Abstract
Purpose
To evaluate the 1-year results of vitrectomy performed in combination with intraoperative dexamethasone implant for tractional and nontractional refractory diabetic macular edema (DME).
Methods
Thirteen eyes from 13 subjects who were diagnosed with tractional DME and 17 eyes from 17 subjects who were diagnosed with nontractional refractory DME underwent vitrectomy and dexamethasone implant injection. Changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT) during the one year following vitrectomy were evaluated in each group. Additionally, changes in intraocular pressure and other complications were investigated postoperatively.
Results
In eyes with tractional DME, a statistically significant improvement in BCVA was noted at 3, 6, and 12 months, and a statistically significant improvement in CMT was noted at 1, 3, 6, and 12 months from baseline after vitrectomy (p < 0.05). In eyes with nontractional refractory DME, a statistically significant improvement in BCVA was noted at 12 months, but there were no significant improvements in CMT despite the tendency to decrease from baseline. Sixteen (53.3%) of the 30 eyes included in this study showed intraocular pressure elevation, which was addressed using antiglaucoma medication, and there were no other severe complications.
Conclusions
Vitrectomy combined with intraoperative dexamethasone implant may be safe and effective in treating DME, especially tractional DME. In this study, patients with nontractional DME required more additional treatments and time for anatomical and functional improvement compared to patients with tractional DME.
Keywords: Dexamethasone implant, Nontractional diabetic macular edema, Tractional diabetic macular edema, Vitrectomy
Diabetic macular edema (DME), which affects approximately 6.8% of the diabetic population, is the leading cause of vision loss in patients with diabetes [1]. Nonsurgical treatments for DME have included focal and grid macular laser photocoagulation [2], posterior sub-tenon injection of triamcinolone acetonide [3,4], intravitreal injection of triamcinolone acetonide (IVTA) [5,6], and intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents [7,8,9,10]. Vitrectomy as a surgical treatment may be considered when there is little to no response to nonsurgical treatments or in cases in which DME is accompanied by definitive vitreomacular traction (VMT) or epiretinal membrane (ERM). Combining vitrectomy and nonsurgical treatments can produce favorable outcomes because these treatments seem to work synergistically [11,12].
The dexamethasone intravitreal implant (Ozurdex; Allergan, Irvine, CA, USA) is a sustained-release device that is currently approved for treatment of macular edema associated with diabetic retinopathy [13], retinal vein occlusions [14], and noninfectious posterior uveitis [15]. The dexamethasone intravitreal implant is able to release medication for up to 6 months and has been shown to have similar pharmacokinetic profiles in both vitrectomized and nonvitrectomized eyes [16,17,18].
We previously reported the favorable effects of triple therapy involving vitrectomy, IVTA, and macular focal laser photocoagulation for DME refractory to conventional treatments [11]. In addition, the long-term stability and efficacy of triple therapy were confirmed [19]. Vitrectomy performed in combination with dexamethasone intravitreal implant instead of IVTA may yield longer-lasting treatment effects, because the half-life of IVTA in vitrectomized eyes was reported to be far shorter [20], and a dexamethasone intravitreal implant can release medication for a longer period than IVTA. Additionally, the dexamethasone implant has been associated with a smaller incidence of increase in intraocular pressure (IOP) compared with the intravitreal fluocinolone acetonide implant or intravitreal triamcinolone acetonide [17]. These characteristics can also be an advantage for treatment of DME of a chronic and recurrent nature.
One previous study reported outcomes of vitrectomy combined with intraoperative dexamethasone for intractable DME. The results indicated that central retinal thickness and best-corrected visual acuity (BCVA) improved significantly and were maintained until 12 months [21]. However, further studies on the efficacy and adverse events of this therapy are needed, including to distinguish between patients with nontractional refractive DME and tractional DME and analyze them through more cases. The objective of the current study was to investigate clinical outcomes of vitrectomy combined with intraoperative dexamethasone implant as a treatment for DME.
Materials and Methods
Ethics statement
This retrospective study was performed at a single center and adhered to the tenets of the Declaration of Helsinki. The study was approved by the institutional review board and ethics committees of Samsung Medical Center in Seoul, Korea (2018-02-129). Informed consent was waived due to the retrospective nature of the study.
Patient selection
This study included patients who underwent vitrectomy combined with intraoperative dexamethasone implant between March 2015 and September 2016 as treatment for nontractional refractory DME or tractional DME. Refractory DME was defined as biomicroscopically, angiographically, and tomographically confirmed diffuse DME that had a central macular thickness (CMT) of 300 microns or more despite repeated nonsurgical treatments including focal and grid macular laser photocoagulation, posterior sub-tenon injection of triamcinolone acetonide, intravitreal injection of triamcinolone acetonide, and intravitreal injection of anti-VEGF agents. Of these patients, tractional DME and nontractional DME were classified according to the presence or absence of traction force like VMT or ERM on optical coherence tomography (OCT). Major exclusion criteria included (1) a postoperative follow-up period less than 12 months, (2) active proliferative diabetic retinopathy, (3) intraocular inflammation, (4) uncontrolled IOP, (5) cataract surgery within the past 6 months, (6) prior history of vitreoretinal surgery, and (7) evidence of any retinal disease that might affect visual acuity or macular microstructure. Eyes subjected to DME treatment such as intravitreal or periocular injection of steroid or anti-VEGF agents within three months were also excluded. When both eyes met the inclusion criteria, the eye that had undergone prior surgery was included.
Preoperative examination
Preoperative ocular examination included BCVA using Snellen visual acuity charts, applanation tonometry, slit-lamp biomicroscopy, and fundus examination. OCT was conducted in each eye using a spectral domain OCT (SD-OCT) system (Spectralis HRA-OCT; Heidelberg Engineering, Heidelberg, Germany) to evaluate abnormalities of the vitreomacular interface and to determine macular thickness. To evaluate the severity of diabetic retinopathy and the type of DME, preoperative fluorescein angiograms and fundus photographs were also obtained.
Surgical procedure
A standard 3-port pars plana vitrectomy was performed by a single surgeon (SWK) using a Constellation (Alcon Laboratories, Fort Worth, TX, USA) or Associate (Dutch Ophthalmic Research Center, Zuidland, The Netherlands) 23-gauge vitrectomy system under local anesthesia. Vitrectomy with removal of the retinal internal limiting membrane (ILM) was conducted in all patients. The retinal ILM was peeled off via careful grasping with intraocular forceps from a round area with a dimension of approximately 2-disc diameters centered on the fovea. In most cases, the retinal ILM was removed with the assistance of indocyanine green staining dye. If a VMT or ERM was present, it was removed. Panretinal endolaser photocoagulation coupled with vitrectomy was performed in cases with extensive retinal capillary dropout or with apparent high-risk characteristics. At the conclusion of the operation, a dexamethasone implant was placed into the vitreous cavity through the 23-gauge vitrectomy port. Patients were advised to maintain a sitting position for a few hours in order to position the dexamethasone implant at the inferior periphery. Combined cataract surgery was performed in patients of older ages (over 50 years) to pretreat for post-vitrectomy lens opacity. According to the judgement of the operator, cataract surgery was performed if necessary.
Postoperative examination
During follow-up visits at 1, 3, 6, and 12 months postoperatively, all patients underwent BCVA measurement, slit-lamp examination, dilated fundus examination with a 90-diopter lens, and SD-OCT. The BCVA was transformed to a logarithmic scale for statistical analysis. Additional treatments for postoperative recurrence of DME and complications were identified.
Statistical analysis
We analyzed changes in BCVA and CMT after vitrectomy in each group. The data were analyzed using repeated measures analysis of variance with the Bonferroni correction. Due to the small sample size of this study, non-parametric statistical analyses including the Mann-Whitney U-test and Fisher's exact test were applied to assess the significance of differences between the two groups. SAS ver. 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analysis of the data, and p-values less than 0.05 were considered statistically significant.
Results
Forty-two eyes of 42 subjects with DME underwent vitrectomy combined with intraoperative dexamethasone implant. Finally, 30 eyes were included in the analysis, with 17 patients composing the nontractional refractory DME group and 13 patients composing the tractional DME group. The other 12 patients were excluded from the study for the following reasons: (1) active proliferative diabetic retinopathy (two patients), (2) prior history of vitreoretinal surgery (two patients), (3) evidence of retinal disease that might affect visual acuity or macular microstructure (four eyes), and (4) follow-up period less than 12 months (four eyes).
The baseline characteristics of the nontractional refractory DME and tractional DME groups are presented in Table 1. The mean age of each group was 56.4 ± 7.6 and 62.6 ± 11.4 years, respectively. The mean durations of diabetes were 11.5 ± 7.7 and 20.0 ± 8.5 years, respectively, and the difference was significant (p = 0.009). In the nontractional refractory DME group, 7 eyes (41.2%) had undergone macular laser photocoagulation, corticosteroid therapy, or both, and 9 eyes (52.9%) had undergone panretinal photocoagulation. In the tractional DME group, 2 eyes (15.4%) had undergone corticosteroid therapy and 11 eyes (84.6%) had undergone panretinal photocoagulation. All eyes in the nontractional refractory DME group had undergone intravitreal bevacizumab injection a mean of 6.0 ± 7.6 times (3 to 32 times), and three eyes in the tractional DME had undergone intravitreal bevacizumab injection a mean of 2.7 ± 2.9 times (1 to 6 times). Preoperatively, the mean logarithm of the minimum angle of resolution (logMAR) BCVA was 0.68 ± 0.37 and 0.55 ± 0.20, and the mean CMT was 470.3 ± 127.6 and 448.2 ± 95.1 µm, respectively. Six eyes in the nontractional DME group and two eyes in the tractional DME group were accompanied by foveolar detachment with exudation.
Table 1. Baseline characteristics of patients with nontractional DME and tractional DME.
Values are presented as number, mean ± standard deviation, or number (%).
DME = diabetic macular edema; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative retinopathy; IVTA = intravitreal triamcinolone acetonide; PSTA = posterior sub-tenon triamcinolone acetonide; PRP = panretinal photocoagulation; logMAR = logarithm of the minimal angle of resolution.
*p < 0.05.
Three of the 17 eyes with nontractional DME and 4 of the 13 eyes with tractional DME presented in a pseudophakic state prior to surgery. Phacoemulsification and implantation of a posterior chamber intraocular lens coupled with vitrectomy were conducted in 11 of 14 eyes with nontractional DME and in five of nine eyes with tractional DME due to patient age (over 50 years), presence of nucleosclerotic cataract, or both.
Treatment outcomes in each group of nontractional refractory DME and tractional DME
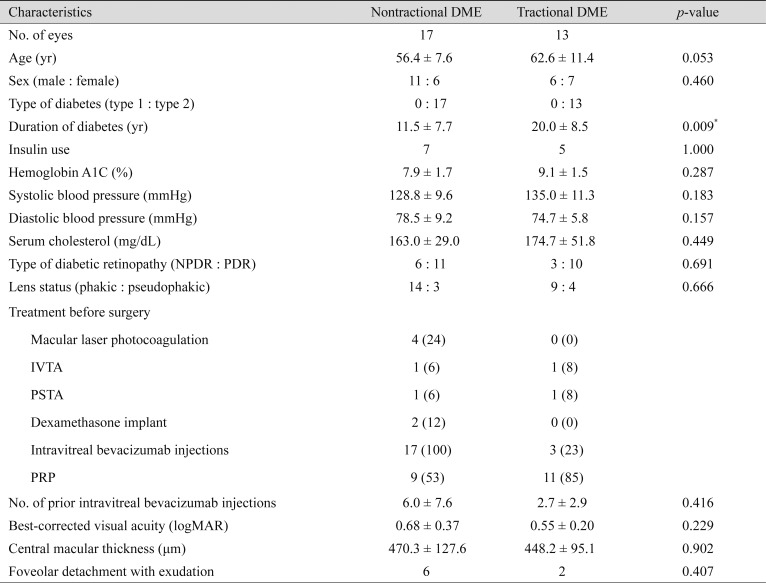
Changes in mean BCVA after vitrectomy combined with intraoperative dexamethasone implant are shown in Fig. 1A and 1B. In the nontractional refractory DME group, the mean logMAR BCVA values before surgery and at one, three, six, and 12 months after vitrectomy were 0.68 ± 0.37, 0.55 ± 0.29, 0.48 ± 0.18, 0.52 ± 0.17, and 0.33 ± 0.13, respectively. Compared with the preoperative value, BCVA was improved significantly at 12 months after vitrectomy (p < 0.002). In the tractional DME group, the mean logMAR BCVA values before surgery and at 1, 3, 6, and 12 months after vitrectomy were 0.55 ± 0.20, 0.52 ± 0.13, 0.42 ± 0.11, 0.38 ± 0.16, and 0.32 ± 0.16, respectively. BCVA improved significantly at three, six, and 12 months after vitrectomy compared with the preoperative value in this group (p = 0.041, p = 0.037, and p < 0.002, respectively).
Fig. 1. Graph illustrating changes in logarithm of the minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) and central macular thickness (CMT) at baseline and 1, 3, 6, and 12 months after vitrectomy combined with intraoperative dexamethasone implant for the treatment of diabetic macular edema (DME). Statistical significance was determined using repeated measures analysis of variance. Asterisks indicate statistically significant results after Bonferroni's correction. (A) The logMAR BCVA was significantly improved at 12 months in nontractional DME and at 3, 6, and 12 months in tractional DME after surgery. (B) The CMT was significantly decreased at 1, 3, 6, and 12 months in tractional DME after surgery, but there was no significant decrease in nontractional DME postoperatively despite the tendency to decrease from baseline. *Statistical significance.
Changes in mean CMT after vitrectomy combined with intraoperative dexamethasone implant are also shown in Fig. 1. In the nontractional refractory DME group, the mean CMT values before surgery and at one, three, six, and 12 months after vitrectomy were 470.3 ± 127.6, 400.1 ± 122.6, 428.6 ± 106.0, 414.1 ± 90.6, and 386.7 ± 103.6 µm, respectively. Compared with the preoperative value, there were no significant improvements in CMT despite the tendency to decrease from baseline. In the tractional DME group, the mean CMT values before surgery and at 1, 3, 6, and 12 months after vitrectomy were 448.2 ± 95.1, 359.2 ± 55.0, 348.4 ± 59.0, 369.2 ± 96.7, and 323.3 ± 59.3 µm, respectively. The CMT value decreased significantly at 1, 3, 6, and 12 months after vitrectomy versus the preoperative value in this group (p = 0.013, p < 0.001, p = 0.006, and p < 0.001, respectively). The mean CMT showed a tendency to decline during the follow-up period in both groups, but increased slightly between 1 and 3 months after surgery in the nontractional DME group and between 3 and 6 months after surgery in the tractional DME group. Additional treatments such as macular laser photocoagulation or corticosteroid therapy were performed in five of 17 eyes (29.4%) with nontractional DME beginning at 3 months after surgery.
On the other hand, two of 13 eyes (15.4%) with tractional DME underwent additional treatments beginning at 6 months after surgery. All cases with foveolar detachment in each group showed a resolution of foveolar detachment after the administration of vitrectomy combined with intraoperative dexamethasone implant. The preoperative and postoperative images of representative cases in our study are displayed in Fig. 2A–2L.
Fig. 2. Color fundus photographs and optical coherence tomography scans. The left column (A–F) represents a case of a 68-year-old male with nontractional diabetic macular edema (DME), and the right column (G–L) represents a case of a 55-year-old male with tractional DME. (A,G) Each of the patients had received multiple intravitreal bevacizumab injections for persistent DME preoperatively. (B) Preoperative central macular thickness (CMT) was 459 µm and best-corrected visual acuity (BCVA) was 0.2 in decimal equivalent. (C) One month postoperatively, CMT decreased to 404 µm. (D) At postoperative month 3, CMT was increased to 482 µm. (E) At postoperative month 6, CMT was 458 µm, and dexamethasone implant injection was conducted. (F) At postoperative month 12, CMT was maintained at 382 µm and BCVA was 0.5. (H) Preoperative CMT was 634 µm and BCVA was 0.1 in decimal equivalent. Vitreomacular traction was observed on optical coherence tomography (arrowhead). (I) One month postoperatively, CMT decreased to 267 µm. (J) At postoperative month 3, CMT increased to 298 µm. (K) At postoperative month 6, CMT was 390 µm and dexamethasone implant injection was conducted. (L) At postoperative month 12, CMT was maintained at 244 µm and BCVA was 0.6.
Adverse events
Four of seven phakic eyes (57.1%)—specifically, two of three phakic eyes in the nontractional DME group and two of four phakic eyes in the tractional DME group—underwent cataract surgery between 6 and 9 months after vitrectomy because of cataract development or progression. Additionally, 16 of 30 eyes (nine of 17 eyes in the nontractional DME group and seven of 13 eyes in the tractional DME group) required antiglaucoma medications because of increased IOP higher than 21 mmHg at any time after vitrectomy. The IOP was 25 mmHg or more after vitrectomy in four of 17 eyes in the nontractional DME group and four of 13 eyes in the tractional group. One eye of each group was still receiving antiglaucoma medications at the last follow-up. In all cases, IOP increased within three months of surgery and was successfully treated with antiglaucoma medications. No patients required trabeculectomy or other filtering surgery to control IOP. Furthermore, there were no severe postoperative complications such as retinal detachment, iris neovascularization, malposition of dexamethasone implant, or endophthalmitis after vitrectomy.
Discussion
Some studies have reported that vitrectomy had a beneficial effect in the management of DME with traction like taut hyaloid, VMT, or ERM [22,23,24,25,26,27]. In two recent studies from the Diabetic Retinopathy Clinical Research Network (DRCR.net) that included patients with and without vitreomacular interface abnormality, vitrectomy was found to be beneficial with regard to reducing CMT [28,29]. However, the efficacy of improving visual acuity was limited. The CMT outcomes of our study were comparable to the results of these two studies and we observed a more favorable visual acuity outcome in our patients. Bonnin et al. [30] investigated the effect of vitrectomy including ILM peeling alone on tractional DME group and nontractional DME group, respectively. The vitrectomy including ILM peeling alone showed anatomically and functionally good effects in both groups. Compared with our study, the degree of improvement in macular edema at 1 year was similar, but improvement in visual acuity was greater in our study. This suggests that intraoperative dexamethasone implant might have a better effect on functional improvement. Thus, we believe that the results of visual outcomes in our patients during the 12-month follow-up period can be attributed to intraoperative dexamethasone implant as well as vitrectomy.
The postulated mechanism of action of vitrectomy includes removal of the tractional component of DME [22], improving transvitreal oxygenation of the retina [31], and removal of chemical mediators that promote vascular permeability [32]. Unlike the mechanisms of action of vitrectomy, steroids such as triamcinolone acetonide or dexamethasone block production of VEGF and inflammatory mediators, inhibit macrophage and leukocyte adhesion and transmigration, and strengthen the tight junctions of the blood-retinal barrier [33]. Because these treatment methods seem to have different mechanisms, vitrectomy combined with intraocular steroid injection was expected to yield more favorable outcomes. Previously, we reported favorable effects of vitrectomy combined with IVTA and macular laser photocoagulation for DME [11,12,19]. Because of the possibility of longer-lasting treatment effects and a reduced incidence of increase in IOP, more beneficial effects for DME could be expected if we conducted combined therapy with dexamethasone intravitreal implant instead of IVTA.
Six eyes in the nontractional DME group and two eyes in the tractional DME group had accompanying foveolar detachment with exudation in our study. Foveolar detachment has been implicated as a predictor of poor response to vitrectomy [34]. All of the cases showed a resolution of foveolar detachment after the administration of vitrectomy combined with intraoperative dexamethasone implant. As for resolution of foveolar detachment with exudation, intraoperative dexamethasone seems to offer improvement compared to vitrectomy alone. Also, two eyes in the tractional DME group underwent additional treatments because of recurrence of macular edema at 6 months after surgery. Through additional dexamethasone implantation, visual acuity and CMT remained stable. These results suggest that vitrectomy alone is not enough to achieve satisfactory results in some tractional DME cases.
In this study, vitrectomy combined with intraocular dexamethasone implant was performed in patients with nontractional refractory DME and patients with tractional DME. In subjects with tractional DME, CMT was markedly reduced and visual acuity was significantly improved after treatment. Despite the increase in CMT value at 6 months, thickness stabilized at 12 months with repeat injection of dexamethasone implant in two eyes of tractional DME at 6 months postoperatively. However, in subjects with nontractional refractory DME, it took a longer time for significant improvement of visual acuity. Although the CMT value tended to decrease during the follow-up periods, there was no statistical significance regarding this reduction. Massin et al. [26] reported that vitrectomy was beneficial in eyes with diffuse DME combined with VMT, but not in eyes without traction. In their study, retinal thickness decreased from 522 ± 103 µm preoperatively to 428 ± 121 µm at the end of follow-up and BCVA changed from 20 / 100 preoperatively to 20 / 200 at the end of follow-up in patients with DME without VMT. These results suggest a relatively less positive effect of vitrectomy for controlling DME of nontractional origin. The fact that tractional DME patients underwent vitrectomy earlier than nontractional DME patients can be considered as one of the reasons why nontractional refractory DME had relatively less obvious effects than tractional DME in our study. In fact, patients refractory to anti-VEGF or steroid injection were included mainly in the nontractional DME group, and this means that cases of nontractional refractory DME included in this study were more chronic in nature. Tractional DME patients had an average of two administrations of anti-VEGF treatment, while nontractional DME patients received six treatments before surgery. In addition to the chronicity of disease, the inclusion of only patients who did not respond to anti-VEGF treatment might be another reason for the relatively less obvious efficacy of visual and anatomical outcomes in nontractional DME patients in the current study. Therefore, the response to combined treatment in nontractional DME would be less than that seen with tractional DME.
One study reported that BCVA and CMT in intractable DME improved significantly throughout the first 12 months after vitrectomy combined with intraocular dexamethasone implant [21]. The study investigated eyes with DME, regardless of traction, which persisted despite previous nonsurgical treatment. In the current study, we compared responses to vitrectomy combined with intraocular dexamethasone implant for DME between tractional and nontractional DME patients at the same time. In addition, this study included and analyzed more cases than the previous study and we tried to reflect a more diverse spectrum of cases, which is important for evaluating the incidence of adverse events. A randomized controlled prospective trial in the future is necessary to clearly define the role of vitrectomy in combination with intraocular dexamethasone implant in diffuse DME associated with and without traction.
The injection of intravitreal triamcinolone acetonide is one commonly used therapy for diffuse DME because of the associated rapid recovery of vision. However, because anti-VEGF or triamcinolone acetonide would be cleared more rapidly in vitrectomized eyes, more frequent injections are required [18]. Studies indicated that triamcinolone acetonide cleared up to six times more quickly in vitrectomized eyes [20,26]. On the other hand, dexamethasone intravitreal implant as a biodegradable drug delivery system is able to release medication for up to 6 months and has been shown to have similar pharmacokinetic profiles in both vitrectomized and nonvitrectomized eyes [16,17,18]. In our study, there were no eyes that required additional treatment before three months after surgery.
Complications such as IOP elevation and cataract formation may occur due to the intraocular use of triamcinolone acetonide or dexamethasone. Some studies have reported that, as dexamethasone has been shown to activate different patterns of gene expression and possesses different lipophilic properties from triamcinolone acetonide, there may be a decreased risk of IOP elevation and cataract progression following the use of dexamethasone [13,21,35,36]. In our study, four of seven phakic eyes (57.1%) from the total of 30 eyes underwent cataract surgery between 6 and 9 months after vitrectomy because of cataract development or progression. Sixteen out of the 30 eyes included in this study (53.3%) required antiglaucoma medications because of IOP higher than 21 mmHg after vitrectomy, and IOP was over 25 mmHg (25 to 30 mmHg) after vitrectomy in eight of 30 eyes (26.7%). The ratio of cataract development or progression was similar to that in previous studies using triamcinolone acetonide (36% and 66%), and the ratio of IOP elevation over 25 mmHg was also similar to that seen in previous studies (14% and 33%). However, although filtering surgery was conducted for eyes that were refractory to antiglaucoma medications in previous studies [11,12], IOP elevation in the current study was successfully treated with antiglaucoma medications without other complications or additional surgery.
In addition to its retrospective nature and relatively small sample size, our study had several limitations. First, there was no control group for vitrectomy combined with intraoperative dexamethasone implant therapy, so we could not clearly demonstrate the role of treatment and any additional effects of this therapy. Second, 16 of the 23 phakic eyes underwent vitrectomy and cataract extraction simultaneously. Thus, pseudophakic macular edema may have had an influence on CMT. However, we believe that the dexamethasone implant might protect against the development of pseudophakic macular edema. Finally, as this was a retrospective study, a controlled prospective trial is necessary to more clearly evaluate the role of this therapy by comparing the group with vitrectomy only and the group with vitrectomy combined with intraoperative dexamethasone in each of the tractional and nontractional DME groups.
In summary, vitrectomy combined with intraoperative dexamethasone implant may be safe and effective in treating both DME of tractional origin and refractory DME of nontractional origin, although patients with nontractional DME needed more additional treatment and time before displaying anatomical and functional improvement than patients with tractional DME.
Footnotes
This paper was presented at the 118th Korean Ophthalmological Society Meeting 2017 in Seoul, Korea.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research Network. Chew E, Strauber S, et al. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology. 2007;114:1190–1196. doi: 10.1016/j.ophtha.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Entezari M, Ahmadieh H, Dehghan MH, et al. Posterior sub-tenon triamcinolone for refractory diabetic macular edema: a randomized clinical trial. Eur J Ophthalmol. 2005;15:746–750. doi: 10.1177/112067210501500614. [DOI] [PubMed] [Google Scholar]
- 5.Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 6.Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–224. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo JF, Sanchez JG, Wu L, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488–1497. doi: 10.1016/j.ophtha.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175–2181. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142–1150. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Kang SW, Park SC, Cho HY, Kang JH. Triple therapy of vitrectomy, intravitreal triamcinolone, and macular laser photocoagulation for intractable diabetic macular edema. Am J Ophthalmol. 2007;144:878–885. doi: 10.1016/j.ajo.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kang SW, Ha HS, Kim JR. Vitrectomy combined with intravitreal triamcinolone acetonide injection and macular laser photocoagulation for non-tractional diabetic macular edema. Korean J Ophthalmol. 2013;27:186–193. doi: 10.3341/kjo.2013.27.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Haller JA, Bandello F, Belfort R, Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Lowder C, Belfort R, Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 16.Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52:4605–4609. doi: 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 17.Kiddee W, Trope GE, Sheng L, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013;58:291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros MD, Alkabes M, Navarro R, et al. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2014;30:709–716. doi: 10.1089/jop.2014.0010. [DOI] [PubMed] [Google Scholar]
- 19.Kim YT, Kang SW, Kim SJ, et al. Combination of vitrectomy, IVTA, and laser photocoagulation for diabetic macular edema unresponsive to prior treatments; 3-year results. Graefes Arch Clin Exp Ophthalmol. 2012;250:679–684. doi: 10.1007/s00417-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 20.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Kim YJ, Yoon YH. Minimally invasive microincision vitrectomy surgery with an intraoperative dexamethasone implant for refractory diabetic macular edema. Ophthalmologica. 2016;235:150–156. doi: 10.1159/000443751. [DOI] [PubMed] [Google Scholar]
- 22.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–759. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 23.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–413. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 24.Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–133. [PubMed] [Google Scholar]
- 25.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–186. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 26.Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–177. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 27.Stolba U, Binder S, Gruber D, et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295–301. doi: 10.1016/j.ajo.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30:1488–1495. doi: 10.1097/IAE.0b013e3181e7974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetic Retinopathy Clinical Research Network Writing Committee. Haller JA, Qin H, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093.e3. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnin S, Sandali O, Bonnel S, et al. Vitrectomy with internal limiting membrane peeling for tractional and nontractional diabetic macular edema: long-term results of a comparative study. Retina. 2015;35:921–928. doi: 10.1097/IAE.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 31.Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy: a theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79:435–440. doi: 10.1034/j.1600-0420.2001.790502.x. [DOI] [PubMed] [Google Scholar]
- 32.Otani T, Kishi S. A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol. 2002;134:214–219. doi: 10.1016/s0002-9394(02)01548-9. [DOI] [PubMed] [Google Scholar]
- 33.Antonetti DA, Wolpert EB, DeMaio L, et al. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677. doi: 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 34.Shah SP, Patel M, Thomas D, et al. Factors predicting outcome of vitrectomy for diabetic macular oedema: results of a prospective study. Br J Ophthalmol. 2006;90:33–36. doi: 10.1136/bjo.2005.072934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129:914–920. doi: 10.1001/archophthalmol.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Bailey C, Loewenstein A, Massin P. Intravitreal corticosteroids in diabetic macular edema: pharmacokinetic considerations. Retina. 2015;35:2440–2449. doi: 10.1097/IAE.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]