MB-231, BT-549, and HCC1937, as well as a primary human dissociated breast tumor culture, DT-28. After pretreatment with or without vitamin C (50 μM) for 72 h, cells were treated with JQ1, IBET-762, I-BET151, and CPI-203 over a 10-point / 3-fold serial dilution with a starting concentration of 10 μM. The mean EC50 values of the four BETi were diminished by vitamin C pretreatment in all 3 TNBC lines and in the DT-28 primary culture (Fig. 1b), demonstrating an increased potency of BETi when combined with vitamin C. In contrast, the antioxidant glutathione (GSH), which has no effect on TET-mediated DNA demethylation [18], exerted no effect on BETi efficacy despite its potent antioxidant property (Fig. 1c-f). To test if the increased efficacy of BETi by vitamin C is dependent on TET-mediated DNA demethylation, the expression of TETs was decreased by siRNAs targeting all three isoforms (TET1, TET2, TET3) (Supplementary Fig. S2). Once the expression of TETs had been silenced, vitamin C no longer affected the EC50 of JQ1 and IBET-762 in treating MDA-MB-231 cells (Supplementary Fig. S3). These results suggest that vitamin C increases the potency and efficacy of BETi by decreasing EC50 values and increasing effectiveness, an action likely independent of its general antioxidant activity but dependent on TET-mediated DNA demethylation.
3.2. Vitamin C suppresses the acetylation of H3 and H4 in TNBC cells
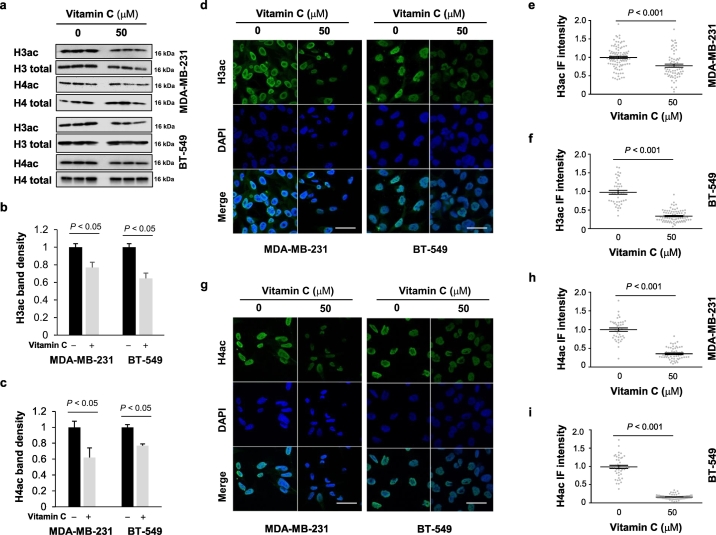
In principle, two potential mechanisms could underlie the enhanced efficacy of BETi upon co-treatment with vitamin C: 1) a reduced expression of BET proteins; or 2) downregulated histone acetylation. Both would result in lower BET protein binding to acetylated histones and therefore yield enhanced BETi efficacy. Examination of our existing RNA-seq data from MDA-MB-231 cells with or without vitamin C [24] showed that BET transcripts remain stable after vitamin C treatment. We thus turned our attention to histone acetylation. By evaluating pan-H3 acetylation (H3ac) and H4 acetylation (H4ac) using Western blot, we found that vitamin C treatment decreased both H3ac and H4ac in MDA-MB-231 cells (Fig. 2a-c). The inhibition of H3ac and H4ac by vitamin C was also observed in a second TNBC line, BT-549 (Fig. 2a-c).
Fig. 2.
Vitamin C treatment reduces H3ac and H4ac. (a) Vitamin C (50 μM) treatment for 72 h reduces H3ac and H4ac in MDA-MB-231 and BT-549 TNBC cells. (b-c) Densitometry analysis shows that both H3ac and H4ac are decreased by vitamin C in MDA-MB-231 and BT-549 TNBC cells. (d-i) Immunofluorescence and quantification further corroborate that vitamin C (50 μM) treatment reduces H3ac and H4ac in MDA-MB-231 and BT-549 cells. All data are presented as mean ± SEM (t-test). (bar = 20 μm).
To corroborate this observation, we used immunofluorescence to visualize intracellular H3ac and H4ac. In both MDA-MB-231 and BT-549 cells, H3ac and H4ac nuclear fluorescence was decreased after vitamin C treatment. Quantification of nuclear H3ac or H4ac immunofluorescence revealed that both H3ac and H4ac are reduced significantly by vitamin C treatment (Fig. 2d-i). Taken together, these results suggest that vitamin C treatment decreases H3ac and H4ac, which would, in turn, decrease BET-histone interactions.
3.3. Vitamin C inhibits histone acetylation by increasing HDAC1 expression
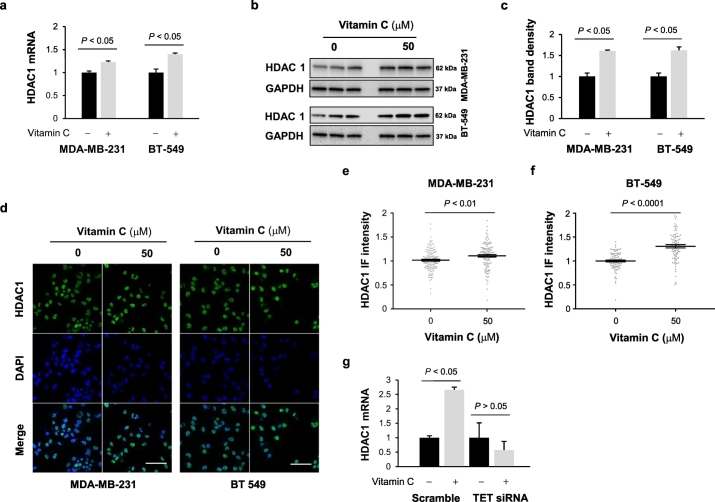
The dynamic H3ac and H4ac levels are regulated primarily by histone acetyltransferases (HAT)-mediated acetylation and by histone deacetylases (HDAC)-mediated deacetylation. To elucidate how vitamin C might suppress H3ac or H4ac, we again investigated our published RNA-seq data [24], which showed that HDAC1 expression is upregulated by vitamin C treatment. Quantitative RT-PCR confirmed that HDAC1 expression levels were increased in MDA-MB-231 and BT-549 cells by vitamin C treatment (Fig. 3a). Western blot, immunofluorescence, and corresponding quantification further confirmed that vitamin C treatment augmented HDAC1 protein levels in MDA-MB-231 and BT-549 cells (Fig. 3b-f).
Fig. 3.
Vitamin C treatment augments HDAC1 expression. (a-b) Vitamin C (50 μM) treatment for 72 h augments HDAC1 mRNA and protein expression in MDA-MB-231 and BT-549 cells. (c) Densitometry analysis of HDAC1 protein after vitamin C (50 μM) treatment in MDA-MB-231 and BT-549 cells. (d-f) Immunofluorescence and quantification further corroborate that vitamin C treatment (50 μM) increases HDAC1 expression in MDA-MB-231 and BT-549 cells. (bar = 20 μm). (g) After vitamin C treatment, the level of HDAC1 mRNA remains unchanged in TETs knockdown MDA-MB-231 cells as shown by qRT-PCR. All data are presented as mean ± SEM (t-test).
To test if the elevation of HDAC1 is induced by TET-mediated DNA demethylation, the expression of TETs was decreased by siRNAs targeting all three isoforms (TET1, TET2, TET3) (Supplementary Fig. S2). After TETs knockdown, HDAC1 mRNA remained largely unchanged by vitamin C (50 μM) treatment. Conversely, the control group (scramble siRNA) showed an elevation in HDAC1 after the same vitamin C treatment in MDA-MB-231 cells (Fig. 3g) and BT-549 cells (Supplementary Fig. S4). These results suggest that the upregulation of HDAC1 by vitamin C treatment is likely mediated by and dependent on TETs.
In addition to HDAC1, the transcript and protein levels of HDAC6 were also increased by vitamin C treatment (Supplementary Fig. S5). Since HDAC1 erases histone acetylation and HDAC6 mainly targets non-histone acetylation [29], the upregulated HDAC1 is more likely to be responsible for the reduced H3ac and H4ac after vitamin C treatment. Unlike in melanoma [26], vitamin C had no obvious effect on HAT1 expression in TNBC cells (Supplementary Fig. S6).
3.4. Vitamin C and BETi cooperatively block the interaction between BRD4 and histones
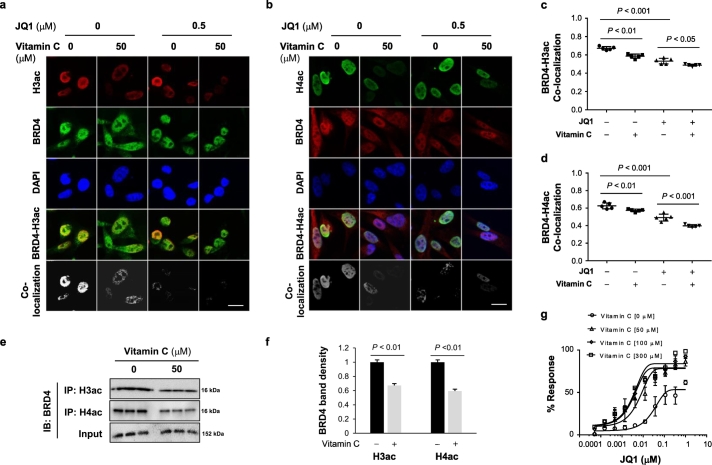
By inhibiting H3ac and H4ac, vitamin C reduces BET protein binding sites, thereby decreasing BET-histone interaction, which may underlie the enhanced BETi efficacy in TNBC cells. Immunofluorescence was used to visualize the combined effect of vitamin C and BETi on co-localization of BRD4 with H3ac or H4ac. In untreated MDA-MB-231 cells, BRD4 co-localized with H3ac or H4ac. Vitamin C (50 μM) alone reduced detectable H3ac and H4ac, and reduced co-localization with BRD4. Treatment of MDA-MB-231 cells with JQ1 (0.5 μM) decreased the interaction of BRD4 with either H3ac or H4ac. Co-treatment with vitamin C and JQ1 almost completely abolished the co-localization signal (Fig. 4a-d) and co-immunoprecipitation (co-IP) confirmed the effect of vitamin C to decrease BRD4 interaction with these histones. Co-IP with either anti-h3 ac or anti-H4ac antibody were resolved and Western blotted with anti-BRD4 antibody. Vitamin C (50 μM) decreased the H3ac and H4ac bands by over 30% (Fig. 4e, f; Supplementary Fig. S7). Collectively, these results suggest that vitamin C enhances the effect of JQ1 by decreasing HDAC1-mediated H3 and H4 deacetylation thereby impairing BRD4 recruitment to these histones.
Fig. 4.
Vitamin C and JQ1 synergistically inhibit the binding of BRD4 to acetylated histones. (a-b) Confocal microscopy images of H3ac, H4ac, BRD4, and their co-localization in MDA-MB-231 cells. (bar = 20 μm). (c-d) Quantification of H3ac and BRD4 co-localization reveals that vitamin C treatment alone reduces both H3ac and H4ac subsequently diminishing H3ac-BRD4 co-localization. The co-localization coefficient of H3ac-BRD4 and H4ac-BRD4 was further decreased by co-treatment with vitamin C and JQ1. (e) ChIP assay of H3ac/H4ac and BRD4 binding in MDA-MB-231 cells. (f) Densitometry analysis of ChIP bands showing a reduction in BRD4 binding to H3ac and H4ac in MDA-MB-231 cells after vitamin C treatment. All data are presented as mean ± SEM (ANOVA). (g) Both 100 μM and 300 μM vitamin C decrease the EC50 of JQ1 further by ~2-fold compared to 50 μM vitamin C.
3.5. Vitamin C and BETi synergistically suppress TNBC malignancy in vitro
To determine if the drug effects of BETi and vitamin C are synergistic or additive, combined treatment effects were analyzed by isobologram and combination index [30]. We analyzed different combinations of vitamin C and JQ1 to achieve 50% and 70% inhibition of MDA-MB-231 cells. All combination data points were shown to fall below corresponding EC50 and EC70 lines, which correspond to combination index (CI) values of <1.0 (Supplementary Fig. S8), indicating a synergistic interaction between vitamin C and BETi in treating TNBC cells.
The average concentration of vitamin C in the plasma of healthy humans is ~50 μM. By diet and oral supplementation, plasma vitamin C can reach up to ~100 μM [31]. To achieve plasma vitamin C levels higher than 200–300 μM would require intravenous infusion or intraperitoneal injection. We then tested the impact of vitamin C at different concentrations on the EC50 of JQ1 in vitro. 50 μM vitamin C decreased the EC50 of JQ1 by ~10-fold and 100 μM vitamin C decreased the EC50 of JQ1 by 2-fold more than did vitamin C at 50 μM. In contrast, vitamin C at 300 μM did not further reduce the EC50 of JQ1 compared to vitamin C at 100 μM (Fig. 4g). These data suggest that 100 μM vitamin C might be sufficient to maximize the efficacy of BETi. The higher dose of 300 μM vitamin C, which would require intravenous infusion or intraperitoneal injection in humans and mice, does not appear to exert additional benefit on BETi efficacy.
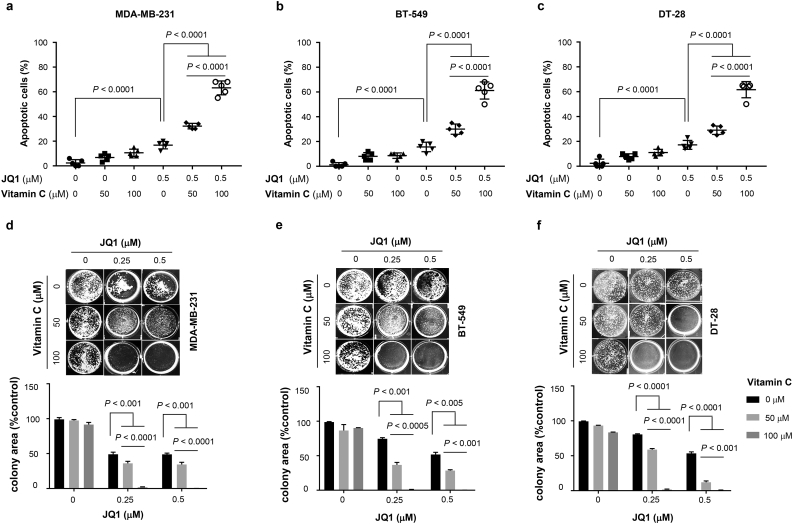
Given the enhancement of BETi efficacy by vitamin C on viability in vitro, we next tested their potential synergy on the malignant phenotypes of cultured TNBC cells. A TUNEL assay, used as a measure of apoptosis, showed vitamin C (50 μM) alone did not induce apoptosis, while vitamin C at 100 μM increased apoptotic cells. JQ1 (0–0.5 μM) dose-dependently promoted apoptosis. In combination, vitamin C enhanced the effect of JQ1 to induce apoptosis in MDA-MB-231, BT-549, and DT-28 cells (Fig. 5a-c). Co-treatment of TNBC cells with 100 μM vitamin C and JQ further induced apoptosis compared to co-treatment with 50 μM vitamin C and JQ1 or JQ1 alone.
Fig. 5.
Vitamin C reinforces the effect of JQ1 to induce apoptosis and inhibit survival of TNBC cells. (a–c) JQ1 (0.5 μM) alone slightly induces apoptosis (~20%) in MDA-MB-231, BT-549, and DT-28 cells (P < .0001) (ANOVA). More apoptosis appears in co-treatment with JQ1 and vitamin C (50, 100 μM) compared to JQ1 alone group (P < .0001). In combination with 100 μM vitamin C, JQ1 induces more apoptosis (~65%) compared to JQ1 with 50 μM vitamin C (~35%) (P < .0001) (ANOVA). (d-f) JQ1 (0.25, 0.5 μM) alone moderately inhibits colony formation by MDA-MB-231, BT-549, and DT-28 cells (P < .01) (ANOVA). Co-treatment with JQ1 and vitamin C (50, 100 μM) further inhibit colony formation compared to JQ1 alone group (P < .001) (ANOVA). In combination with 100 μM vitamin C, JQ1 almost completely blocks colony formation.
We then examined vitamin C and JQ1 co-treatment on TNBC colony formation. JQ1 (0–0.5 μM) dose-dependently inhibited TNBC colony formation while vitamin C alone had no obvious effect. Combined treatment with JQ1 and vitamin C further decreased colony formation (Fig. 5d-f). Comparatively, co-treatment with 100 μM vitamin C and JQ1 more effectively inhibited colony formation compared to co-treatment with 50 μM vitamin C with JQ1 or treatment with JQ1 alone. Thus, vitamin C at 100 μM, which is achievable by oral supplementation, significantly enhances the effect of JQ1 to suppress TNBC malignancy in vitro.
3.6. Oral vitamin C improves the response of TNBC to BETi in vivo
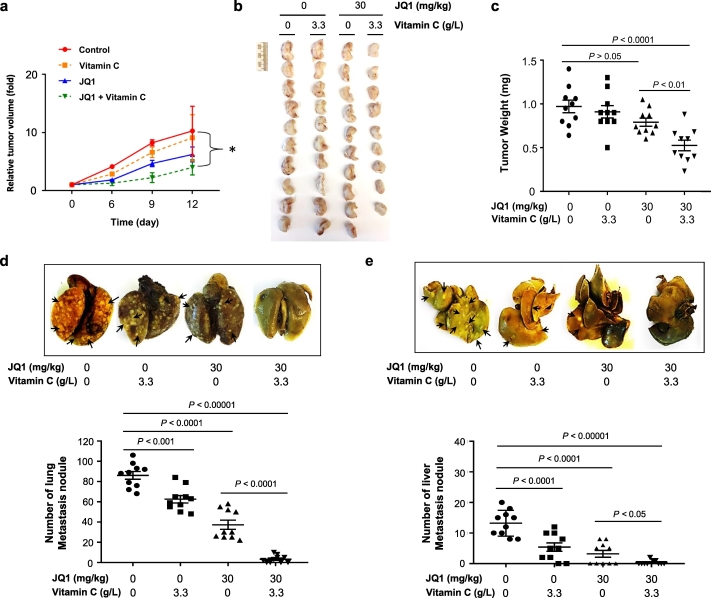
Humans as well as other primates cannot synthesize vitamin C due to a mutant and nonfunctional gulonolactone oxidase (Gulo), the enzyme catalyzing the final step of vitamin C biosynthesis. For humans, vitamin C must be supplied in the diet or via dietary supplements. In contrast, mice synthesize vitamin C de novo in the liver. We examined TNBC xenograft in immune-deficient NSG mice, whose endogenous vitamin C plasma concentration is around 50 μM, similar to the level observed in healthy humans [31,32]. Our cell-based experiments clearly suggested that 100 μM vitamin C treatment is superior to 50 μM for enhancing the effect of JQ1. To reach ~100 μM vitamin C in the plasma, additional vitamin C (3.3 g/L) was provided in the drinking water of NSG mice as previously described [32]. For our first in vivo test of JQ1 and vitamin C dual therapy, we applied the therapeutic dose of JQ1 (50 mg/kg body weight) that has been used in previous studies [[4], [5], [6], [7], [8]]. We tested the effects of JQ1 and vitamin C co-treatment on in vivo tumor growth. Starting five days after MDA-MB-231 cell implantation, JQ1 (50 mg/kg body weight) was administrated intraperitoneally (IP) daily for 2 weeks. JQ1 alone inhibited xenograft growth. With vitamin C supplementation, JQ1 mediated inhibition of TNBC xenograft growth was greater than that observed with JQ1 alone group (P < .001) (ANOVA) (Supplementary Fig. S9), suggesting that vitamin C complements the anti-tumor effect of JQ1.
We then tested the impact of vitamin C supplementation and a dose of JQ1 previously shown to be sub-therapeutic in xenograft models [[4], [5], [6], [7], [8]] on TNBC xenograft growth. After palpable xenografts (3–5 mm diameter) formed, JQ1 (30 mg/kg body weight IP daily for 2 weeks) was administrated and vitamin C supplementation (3.3 g/L) was provided in the drinking water. In vivo measurement of tumor by caliper showed that at day 9 and day 12, tumor volumes were smaller in JQ1 and vitamin C co-treatment group compared to non-treated group (Fig. 6a). Mice were then sacrificed for direct examination of xenografts. Vitamin C (3.3 g/L) showed no obvious tumoristatic effect when administered alone (P > .05) (ANOVA). JQ1 at this sub-therapeutic dose caused a small but non-significant inhibition of xenograft growth (P > .05) (ANOVA). Only JQ1 in combination with vitamin C measurably attenuated xenograft growth compared to non-treated controls (P < .0001) (ANOVA) or compared to the JQ1 alone group (P < .01) (ANOVA) (Fig. 6b, c). Thus, oral vitamin C supplementation, at a dose achievable in mice and humans, renders a dose of JQ1 that is on its own sub-therapeutic capable of effective antitumor activity in vivo. Notably, the animals showed no excess toxicity and weight gain was not altered compared to controls during the course of the experiment.
Fig. 6.
Vitamin C supplementation renders JQ1 at a sub-therapeutic dose to inhibit TNBC xenograft and metastasis. (a) At day 9 and day 12, tumor volumes measured by caliper are smaller in JQ1 and vitamin C co-treatment group compared to non-treated group (* P < .05) (ANOVA). (b) Photograph of human MDA-MB-231 TNBC xenografts from female NSG mice treated with or without JQ1 (30 mg/kg body weight) and supplemented with or without vitamin C (3.3 g/L) in the drinking water. (c) Quantification of xenograft weights shows that xenografts in JQ1 alone group are similar to non-treated group (P > .05) (ANOVA). Xenografts in JQ1 and vitamin C co-treatment group are smaller compared to non-treated group (P < .0001) (ANOVA) or compared to JQ1 alone group (P < .01) (ANOVA). (d) Representative lung images and quantification show metastatic nodules in JQ1 alone group (P < .0001) (ANOVA) or vitamin C alone group (P < .001) (ANOVA) are less than non-treated group. Metastatic nodules are fewer in JQ1 and vitamin C co-treatment group compared to non-treated group (P < .0001) (ANOVA) or compared to JQ1 alone group (P < .0001) (ANOVA). (e) Representative liver images and quantification show metastatic nodules in JQ1 alone group or vitamin C alone group are less compared to non-treated group (P < .0001) (ANOVA). Co-treatment with JQ1 and vitamin C further reduces metastatic nodules in the liver compared to non-treated group (P < .00001) (ANOVA) or compared to JQ1 alone group (P < .05) (ANOVA).
The improved efficacy of JQ1 by vitamin C supplementation was further revealed in a reduction in tumor metastasis to other organs. Oral vitamin C alone (P < .001) (ANOVA) or JQ1 alone (P < .0001) (ANOVA) reduced the formation of lung metastasis. The combination of JQ1 and vitamin C further decreased metastatic lung nodules compared to non-treated controls (P < .0001) (ANOVA) or compared to the JQ1 alone group (P < .0001) (ANOVA) (Fig. 6d). Similarly, JQ1 alone and vitamin C alone attenuated liver metastasis (P < .0001) (ANOVA), co-treatment with JQ1 and vitamin C caused the greatest decrease in liver metastasis (dual therapy compared to non-treated group (P < .00001) (ANOVA); dual therapy compared to JQ1 alone group (P < .05) (ANOVA) (Fig. 6e). Taken together, these results suggest that vitamin C supplementation might expand the therapeutic window of JQ1, rendering a JQ1 dose that is a sub-therapeutic when given alone capable of significantly inhibiting TNBC xenograft growth and metastasis.
4. Discussion
Rapid progress has recently been made in preclinical and clinical studies characterizing the anti-cancer properties of BETi. There are currently over twenty clinical trials underway evaluating various BETi for treatment of human malignancies, including TNBC. Unfortunately, significant toxicities, most of which are dose-dependent, have been observed in phase I trials [[14], [15], [16]]. The relatively narrow therapeutic window jeopardizes the prospect of subsequent BETi clinical trials and the ultimate implementation of these drugs in patient care.
The current preclinical study provides evidence for a potential solution that would expand the therapeutic window of BETi. We show that vitamin C improves the efficacy of BETi in treating TNBC in vitro. Vitamin C at 100 μM, a dose achievable in vivo by oral supplementation, is more potent than 50 μM, and effectively promotes the effect of JQ1 to induce apoptosis and to inhibit survival and colony formation. In contrast, vitamin C at 300 μM, a dose that requires intravenous infusion or intraperitoneal injection, does not further enhance BETi efficacy. With co-administration of oral vitamin C, a JQ1 dose that had limited anti-tumor activity on its own was shown to inhibit growth of both primary xenografts. Furthermore, co-treatment with JQ1 and vitamin C almost completely blocks visible metastasis in the lung and liver. Overall, in vitro and in vivo data suggest that vitamin C can extend the therapeutic window for TNBC.
The response to therapeutic drugs can be improved by altering certain epigenetic modifications in a specific cancer type [17]. The epigenetic role of vitamin C, particularly its enhancement of TET-mediated DNA demethylation, appears to mediate the induction of HDAC1, thereby decreasing H3ac and H4ac to enhance BETi efficacy. Other functions of vitamin C, including its actions as an antioxidant or collagen hydroxylase cofactor, are less likely to play key roles in drug responses. GSH is a potent antioxidant that enters cells through GSH transporters and connexin hemichannels [33,34]. Our prior work showed that GSH does not affect 5-hydroxymethylcytosine (5hmC) generation [17]. Despite its potent antioxidant action, GSH does not alter the response of TNBC cells to BETi while vitamin C does. Thus, the antioxidant effect of vitamin C alone is not likely to play an important role in mediating its action on BETi.
Vitamin C promotes TET-mediated DNA demethylation, which is initiated by oxidizing 5-methylcytosine (5mC) to 5hmC, as discovered by our group and others [[18], [19], [20], [21], [22]]. Genomic loss of 5hmC has been recognized as an epigenetic hallmark in most, if not all, types of cancer including TNBC [35]. We've previously shown that addition of vitamin C increases 5hmC content and significantly alters the transcriptome in TNBC cells [24]. Vitamin C increases HDAC1 expression, leading to a loss of H3ac and H4ac. Upon TETs depletion, vitamin C no longer promotes HDAC1 expression, suggesting that vitamin C induced HDAC1 is largely mediated by TETs. Vitamin C increases HDAC1 expression and decreases H3ac and H4ac in TNBC cells, which reduces BET protein binding sites. BETi attenuate BET proteins by structurally mimicking acetylated lysine. Furthermore, vitamin C is also a known cell differentiation factor which alters other epigenetics features in cancer [36,37]. Through these mechanisms, vitamin C synergistically sensitizes TNBC cells to BETi. Vitamin C is a safe, well-tolerated micronutrient that is readily available and could be implemented in patient care. Through co-administration with vitamin C at well-tolerated non-toxic doses, lower doses of BETi could be used to achieve an increased therapeutic index, which will translate to a reduced side effect profile in patients.
In conclusion, our study suggests that vitamin C expands the therapeutic window of BETi for TNBC by reducing H3ac and H4ac. The efficacy of BETi monotherapy might be affected by a patient's endogenous vitamin C levels. Future clinical trials of BETi monotherapy should consider incorporating patient vitamin C levels in the study design. A therapeutic strategy of combining vitamin C supplementation with BETi might provide a novel opportunity to expand the utility of these promising yet toxic cancer drugs for patient care.
Funding sources
This work is supported by University of Miami Sylvester Comprehensive Cancer Center, Bankhead Coley Cancer Research Program (7BC10), Flight Attendant Medical Research Institute, and NIH grants R21CA191668 (to GW) and 1R56AG061911 (to CW and CHV).
Declaration of interests
The authors declare no competing interests.
Authors' contributions
G.W. designed the study. J.S. and C.W. contributed to data interpretation and manuscript edition. S.M., C-H.V., and S.P·B conducted high-throughput compound screen. S.M., T.C.H., D.W.S. and L.Z. performed cell-based experiments. S.M., V.C., R.Q., and H.Y. conducted animal experiments. S.M. and G.W. drafted the manuscript. All authors edited, commented and approved the manuscript.
Acknowledgments
The authors sincerely thank the Bradner lab and Dr. Jun Qi at Dana-Farber Cancer Institute, Harvard University for providing JQ1 used in this research. We also thank Dr. Dorraya El-Ashry at University of Minnesota for providing DT-28 cells. This study was also supported in part by the Molecular Therapeutics Shared Resource (MTSR) at the Sylvester Comprehensive Cancer Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.04.006.
Appendix A. Supplementary data
Supplementary material
References
- 1.Jain A.K., Barton M.C. Bromodomain histone readers and cancer. J Mol Biol. 2017;429:2003–2010. doi: 10.1016/j.jmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Filippakopoulos P., Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13:337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- 3.Filippakopoulos P., Qi J., Picaud S. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu S., Lin C.Y., He H.H. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahni J.M., Gayle S.S., Bonk K.L. Bromodomain and extraterminal protein inhibition blocks growth of triple-negative breast cancers through the suppression of aurora kinases. J Biol Chem. 2016;291:23756–23768. doi: 10.1074/jbc.M116.738666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Peña J., Serrano-Heras G., Montero J.C., Corrales-Sánchez V., Pandiella A., Ocaña A. In silico analysis guides selection of BET inhibitors for triple-negative breast cancer treatment. Mol Cancer Ther. 2016;15:1823–1833. doi: 10.1158/1535-7163.MCT-16-0004. [DOI] [PubMed] [Google Scholar]
- 7.Sahni J.M., Gayle S.S., Webb B.M. Mitotic vulnerability in triple-negative breast cancer associated with LIN9 is targetable with BET inhibitors. Cancer Res. 2017;77:5395–5408. doi: 10.1158/0008-5472.CAN-17-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieto-Jiménez C., Alcaraz-Sanabria A., Pérez-Peña J. Targeting basal-like breast tumors with bromodomain and extraterminal domain (BET) and polo-like kinase inhibitors. Oncotarget. 2017;8:19478–19490. doi: 10.18632/oncotarget.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Motta L.L., Ledaki I., Purshouse K. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene. 2017;36:122–132. doi: 10.1038/onc.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai L., Zhou B., Yang C.Y. Targeted degradation of BET proteins in triple-negative breast cancer. Cancer Res. 2017;77:2476–2487. doi: 10.1158/0008-5472.CAN-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratikopoulos E.E., Dendy M., Szabolcs M. Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell. 2015;27:837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borbely G., Haldosen L.A., Dahlman-Wright K., Zhao C. Induction of USP17 by combining BET and HDAC inhibitors in breast cancer cells. Oncotarget. 2015;6:33623–33635. doi: 10.18632/oncotarget.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez R., Riveiro M.E., Astorgues-Xerri L. The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. Oncotarget. 2017;8:7598–7613. doi: 10.18632/oncotarget.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthon C., Raffoux E., Thomas X. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3:e186–e195. doi: 10.1016/S2352-3026(15)00247-1. [DOI] [PubMed] [Google Scholar]
- 15.Amorim S., Stathis A., Gleeson M. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3:e196–e204. doi: 10.1016/S2352-3026(16)00021-1. [DOI] [PubMed] [Google Scholar]
- 16.Stathis A., Zucca E., Bekradda M. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov M., Barragan I., Ingelman-Sundberg M. Epigenetic mechanisms of importance for drug treatment. Trends Pharmacol Sci. 2014;35:384–396. doi: 10.1016/j.tips.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Minor E.A., Court B.L., Young J.I., Wang G. Ascorbate induces Ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson K.M., Gustafson C.B., Young J.I., Züchner S., Wang G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem Biophys Res Commun. 2013;439:522–527. doi: 10.1016/j.bbrc.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin R., Mao S.Q., Zhao B., Chong Z., Yang Y., Zhao C. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135:10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 21.Blaschke K., Ebata K.T., Karimi M.M. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Guo L., Zhang L. Vitamin C modulates Tet1 function during somatic cell reprogramming. Nat Genet. 2013;45:1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 23.Wilson J.X. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 24.Sant D.W., Mustafi S., Gustafson C.B., Chen J., Slingerland J.M., Wang G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci Rep. 2018;8:5306. doi: 10.1038/s41598-018-23714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monfort A., Wutz A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013;14:337–346. doi: 10.1038/embor.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafi S., Camarena V., Volmar C.H. Vitamin C sensitizes melanoma to BET inhibitors. Cancer Res. 2018;78:572–583. doi: 10.1158/0008-5472.CAN-17-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drews-Elger K., Brinkman J.A., Miller P. Primary breast tumor-derived cellular models: characterization of tumorigenic, metastatic, and cancer-associated fibroblasts in dissociated tumor (DT) cultures. Breast Cancer Res Treat. 2014;144:503–517. doi: 10.1007/s10549-014-2887-9. [DOI] [PubMed] [Google Scholar]
- 28.Andrieu G., Tran A.H., Strissel K.J., Denis G.V. BRD4 regulates breast cancer dissemination through Jagged1/Notch1 signaling. Cancer Res. 2016;76:6555–6567. doi: 10.1158/0008-5472.CAN-16-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seto E., Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 31.Levine M., Padayatty S.J., Espey M.G. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2:78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsao C.S., Leung P.Y., Young M. Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J Nutrition. 1997;117:291–297. doi: 10.1093/jn/117.2.291. [DOI] [PubMed] [Google Scholar]
- 33.Bachhawat A.K., Thakur A., Kaur J., Zulkifli M. Glutathione transporters. Biochim Biophys Acta. 2013;1830:3154–3164. doi: 10.1016/j.bbagen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Shi W., Riquelme M.A., Gu S., Jiang J.X. Connexin hemichannels mediate glutathione transport and protect lens fiber cells from oxidative stress. J Cell Sci. 2018;131 doi: 10.1242/jcs.212506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeifer G.P., Xiong W., Hahn M.A., Jin S.G. The role of 5-hydroxymethylcytosine in human cancer. Cell Tissue Res. 2014;356:631–641. doi: 10.1007/s00441-014-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillberg L., Orskov A.D., Liu M., Harslof L.B.S., Jones P.A., Gronbaek K. Vitamin C—a new player in regulation of the cancer epigenome. Semin Cancer Biol. 2018;51:59–67. doi: 10.1016/j.semcancer.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Venturelli S., Sinnberg T.W., Berger A. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front Oncol. 2014;4:227. doi: 10.3389/fonc.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material