Abstract
The pace of transmission of domesticated cereals, including millet from China as well as wheat and barley from southwest Asia, throughout the vast pastoralist landscapes of the Eurasian Steppe (ES) is unclear. The rich monumental record of the ES preserves abundant human remains that provide a temporally deep and spatially broad record of pastoralist dietary intake. Calibration of human δ13C and δ15N values against isotope ratios derived from co-occurring livestock distinguish pastoralist consumption of millet from the products of livestock and, in some regions, identify a considerable reliance by pastoralists on C3 crops. We suggest that the adoption of millet was initially sporadic and consumed at low intensities during the Bronze Age, with the low-level consumption of millet possibly taking place in the Minusinsk Basin perhaps as early as the late third millennium cal BC. Starting in the mid-second millennium cal BC, millet consumption intensified dramatically throughout the ES with the exception of both the Mongolian steppe where millet uptake was strongly delayed until the end of first millennium cal BC and the Trans-Urals where instead barley or wheat gained dietary prominence. The emergence of complex, trans-regional political networks likely facilitated the rapid transfer of cultivars across the steppe during the transition to the Iron Age.
Subject terms: Proteins, Stable isotope analysis
Introduction
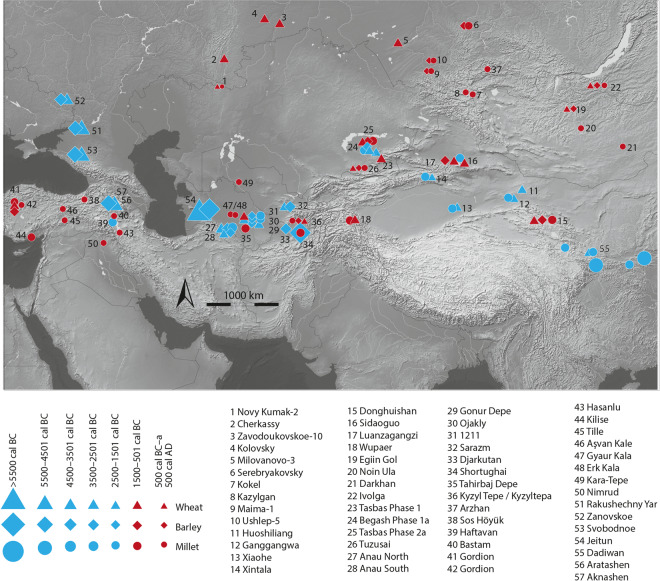
The Eurasian Steppe (ES) is a vast cultural crossroads situated between two independent centers of crop domestication of southwest Asia and China and is a region key to understanding the spread of domesticated plants and animals (Fig. 1)1–4. Despite the expansive distances that characterize the steppe, the ES served for millennia as a dynamic socio-economic intersection through which commodities and technologies flowed via pastoralist communities who relied on domesticated livestock for subsistence. The transmission of cultivars across the ES was also facilitated by pastoralists who exchanged and, in some regions, cultivated cereals for subsistence and ritual use3,5,6. However, the pace of cultivar uptake and the intensity of cereal consumption by pastoralists remains poorly understood during two critical periods, from the third to second millennia cal BC when the translocation of wheat, barley, and millet grains into the ES first took place, and the mid-second through first millennium cal BC when inter-regional trade and exchange networks intensified and complex political structures emerged across the steppe7–11.
Figure 1.
Distribution of millet, wheat and barley in the Eurasian steppe across time and space as identified in the carbonized seed record. Basemap constructed in ArcGIS 10 with hillshade generated using, http://www.naturalearthdata.com (freely available). Seed locations were generated based on published data using Adobe Illustrator CS.
The paleobotanical record has provided important glimpses into the translocation of cultivars across the ES. After the initial domestication of wheat (Triticum sp.) and barley (Hordeum sp.) in southwest Asia by 8,500 cal BC, these cultivars spread into the Iranian Plateau by 6000 cal BC and into Pakistan by c. 5500 cal BC12–15. In southern Central Asia, domesticated forms of barley and wheat identified in Jeitun cultural contexts in Turkmenistan suggests these cultivars had spread to western Central Asia around 6100 cal BC16,17. Domesticated wheat and barley were cultivated in the southern Caucasus by c. 6000 cal BC18,19 and were present in the northern Caucasus by c. 4500 cal BC20. Wheat and barley spread into eastern Ukraine by 4000 cal BC and into the Crimean steppe by 3400 cal BC19. Notably, the carbonized seed record suggests there was a significant delay in the introduction of wheat and barley into the central ES and western China, which did not begin until the mid-third millennium cal BC21–26. The earliest evidence for the use of wheat and barley in the central ES is in the form of carbonized grains from southern Kazakhstan directly dated to c. 2500 cal BC25. In China, barley and wheat were present in Xinjiang by c. 2000 cal BC23,26 and in the Hexi corridor at 1700 cal BC24.
A considerable lag between initial millet domestication and its spread throughout the ES is also evident. Domesticated broomcorn millet (Panicum miliaceum) and foxtail millet (Setaria italica) seeds recovered from Neolithic agricultural settlements located in northeastern China dating to the early sixth millennium cal BC strongly suggests this region was a center for initial millet domestication27,28. Domesticated broomcorn and foxtail millet grains from Inner Mongolia directly dated to the mid-sixth millennium cal BC suggests that millet flowed relatively rapidly out of China to the northeast29. However, the Xinjiang region and ES remained devoid of broomcorn millet until the early second millennium cal BC2,5,30. Broomcorn millet was present on the western edges of the ES in southeastern Kazakhstan by 2200 cal BC, somewhat later than wheat5, and in Turkmenistan and Afghanistan by the mid-second millennium cal BC3,6,31. Overall, evidence for broomcorn millet in the ES at this time is sparse, limited to paleobotanical remains and impressions visible in ceramics from the Trans-Urals (late 2nd to early 1st mil. BC), Southern Siberia and the Altai (early 1st mil. BC to 1st cent. AD), and from Mongolia (late 1st mil. BC)3 (Fig. 1). Recent direct dating of charred seed remains recovered from central and southeast Europe indicate that millet was present in the region by the mid second millennium cal BC19. In the Caucasus region, evidence for millet is limited to a single impression on pottery obtained from a broadly dated context dating to 6200–3300 cal BC19.
While the carbonized seed record has been instrumental in tracing the spread of millet, wheat, and barley throughout the ES, the temporal correspondence between initial use of cultivars and their incorporation into pastoralist diets remains under-defined, as does the degree to which the intensity of pastoralist cereal exploitation articulated with socio-political developments across the steppe. Dietary intake recorded in the isotope record of ancient human tissues have previously provided evidence for the spread of domesticated crops both westward across China32 and out of Mesoamerica into North America and South America33,34. We employ a similar approach to examine the spread of isotopically distinct C4 millet and C3 barley and wheat throughout the ES, but we further refine the approach by calibrating the distribution of human carbon and nitrogen isotopes against the isotopic composition of animals commonly exploited by pastoralists including domesticated sheep, goats, cattle and horses. This method takes into consideration the diverse environmental inputs that affect the isotopic composition of steppe foodwebs, in particular aridity and water availability which influences the natural abundance of C3 and C4 flora in steppic vegetational biomes and the carbon isotopic composition of C3 plants which underscore the isotopic composition of the animal-based portion of pastoralist diets.
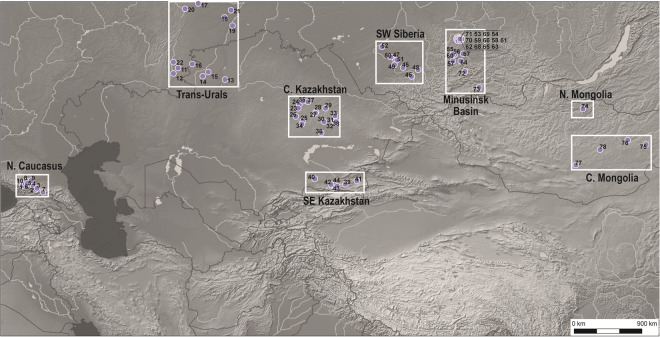
We report here a comprehensive meta-analysis of previously published carbon and nitrogen isotope values from humans and co-occurring livestock from Bronze Age and Iron Age sites located in the ES dating to ca 5000 cal BC to 400 AD (Fig. 2)11,35–58. The precise chronology of each cultural period varies across the ES reflecting regional differences in the pace of local social, economic, and political developments as well as the robustness of local radiocarbon chronologies. Here, we use previously established cultural-chronological sequences constructed from combined cultural materials and radiocarbon analyses (Table S1); no further modeling of radiocarbon determinations were undertaken due to the lack of information available regarding the type of material analyzed (e.g., wood charcoal, carbonized seeds, or human or animal bone collagen) and, in the case of human remains, the potential impact of freshwater reservoir effects on dates continue to be unresolved for the steppe (see discussion in methods). Carbon and nitrogen isotopic data sets are derived from the collagenous fraction of human skeletal remains from socially distinguished individuals interred in monumental mortuary structures that were a ubiquitous feature of ancient ES landscapes and considered to be those of steppe pastoralists8,11,35. These mortuary features maintained regionally distinctive styles and functions as commemorative spaces59, social integrative elements60,61, and focal points for political action11,62.
Figure 2.
Locations of sites used in stable isotope meta-analysis: 1 Kabardinka, 2 Kudachurt, 3 Zaragizh, 4 Baksanyonok, 5 Gorjachevodskii 2, 6 Nezhinskaya, 7 Zamankul, 8 Zanozina Balka, 9 Inosemsevo, 10 Klin-Yar, 11 Kamennyi Ambar V, 12 Bolshekaragansky, 13 Bestamak, 14 Novoilinka, 15 Lisakovsk, 16 Isiney, 17 Kurtuguz I, 18 Murzino 1, 19 Skaty 1, 20 Shaidurikha, 21 Gayovsky I, 22 Pobedy, 23 Tengiszhol, 24 Temirkash, 25 Nurtaldy, 26 Dariya, 27 Tashik, 28 Aschisu, 29 Ayap-bergen, 30 Kopa-1, 31 Akimbek, 32 Tasyrbai 2, 33 Kyzylkol, 34 Kairakty, 35 Kudryavaya Sopka-1, 36 Kyzyl, 37 Karatugai, 38 Kent, 39 Kyzyl Bulak, 40 Oi-Dzailau VII, 41 Karatuma, 42 Kargaly 1, 43 Alatau 1, 44 Kamenka, 45 Itkul, 46 Ust-Isha, 47 Firsovo XI, 48 Solnts 5, 49 Tuzovskiye, 50 Teleutsky Vzvoz, 51 Firsovo XIV, 52 Chicha, 53 Afanasyeva Gora, 54 Karasuk III, 55 Uibat III, 56 Uibat V, 57 Verhniy Askiz I, 58 Bateni, 59 Melnichniy Log, 60 Okunev Ulus, 61 Solnechniy Log, 62 Upper Karasuk River, 63 Yarki, 64 Chernoye Ozero I, 65 Grishkin Log, 66 Lepeshki, 67 Nurilkov Ulus, 68 Podgornoye Ozero, 69 Saragash, 70 Saragshinskoe Ozero, 71 Saragashinskiy Spusk, 72 Ai-Dai, 73 Amyrlyg, 74 Egiin Gol, 75 Ulaanzuukh, 76 Daram uul, 77 Tevsh, 78 Baga Gazaryn Chuluu. Basemap constructed in ArcGIS 10 with hillshade generated using, http://www.naturalearthdata.com (freely available). Site locations generated in Adobe Illustrator CS6.
Significant Differences in Linear Regression Slopes Between Humans and Livestock Indicate Pastoralist Cereal Consumption
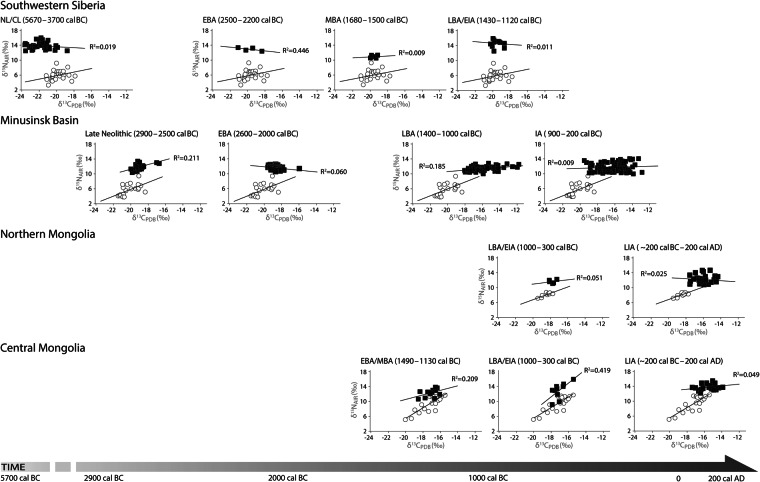
Pronounced changes in the carbon and nitrogen isotopic composition of humans relative to co-occurring livestock indicate that a significant human dietary transition was underway by the mid-second millennium cal BC. Across the ES, Bronze Age human groups share carbon isospace with regionally corresponding livestock and exhibit a positive correlation between nitrogen and carbon isotope values, a pattern also observed in livestock (Figs 3 and 4). Bronze Age humans and livestock exhibit similar regression line slopes across the ES with the exception of paired groups from the Trans-Urals and Minusinsk Basin (Table S2). Isotopic congruence between humans and livestock indicate that the Bronze Age human dietome was primarily structured by the isotopic composition of steppe and forest steppe flora ingested by grazing livestock and, after accounting for trophic level fractionation effects, strongly suggests that Bronze Age pastoralist dietary intake in most regions relied heavily on animal products.
Figure 3.
Carbon (δ13C) and nitrogen (δ15N) isotope values of humans (black squares) and livestock (open circles) for the Trans-Urals, Central Kazakhstan, Southeastern Kazakhstan and the Northern Caucasus.
Figure 4.
Carbon (δ13C) and nitrogen (δ15N) isotope values of humans (black squares) and livestock (open circles) for Southwestern Siberia, the Minusinsk Basin, Northern Mongolia and Central Mongolia.
The shift in pastoralist food intake from diets based on the products of livestock including meat and milk as well as fish in some regions to diets that included an appreciable amount of cereals occurred at various timings and intensities across the ES. This dietary transition was likely underway throughout the mid-third and early second millennium cal BC in some regions, followed by relatively rapid uptake throughout the ES of cereal inclusive diets during the Iron Age starting in the mid-second millennium cal BC. This major dietary switchover to dedicated cereal consumption is evidenced in the isotopic record by (i) a continued positive correlation between δ13C and δ15N values exhibited in livestock but a breakdown in this isotopic relationship observed in contemporaneous humans indicating a dramatic departure from regional steppe dietomes (Figs 3 and 4), (ii) a significant shift in average δ13C values of Iron Age humans relative to Bronze Age humans (Table S3), and (iii) wide isotopic spacing between the carbon isotopes of Iron Age humans and reference livestock in most regions (Fig. S1). The contribution of wheat and barley to pastoralist diets that may also include millet remain invisible using this approach. We also calculated isotopic niches for each time period and region, to identify shifts in niche size and overlap, in an effort to clarify the introduction of new dietary inputs. An increase in isotopic niche size over time, as seen in density plots (S6), indicate an expansion of dietary diversity, in most regions, to include a 13C-enriched food source. The maximum percent overlap of Bayesian standard ellipse areas were plotted at a 99% confidence interval (CI) to highlight chronological variation in isotopic niches. Regionally, there was a decrease in the overlap of isotopic niches over time further supporting the introduction of a new food source in later periods.
Regional Variation in the Intensification in Cereal Consumption
Southwestern Siberia
Pastoralists in southwest Siberia appear to have consumed both quantities of fish and terrestrial animal protein in the Late Bronze and Early Iron Ages (LBA/EIA) during the mid to late second millennium cal BC indicated by high human bone collagen δ15N values averaging 15‰ and a c. 9‰ enrichment in human δ15N values compared to livestock38 (Figs 4, S1; Table S2). Bayesian ellipses and isotopic niches demonstrate a decrease of the isospace occupied by humans over time (Figs S2, S6.1, S6.2), suggesting that hunter-gatherer diets were more diverse than later pastoralist diets. The stable isotopic data do not confirm or reject the low-level consumption of cereals in later periods due to the high contribution of fish to diets in SW Siberia. Flotation at the LBA/EIA site confirms the presence of plant remains, but an absence of grains from domesticated cultivars, indicating that cereal crops did not figure into pastoralist diets in SW Siberia38.
Minusinsk Basin
Low-level millet consumption by hunter-gatherer populations in the ES may have taken place in the Early Bronze Age (EBA) sometime during the late third to early second millennium cal BC suggested by i) the combination of a 1.6‰ increase on average in δ13C values (Table S3), ii) low δ13C values (c. −24‰) exhibited by Minusinsk Basin fish49, and iii) the breakdown of the positive linear relationship between carbon and nitrogen isotopes in EBA human collagens and livestock caused by the incorporation a 13C-enriched food source into the diet (Fig. 4; Table S2) (c.f.49). The absence of 13C-enriched fish in local waterways or indigenous C4 flora, indicated by low livestock δ13C values averaging 20‰, may indicate that low-level millet consumption is responsible for the isotopic patterns visible in EBA humans. We recognize that freshwater fish may have varied in isotopic composition and that fish could have been enriched in 13C relative to modern species. It may be that the consumption of fish, rather than millet, is responsible for the carbon isotopic pattern observed for this region and time period. Furthermore, in the absence of radiocarbon determinations from human individuals analyzed for carbon and nitrogen isotopes, some individuals in the EBA group could date to as late as c. 2000 cal BC. Millet was clearly an important part of pastoralist diets by the mid-second millennium cal BC in the Minusinsk Basin. This is indicated by the pronounced 2.7‰ increase, on average, in human δ13C values from the EBA to LBA (Table S3) and a shift in the carbon isospace occupied by LBA humans (EBA = −19.5‰ to −15.9‰; LBA = −18‰ to −11.8‰) confirming previous findings for the region49. High levels of millet consumption were maintained through the Iron Age, while fish regained dietary importance. Differences in the isospace occupied by temporally varied human groups were compared by plotting standard ellipse areas (SEA; Fig. S2) and as Bayesian permutations of standard ellipse areas (SEAB; Fig. S6). The maximum percent overlap of SEAB’s of LN and EBA groups has a 99% probability of being below 60% (S6) while the EBA ellipse slope varies from the earlier period. The overlap visible in the EBA ellipse with the ellipses of 13C enriched LBA and IA humans (Figs S2 and S6) further suggests that dietary intake differed between these periods and that humans incorporated low levels of a 13C-enriched food source, such as millet, into the EBA human diet. A density plot demonstrates the confidence intervals of the different ellipse areas, demonstrating the expansion of isotopic niches over time. Isotopic niche sizes varied notably between the early (LN, EBA) and later (LBA, IA) groups, increasing in the later periods (S6.3-S6.5). The LBA and IA niches expanded greatly compared to earlier time slices, indicating the consumption of a 13C-enriched food source, which lends further support for the millet consumption in these periods.
Southeastern Kazakhstan
No isotopic evidence for cereal consumption is visible in the remains of pastoralists inhabiting SE Kazakhstan in the early second millennium, although sample sizes are small (Fig. 3, LBA1) (Sensu55). By the mid-second millennium cal BC, the combination of a 2.3‰ increase on average in LBA2 human carbon isotope values and loss of a positive linear relationship between carbon and nitrogen isotope values in humans and livestock indicates that millet was incorporated into pastoralist diets at this time. The overlap in dating for the two LBA time slices suggests that communities had varied dietary intake in this region. Moderately high human δ13C values averaging 15.4‰ and a breakdown in the positive correlation between regression slopes of EIA humans and livestock (Fig. 3) indicates moderate levels of millet consumption continued through the end of first millennium cal BC. These findings are supported by Bayesian ellipses of humans (Fig. S2 and S6.6–S6.8) which demonstrate incomplete overlap (<40%) between LBA1 and LBA2. The separation of isotopic niches between LBA1 and LBA2 suggests dietary distinctions between pastoralist groups, while the overlap between ellipses for LBA2 and 13C enriched EIA groups suggests new dietary inputs, such as millet, for both groups.
Central Kazakhstan
Low-level millet consumption is evident in pastoralist diets in Central Kazakhstan during the late second millennium cal BC at the tail end of the Bronze Age (Figs 2 and 3). Late Bronze Age (LBA) humans and livestock exhibit similar positive regression line slopes, while later Final Bronze Age (FBA) humans and livestock exhibit significantly different regression slopes (Table S2). This, in addition to the significant 0.6‰ enrichment in FBA human δ13C values relative to LBA humans, and moderate increase in the carbon isotopic spacing between humans and livestock during the FBA (Figs 3, S1; Tables S2, S3), suggest the addition to FBA pastoralist diets of a C4 food source outside of that typically available in this steppe environment. High δ15N values of humans, averaging 13.5‰, were maintained throughout the Bronze Age reflecting considerable animal protein intake. The maximum percent overlap of SEAB’s (LBA and FBA groups) have a 99% probability of being below 60% (S6) suggesting a change in human dietary intake over time. The expansion of the isotopic niche for FBA humans indicates that dietary intake included new dietary inputs, likely low levels of a 13C-enriched food source such as millet (Figs S6.9–S6.11).
Mongolia
Millet appears to have circumvented the Mongolian steppe throughout much of the Iron Age. In N. Mongolia, the combination of a significant difference in regression line slopes computed for LIA humans and herbivores (Table S2), a significant 1.7‰ enrichment in the δ13C values of LIA humans relative to LBA/EIA humans (Table S3), and a wider 2.4‰ carbon isotopic spacing between humans and livestock (Fig. S1) together indicates that the incorporation of millet into pastoralist diets did not take place until the end of the first millennium cal BC. The absence of nitrogen isotopic change between LBA/EIA and LIA humans suggests little change in animal protein intake over time (Table S3). These findings are further supported by Bayesian standard ellipses that show the LBA/EIA and LIA overlapped less than 40%. Furthermore, the LIA ellipse demonstrates a greatly expanded niche, indicating a significant dietary change in this period that included the consumption of millet (Figs S2, S6.12–S6.14).
Millet appears to have been consumed in central Mongolia during the Late Iron Age. In contrast to similar regression line slopes calculated for Bronze Age humans, LIA humans yield a different carbon and nitrogen isotopic relationship. Regression line slopes of LIA humans were significantly different than those of earlier humans as well as livestock (Fig. 4, Table S2). The moderate, albeit significant, 1.6‰ enrichment in LIA human δ13C values relative to MBA humans, and moderate 1.5‰ carbon isotope spacing between LIA humans and livestock suggests low level incorporation of millet into the diets of pastoralists inhabiting the northern Gobi. The increase in the nitrogen isotopic spacing between humans and livestock over time, from 2.8 to 4.3‰ in the water poor Gobi steppe desert, likely reflects a shift in animal management strategies to include grazing of livestock on 15N enriched winter pastures during the LIA53. Bayesian standard ellipses (99%) indicate considerable overlap between the EBA/MBA and LBA/EIA (70%), demonstrating only slight variation in dietary intake. A decrease in the overlap between LBA/EIA and LIA ellipses (<45%) coincide with an expansion of the LIA ellipse toward higher carbon isotope values suggesting that dietary intake shifted in the later period to include a 13C-enriched food source such as millet (Figs S2, S6.15–S6.17).
Trans-Urals
Millet contributed little, if at all, to pastoralist diets in the Trans-Urals. Instead, C3 cultivars such as wheat and barley appear to have been gradually incorporated into pastoralist diets during the mid-second millennium cal BC and became a dietary focus by the mid-first millennium cal BC during the Iron Age. During the early second millennium cal BC, the combination of high δ15N values averaging 12.3‰ and significant differences between positive regression slopes visible in human and livestock isospace indicates fish were an important part of the MBA human diet (Fig. 3). During the subsequent LBA 1 and LBA 2, which are contemporaneous periods, variation in dietary strategies emerges. During the LBA 1, at the site of Lisakovsk, the reduction in the range of nitrogen isotopes weighted toward lower δ15N values in humans combined with similar regression slopes exhibited by humans and livestock suggest that fish consumption decreased considerably by the early second millennium cal BC and was at least partially replaced by animal products derived from livestock. Pastoralist dietary intake appears to have shifted at the LBA 2 site of Isiney indicated by a 0.6‰ decrease in human δ15N values from the MBA, suggestive of a further reduction in fish intake. Significant differences between human and livestock regression slopes indicate Trans-Urals pastoralist diets included some cereals by the mid second-millennium cal BC (LBA 2, Fig. 3; Table S2). C3 cereals became an important part of pastoralist diets by the mid-first millennium cal BC during the Iron Age (IA) indicated by (i) the significant 0.8‰ depletion in human δ13C values relative to the preceding LBA 2 (Table S3), (ii) an expanded range of IA carbon isotopes heavily weighed to lower δ13C values between −17.5‰ to −22.8‰ (compared to all earlier periods which exhibit a restricted range of δ13C values from c. −16.8‰ to −20.3‰) (Tables S2, S3), and (iii) the absence of a positive linear relationship between carbon and nitrogen isotopes expressed in human collagens (Fig. 3). These findings are supported by differences between Bayesian standard ellipse areas. Overlap between the MBA and LBA 1 ellipses are high, reaching 85% (Figs S2, S6.18–S6.20) indicating similar dietary niches. Ellipses for LBA 1 (Lisakovsk) and LBA 2 (Isiney) have less of an overlap (60%), and the latter has an ellipse with an expanding niche width, suggesting a new dietary input (S6.19, S6.20). Dietary change is clear by the IA, where the isotopic niche has expanded to include a 13C-depleted food source, such as domesticated wheat or barley.
The Northern Caucasus
The incorporation of millet into pastoralist diets took place in the Early Iron Age (EIA) during the late second millennium cal BC in the N. Caucasus, indicated by similar positive regression slopes exhibited by Bronze Age humans and livestock followed by a significant difference in this relationship during the Iron Age (Fig. 3). In addition, MBA and MBA/LBA humans exhibited only a 0.6‰ spacing between human and livestock δ13C values, reflecting pastoralist dietary intake driven by carbon isotopic variation in local pastures. A subsequent 5.0‰ and 2.7‰ spacing between LBA/EIA and IA humans and livestock, respectively, is consistent with the pronounced contribution of a C4 food source to human diets (Fig. S1). The significant c. 0.7‰ enrichment in 15N and depletion in 13C of IA humans relative to preceding LBA/EIA humans may suggest dietary diversification in the later period and the initial contribution of a C3 food source such as wheat or barley (Tables S2; Fig. S1). Isotopic niche widths were plotted as standard ellipse areas (SEA; Fig. S2) and as Bayesian permutations of standard ellipse areas (SEAB; Fig. S6.21). SEAs demonstrate strongly overlapping ellipses for MBA and MBA/LBA groups, with low overlap between these groups and the IA (Fig. S2). There is also low overlap between the LBA/EIA and the IA suggesting significant variation in dietary intake between these periods. Plots of SEAB for humans and animals through time further demonstrate differences between early (MBA, MBA/LBA) and later (LBA/EIA, IA) populations in the region (Fig. S6.21, S6.22). The expansion of isotopic niches in the later periods indicates that dietary intake differed between periods and that humans incorporated a 13C-enriched food source, such as millet, into their diets.
Temporal disparity between first evidence of cereals, subsequent incorporation into pastoralist diets, and intensification of cereal consumption in the ES
A substantial lag time between initial translocation and intensive consumption of cultivars is evidenced in both the carbonized seed record and the stable isotopic record in several regions of the ES (Figs 1; S2, S3, S4). The earliest millet was present in SE Kazakhstan by 2200 cal BC5 but, as indicated in the human isotopic record, did not have a significant dietary impact until c. 1500 cal BC. Similarly, in the Trans-Urals indirectly dated wheat grains suggests the cultivar was present c. 1750 cal BC but was not an important part of the pastoralist diet until c. 1600 cal BC. This disparity in uptake could indicate the conversion of cereals from an exotic cultigen used in ceremonial contexts during the Bronze Age, as seen at the pastoralist settlement of Begash (where millet grains recovered from human burial cists points toward the ritual use of cereals in mortuary contexts)5, to a subsistence food that was consumed at varied intensities by different pastoral populations across the ES.
Participation in trans-regional political exchange networks redirected pastoralist dietary intake
The marked intensification in the consumption of grains by pastoralists coincided with a shift in the configuration of socio-political relationships from internally-focused, community oriented interaction spheres, to externally oriented networks that facilitated the creation and maintenance of trans-regional political relationships across the ES. During the third and second millennia BC, Bronze Age interaction spheres facilitated the flow of locally available prestige goods and operated at regional scales8,11,63,64. Burial practices emphasized the collective, perhaps kin-based, interment of multiple individuals from the same community8,65 and monument construction facilitated local community cohesion and integration11,66. The emergence of trans-regional connections during the first millennium cal BC culminated in the formation of socio-political confederations in multiple regions7–11,67. This process was evidenced by a marked increase in the exchange and use of costly prestige goods sourced from diverse locales across Eurasia and China11,68, the construction of ostentatious mortuary monuments memorializing individuals, and the spread of horse-riding paraphernalia7,35,69. Furthermore, as horseback riding became the norm, based on the regular apperance of bits and bridles in mortuary contexts35,70,71, individuals and small groups were able to move at the speed of the horse rather than the herd, increasing the frequency and regularity of contact between communities as well as regions, facilitating the growth of networks, and amplifying the flows of goods and technologies. While Iron Age groups have generally been cast as highly nomadic pastoralists, it remains to be seen if they engaged in intensive agricultural production. Our findings indicate that it is precisely during this period of proposed nomadism when the consumption of cereals intensifies across Eurasia.
Considerable regional variation in the intensity of millet consumption by pastoralists across the ES is likely due to differences in the intensity of local cultivation, degree of local participation in trans-regional networks, and importance of access to cultivars in structuring socio-political relationships. In the case of the Minusinsk Basin during the late third millennium cal BC, possible low-level millet consumption (Fig. S3) coincided with the intensification of trans-regional interactions with communities associated with the Chemurchek (Qiemu’erqieke) tradition located across the Russian Altai, western Mongolia, and NW Xinjiang72, where similar flat bottomed jars, stone cist burials, and anthropomorphic stone stelae were recovered73–75. However, the carbonized seed record indicates millet was not used in Xinjiang until the early second millennium cal BC23,76,77 while high carbon isotope values associated with intensive millet consumption is not evident in the region until 900 cal BC78,79. The combined archaeological and isotopic data from the Minusinsk Basin may indicate millet was cultivated at an earlier date in Xinjiang or alternatively, albeit less likely given the absence of millet in the Baikal region, was introduced into the Minusinsk from sources in northeast China, where charred millet grains are present at c. 6000 cal BC28. Bronze daggers and socketed axes associated with the Andronovo or Karasuk cultures that are typical for the Minusinsk Basin were present in northeast China and indicate interactions between these regions during the late second millennium cal BC80–83.
The first isotopic evidence for low-level wheat or barley consumption occurs in the Trans-Urals and is visible at the beginning of the second millennium cal BC (LBA 2, Figs 3; S3). The chronological overlap between LBA 1 and LBA 2, but variation in dietary intake between humans assigned to each group suggests that Andronovo communities had diverse subsistence strategies. The initial influx of grains may have come from the southwest (Caucasus) region or from southern Central Asia, with the latter more likely as limited numbers of exotic items found in Andronovo sites are often connected to southern regions8,82,84. Intensification of wheat and barley consumption in the Trans-Urals occurred during the Iron Age when Sauro-Sarmatian and Sargat interaction spheres spread across large swaths of the Eurasian steppe, evident in shared prestige good assemblages, warrior equipment, and mortuary rituals35. This region was deeply involved in exchange with areas in central and southeastern Kazakhstan that consumed millet, yet Trans-Ural groups opted instead to grow wheat and/or barley. Early evidence for a dietary focus on wheat and/or barley, rather than millet, suggests that Trans-Urals populations may have been trading these cultivars to other areas of the steppe that lacked the water resources to cultivate these crops. Investments in different farming technique or a higher value placed on wheat and/or barley may also have been factors affecting the decision to focus on the production of these grains.
In SE Kazakhstan, diversification of pastoralism to include dedicated cultivation appears to have taken place at c. 1500 cal BC, indicated by cropping of millet, wheat, barley and peas and the local processing of harvested crops1 which roughly coincides with increased millet consumption seen in the stable isotopic record (Figs 3; S4). Early evidence for millet in SE Kazakhstan may have been introduced from western China, while wheat and barley were introduced from southern Central Asia, as interactions along the mountain corridor were longstanding85. During the second millennium cal BC, durable networks of interaction linked western China and southern Central Asia through SE Kazakhstan1,85.
Low–level consumption of millet in Central Kazakhstan, which occured several hundred years later, is associated with the emergence of regional centers and participation in pan-regional interaction spheres, from the Urals to the Altai, which may have facilitated access to cultivars8,86. The incorporation of millet into pastoralist diets, albeit at low-levels, in C. Kazakhstan by c. 1050 cal BC also coincided with a shift in socio-political relationships (Fig. S4). Late Bronze Age interactions were locally oriented, evidenced by a lack of imported goods and diverse mortuary rituals, while participation in wider trans-regional networks occurred during the FBA, specifically with areas of southeastern Kazakhstan where similar ceramic ornamentation and bronze ornamental goods have been identified8,25,86,87.
The intensification in millet consumption in the northern Caucasus by c. 1200 cal BC (Fig. S4) coincided with the emergence of an extensive exchange system centered in the Caucasus that facilitated movement of raw materials required for tin-bronze metal working and finished metal goods88. While millet has been identified in the southern Caucasus as early as c. 1600 cal BC, the introduction of this crop to mountainous zones only occurred as network interactions became externally focused. This only occurred after 1200 cal BC when imported goods such as Assyrian helmets were recovered from local burial contexts89 and locally made weapons and armor were exported as prestige goods7.
In the Mongolian steppe, millet consumption levels were the lowest in the ES for the Iron Age (Fig. S5) and suggests either local cultivation activities were small in scale or the Xiongnu elite engaged in indigenous forms of ideological expression centered on domesticated livestock and steppe nomadism11,68, or a combination of both. The late incorporation of millet into pastoralist diets in the Mongolian steppe coincided with macro-regional integrations of the Xiongnu political confederation at the end of the first millennium cal BC10,11. Notably, although millet was an important part of Iron Age steppic communities, nowhere in the ES did pastoralist millet intake approach the intensive levels of millet consumption seen in contemporaneous agricultural societies in China, where high δ13C values observed in human collagens ranging from −6.5 to −11‰ are common90–92.
Discussion
The transformation of pastoralist dietary intake from a heavy reliance on livestock products, or hunting-fishing-gathering in some regions, during the Bronze Age to consumption of cereals during the transition to the Iron Age took place alongside a radical change in the scale of pastoralist interaction from inward focused interaction spheres to trans-regional, externally-oriented networks during the mid-second millennium BC. The apparently substantial temporal lag between the first appearance of cereals visible in the carbonized seed record and the regular inclusion of cereals into human diets indicated in the bone collagen isotopic record likely reflects the conversion of cereals from an exotic and rare cultigen, used in Bronze Age ritual contexts, to a subsistence food possibly used to demarcate elite status in pastoralist societies participating in increasingly interconnected trans-regional political networks. The first evidence for a dietary transition, and possibly millet consumption, in the ES appears to occur in the Minusinsk Basin which would suggest a route of grain translocation through western China (Xinjiang) or, less likely, across southern Russia originating in far northeastern China (Fig. S2). As millet next appears in SE Kazakhstan, the route through Xinjiang seems more likely (Fig. S4). Millet was then slowly incorporated into pastoral diets in central Kazakhstan and the N. Caucasus, while groups in the Trans-Urals region either consumed millet in very small amounts or opted to focus on wheat and barley (Figs S2–S4). The relatively minor contribution of millet to the diets of pastoralists inhabiting the Mongolian steppe throughout the first millennium AD (Figs S3, S4) suggests that populations maintained pastoralist lifeways connected to indigenous ideologies centered on human-animal relationships.
Methods
Previously published human and livestock δ13C and δ15N values obtained from bone collagen were grouped according to broad environmental zones in order to account for variation in the isotopic composition of locally available dietary resources. Published human isotopic data sets that did not include paired livestock values were not included in this study. The ES is broadly divided into two environmental zones, the forest-steppe and grassland steppe which support C3 vegetation and mixed C3/C4 vegetation, respectively93–95. The overall carbon isotope composition of steppe and forest-steppe floral biomes varies according to regional precipitation levels, temperature, aridity, and topography which together influence the relative abundance of 13C depleted C3 and 13C enriched C4 flora96 and modify the carbon isotope composition of C3 plants. Here, the ES is divided into 8 zones according to location of sampled sites and regional differences in phytogeographic and climatic conditions that broadly determine the carbon isotope composition of regional floral biomes (Fig. 2). These zones include the (1) C3 mountainous uplands of the northern Caucasus, (2) largely C3 foothills and steppe of the Trans-Urals, (3) C3/C4 steppe of central Kazakhstan, (4) the C3/C4 steppe of southern Kazakhstan, (5) mainly C3 Baraba forest-steppe of SW Siberia, (6) largely C3 steppe/forest-steppe of the Minusinsk Basin, surrounded by high, densely forested mountains, (7) primarily C3 forest-steppe of northern Mongolia, and (8) C3/C4 Gobi steppe desert of south central Mongolia.
The broad differences in vegetation composition, aridity levels and topography characterizing each of these eight zones together influence the carbon and nitrogen isotope composition of the floral base of the foodweb. This is further reflected in the isotope composition of herbivorous consumers, in this case grazing and browsing livestock97–99. In order to distinguish the contribution of 13C enriched or 13C depleted agricultural cereals to pastoralist diets from foodstuffs that derive from steppe food resource pools, which include milk and meat products obtained from livestock grazing on 13C enriched C4 and water-stressed C3 graze, we used independent isotope reference sets that measure isotope variation in local foodwebs using δ13C and δ15N values of livestock that are spatially and temporally congruent to the human sample set. The δ13C and δ15N values of pastoralists whose dietary intake draws heavily from animal products should closely track those of domesticated herbivores alongside a trophic fractionation factor of + 3–6‰ in δ15N100–102 and +1‰ in δ13C103,104, represented by positive regression lines similar to those exhibited by co-occurring livestock ingesting local floral resources. Conversely, pastoralist diets characterized by a sustained consumption of 13C enriched millet or 13C depleted barley and wheat no longer track herbivores and instead exhibit significantly different regression lines from co-occurring livestock indicating consumption of dietary resources typically not available on steppe landscapes.
Average δ13C and δ15N values of humans grouped by region and chronological period were compared using an analysis of variance coupled with Tukey’s test105 to determine the statistical significance of differences between human diets across time and space (Table S3). T-tests of the slopes of regression lines, which indicate a relationship between two variables (δ13C, δ15N), were used to compare humans to livestock by region (Table S2). Inter-regional comparisons of the intensity of pastoralist cereal consumption were carried out by standardizing regional human collagen δ13C values against the δ13C values obtained from co-localized livestock (Fig. S1); this approach removes regionally specific environmental isotopic overprints that are incorporated into human tissue via ingestion of animal products. Similar standardization was carried out for human collagen δ15N values against those of regional livestock to evaluate variation in the nitrogen isotopic composition of human diets over time (Fig. S1). Standardization, or the magnitude of isotopic spacing, was calculated between herbivore and human values (∆herbivore − human) for each time period (Fig. S1).
Time slices within each region were established based on accepted chronological brackets defined by material culture types and stylistic forms associated with radiocarbon dates. The construction of robust radiocarbon chronologies in the Eurasian steppe is so far constrained as there are relatively few radiocarbon determinations available for this broad region. For example, recent Bayesian modeling of radiocarbon dates from Bronze Age and Early Iron Age106 Mongolia encompasses 1500 years of prehistory, yet is based on a sample of only 45 dates. This is one of the most robust chronological datasets currently available for Eurasia106 and similar in size to a dataset (n = 40) analyzed a decade ago for the Trans-Urals region39. Reported radiocarbon determinations are also frequently under-described as to the material dated which may have included wood charcoal, carbonized seeds, as well as human and faunal bone, substrates that may yield incongruent radiocarbon determinations depending on the biological history of the dated sample. Many of the sites in the Eurasian steppe are located alongside rivers and lakes, and human diets may have included freshwater resources which potentially result in anomalously old radiocarbon dates. The impact of the freshwater reservoir effect (FRE) on radiocarbon dates derived from human bone collagen in the study region has been extensively studied41,107–109 with results indicating substantial variation in FRE neccesitating assessment of radiocarbon dates on a case by case basis108,110. Resolving issues of small sample sizes, poor reporting of dated materials, and a full consideration of modern and archaeological isotope values to deal with the reservoir effect are well beyond the scope of this paper. Therefore, we used established radiocarbon date ranges from scholars that are specialists in their region and time period to determine date ranges for each time slice.
We chose to retain outliers present in stable isotopic datasets as they convey meaningful information regarding intra-community variation. In order to overcome issues with small sample sizes we employed SIBER (Stable Isotope Bayesian Ellipses in R) to construct Bayesian ellipse standard areas (SEAB) at a 99% confidence interval (CI) for each time period. In a Bayesian approach, a set of iterative draws (1010) from a simulation is used to construct an ellipse and derive metrics, such as the area, which is referred to as SEAB. This process is repeated for all simulated values, producing a range of probable values for the calculated metric. Bayesian ellipses take into account uncertainty in the sampled data and incorporate error arising from the sampling process. Ellipses are unbiased with respect to sample size and robust comparisons can be made between data sets111. We also used density plots by region that indicate the confidence intervals of standard ellipse areas for each human group respectively. Further, we computed the percent overlap for ellipses between periods when dietary shifts were occurring. These were computed at 99% confidence and represent the maximum amount of overlap of two Bayesian standard ellipse areas (Fig. S6).
Electronic supplementary material
Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Table S1, Table S2, Table S3, Table S4, Table S5
Acknowledgements
The authors would like to thank colleagues who have commented on our manuscript at multiple stages, including Bryan Hanks, Isabella von Holstein, Bryan Miller and Josh Wright. The authors would like to thank Ines Reese for preparing figures for this manuscript. This manuscript was first conceptualized as part of discussions during meetings of the Archaeological Stable Isotope Laboratory (ASIL) at CAU Kiel.
Author Contributions
A.V.M. desinged and intiated the research. A.V.M. compiled all stable isotope data and conducted data analyses. The manuscript text was written by A.V.M. and C.A.M. A.V.M. and C.A.M. developed the figure content. Supplemental data and figures were prepared by A.V.M.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/1/2022
A Correction to this paper has been published: 10.1038/s41598-022-19230-4
Contributor Information
Alicia R. Ventresca Miller, Email: ventrescamiller@shh.mpg.de
Cheryl A. Makarewicz, Email: c.makarewicz@ufg.uni-kiel.de
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35758-w.
References
- 1.Spengler RN. Agriculture in the Central Asian Bronze Age. Journal of World Prehistory. 2015;28:215–253. doi: 10.1007/s10963-015-9087-3. [DOI] [Google Scholar]
- 2.Spengler RN, III., Ryabogina N, Tarasov PE, Wagner M. The spread of agriculture into northern Central Asia: Timing, pathways, and environmental feedbacks. The Holocene. 2016;26:1527–1540. doi: 10.1177/0959683616641739. [DOI] [Google Scholar]
- 3.Miller NF, Spengler RN, Frachetti M. Millet cultivation across Eurasia: Origins, spread, and the influence of seasonal climate. The Holocene. 2016;26:1566–1575. doi: 10.1177/0959683616641742. [DOI] [Google Scholar]
- 4.Stevens CJ, et al. Between China and South Asia: A Middle Asian corridor of crop dispersal and agricultural innovation in the Bronze Age. The Holocene. 2016;26:1541–1555. doi: 10.1177/0959683616650268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frachetti MD, Spengler RN, Fritz GJ, Mar’yashev AN. Earliest direct evidence for broomcorn millet and wheat in the central Eurasian steppe region. Antiquity. 2010;84:993–1010. doi: 10.1017/S0003598X0006703X. [DOI] [Google Scholar]
- 6.Spengler RN, Cerasetti B, Tengberg M, Cattani M, Rouse LM. Agriculturalists and pastoralists: Bronze Age economy of the Murghab alluvial fan, southern Central Asia. Vegetation history and archaeobotany. 2014;23:805–820. doi: 10.1007/s00334-014-0448-0. [DOI] [Google Scholar]
- 7.Reinhold S. Engendering cultural communication networks: Gender related exchange systems of North Caucasian Iron Age societies between high mountains, piedmonts and the steppe. Gender Locales and Local Genders in Archaeology, Bar International Series (ed. Hjørungdal, T.)25 (2005).
- 8.Koryakova, L. & Epimakhov, A. V. The Urals and western Siberia in the Bronze and Iron ages. (Cambridge University Press, 2014).
- 9.Hanks B. Archaeology of the Eurasian steppes and Mongolia. Annual Review of Anthropology. 2010;39:469–486. doi: 10.1146/annurev.anthro.012809.105110. [DOI] [Google Scholar]
- 10.Miller BK. Xiongnu ‘kings’ and the political order of the steppe empire. Journal of the Economic and Social History of the Orient. 2014;57:1–43. doi: 10.1163/15685209-12341340. [DOI] [Google Scholar]
- 11.Honeychurch, W. Inner Asia and the Spatial Politics of Empire. (Springer, 2015).
- 12.Zohary, D., Hopf, M. & Weiss, E. Domestication of Plants in the Old World: The origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin. (Oxford University Press on Demand, 2012).
- 13.Pashkevich, G. Paleoethnobotanical evidence of agriculture in the steppe and forest-steppe of east Europe in the Late Neolithic and BronzeAge, in Prehistoric steppe adaptation and the horse 287–97 (2003).
- 14.Harris, D. R. & Gosden, C. The beginnings of agriculture in western Central Asia. The origins and spread of agriculture and pastoralism in Eurasia 370–389 (1996).
- 15.Miller NF. Agricultural development in western Central Asia in the Chalcolithic and Bronze Ages. Vegetation History and Archaeobotany. 1999;8:13–19. doi: 10.1007/BF02042837. [DOI] [Google Scholar]
- 16.Miller, N. F. The Use of Plants at Anau North. A Central Asian Village at the Dawn of Civilization, Excavations at Anau, Turkmenistan, in A Central Asian Village at the Dawn of Civilization, Excavations at Anau, Turkmenistan 127–138 (University of PennsylvaniaMuseum, 2003).
- 17. Harris, D. R. Origins of agriculture in western Central Asia: an environmental-archaeological study. (University of Pennsylvania Press, 2010).
- 18.Hovsepyan R, Willcox G. The earliest finds of cultivated plants in Armenia: evidence from charred remains and crop processing residues in pisé from the Neolithic settlements of Aratashen and Aknashen. Vegetation History and Archaeobotany. 2008;17:63–71. doi: 10.1007/s00334-008-0158-6. [DOI] [Google Scholar]
- 19.Motuzaite-Matuzeviciute G. The earliest appearance of domesticated plant species and their origins on the western fringes of the Eurasian Steppe. Documenta Praehistorica. 2012;39:1–21. doi: 10.4312/dp.39.1. [DOI] [Google Scholar]
- 20.Lebedeva E. First results of archaeobotanical investigations at the archaeological site of Adygei. Analytical studies in the laboratory for natural science methods. 2011;2:244–257. [Google Scholar]
- 21.Betts A, Jia PW, Dodson J. The origins of wheat in China and potential pathways for its introduction: A review. Quaternary International. 2014;348:158–168. doi: 10.1016/j.quaint.2013.07.044. [DOI] [Google Scholar]
- 22.Lebedeva EY. Archaeobotany and study of the Bronze Age agriculture in Eastern Europe. OPUS: Mezhdistsiplinarnye issledovaniia v arkheologii. 2005;4:50–68. [Google Scholar]
- 23.CRAIXAR (The Cultural Relics and Archaeological Institute of Xinjiang Autonomous Regions). A brief excavation report on Xiaohe graveyard located in Luobupo, Xinjiang Autonomous Region. Cultural Relics10, 4–42 (2007). Chinese.
- 24.Flad R, Shuicheng L, Xiaohong W, Zhijun Z. Early wheat in China: Results from new studies at Donghuishan in the Hexi Corridor. The Holocene. 2010;20:955–965. doi: 10.1177/0959683609358914. [DOI] [Google Scholar]
- 25.Doumani PN, et al. Burial ritual, agriculture, and craft production among Bronze Age pastoralists at Tasbas (Kazakhstan) Archaeological Research in Asia. 2015;1:17–32. doi: 10.1016/j.ara.2015.01.001. [DOI] [Google Scholar]
- 26.Liu X, et al. The virtues of small grain size: Potential pathways to a distinguishing feature of Asian wheats. Quaternary International. 2016;426:107–119. doi: 10.1016/j.quaint.2016.02.059. [DOI] [Google Scholar]
- 27.Crawford, G. W. East Asian plant domestication, in Archaeology of Asia (ed. Stark, M.) 77–95 (Blackwell Publishing Ltd, 2006).
- 28. Liu, X., Hunt, H. V. & Jones, M. K. River valleys and foothills: changing archaeological perceptions of North China’s earliest farms. Antiquity83, 82–95 (2009).
- 29.Zhao Z. New archaeobotanic data for the study of the origins of agriculture in China. Current Anthropology. 2011;52:S295–S306. doi: 10.1086/659308. [DOI] [Google Scholar]
- 30.Li C, et al. Ancient DNA analysis of desiccated wheat grains excavated from a Bronze Age cemetery in Xinjiang. Journal of Archaeological Science. 2011;38:115–119. doi: 10.1016/j.jas.2010.08.016. [DOI] [Google Scholar]
- 31.Willcox, G. In New light on early farming. Recent developments in palaeoethnobotany (ed. Renfrew, J.) 139–153 (1991).
- 32.Liu X, Reid RE, Lightfoot E, Matuzeviciute GM, Jones MK. Radical change and dietary conservatism: Mixing model estimates of human diets along the Inner Asia and China’s mountain corridors. The Holocene. 2016;26:1556–1565. doi: 10.1177/0959683616646842. [DOI] [Google Scholar]
- 33.Van der Merwe NJ, Vogel JC. 13C content of human collagen as a measure of prehistoric diet in woodland North America. Nature. 1978;276:815–816. doi: 10.1038/276815a0. [DOI] [PubMed] [Google Scholar]
- 34.Schoeninger MJ. Stable isotope evidence for the adoption of maize agriculture. Current Anthropology. 2009;50:633–640. doi: 10.1086/605111. [DOI] [PubMed] [Google Scholar]
- 35.Hanks, B. The Eurasian steppe ‘nomadic world’ of the first millennium BC: Inherent problems within the study of Iron Age nomadic groups in Ancient Interactions: East and West in Eurasia (eds Boyle, K., Renfrew, C. & Levine, M.) 183–197 (2002).
- 36.Chang C, Benecke N, Grigoriev FP, Rosen AM, Tourtellotte PA. Iron Age society and chronology in South-east Kazakhstan. Antiquity. 2003;77:298–312. doi: 10.1017/S0003598X00092280. [DOI] [Google Scholar]
- 37.Privat, K. Palaeoeconomy of the Eurasian steppe: biomolecular studies. (Doctoral dissertation, University of Oxford) (2004).
- 38.Privat K, et al. Economy and diet at the Late Bronze Age/Iron Age site of Cica. Artefactual, archaeozoological and biochemical analyses. Eurasia antiqua: Zeitschrift für Archäologie Eurasiens. 2005;11:419–448. [Google Scholar]
- 39.Hanks BK, Epimakhov A, Renfrew A. Towards a refined chronology for the Bronze Age of the southern Urals, Russia. Antiquity. 2007;81:353–367. doi: 10.1017/S0003598X00095235. [DOI] [Google Scholar]
- 40.Svyatko, S., Murphy, E., Schulting, R. & Mallory, J. Environment, lifestyle and diet of prehistoric populations from the Minusinsk Basin, Southern Siberia, Russia in Eurasian perspectives on environmental archaeology (eds Makohonienko, M., Makowiecki, D. & Czerniawska, J.) 3, 139–142 (Bogucki Wydawnictwo Naukowe, 2007).
- 41.Svyatko SV, et al. New radiocarbon dates and a review of the chronology of prehistoric populations from the Minusinsk Basin, southern Siberia, Russia. Radiocarbon. 2009;51:243–273. doi: 10.1017/S0033822200033798. [DOI] [Google Scholar]
- 42.Higham T, Warren R, Belinskij A, Härke H, Wood R. Radiocarbon Dating, Stable Isotope Analysis, and Diet-Derived Offsets in 14 C Ages from the Klin-Yar Site, Russian North Caucasus. Radiocarbon. 2010;52:653–670. doi: 10.1017/S0033822200045689. [DOI] [Google Scholar]
- 43.Hollund H, Higham T, Belinskij A, Korenevskij S. Investigation of palaeodiet in the North Caucasus (South Russia) Bronze Age using stable isotope analysis and AMS dating of human and animal bones. Journal of Archaeological Science. 2010;37:2971–2983. doi: 10.1016/j.jas.2010.08.009. [DOI] [Google Scholar]
- 44.Machicek, M. L. Reconstructing past diet in Mongolia from stable isotope analysis in Dundgov’ aimagt khiisen arkheologiin sudalgaa: Baga Gazryn Chuluu (eds Amartuvshin, C. & Honeychurch, W.) 430–435 (2010).
- 45.Panyushkina IP, Mills BJ, Usmanova ER, Cheng L. Calendar age of Lisakovsky timbers attributed to Andronovo community of Bronze Age in Eurasia. Radiocarbon. 2008;50:459–469. doi: 10.1017/S0033822200053558. [DOI] [Google Scholar]
- 46.Machicek, M. L. Reconstructing diet, health and activity patterns in early nomadic pastoralist communities of inner Asia. (Doctoral dissertation, University of Sheffield) (2011).
- 47.Murphy EM, et al. Iron Age pastoral nomadism and agriculture in the eastern Eurasian steppe: implications from dental palaeopathology and stable carbon and nitrogen isotopes. Journal of Archaeological Science. 2013;40:2547–2560. doi: 10.1016/j.jas.2012.09.038. [DOI] [Google Scholar]
- 48.Logvin, A. & Ševnina, I. Die Nekropole von Bestamak in Unbekanntes Kasachstan. Archäologie im Herzen Asiens. Katalog der Ausstellung des Deutschen Bergbau–Museums Bochum(eds Stӧllner, T. & Samašev, Z.) 231–244 (2013).
- 49.Svyatko SV, et al. Stable isotope dietary analysis of prehistoric populations from the Minusinsk Basin, Southern Siberia, Russia: a new chronological framework for the introduction of millet to the eastern Eurasian steppe. Journal of Archaeological Science. 2013;40:3936–3945. doi: 10.1016/j.jas.2013.05.005. [DOI] [Google Scholar]
- 50.Fenner JN, Tumen D, Khatanbaatar D. Food fit for a Khan: stable isotope analysis of the elite Mongol Empire cemetery at Tavan Tolgoi, Mongolia. Journal of Archaeological Science. 2014;46:231–244. doi: 10.1016/j.jas.2014.03.017. [DOI] [Google Scholar]
- 51.Ventresca Miller A, et al. Subsistence and social change in central Eurasia: stable isotope analysis of populations spanning the Bronze Age transition. Journal of Archaeological Science. 2014;42:525–538. doi: 10.1016/j.jas.2013.11.012. [DOI] [Google Scholar]
- 52.Lightfoot E, et al. How ‘pastoralist’ is pastoralism? Dietary diversity in Bronze Age communities in the central Kazakhstan steppes. Archaeometry. 2015;57:232–249. doi: 10.1111/arcm.12123. [DOI] [Google Scholar]
- 53.Makarewicz CA. Winter pasturing practices and variable fodder provisioning detected in nitrogen (δ 15 N) and carbon (δ 13 C) isotopes in sheep dentinal collagen. Journal of Archaeological Science. 2014;41:502–510. doi: 10.1016/j.jas.2013.09.016. [DOI] [Google Scholar]
- 54.Motuzaite Matuzeviciute G, et al. The extent of cereal cultivation among the Bronze Age to Turkic period societies of Kazakhstan determined using stable isotope analysis of bone collagen. Journal of Archaeological Science. 2015;59:23–34. doi: 10.1016/j.jas.2015.03.029. [DOI] [Google Scholar]
- 55.Motuzaite Matuzeviciute G, et al. Climatic or dietary change? Stable isotope analysis of Neolithic–Bronze Age populations from the Upper Ob and Tobol River basins. The Holocene. 2016;26:1711–1721. doi: 10.1177/0959683616646843. [DOI] [Google Scholar]
- 56.Yoneda, M. et al. Carbon and nitrogen stable isotope ratios and radiocarbon ages on the skeletal remains from Daram and Tevsh Sites of the Bronze Age, Mongolia in Excavations at Daram and Tevsh Sites: A Report on Joint Mongolian-Japanese Excavations in Outer Mongolia (eds Miyamoto, K. & Obata, H.) 63–66 (2016).
- 57. Hanks, B. et al. Bronze Age Diet and Economy: New Stable Isotope Data from the Steppes of Central Eurasia (2100–1700 BC). Journal of Archaeological Science (2018).
- 58.Knipper, C. et al. Economic strategies at Bronze Age and Early Iron Age upland sites in the North Caucasus: Archaeological and stable isotope investigations. Isotopic Investigations of Pastoralism (eds Ventresca Miller, A. R. & Makarewicz, C. A.) (Taylor & Francis, in press).
- 59.Hanks, B. K. Late Prehistoric Mining, Metallurgy, and Social Organization in North Central Eurasia in Social complexity in prehistoric Eurasia: Monuments, metals and mobility. (eds Hanks, B. K. & Linduff, K.) 146–167 (Cambridge University Press, 2009).
- 60.Houle, J. -L. (2009) Socially integrative facilities and the emergence of societal complexity on the Mongolian steppe in Social complexity in prehistoric Eurasia: Monuments, metals and mobility. (eds Hanks, B. K. & Linduff, K.) 358–377 (Cambridge University Press, 2009).
- 61.Jackson SE, Wright J. The Work of Monuments: Reflections on Spatial, Temporal and Social Orientations in Mongolia and the Maya Lowlands. Cambridge Archaeological Journal. 2014;24:117–140. doi: 10.1017/S0959774314000018. [DOI] [Google Scholar]
- 62.Reinhold S, Korobov DS. The Kislovodsk basin in the North Caucasian piedmonts–archaeology and GIS studies in a mountain cultural landscape. Preistoria Alpina. 2007;42:183–207. [Google Scholar]
- 63.Parzinger, H. Die frühen Völker Eurasiens: Von Neolithikum bis zum Mittelalter German (Verlag C. H. Beck, 2006).
- 64.Johnson, J. & Hanks, B. Society, demography and community: reassessing BronzeAge Sintashta populations in the southern Urals, Russia (2100–1700 BC) in Beyond Elites: Alternatives to Hierarchical Systemsin Modelling Social Formations (eds Kienlin, T. & Zimmermann, A.) 355–65 (2012).
- 65.Usmanova, E. R. Monuments of the Lisakovsk Area: Archaeological subjects. (ed. Usmanova, E. R.) (Tengri Ltd, 2013).
- 66.Wright J. Organizational principles of Khirigsuur monuments in the lower Egiin Gol valley, Mongolia. Journal of anthropological archaeology. 2007;26:350–365. doi: 10.1016/j.jaa.2007.04.001. [DOI] [Google Scholar]
- 67.Brosseder, U. A study on the complexity and dynamics of interaction and exchange in late Iron Age Eurasia in Complexity of Interaction Along the Eurasian Steppe Zone in the First Millennium CE (eds Bemmann, J. & Schmauder, M.) 349–424 (Bonn Contributions to Asian Archaeology vol. 5, 2015).
- 68.Miller, B. & Brosseder, U. B. Global dynamics in local processes of Iron Age Inner Asia in The Routledge Handbook of Archaeology and Globalization (ed. Hodos, T.) 470–487 (Routledge, 2017).
- 69.Kiryushin, J. F., Tishkin, A. A., & Molodin, V. I. Scythian epoch of Mountainous Altai: Culture of the population in early Scythian times (Izd-Vo Altajskogo Gos. Universiteta, 1997).
- 70.Renfrew, C. Pastoralism and Interaction: Some introductory questions in Ancient Interactions: East and West in Eurasia (eds Boyle, K., Renfrew, C., & Levine, M.) 1–20 (McDonald Institute for Archaeological Research, 2002).
- 71.Olsen, S. L. Early horse domestication on the Eurasian steppe in Documenting domestication: new genetic and archaeological paradigms (eds Zeder, M. A., Bradley, D. G., Emshwiller, E., & Smith, B. D.) 245–269 (University of California Press, 2006).
- 72.Turbat, T. Reconsidering the name of the archaeological culture ‘Khemcek’. Arheologijn Sydlal XXXIV9, 109–122 (2014).
- 73.Kovalev, A. A. & Erdenebaatar, D. Discovery of new cultures of the Bronze Age in Mongolia according to the data obtained by the International Central Asian Archaeological Expedition in Current archaeological research in Mongolia (eds Bemmann, J., Parzinger, H., Pohl, E., & Tseveendorzh, D.) 149–170 (Bonn Contributions to Asian Archaeology, 2009).
- 74.Jia PWM, Betts AV. A re-analysis of the Qiemu’erqieke (Shamirshak) cemeteries, Xinjiang, China. Journal of Indo-European Studies. 2010;38:275. [Google Scholar]
- 75.Kovalev, A. A. ed. The oldest Europeans in the heart ofAsia: The Chemurchek cultural phenomenon (Izd-vo LEMA, 2015).
- 76.CRAIXAR (The Cultural Relics and Archaeological Institute of Xinjiang Autonomous Regions) The Excavation Report of Xiaohe Cemetery. RCCFAJU. Frontier Archaeology3, 336–385 (2004).
- 77.Yang R, et al. Investigation of cereal remains at the Xiaohe Cemetery in Xinjiang, China. Journal of Archaeological Science. 2014;49:42–47. doi: 10.1016/j.jas.2014.04.020. [DOI] [Google Scholar]
- 78.Si Y, et al. Discussion of the food structure and population composition of the ancestral people of the Yanghai Cemetery, Xinjiang. Kexue Tongbao. 2013;15:1422–1429. [Google Scholar]
- 79.Zhang X, Chou S, Zhang J, Guo W. Carbon and Nitrogen stable isotope analyses of human bone unearthed from Duogang cemetery, Xinjiang. Nanfang Wenwu. 2014;3:79–91. [Google Scholar]
- 80.Okladnikov, A. ed. History of Siberia from ancient times to our days. (Izdatel’stvo Nauka: Leningrad, 1968).
- 81.Mei J, Shell C. The existence of Andronovo cultural influence in Xinjiang during the 2nd millennium BC. Antiquity. 1999;73:570–578. doi: 10.1017/S0003598X00065121. [DOI] [Google Scholar]
- 82.Kohl, P. L. The making of bronze age Eurasia. (Cambridge University Press, 2007).
- 83.Shelach-Lavi, G. Steppe Land Interactions and Their Effects on Chinese Cultures during the Second and Early First Millennia BCE in Nomads as agents of cultural change: the Mongols and their Eurasian predecessors (eds Amitai, R. & Biran, M.) 10–31 (University of Hawai’i Press, 2015).
- 84.Anthony, D. W. The horse, the wheel, and language: how Bronze-Age riders from the Eurasian steppes shaped the modern world. (Princeton University Press, 2010).
- 85.Frachetti MD, et al. Multiregional emergence of mobile pastoralism and nonuniform institutional complexity across Eurasia. Current Anthropology. 2012;53:000–000. doi: 10.1086/663692. [DOI] [Google Scholar]
- 86.Vinogradov, N. & Epimakhov, A. From a settled way of life to nomadism: Variances in models of transition in Kurgans, ritual sites, and settlements Eurasian Bronze and Iron Age (eds Davis-Kimball, J., Murphy, E., Koryakova, L., & Yablonsky, L.) 41–45 (BAR International Series 890, Archaeopress, 2000).
- 87.Frachetti MD, Mar’yashev AN. Long-term occupation and seasonal settlement of eastern Eurasian pastoralists at Begash, Kazakhstan. Journal of Field Archaeology. 2007;32:221–242. doi: 10.1179/009346907791071520. [DOI] [Google Scholar]
- 88.Kohl, P. & Trifonov, V. The Prehistory of the Caucasus: Internal Developments and External Interactions in The Cambridge World Prehistory 3 (eds Renfrew, C. & Bahn, P.) 1571–1595 (Cambridge University Press, 2014).
- 89.Härke, H. & Belinskij, A. Causes and contexts of long-term ritual change in Death and Changing Rituals: Function and meaning in ancient funerary practices 7 (Brandt, J. R., Ingvaldsen, Hî., & Prusac, M.) 93–104 (Oxbow Books, 2014).
- 90.Pechenkina EA, Ambrose SH, Xiaolin M, Benfer RA. Reconstructing northern Chinese Neolithic subsistence practices by isotopic analysis. Journal of Archaeological Science. 2005;32:1176–1189. doi: 10.1016/j.jas.2005.02.015. [DOI] [Google Scholar]
- 91.Ma M, et al. Stable isotope analysis of human and faunal remains in the Western Loess Plateau, approximately 2000 cal BC. Archaeometry. 2014;56:237–255. doi: 10.1111/arcm.12071. [DOI] [Google Scholar]
- 92.Ma M, et al. Stable isotope analysis of human and animal remains at the Qijiaping site in middle Gansu, China. International Journal of Osteoarchaeology. 2015;25:923–934. doi: 10.1002/oa.2379. [DOI] [Google Scholar]
- 93.Auerswald K, et al. Large regional-scale variation in C3/C4 distribution pattern of Inner Mongolia steppe is revealed by grazer wool carbon isotope composition. Biogeosciences. 2009;6:795–805. doi: 10.5194/bg-6-795-2009. [DOI] [Google Scholar]
- 94.Rachkovskaya, E. & Bragina, T. Steppes of Kazakhstan: diversity and present state in Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World (eds Werger, M. J. A. & Staalduinen, M. A.) 103–148 (Springer, 2012).
- 95.Smelansky, I. E, & Tishkov, A. A. The steppe biome in Russia: ecosystem services, conservation status, and actual challenges in Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World (eds Werger, M. J. A. & Staalduinen, M. A.) 45–101 (Springer, 2012).
- 96.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual review of plant biology. 1989;40:503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- 97.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochimica et cosmochimica acta. 1978;42:495–506. doi: 10.1016/0016-7037(78)90199-0. [DOI] [Google Scholar]
- 98.Ambrose SH, DeNiro MJ. The isotopic ecology of East African mammals. Oecologia. 1986;69:395–406. doi: 10.1007/BF00377062. [DOI] [PubMed] [Google Scholar]
- 99.Koch P, et al. Isotopic Tracking of Change in Diet and Habitat Use in African Elephants. Science. 1995;267:1340–1343. doi: 10.1126/science.267.5202.1340. [DOI] [PubMed] [Google Scholar]
- 100.DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et cosmochimica acta. 1981;45:341–351. doi: 10.1016/0016-7037(81)90244-1. [DOI] [Google Scholar]
- 101.Minagawa M, Wada E. Stepwise enrichment of 15 N along food chains: further evidence and the relation between δ 15 N and animal age. Geochimica et cosmochimica acta. 1984;48:1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- 102.O’Connell TC, Kneale CJ, Tasevska N, Kuhnle GG. The diet‐body offset in human nitrogen isotopic values: A controlled dietary study. American journal of physical anthropology. 2012;149:426–434. doi: 10.1002/ajpa.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krueger, H. W. & Sullivan, C. H. Models for carbon isotope fractionation between diet and bone in Stable isotopes in nutrition (eds Turnlund, J. R. & Johnson, P. E.) 205–220 (ACS Publications, 1984).
- 104.Ambrose, S. H. & Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate in Prehistoric human bone (eds Lambert, J. B. & Grupe, G.) 1–37 (Springer, 1993).
- 105.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, http://www.R-project.org/ (Vienna, 2016).
- 106.Taylor WTT, et al. Bayesian chronology for early domestic horse use in the Eastern Steppe. Journal of Archaeological Science. 2017;81:49–58. doi: 10.1016/j.jas.2017.03.006. [DOI] [Google Scholar]
- 107.Schulting RJ, Ramsey CB, Bazaliiskii VI, Goriunova OI, Weber A. Freshwater reservoir offsets investigated through paired human-faunal 14 C dating and stable carbon and nitrogen isotope analysis at Lake Baikal, Siberia. Radiocarbon. 2014;56:991–1008. doi: 10.2458/56.17963. [DOI] [Google Scholar]
- 108.Svyatko SV, Mertz IV, Reimer PJ. Freshwater reservoir effect on redating of Eurasian steppe cultures: First results for Eneolithic and Early Bronze Age Northeast Kazakhstan. Radiocarbon. 2015;57:625–644. doi: 10.2458/azu_rc.57.18431. [DOI] [Google Scholar]
- 109.Schulting RJ, Ramsey CB, Bazaliiskii VI, Weber A. Highly Variable Freshwater Reservoir Offsets Found along the Upper Lena Watershed, Cis-Baikal, Southeast Siberia. Radiocarbon. 2015;57:581–593. doi: 10.2458/azu_rc.57.18458. [DOI] [Google Scholar]
- 110.Wood R, et al. Freshwater radiocarbon reservoir effects at the burial ground of Minino, northwest Russia. Radiocarbon. 2013;55:163–177. doi: 10.2458/azu_js_rc.v55i1.16448. [DOI] [Google Scholar]
- 111.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology. 2011;80:595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Table S1, Table S2, Table S3, Table S4, Table S5