Abstract
Arabidopsis pumila, an annual ephemeral plant, plays important roles in preventing wind and sand erosion, water and soil conservation, and microhabitat improvement in the North of Xinjiang, China. Studies of adaptive mechanisms in harsh desert environments at the genetic and genomic levels can be used to more effectively develop and protect this species. The quantitative real-time polymerase chain reaction (qRT-PCR) method is one of the essential means to achieve these goals, and the selection of an appropriate reference gene is the prerequisite for qRT-PCR. In this study, 10 candidate reference genes were identified from the full-length transcriptome data of A. pumila, and their expression stabilities under four abiotic stresses (drought, heat, cold and salt) and in seven different tissues (roots, hypocotyl, cotyledon, leaves, stems, flowers and siliques) were evaluated with four programmes geNorm, NormFinder, Bestkeeper and RefFinder. Although the most stable reference genes were variable under different treatments using different software, comprehensive ranking revealed that UEP and HAF1 under drought stress, UBQ9 and GAPDH under heat stress, UBC35 and GAPDH under cold stress, GAPDH and ACT1 under salt stress, and ACT1 and GAPDH in different tissues were the most stable reference genes. Moreover, GAPDH and UBQ9 were the most suitable reference gene combinations for all samples. The expression pattern of the K+ uptake permease gene KUP9 further validated that the selected reference genes were suitable for normalization of gene expression. The identification of reliable reference genes guarantees more accurate qRT-PCR quantification for A. pumila and facilitates functional genomics studies of ephemeral plants.
Subject terms: Reverse transcription polymerase chain reaction, Plant sciences
Introduction
Ephemeral comes from Greek “ephémeros”, which means transitory and quickly fading, lasting only one day. In botany, an ephemeral plant is a short-lived plant that has one or more generations per year, growing only during favourable periods (e.g., when adequate water is available), and surviving the unfavourable periods in the form of seeds1. In China, ephemeral plants are mainly distributed in the desert environments of the Junggar Basin and the Yili River valley in northern Xinjiang, with the eastern margin of the Junggar Basin as the boundary. Ephemeral plants sprout in mid-March or early April each year, making use of rain and snow water melt from Tianshan Mountain, and complete their life-cycle within approximately two months by mid-June, a period with the strongest wind force in the desert regions of northern Xinjiang1,2. Accordingly, ephemeral plants play important roles as windbreaks and in the stabilization of sand, water and soil conservation, and microhabitat improvement. Arabidopsis pumila (synonym: Olimarabidopsis pumila), an ephemeral plant, is well adapted to take advantage of the short favourable seasons in desert areas (Supplementary Fig. S1)2,3. In addition to fast growth, it also has the advantages of high yield, high photosynthetic efficiency and stress tolerance3,4. To utilize and protect this ephemeral plant, it is of great significance to elucidate the molecular mechanism of rapid growth and adversity adaptation. Based on the above reasons, our group generated a full-length transcriptome database of A. pumila seedlings using a combination of single-molecule real-time (SMRT) sequencing and Illumina sequencing3.
Quantitative real-time polymerase chain reaction (qRT-PCR) is a commonly used and powerful tool for accurately analysing gene expression levels in different samples or under different experimental conditions in a wide range of biological experiments5. Compared with traditional quantitative analysis techniques of gene expression, qRT-PCR has the advantages of simplicity, precision, specificity, sensitivity, flexibility, scalability and high throughput capacity5–8. It is well known that the appropriate choice of reference genes for normalizing expression levels of a test gene is crucial to the accuracy and reliability of qRT-PCR data. The ideal reference genes should have a moderate and stable expression level in different tissues at different developmental stages and under different experimental treatments9–11. Some of the most frequently used reference genes for qRT-PCR in animals and plants are 18S rRNA, actin (ACT1 and ACT2), α-tubulin and β-tubulin (TUA and TUB), cyclophilin (CYP1 and CYP2), elongation factor-1α (EF-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), polyubiquitin (UBQ), and ribosomal protein L (RPL1 and RPL2)9,12–17. Unfortunately, several studies have shown that the transcripts of these reference genes are not always stable when subjected to various experimental conditions18–20. The screening of stable reference genes is now gradually attracting scientists’ attention. A wiki-driven database of internal control genes (ICG: http://icg.big.ac.cn) has been constructed for qRT-PCR normalization involving a wide range of specific tissues, development stages and experimental treatments. ICG has integrated 209 species, including 73 animals, 115 plants, 12 fungi and 9 bacteria, and has curated 757 internal control genes with validation under more than 70 types of experimental conditions, such as salinity, heat and insecticide treatment21.
However, no reference genes are available for transcript normalization in A. pumila, so we were unable to validate our transcriptome sequencing data, analyse the expression profiles of salt or other stress-related genes, or further reveal the adaptive growth mechanism of A. pumila to desertification. The objective of this study was to identify stable reference genes in A. pumila subjected to various abiotic stresses as well as different developmental tissues.
Results
Amplification specificity and amplification efficiency of the candidate reference genes and target genes
A total of 10 candidate reference genes and one target gene from the A. pumila full-length transcriptome database were selected for qRT-PCR normalization. The unigene name, gene symbol, homologue locus and E value compared with those of the homologous genes are summarized in Table 1. The primer sequences, amplicon size, product Tm, and amplification efficiencies are shown in Table 2. The size of PCR products for each reference gene was tested by 2.0% agarose gel, which provided the expected amplicon length (Supplementary Fig. S2). The specificity for each primer pair was verified by melting curve analysis, which revealed that each gene had a single amplification peak (Supplementary Fig. S3). qRT-PCR efficiency for all 10 candidate reference genes ranged from 90.3% for EF1B to 99.1% for UEP, and correlation coefficients varied from 0.988 (ACT2) to 0.999 (LOS1) (Table 2).
Table 1.
Description of candidate reference genes and target genes.
| Gene name | Gene symbol | Homolog locus | Gene ID | E value |
|---|---|---|---|---|
| Actin-1 | ACT1 | AT2G37620.1 | 2–3k.c16834/1/1406 | 0.0 |
| Actin-2 | ACT2 | AT3G18780.2 | 2–3k.c6923/1/1569 | 0.0 |
| Aldehyde dehydrogenase | ALDH | AT3G66658.1 | 1–2k.c31292/1/2035 | 0.0 |
| Translation elongation factor | EF1B | AT5G19510.1 | 2–3k.c8091/1/833 | e-168 |
| Histone acetyltransferase | HAF1 | AT1G32750.1 | 3–6k.c17817/1/4957 | 0.0 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | AT1G13440.1 | 2–3k.c18384/1/1353 | 0.0 |
| Low expression of osmotically responsive genes 1 | LOS1 | AT1G56070.1 | 1–2k.c36904/1/2756 | 0.0 |
| Polyubiquitin 9 | UBQ9 | AT5G37640.1 | 1–2k.c36785/1/1730.1 | 1e-61 |
| Ubiquitin-conjugating enzyme 35 | UBC35 | AT1G78870.1 | 1–2k.c18460/1/1770.2 | 3e-84 |
| Ubiquitin extension protein | UEP | AT3G52590.1 | 1–2k.c21768/1/663 | 0.0 |
| K+ uptake permease 9 | KUP9 | AT4G19960.1 | 2–3k.c27754/1/2830 | 0.0 |
Table 2.
Primers used in this study and their amplification efficiency and amplification characteristics.
| Genes | Primer sequence (5′-3′) | Tm (°C) | R2 | E (%) | Amplicon size (bp) |
|---|---|---|---|---|---|
| ACT1 | F: CGACAATGGAACTGGAATGGTTA | 59 | 0.997 | 0.936 | 296 |
| R: GTGCCTCGGTAAGTAGAATAGGA | 59 | ||||
| ACT2 | F: TGTGCCAATCTACGAGGGTT | 59 | 0.988 | 0.934 | 137 |
| R:TTTCCCGTTCTGCTGTTGTG | 59 | ||||
| ALDH | F: ACAACGGCACTACTGGATCA | 59 | 0.994 | 0.920 | 224 |
| R: CCAATGCAAGCCTGTCCATT | 59 | ||||
| EF1B | F: GCAACAATGGCCGTTACCT | 59 | 0.996 | 0.903 | 153 |
| R: ATCGCTGGGTTTCACTGGA | 59 | ||||
| GAPDH | F:CTCCCATGTTTGTTGTTGGTGTCA | 62 | 0.997 | 0.951 | 217 |
| R: CTTCCACCTCTCCAGTCCTTCATT | 62 | ||||
| HAF1 | F: TAAGTCCACGTCTAAGATCAGCA | 59 | 0.997 | 0.938 | 186 |
| R: GCCTGGTTTCTCTCTGTACTAGA | 59 | ||||
| LOS1 | F: GGCAAGAGACTGTGGAGGA | 59 | 0.999 | 0.909 | 162 |
| R: CAGCAACACGAACAACAGGA | 59 | ||||
| UBC35 | F: GAGGATCACGTCAGATTCGAG | 58 | 0.998 | 0.907 | 163 |
| R: AGTTTCCTTGATGATTCTTCGCG | 60 | ||||
| UBQ9 | F: GTGCTGAGAGATGCGGATTG | 59 | 0.998 | 0.908 | 252 |
| R: CCTCTCCTCCTCCAACAGTC | 59 | ||||
| UEP | F: GAGGTCGAGAGCAGTGACAC | 60 | 0.998 | 0.991 | 178 |
| R: CCCTGAGCCTCAACACAAGA | 60 | ||||
| KUP9 | F: TGTTCTTTCGGCAACTGGTG | 59 | 1.000 | 0.979 | 284 |
| R: AAATCCAACCATCCCGACCT | 59 |
Expression profiles of the candidate reference genes
Boxplot analysis of Ct values of the 10 different reference genes in all experimental samples showed the means and standard deviation of the Ct values for each reference gene (Fig. 1). The results showed that the reference genes displayed a wide range of transcription levels across all test samples, with average Ct values ranging from 22.13 to 28.89 (Supplementary Table S1). Because gene expression levels are negatively correlated with Ct values, ACT2 exhibited the highest expression abundance, with the lowest mean Ct values. Both GAPDH and UEP showed relatively high expression, with mean Ct values of 22.63 and 22.91, respectively, whereas ACT1 and ALDH showed relatively low expression (mean Ct = 28.79 and 28.89). The expression levels of other genes were at the mean level.
Figure 1.
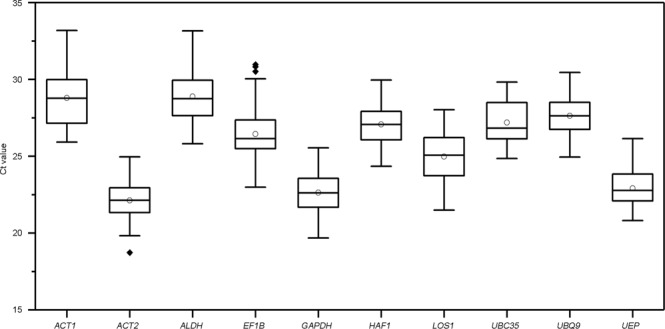
Distribution of Ct values for 10 candidate reference genes across all A. pumila samples using qRT-PCR data. The solid line within each box represents the median Ct values, and boxes denote the 25th and 75th percentiles. Circles and black dots represent the mean Ct values and potential outliers, respectively.
Analysis of expression stability of the candidate reference genes
Expression stability of the 10 reference genes was carried out using geNorm22, NormFinder14, BestKeeper23 and RefFinder24,25 software.
GeNorm analysis
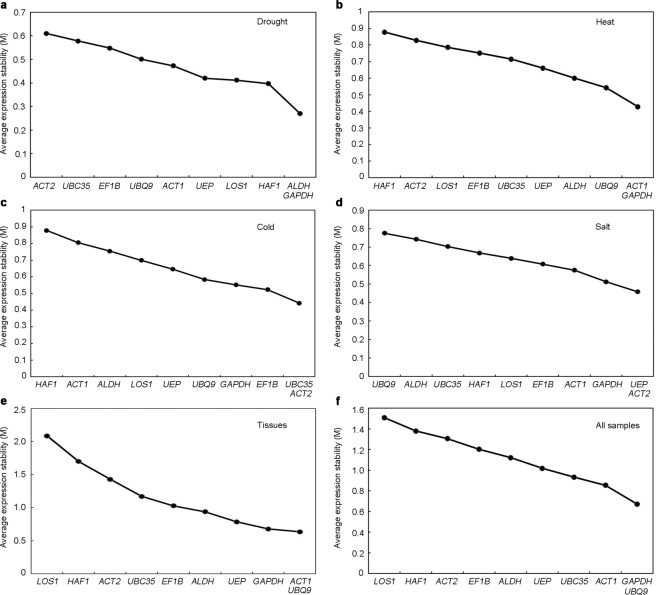
Gene expression stability was evaluated by the M value using geNorm analysis. The lower the M value, the higher the gene expression stability22. We first calculated the stable value M of the reference gene and sorted the M value from high to low (Fig. 2). We found that all 10 reference genes under abiotic stresses and in different tissues had M values below 1.5, suggesting that these genes were stable across all these experimental sets. ALDH and GAPDH under drought stress (10% PEG-6000), ACT1 and GAPDH under heat stress (40 °C), and ACT2 and UBC35 under cold stress (4 °C) were the most stable reference genes, with the lowest M values of 0.250, 0.428 and 0.442, respectively (Fig. 2a–c). At the same time, UEP and ACT2 were considered to be the most stable genes under the treatment of salt stress (250 mM NaCl) with an M value of 0.458 (Fig. 2d). In the experimental developmental tissue group, ACT1 and UBQ9 with the lowest M value of 0.635 were ranked as the most stable genes, while LOS1 (2.085) was ranked as least stable (Fig. 2e). In addition, GAPDH and UBQ9 were suggested to be the two most stable genes with the lowest M value of 0.673 across all samples (Fig. 2f). In contrast, LOS1 was the least stable gene in all treatments, with the highest M value of 1.508.
Figure 2.
geNorm analysis of the stability values (M) of 10 candidate reference genes under different treatments and in different tissues. (a) Drought treatment. (b) Heat treatment. (c) Cold treatment. (d) Salt treatment. (e) Tissues. (f) All samples. The most stable genes are arranged on the right, while the least stable genes are on the left.
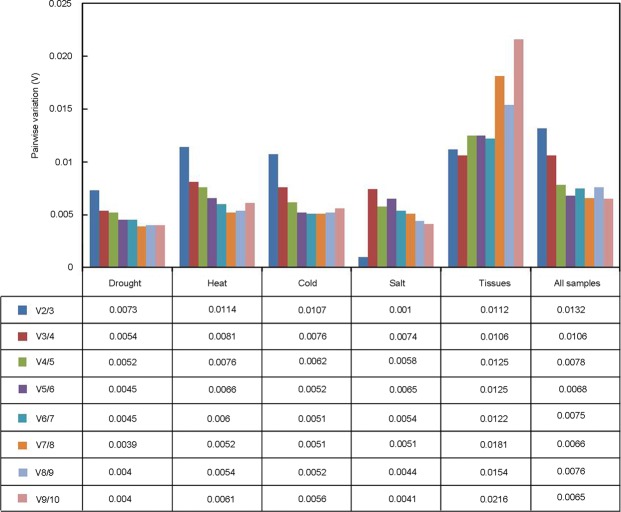
To determine the optimal number of reference genes for qRT-PCR normalization, the pairwise variation value (Vn/Vn+1, n represents the reference gene number) between sequential normalization factors was also calculated by geNorm for all sample sets22. The results showed that the Vn/Vn+1 values for different experimental groups were below the cut-off value of 0.15 (Fig. 3), which indicated that two reference genes would be sufficient for normalization of qRT-PCR analysis in A. pumila.
Figure 3.
Optimal number of reference genes for normalization under the test experimental conditions. geNorm was used to calculate the pairwise variation (Vn/Vn+1, n represents reference gene numbers) to evaluate the number of reference genes in each experimental group.
NormFinder analysis
Expression stability values for each candidate reference gene were further analysed by NormFinder, which is based on variance analysis to calculate the stable value of each gene14. Similar to geNorm, a higher stable value indicates a more unstable gene, whereas a lower stable value indicates greater gene stability. According to the stability values calculated by NormFinder (Table 3), UEP (0.214) and HAF1 (0.271) were the two most stable genes under drought stress conditions; UBQ9 (0.196) and GAPDH (0.451) were the two most stable genes under heat treatment; GAPDH (0.269) and EF1B (0.291) were the two most stable genes under cold treatment; and GAPDH (0.162) and ACT1 (0.380) were the two most stable genes in the salt stress group. Among different developmental tissues, UBC35 and ACT1 were the two most stable reference genes with the lowest stability values of 0.299 and 0.375, respectively. In addition, GAPDH and UBQ9 were the most stable reference genes across all samples, with the lowest stability values of 0.479 and 0.601, respectively, and LOS1 was the most unstable gene with the highest stability value of 1.777, which is completely consistent with the results determined through geNorm.
Table 3.
Expression stability values (M) of 10 candidate reference genes calculated by NormFinder.
| Rank | Drought | Heat | Cold | Salt | Tissues | All samples |
|---|---|---|---|---|---|---|
| 1 | UEP | UBQ9 | GAPDH | GAPDH | UBC35 | GAPDH |
| Stability | 0.214 | 0.196 | 0.269 | 0.162 | 0.299 | 0.479 |
| 2 | HAF1 | GAPDH | EF1B | ACT1 | ACT1 | UBQ9 |
| Stability | 0.271 | 0.451 | 0.291 | 0.380 | 0.375 | 0.601 |
| 3 | LOS1 | ALDH | UBC35 | HAF1 | UBQ9 | UBC35 |
| Stability | 0.323 | 0.463 | 0.295 | 0.439 | 0.442 | 0.670 |
| 4 | UBQ9 | ACT1 | ACT2 | ACT2 | GAPDH | UEP |
| Stability | 0.398 | 0.556 | 0.421 | 0.507 | 0.445 | 0.858 |
| 5 | ACT1 | EF1B | UBQ9 | UEP | ALDH | ACT1 |
| Stability | 0.451 | 0.610 | 0.519 | 0.557 | 0.560 | 0.871 |
| 6 | ALDH | UEP | LOS1 | LOS1 | UEP | EF1B |
| Stability | 0.474 | 0.612 | 0.698 | 0.603 | 0.612 | 1.173 |
| 7 | EF1B | UBC35 | UEP | UBC35 | EF1B | ACT2 |
| Stability | 0.490 | 0.673 | 0.725 | 0.640 | 0.617 | 1.253 |
| 8 | GAPDH | LOS1 | ALDH | EF1B | ACT2 | HAF1 |
| Stability | 0.513 | 0.715 | 0.780 | 0.647 | 0.773 | 1.340 |
| 9 | UBC35 | ACT2 | ACT1 | UBQ9 | HAF1 | ALDH |
| Stability | 0.529 | 0.868 | 0.917 | 0.729 | 0.911 | 1.344 |
| 10 | ACT2 | HAF1 | HAF1 | ALDH | LOS1 | LOS1 |
| Stability | 0.629 | 0.922 | 1.047 | 0.735 | 1.492 | 1.777 |
BestKeeper analysis
We then used another programme, BestKeeper, to reanalyse the expression stability of the candidate reference genes. In our experiment, the r value was used to assess the stability of the reference genes. The higher the r value, the more stable the expression of the reference gene23. As shown in Table 4, under drought stress, LOS1 (r = 0.983) and UEP (r = 0.980) were considered to be the two most stable reference genes, which is not consistent with geNorm and NormFinder. Under heat stress, UBQ9 (r = 0.956) had the highest stability, which is consistent with the result of NormFinder. Under cold stress, GAPDH (r = 0.966) and EF1B (0.896) were the two most stable genes, which is completely consistent with the result of NormFinder. Similarly, the highest r value of 0.966 was observed under salt stress and for all developmental tissues, showing GAPDH and UBC35 as the most stable reference genes, respectively. The most unstable genes determined by BestKeeper were also consistent with the results of NormFinder: ACT2 under drought, HAF1 in the heat and cold groups, ALDH under salt stress and LOS1 in all tissues. In addition, GAPDH (r = 0.929) and ACT1 (0.916) were the two most stable reference genes for all samples, and LOS1 (r = 0.563) was the most unstable gene.
Table 4.
Expression stability values (M) of 10 candidate reference genes calculated by BestKeeper.
| Rank | Drought | Heat | Cold | Salt | Tissues | All samples |
|---|---|---|---|---|---|---|
| 1 | LOS1 | UBQ9 | GAPDH | GAPDH | UBC35 | GAPDH |
| r value | 0.983 | 0.956 | 0.966 | 0.966 | 0.966 | 0.929 |
| 2 | UEP | ACT1 | EF1B | ACT1 | UEP | ACT1 |
| r value | 0.980 | 0.935 | 0.896 | 0.952 | 0.965 | 0.916 |
| 3 | HAF1 | GAPDH | ALDH | LOS1 | ACT1 | UBQ9 |
| r value | 0.975 | 0.910 | 0.895 | 0.934 | 0.933 | 0.909 |
| 4 | ACT1 | EF1B | LOS1 | UBQ9 | UBQ9 | UEP |
| r value | 0.971 | 0.901 | 0.873 | 0.919 | 0.921 | 0.901 |
| 5 | ALDH | ALDH | UBC35 | EF1B | GAPDH | UBC35 |
| r value | 0.955 | 0.882 | 0.869 | 0.916 | 0.910 | 0.892 |
| 6 | GAPDH | LOS1 | ACT1 | UEP | HAF1 | HAF1 |
| r value | 0.950 | 0.802 | 0.862 | 0.874 | 0.878 | 0.788 |
| 7 | UBQ9 | UEP | ACT2 | HAF1 | ALDH | EF1B |
| r value | 0.947 | 0.792 | 0.837 | 0.866 | 0.853 | 0.782 |
| 8 | EF1B | ACT2 | UBQ9 | ACT2 | EF1B | ALDH |
| r value | 0.925 | 0.775 | 0.746 | 0.846 | 0.833 | 0.751 |
| 9 | UBC35 | UBC35 | UEP | UBC35 | ACT2 | ACT2 |
| r value | 0.908 | 0.752 | 0.584 | 0.730 | 0.746 | 0.748 |
| 10 | ACT2 | HAF1 | HAF1 | ALDH | LOS1 | LOS1 |
| r value | 0.871 | 0.727 | 0.001 | 0.717 | 0.001 | 0.563 |
ΔCt analysis
According to the analysis of the ΔCt method calculated by RefFinder24,25 software (Supplementary Table S2), UEP and HAF1 were the two most stable reference genes under drought stress, while ACT2 was the most unstable gene, which is consistent with the analysis of NormFinder. UBQ9 under heat stress, GAPDH under cold stress, and GAPDH under salt stress were the most stable genes, which is also completely consistent with the NormFinder and Bestkeeper analyses. In addition, the most stable reference gene in different tissues was ACT1, which is consistent with geNorm analysis but not with NormFinder and Bestkeeper; the most unstable gene was LOS1, which is consistent with the analysis determined using the three other programmes.
Comprehensive ranking of the candidate reference genes
The four different programmes yielded the same or divergent ranking for the stable reference genes, and this variation has also been reported in several studies26–30. To rank the reference genes under different stress conditions and in different experimental tissues, we further carried out comprehensive stability analysis for all 10 candidate reference genes. The final ranking result of expression stability for all reference genes was sorted according to the geometric mean of the reference genes (Table 5)22,26. According to the analysis results, UEP and HAF1, UBQ9 and GAPDH, UBC35 and GAPDH were the most stable genes under drought stress, heat stress and cold stress, respectively. The most stable reference genes under salt stress and in different tissues were both GAPDH and ACT1. Under all of the experimental conditions, GAPDH and UBQ9 were the most stable reference genes, and LOS1 was the most unstable reference gene.
Table 5.
Ranking of expression stability for the 10 candidate reference genes.
| Methods | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| A. Drought stress (Ranking order from better-good-average) | ||||||||||
| geNorm | GAPDH/ALDH | HAF1 | LOS1 | UEP | ACT1 | UBQ9 | EF1B | UBC35 | ACT2 | |
| NormFinder | UEP | HAF1 | LOS1 | UBQ9 | ACT1 | ALDH | EF1B | GAPDH | UBC35 | ACT2 |
| Bestkeeper | LOS1 | UEP | HAF1 | ACT1 | ALDH | GAPDH | UBQ9 | EF1B | UBC35 | ACT2 |
| ∆CT | UEP | HAF1 | LOS1 | UBQ9 | ALDH | ACT1 | GAPDH | EF1B | UBC35 | ACT2 |
| Comprehensive | UEP | HAF1 | UBQ9 | LOS1 | ALDH | GAPDH | UBC35 | ACT1 | EF1B | ACT2 |
| B. Heat stress (Ranking order from better-good-average) | ||||||||||
| geNorm | ACT1/GAPDH | UBQ9 | ALDH | UEP | UBC35 | EF1B | LOS1 | ACT2 | HAF1 | |
| NormFinder | UBQ9 | GAPDH | ALDH | ACT1 | EF1B | UEP | UBC35 | LOS1 | ACT2 | HAF1 |
| Bestkeeper | UBQ9 | ACT1 | GAPDH | EF1B | ALDH | LOS1 | UEP | ACT2 | UBC35 | HAF1 |
| ∆CT | UBQ9 | GAPDH | ALDH | ACT1 | UEP | EF1B | UBC35 | LOS1 | ACT2 | HAF1 |
| Comprehensive | UBQ9 | GAPDH | ACT1 | UEP | ALDH | UBC35 | EF1B | LOS1 | ACT2 | HAF1 |
| C. Cold stress (Ranking order from better-good-average) | ||||||||||
| geNorm | UBC35/ACT2 | EF1B | GAPDH | UBQ9 | UEP | LOS1 | ALDH | ACT1 | HAF1 | |
| NormFinder | GAPDH | EF1B | UBC35 | ACT2 | UBQ9 | LOS1 | UEP | ALDH | ACT1 | HAF1 |
| Bestkeeper | GAPDH | EF1B | ALDH | LOS1 | UBC35 | ACT1 | ACT2 | UBQ9 | UEP | HAF1 |
| ∆CT | GAPDH | EF1B | UBC35 | ACT2 | UBQ9 | LOS1 | UEP | ALDH | ACT1 | HAF1 |
| Comprehensive | UBC35 | GAPDH | EF1B | ACT2 | UBQ9 | UEP | LOS1 | ALDH | ACT1 | HAF1 |
| D. Salt stress (Ranking order from better-good-average) | ||||||||||
| geNorm | ACT2/UEP | GAPDH | ACT1 | EF1B | LOS1 | HAF1 | UBC35 | ALDH | UBQ9 | |
| NormFinder | GAPDH | ACT1 | HAF1 | ACT2 | UEP | LOS1 | UBC35 | EF1B | UBQ9 | ALDH |
| Bestkeeper | GAPDH | ACT1 | LOS1 | UBQ9 | EF1B | UEP | HAF1 | ACT2 | UBC35 | ALDH |
| ∆CT | GAPDH | ACT1 | HAF1 | ACT2 | UEP | LOS1 | UBC35 | EF1B | ALDH | UBQ9 |
| Comprehensive | GAPDH | ACT1 | ACT2 | UEP | HAF1 | UBC35 | LOS1 | EF1B | ALDH | UBQ9 |
| E. Tissues (Ranking order from better-good-average) | ||||||||||
| geNorm | ACT1/UBQ9 | GAPDH | UEP | ALDH | EF1B | UBC35 | ACT2 | HAF1 | LOS1 | |
| NormFinder | UBC35 | GAPDH | ACT1 | UEP | UBQ9 | EF1B | ACT2 | ALDH | HAF1 | LOS1 |
| Bestkeeper | UBC35 | UEP | ACT1 | UBQ9 | GAPDH | HAF1 | ALDH | EF1B | ACT2 | LOS1 |
| ∆CT | ACT1 | GAPDH | UBQ9 | UEP | UBC35 | EF1B | ALDH | ACT2 | HAF1 | LOS1 |
| Comprehensive | ACT1 | GAPDH | UBC35 | UBQ9 | ACT2 | UEP | EF1B | ALDH | HAF1 | LOS1 |
| F. All samples (Ranking order from better-good-average) | ||||||||||
| geNorm | GAPDH/UBQ9 | ACT1 | UBC35 | UEP | ALDH | EF1B | ACT2 | HAF1 | LOS1 | |
| NormFinder | GAPDH | UBQ9 | UBC35 | UEP | ACT1 | EF1B | ACT2 | HAF1 | ALDH | LOS1 |
| Bestkeeper | GAPDH | UBQ9 | UBC35 | ACT2 | UEP | EF1B | HAF1 | LOS1 | ACT1 | ALDH |
| ∆CT | GAPDH | UBQ9 | UBC35 | UEP | ACT1 | EF1B | ACT2 | ALDH | HAF1 | LOS1 |
| Comprehensive | GAPDH | UBQ9 | UBC35 | UEP | ACT1 | EF1B | ACT2 | ALDH | HAF1 | LOS1 |
Reference gene validation
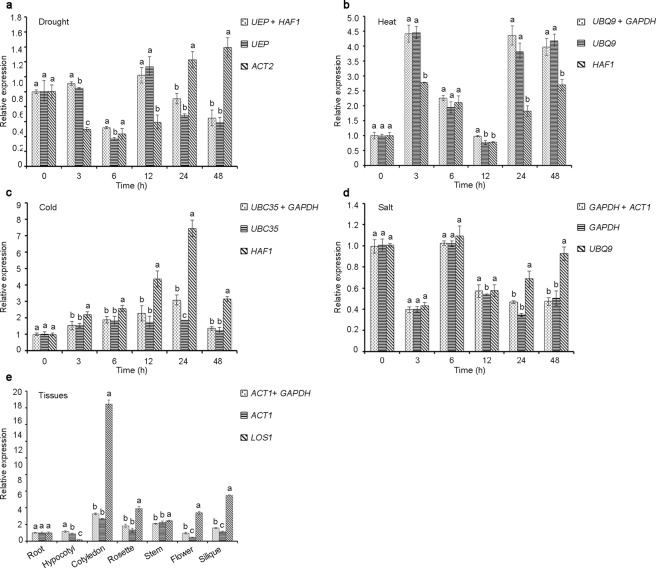
The K+ uptake permease 9 (KUP9) gene, which functions in the potassium ion transporter, was used for reference gene validation in this study. As shown in Fig. 4, when the best reference gene combinations and the most stable genes were used for normalization analysis, the expression profiles of KUP9 obtained through qRT-PCR were similar in all experimental groups. Under drought stress conditions, KUP9 expression fluctuated significantly (Fig. 4a). The expression of KUP9 was downregulated after 6 h of drought stress, upregulated quickly at 12 h and was then continuously downregulated. However, KUP9 expression began to increase continuously after 12 h of drought stress, when the least stable gene was selected as the reference gene. When the optimal internal reference gene combination UEP and HAF1 and the most stable internal reference gene UEP were used to normalize the relative expression changes of KUP9, the expression changes of KUP9 were basically the same, especially at 12 h and later (P > 0.05), but when the least stable reference gene ACT2 was used, the expression of KUP9 was significantly changed compared with the former two results (P < 0.05) (Fig. 4a). Under heat stress conditions, when the most stable genes, i.e., the UBQ9 and GAPDH reference gene combination, were used for normalization, the expression level of KUP9 rose sharply to the highest point (approximately 4.5-fold higher than the control group) after 3 h of stress, then gradually decreased to the level of the control group from 6 h to 12 h, and finally KUP9 showed a high level of expression at 24–48 h of stress (Fig. 4b). Furthermore, when the most stable gene, UBQ9, was used as an internal reference, the expression trend of KUP9 was basically consistent with the previous analysis. However, when the most unstable gene, HAF1, was used as a reference gene, the expression of KUP9 did not show a clear expression trend. For cold stress, when the best reference gene combination, UBC35 and GAPDH, was used for normalization, KUP9 expression only increased slightly and the same result also occurred with the most stable reference gene UBC35 (Fig. 4c). When the least stable gene HAF1 was used for normalization, a sustained increase in the expression level of KUP9 was observed after stress, and a peak point was observed at 24 h, which clearly exhibited an overestimation of expressionand compared with the results of the expression changes of the former two, the relative expression of KUP9 has changed significantly (P < 0.05).
Figure 4.
Relative expression levels of KUP9 across all experimental sets normalized by the most stable reference gene combination, the most stable gene and the most unstable gene. (a) Drought stress. (b) Heat stress. (c) Cold stress (d) Salt stress. (e) Tissues. Error bars represent the standard deviation of three biological replicates. Different lowercase letters represent statistically significant differences as determined by one-way ANOVA (P < 0.05, Duncan’s multiple range tests).
Under salt stress, the expression of KUP9 first decreased, and after increasing at 6 h, decreased again (Fig. 4d). KUP9 showed consistent expression profiles when a combination of reference genes (GAPDH and ACT1) or the single most stable gene (GAPDH) was used to normalize the expression. However, when UBQ9 (unstable gene) was used for normalization, KUP9 expression was significantly affected at 24 h and 48 h of salt stress (Fig. 4d). Similarly, KUP9 showed similar expression patterns when a combination of the most stable reference genes (ACT1 and GAPDH) or the single most stable gene (ACT1) was used to normalize the expression (Fig. 4e) in different tissues. The expression level of KUP9 in cotyledons was over stated when the most unstable gene, LOS1, was used for normalization compared with the results of most reference gene and the combination of reference genes (P < 0.05).
Discussion
In recent years, our group has been trying to demystify the gene expression dynamics of A. pumila adaptation to adversity. However, the application of qRT-PCR in A. pumila has been limited due to a lack of reference gene information for a variety of experimental contexts. Therefore, suitable reference genes should first be screened prior to large-scale analysis of gene expression profiles under various stress conditions and in different developmental tissues in A. pumila. As a result, in our analysis of potential candidate reference genes in A. pumila, we noted that there was no single reference gene that exhibited a constant expression level in all the samples under various experimental conditions, similar to the findings for a number of plant species such as Arabidopsis thaliana17, Caragana intermedia31, Gossypium hirsutum32, Pennisetum glaucum33.
In the present study, 10 candidate internal reference genes (ACT1, ACT2, ALDH, EF1B, HAF1, GAPDH, LOS1, UBQ9, UBC35 and UEP) were identified from our previous transcriptome data3. The PCR results showed that each pair of primers had a specific band (Supplementary Fig. S2), and the dissolution curve had only a single peak (Supplementary Fig. S3). The amplification efficiency of the primer was between 90.3% and 99.1% (Table 2).
The stability of the candidate reference genes was calculated and analysed using four different analysis software packages, geNorm22, NormFinder14, Bestkeeper23 and RefFinder24,25, to evaluate the best reference genes among 93 samples under different experimental conditions and tissues. We noted that the 10 reference genes exhibited variable expression stability in response to different stresses. For example, under drought stress conditions, geNorm software ranked GAPDH/ALDH as the best reference genes (Fig. 2a), and NormFinder determined UEP as the best reference gene (Table 3). BestKeeper regarded LOS1 as the most stable reference gene (Table 4), and ΔCt inferred that UEP was the most stable reference gene according to its lowest mean SD value (Supplementary Table S2). Under heat stress, UBQ9 and GAPDH were ranked as the top one and two positions by NormFinder and ΔCt, respectively. GeNorm ranked ACT1 and GAPDH as the most stable and UBQ9 as the second most stable. Both NormFinder and Bestkeeper ranked UBQ9 as the most stable gene. As a result, we inferred that UBQ9 and GAPDH are the best combination of stable reference genes for qRT-PCR in response to heat stress. Based on the above results, we found that it was difficult to determine stable reference genes in A. pumila using only one programme. Therefore, we comprehensively evaluated the results of all reference gene analyses by RefFinder and finally determined the stability orders for all reference genes under different experimental conditions.
Ubiquitin is a small regulatory protein found in all eukaryotes34. Genes encoding ubiquitin-like proteins that have a remarkably conserved gene structure among higher plants, animals and fungi are often used as reference genes for normalization in many reports. According to the RefFinder analysis, we found that UEP, UBQ9, and UBC35 were the most stable reference genes under drought stress, heat stress and cold stress, respectively (Table 5). Although the most stable reference gene or reference gene combinations showed differences under different stresses, GAPDH exhibited more stable expression under all abiotic stresses. For example, under salt treatment, GAPDH was ranked in the top position by NormFinder, Bestkeeper and RefFinder. GeNorm determined that GAPDH was the most stable reference gene under drought stress and heat stress. Similarly, GAPDH was the best reference gene under cold stress according to NormFinder, Bestkeeper and RefFinder.
ACT has been considered to be the reference gene in some plants in response to drought and salt stress35,36 and in different tissues37. However, in the present study, ACT2 was ranked in the last position according to the comprehensive ranking results under drought stress (Table 5). When ACT2 was used as a reference gene to detect the expression pattern of KUP9 under drought stress, obviously different expression profiles appeared (Fig. 4).
KUP9 encoding a potassium ion transmembrane transporter was used as a target gene to verify the stability of the reference genes under various stress conditions. A. thaliana AtKUP9 is expressed most highly in young leaves and can be regulated by potassium (K+)38. Our experiments indicated that the expression of the A. pumila KUP9 gene changed significantly under various stress conditions, and its molecular mechanisms of stress tolerance deserve further study. Validation of gene expression revealed that KUP9 showed similar expression patterns when normalized by the most stable reference gene combinations and the single most stable reference gene (Fig. 4). However, when the most unstable reference gene was used for normalization analysis, the expression level of KUP9 was exaggerated or the expression level of KUP9 was underestimated, suggesting that the identified reference genes are reliable.
In summary, we recommend the use of different reference genes or reference gene combinations to obtain more accurate and reliable results for qRT-PCR in A. pumila. Furthermore, two reference gene combinations had V2/3 values less than 0.15 across all experimental subsets (Fig. 3); consequently, UEP and HAF1 under drought stress, UBQ9 and GAPDH under heat stress, UBC35 and GAPDH under cold stress, GAPDH and ACT1 under salt stress, and ACT1 and GAPDH in different tissues are suggested for the accurate normalization of target gene expression in A. pumila. To our knowledge, this is the first report on the identification and validation of suitable reference genes for qRT-PCR analysis in A. pumila under various abiotic stresses and developmental tissues. The identified reference genes can facilitate accurate gene expression analysis by qRT-PCR in A. pumila and other ephemeral species for functional genomics studies.
Methods
Plant materials and stress treatments
The cultivation methods and growth conditions for A. pumila used in this study were the same as those published in our previous reports2,3. For the salinity treatment, four-week-old seedlings were transferred to a 100-mL conical flask containing Hoagland’s nutrient solution for a whole day and were then moved to a fresh Hoagland’s nutrient solution containing 250 mM NaCl. For drought treatments, 10% PEG-6000 (w/v, Shanpu, Shanghai, China) was used to water the four-week-old seedlings. For cold and heat treatments, the four-week-old seedlings were placed in an illumination incubator at 4 °C and 40 °C, respectively. Leaf samples of each individual were carefully collected at 0, 3, 6, 12, 24 and 48 h after treatments.
Tissue samples of A. pumila were collected at different development stages. Roots, hypocotyls and cotyledons were collected from two-week-old seedlings. Rosette leaves and stems were sampled from the vegetative growth phase (six weeks after planting). Flowers and young siliques were collected at the maturity stage (three months after planting). All collected samples were immediately frozen in liquid nitrogen and were stored at −80 °C for follow-up experiments.
RNA isolation and cDNA synthesis
The total RNA for each sample was extracted with the RNAprep Pure Plant Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. RNA was further purified using RNase-free DNase (TIANGEN) according to the manufacturer’s guidelines. The quality, quantity and integrity of the RNA samples were assessed as described previously3. cDNA was synthesized from 300 ng RNA using the PrimeScript II First Strand cDNA Synthesis Kit MIX (Bioteke, Beijing, China) with oligo (dT) primers in a final volume of 20 μL according to the manufacturer’s instructions.
Selection of candidate reference genes
Due to a lack of A. pumila genome information, we generated a full-length transcriptome of leaf tissues for this species using SMRT carried out on a Pacific Biosciences (PacBio, Menlo Park, CA, USA) sequencing platform3. Based on these transcriptome datasets, 10 reference genes with relatively stable expression (based on their FPKM and fold change values) were selected to screen the most reliable reference genes for target gene expression via qRT-PCR experiments (Table 1).
Primer design and amplification efficiency analysis for qRT-PCR
Gene-specific primers for qRT-PCR analysis were designed using the primer 3.0 online tool (http://bioinfo.ut.ee/primer3/) according to the sequences of 10 candidate reference genes and a target gene. Primers were synthesized by the Beijing Genomics Institute (Beijing, China) with the following parameters: Tm values ranging from 50 to 65 °C, GC percent of 45–55%, primer lengths of 17–25 bp and product lengths of 80–300 bp (Table 2).
To detect the specificity and amplification efficiency of each pair of primers that we designed, we first performed RT-PCR in a 20-μL system using a Mastercycler nexus GSX1 PCR apparatus (Eppendorf AG, Hamburg, Germany) containing 60 ng of the synthesized cDNA, 2 μL rTaq buffer (TakaRa, Dalian, China), 0.2 μM of each of the primers, 200 μM each dNTP and 1 U rTaq (TakaRa). The RT-PCR amplification programme consisted of 2 min of initial denaturation at 94 °C, followed by 30 cycles of 30 s at 94 °C, 45 s at 62 °C, 30 s at 72 °C, and a final 5-min extension at 72 °C. The amplification products were evaluated using 2.5% (w/v) agarose gel electrophoresis, and the PCR products were sequenced to validate primer specificity.
qRT-PCR was carried out with the SYBR Green PCR Master Mix system (CWBIO, Beijing, China) on an Applied Biosystems 7500/7500 Fast Real-time PCR System (ABI, Foster City, CA, USA). The PCR amplification system and programme were performed as described previously2. Three biological duplications were performed with independently isolated RNA in all qRT-PCR. Relative gene expression levels were calculated using the 2−ΔΔCt method39.
Determination and validation of expression stability of reference genes
The average Ct values calculated from qRT-PCR data for all samples or genes were used for further analyses. Four different types of statistical software, geNorm22, NormFinder14, BestKeeper23 and RefFinder24,25, were applied to evaluate the expression stability of the reference genes across all experimental sets.
The geNorm programme calculates the gene expression stability value (M value) and ranks the reference genes22. This programme also evaluates the pairwise variation (Vn/Vn+1) to determine the optimal number of genes required for accurate normalization of qRT-PCR data. NormFinder software evaluates the expression stability of each gene through an assessment of within-and between-group variations using a variance analysis-based approach14. A higher stable value calculated by geNorm or NormFinder indicates a more unstable gene, whereas the lower the stable value, the better the stability of the gene. The Ct value of each gene was input into BestKeeper software to calculate the correlation coefficient (r), standard deviation (SD) and coefficient of variation (CV) of each sample across five experimental sets. The closer the r value is to 1, the more stable the gene expression. The comparative ΔCt analysis was assessed via the commonly used RefFinder online software (http://150.216.56.64/referencegene.php?type=reference). Furthermore, we used RefFinder to comprehensively rank the expression stability by analysing the geometric mean of Ct values of all candidate reference genes26,40.
Validation of reference genes
To verify the results of our experiments, the combination of the top two best reference genes, the most stable and most unstable reference genes were used to normalize the expression of one target gene, KUP9, under different experimental conditions and in different tissues. The qRT-PCR experimental methods were the same as those above. Statistical significance analysis was performed using IBM SPSS statistics 19.0 (https://www.ibm.com/products/spss-statistics) based on one-way ANOVA and further evaluated using Duncan’s multiple comparison (P < 0.05)3.
Supplementary information
Identification of reliable reference genes for qRT-PCR in ephemeral plant Arabidopsis pumila based on full-length transcriptome data
Acknowledgements
This project is supported by the grants from the National Natural Science Foundation of China (U1303302, 31060149), the open funds of the State Key Laboratory of Plant Physiology and Biochemistry (SKLPPBKF1807), the Scientific and Technological Innovation Leading Talents of Xinjiang Production and Construction Corps (2006BC001), and the Innovation Team Project for Xinjiang Production and Construction Corps (2014CC005). The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Author Contributions
X.H. and Y.J. conceived the experiments, Y.J. and F.L. conducted the experiments, W.H. and Q.S. analysed the results, X.H. and Y.J. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44849-1.
References
- 1.Shi ZY, Feng G, Christie P, Li XL. Arbuscular mycorrhizal status of spring ephemerals in the desert ecosystem of Junggar Basin, China. Mycorrhiza. 2006;16:269–275. doi: 10.1007/s00572-006-0041-1. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Yang L, Jin Y, Lin J, Liu F. Generation, annotation, and analysis of a large-scale expressed sequence tag library from Arabidopsis pumila to explore salt-responsive genes. Front. Plant Sci. 2017;8:955. doi: 10.3389/fpls.2017.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, et al. Full-length transcriptome sequences of ephemeral plant Arabidopsis pumila provides insight into gene expression dynamics during continuous salt stress. BMC Genomics. 2018;19:717. doi: 10.1186/s12864-018-5106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu W, et al. Spring ephemerals adapt to extremely high light conditions via an unusual stabilization of photosystem II. Front. Plant Sci. 2016;6:1189. doi: 10.3389/fpls.2015.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson HS, et al. Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat. Protoc. 2009;4:902–922. doi: 10.1038/nprot.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 7.Gachon C, Mingam A, Charrier B. Real-time PCR: what relevance to plant studies? J. Exp. Bot. 2004;55:1445–1454. doi: 10.1093/jxb/erh181. [DOI] [PubMed] [Google Scholar]
- 8.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR-a perspective. J. Mol. Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- 9.Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalization; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 11.Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BR, Nam HY, Kim SU, Kim SI, Chang YJ. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol. Lett. 2003;25:1869–1872. doi: 10.1023/A:1026298032009. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CL, Jensen JK, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 14.Dheda K, et al. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- 15.Radonić A, et al. Guideline for reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 16.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazari F, Parham A, Maleki AF. GAPDH, β-actin and β2-microglobulin, as three common reference genes, are not reliable for gene expression studies in equine adipose- and marrow-derived mesenchymal stem cells. J. Anim. Sci. Technol. 2015;57:18. doi: 10.1186/s40781-015-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubie C, et al. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol. Cell Probes. 2005;19:101–109. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Hong SY, Seo PJ, Yang MS, Xiang F, Park CM. Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol. 2008;8:112. doi: 10.1186/1471-2229-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez L, et al. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008;6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 21.Sang J, et al. ICG: a wiki-driven knowledgebase of internal control genes for RT-qPCR normalization. Nucleic. Acids. Res. 2018;46:D121–D126. doi: 10.1093/nar/gkx875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 24.Shakeel M, Rodriguez A, Tahir UB, Jin F. Gene expression studies of reference genes for quantitative real-time PCR: an overview in insects. Biotechnol. Lett. 2018;40:227–236. doi: 10.1007/s10529-017-2465-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Chen F, Ye J, Pan H. Selection of reliable reference genes for RT-qPCR analysis of Bursaphelenchus mucronatus gene expression from different habitats and developmental stages. Front. Genet. 2018;9:269. doi: 10.3389/fgene.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saddhe AA, Malvankar MR, Kumar K. Selection of reference genes for quantitative real-time PCR analysis in halophytic plant Rhizophora apiculata. PeerJ. 2018;6:e5226. doi: 10.7717/peerj.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia W, et al. Analysis of multiple transcriptomes of the African oil palm (Elaeis guineensis) to identify reference genes for RT-qPCR. J. Biotechnol. 2014;184:63–73. doi: 10.1016/j.jbiotec.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Fausto AKS, Silva TDF, Romanel E, Vaslin MFS. microRNAs as reference genes for quantitative PCR in cotton. PLoS One. 2017;12:e0174722. doi: 10.1371/journal.pone.0174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. Reference gene selection for qRT-PCR assays in Stellera chamaejasme subjected to abiotic stresses and hormone treatments based on transcriptome datasets. Peer J. 2018;6:e4535. doi: 10.7717/peerj.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarker N, et al. Identifcation of stable reference genes for quantitative PCR in koalas. Sci. Rep. 2018;8:3364. doi: 10.1038/s41598-018-21723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, et al. Reference gene selection for quantitative real-time PCR normalization in Caragana intermedia under different abiotic stress conditions. PLoS One. 2013;8:e53196. doi: 10.1371/journal.pone.0053196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M. Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol. 2010;10:49. doi: 10.1186/1471-2229-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivhare R, Lata C. Selection of suitable reference genes for assessing gene expression in pearl millet under different abiotic stresses and their combinations. Sci. Rep. 2016;6:23036. doi: 10.1038/srep23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmair A, Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989;56:1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 35.Saha P, Blumwald E. Assessing reference genes for accurate transcript normalization using quantitative real-time PCR in pearl millet [Pennisetum glaucum (L.) R. Br] PLoS One. 2014;9:e106308. doi: 10.1371/journal.pone.0106308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao X, et al. Validation of suitable reference genes for gene expression analysis in the halophyte Salicornia europaea by real-time quantitative PCR. Front. Plant Sci. 2015;5:788. doi: 10.3389/fpls.2014.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, et al. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza. Mol. Biol. Rep. 2010;37:507–513. doi: 10.1007/s11033-009-9703-3. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SJ, Shin R, Schachtman DP. Expression of KT/KUP Genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004;134:1135–1145. doi: 10.1104/pp.103.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of reliable reference genes for qRT-PCR in ephemeral plant Arabidopsis pumila based on full-length transcriptome data