Abstract
To investigate the conjunctival microbiota and the association between the development of conjunctival mucosa-associated lymphoid tissue (MALT) lymphoma and dysbiosis, DNA samples were collected from 25 conjunctival MALT lymphoma patients and 25 healthy controls. To compare the microbiota, samples were collected from the following four body locations: conjunctiva, meibomian gland, periocular skin and hand. Extracted DNA was analyzed by 16S rRNA sequences, and libraries were sequenced on an Illumina MiSeq sequencer. The differences in bacteria were characterized by using principal coordinate analysis of metagenomics data, and the differences in bacterial compositions were evaluated by linear discriminant analysis effect size. The conjunctival microbiota of MALT lymphoma patients was compositionally different from that of healthy controls. For the conjunctival MALT lymphoma patients, alterations in the microbial composition were detected, and a remarkable change was detected at the conjunctiva. Detailed analysis showed that a specific population of the microbiota, the genus Delftia, was significantly more abundant in conjunctival MALT lymphoma patients, and the genera Bacteroides and Clostridium were less abundant in the MALT lymphoma patients. A specific microbiota on the ocular surface in conjunctival MALT lymphoma patients was detected, and dysbiosis may play an important role in the pathophysiology of conjunctival MALT lymphoma.
Subject terms: Bacterial pathogenesis, Bacterial genetics, Pathogens, Infection
Introduction
Conjunctival mucosa-associated lymphoid tissue (MALT) lymphomas are known as localized, low-grade tumors, and extranodal marginal zone B-cell MALT lymphoma is the most common histological subtype1–5. The frequency of ocular adnexal lymphoma is estimated to be approximately 8% of extranodal lymphomas3. For patients with primary lymphoma, the prognosis of primary conjunctival lymphomas is reported to be good, resulting in long-term survival3. Histologically, conjunctival MALT lymphoma has similar characteristics to gastric MALT lymphoma in the stomach, and it is thought to be caused by a chronic inflammatory response6. Several studies have reported the detection of Helicobacter pylori (H. pylori) DNA in some cases of conjunctival MALT lymphoma and the association of C. psittaci in its development, and therefore, these microorganisms were thought to be causative pathogens7,8. However, the pathophysiology of conjunctival MALT lymphoma has not been fully investigated.
In the human body, a great number and variety of commensal bacteria populate and regulate the homeostatic balance of the host9. The microbiota in the human body, including oral, skin, conjunctival, vaginal and respiratory tract microbes, plays an important role in the maintenance of health and disease development10. Dysbiosis, or an abnormality of homeostasis in the microbiota, causes several systemic disorders, such as inflammatory bowel diseases, obesity and cardiovascular diseases11–13. Previous reports have shown the relationships between the immune system and the commensal microbiota in host defense and tissue repair14. Pathological changes in the microbiota, such as Staphylococcus epidermidis, a commensal skin bacterium, has been demonstrated to cause opportunistic infections and result in the development of catheter infection, prosthetic valve endocarditis and endophthalmitis15–18. The association of dysbiosis with central nervous disorders, such as autism, multiple sclerosis, anxiety-depressive behaviors and functional gastrointestinal disorders, is reported in clinical practice, suggesting the possibility of microbe-based therapies to treat symptoms15–18. Thus, alterations of the residual microbiota lead to pathogenic infections or inflammation in the host and even result in fatal conditions9–18.
The ocular surface, a part of the conjunctiva-associated lymphoid tissue (CALT), is continuously exposed to the external environment, such as temperature changes, ultraviolet light and oxidative stress19. This stress has been implicated in the development of pterygium, dry eye, corneal dystrophy and Fuch’s endothelial dystrophy19,20. It is likely that changes in the microenvironment at the ocular surface lead to alteration of the microbiota, resulting in disease development. We hypothesized that mucosal microbial dysbiosis could contribute to immunological changes in the conjunctival mucosa and might be associated with the development of conjunctival MALT lymphoma. To verify this hypothesis, we investigated the microbial diversity in conjunctival MALT lymphoma and healthy controls and compared four body locations to detect their specific microbiota.
In the current study, we detected differences in the microbiota of the conjunctiva between conjunctival MALT lymphoma patients and healthy controls and discussed the role of the microbiota in the pathogenesis of conjunctival MALT lymphoma.
Results
A total of 50 persons (25 patients, 25 healthy controls) were enrolled in this study, and samples were collected at four locations (conjunctiva, meibomian gland, periocular skin and hand) and at two time points (baseline and 1 month later). After DNA extraction, the DNA was analyzed by 16S rRNA sequencing, and libraries were sequenced on an Illumina MiSeq sequencer. The relative abundances of bacteria in the four locations were compared using JMP and R software. The detailed microbacterial differences were shown using the LefSe program.
Clinical data of conjunctival MALT lymphoma patients
The clinical data and background for each patient are shown in Table 1. Of 25 patients, 6 patients had a previous history of chemotherapy, i.e. rituximab, R-THP-COP (rituximab, tetrahydropyranyladriamycin, cyclophosphamide, vincristine, and prednisolone) or R-CHOP (rituximab, adriamycin, cyclophosphamide, vincristine, and prednisolone), 3 patients received radiotherapy, and 5 patients received both. Concerning the sample collections during the treatments, we divided samples into three subgroups before treatment (6 patients), under treatment (14 patients), and after treatment (5 patients). Five patients had gastric lesions; numbers of gastric MALT lymphoma, gastric polyp, gastric ulcer and gastric cancer were 2, 1, 1 and 1, respectively. The mean follow-up period was 50.0 ± 6.2 months (range, 5–93 months). In this study, the biopsy after diagnosis and subsequent histological analyses revealed that the type for all patients was extranodal marginal zone B-cell lymphoma of MALT-type lymphoma. And, all cases were B-cell co-receptor markers (CD20) positive immunologically. Additionally, we performed Kappa light chain restriction analyses on two patients, and confirmed monoclonal proliferation in those two cases.
Table 1.
Demographic data of conjunctival MALT lymphoma patients.
| Age | Sex | Diagnosis | Laterality | Involvements | Treatments | Gastric lesions | Follow-up period (month) |
|---|---|---|---|---|---|---|---|
| 42 | F | MALT | unilateral | none | biopsy | none | 48 |
| 78 | F | MALT | bilateral | none | biopsy | none | 22 |
| 26 | F | MALT | bilateral | Orbita | biopsy | none | 18 |
| 91 | F | MALT | bilateral | Lacrimal gland | Rituximab, radiotherapy | none | 93 |
| 45 | F | MALT | bilateral | none | Rituximab | none | 20 |
| 79 | F | MALT | bilateral | none | biopsy | MALT lymphoma | 7 |
| 73 | F | MALT | unilateral | none | Rituximab, radiotherapy | none | 23 |
| 85 | F | MALT | unilateral | none | R-THP-COP | none | 63 |
| 58 | F | MALT | unilateral | none | biopsy | none | 74 |
| 78 | M | MALT | unilateral | none | Rituximab | gastric cancer | 86 |
| 51 | M | MALT | unilateral | none | Radiotherapy | gastric ulcer | 57 |
| 72 | M | MALT | unilateral | Lacrimal gland | Radiotherapy | none | 53 |
| 74 | F | MALT | bilateral | Orbita | R-CHOP, radiotherapy | none | 89 |
| 78 | M | MALT | bilateral | Orbita | Rituximab | gastric polyp | 64 |
| 52 | F | MALT | unilateral | none | biopsy | none | 66 |
| 50 | F | MALT | unilateral | none | biopsy | none | 15 |
| 63 | M | MALT | unilateral | none | R-CHOP, radiotherapy | none | 90 |
| 61 | F | MALT | bilateral | none | Radiotherapy | none | 85 |
| 57 | M | MALT | bilateral | none | R-CHOP, radiotherapy | MALT lymphoma | 93 |
| 47 | F | MALT | unilateral | none | Rituximab | none | 48 |
| 42 | F | MALT | unilateral | none | biopsy | none | 7 |
| 65 | M | MALT | unilateral | none | R-CHOP | none | 55 |
| 67 | F | MALT | unilateral | none | biopsy | none | 65 |
| 51 | F | MALT | unilateral | none | biopsy | none | 5 |
| 58 | F | MALT | unilateral | none | biopsy | none | 5 |
F = female; M = male; MALT = extranodal marginal zone B-cell MALT lymphoma.
R-THP-COP = rituximab, tetrahydropyranyladriamycin, cyclophosphamide, vincristine, and prednisolone.
R-CHOP = rituximab, adriamycin, cyclophosphamide, vincristine, and prednisolone.
DNA sequencing, data processing and stability
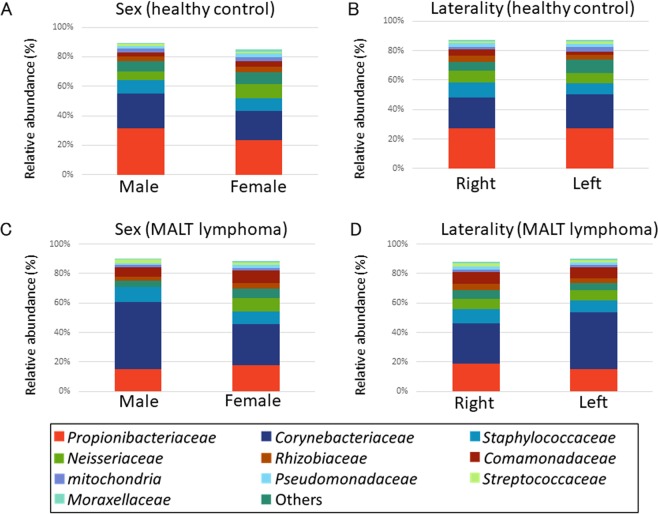
A total of 18,851,375 raw 16S rRNA gene sequences were obtained, which, after quality filtering, resulted in a total of 13,094,927 paired end sequences with an average of 53,231 sequences per sample. To determine the stability of the microbiota among the four locations, DNA sampling was performed one month after the baseline for healthy controls (Supplemental Fig. 1). There was no significant variation between sampling points regarding the microbacterial composition. The influences of sex and laterality on the microbial compositions of the conjunctiva were evaluated in healthy controls (Fig. 1A,B) and conjunctival MALT lymphoma patients (Fig. 1C,D). There were no significant variations in the groups for laterality or sex.
Figure 1.
The differences by sex and laterality in the conjunctiva of healthy controls and patients with MALT lymphoma. (A,B) Relative abundance of top11 compositions in sex and laterality showing that there were no significant variations in the healthy control. (C,D) For conjunctival MALT lymphoma patients, the differences of sex and laterality on the microbacterial composition of the conjunctiva were not detected. “Others” denotes that this part sums up the microbiota that are not recognized the taxometric classification at the family level.
Species richness and similarities of microbacterial groups with α diversities
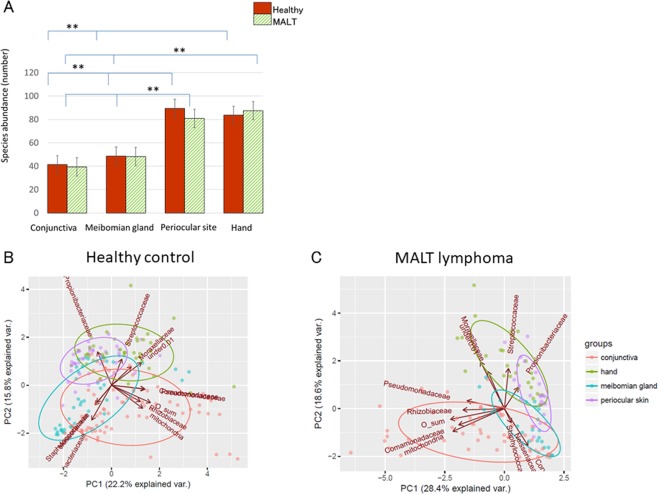
Microbacterial diversity among the four body locations in patients with conjunctival MALT lymphoma and healthy controls was investigated at the family level (Fig. 2A). The data are presented as the mean ± standard error (SE). The numbers of bacteria in the conjunctiva, meibomian gland, periocular skin, and hand of healthy controls were 41.2 ± 0.86 (range, 33–52), 48.6 ± 2.31 (range, 32–65), 89.4 ± 5.14 (range, 50–131), and 83.5 ± 5.11 (range, 38–137), respectively. The numbers of bacteria in the conjunctiva, meibomian gland, periocular skin, and hand of conjunctival MALT lymphoma patients were 39.4 ± 0.86 (range, 30–51), 48.2 ± 2.48 (range, 31–79), 80.8 ± 6.09 (range, 47–187), and 87.4 ± 6.57 (range, 55–140), respectively. The statistical analysis between healthy controls and conjunctival MALT lymphoma patients was performed using two-way ANOVA with Tukey post hoc. There were no significant differences in bacterial α diversity in each location (conjunctiva: P = 1.0, meibomian gland: P = 1.0, periocular skin: P = 0.12, hand: P = 0.93) between healthy controls and conjunctival MALT lymphoma patients. The conjunctival and meibomian samples showed lower α diversities than the periocular skin and hand samples (P < 0.01).
Figure 2.
Commensal microbiota at four locations, abundance and principal component analysis (PCA). (A) The statistical analyses of microbacterial α diversity at four body locations show that there were no significant differences between the conjunctiva of healthy controls and those of conjunctival MALT lymphoma patients. The conjunctival and meibomian samples show lower α diversity than the periocular skin and hand samples (P < 0.01). “**” means significant differences (P < 0.05). (B,C). The similarities of the bacterial groups in β diversity with PCA showing the distances of the four body locations (conjunctiva (red), meibomian gland (blue), periocular skin (purple) and hand (green)) in healthy controls (B) and conjunctival MALT lymphoma patients (C).
The main microorganisms composing the conjunctival microbiota of healthy and MALT lymphoma subjects are shown in Fig. 1. To investigate the similarities in the β diversity among microbiota groups, principal component analysis (PCA) was performed, and four clusters with top 11 compositions were revealed. The distances between the four body locations in healthy controls and conjunctival MALT lymphoma patients are shown in Fig. 2B,C at the family level. There was a relatively small distance between the conjunctiva (red) and the meibomian gland (blue), periocular skin (purple) and hand (green) in healthy controls and conjunctival MALT lymphoma patients. There was a large distance between the conjunctiva and the hand. The separation between the conjunctiva and hand was apparent in the PCA plot.
In addition, we investigated the microbacterial fluctuations provoked by treatments with the permutation analysis of variance (PERMANOVA). However, we could not find any significant differences between before, under, or after treatment.
The differences of the bacterial compositions between healthy controls and patients with conjunctival MALT lymphoma at the genus level
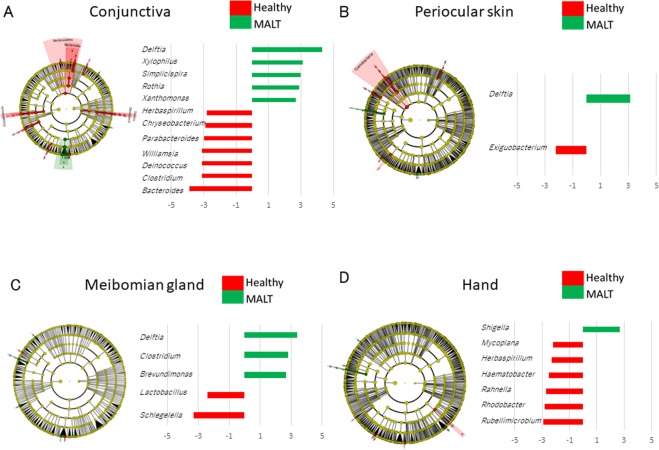
The microorganisms that were significantly increased (green) or decreased (red) in the conjunctival MALT lymphoma subjects were found among the four body locations by an algorism called LefSe (linear discriminant analysis effect size)21 with the data classified by genus level. These linear discriminant analysis (LDA) scores are plotted in Fig. 3. A significantly greater abundance of Delftia, Xylophilus, Simplicispira, Rothia and Xanthomonas and a lower abundance of Bacteroides, Clostridium, Deinococcus, Williamsia, Parabacteroides, Chryseobacterium and Herbaspirillum were detected at the conjunctiva in the conjunctival MALT lymphoma group compared to healthy controls (Fig. 3A). On the periocular skin, a significantly greater abundance of Delftia and a lower abundance of Exiguobacterium were detected (Fig. 3B). At the meibomian gland, a significantly greater abundance of Delftia, Clostridium and Brevundimonas and a lower abundance of Schlegelella and Lactobacillus were detected (Fig. 3C). On the hand, only Shigella was detected in higher abundance and the other microorganisms such as Mycoplana, Herbaspirillum, Haematobacter, Rahnella, Rhodobacter and Rubellimicrobium were lower in abundance in the conjunctival MALT lymphoma patients compared to healthy controls (Fig. 3D).
Figure 3.
The microbial differences between conjunctival MALT lymphoma patients and healthy controls at four locations analyzed by a linear discriminant analysis effect size program (LEfSe). LefSe data and LDA scores showing a significant increase (green) or decrease (red) at four body locations. A significantly greater abundance of microorganism and a lower abundance of them were observed each in conjunctiva (A), periocular skin (B), meibomian gland (C), and hand (D), respectively.
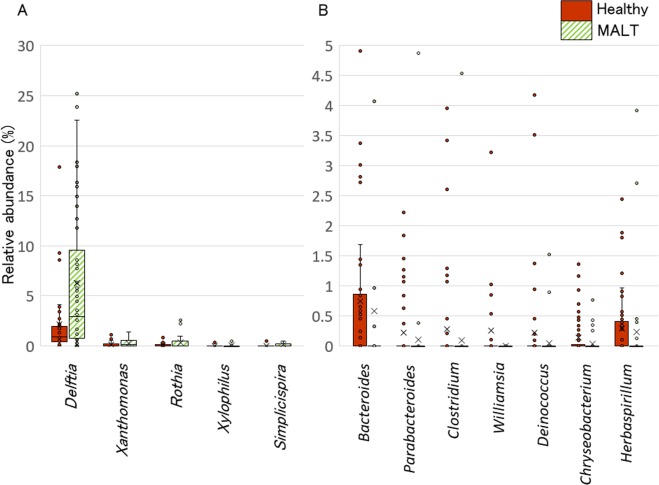
Box plots showed the bacterial prevalence at the conjunctiva in healthy controls and conjunctival MALT lymphoma patients (Fig. 4). The prevalence of Delftia was detected in many healthy controls and conjunctival MALT lymphoma patients, and the presence of Xylophilus, Simplicispira, Rothia and Xanthomonas was very rare in both groups (Fig. 4A). In contrast, the presence of Bacteroides, Parabacteroides, Clostridium, Williamsia, Deinococcus, Chryseobacterium and Herbaspirillum was detected in healthy controls and was rare in conjunctival MALT lymphoma patients (Fig. 4B). The prevalences of seven microorganisms in conjunctival MALT lymphoma were statistically lower than those of healthy controls.
Figure 4.
Microbial differences at the conjunctiva between conjunctival MALT lymphoma patients and healthy controls. Box plots showing the bacterial abundance at the conjunctiva between healthy controls (red) and conjunctival MALT lymphoma patients (green). (A) Delftia was the most prevalent microorganism in MALT lymphoma patients, however; others were detected as significantly prevalent but very rare. (B) The prevalence of seven microorganisms were revealed statistically lower in conjunctival MALT lymphoma patients. They were detected mainly in healthy controls and rare in conjunctival MALT cases.
For Delftia, Bacteroides and Clostridium, in conjunctival MALT lymphoma patients, multivariate analysis was performed on factors such as age, gender, chemotherapy, radiotherapy, laterality, other (orbita and lacrimal gland) involvements (Table 2). There were statistically significant differences for Delftia according to other involvements (orbita and lacrimal gland) (P = 0.022) and for Bacteroides according to chemotherapy (P = 0.018). There were no correlations observed in the three microorganisms at the conjunctiva of MALT lymphoma patients and healthy controls.
Table 2.
Multivariate analysis of three microorganism.
| Clinical Parameters | Microorganism | P value |
|---|---|---|
| Age (under 60’s vs over 60’s) | Delftia | 0.6052 |
| Bacteroides | 0.8175 | |
| Clostridium | 0.6022 | |
| Gender (male vs female) | Delftia | 0.9565 |
| Bacteroides | 0.0842 | |
| Clostridium | 0.9744 | |
| Laterality (bilateral vs unilateral) | Delftia | 0.1138 |
| Bacteroides | 0.3618 | |
| Clostridium | 0.3944 | |
| Involvement (orbita or lacrimal gland vs no involvement) | Delftia | 0.0220 |
| Bacteroides | 0.7983 | |
| Clostridium | 0.8189 | |
| Chemotherapy (performed vs not performed) | Delftia | 0.1378 |
| Bacteroides | 0.0182 | |
| Clostridium | 0.2902 | |
| Radiotherapy (performed vs not performed) | Delftia | 0.7386 |
| Bacteroides | 0.4528 | |
| Clostridium | 0.8101 | |
| Gastric lesion (positive vs negative) | Delftia | 0.4624 |
| Bacteroides | 0.4020 | |
| Clostridium | 0.8412 |
Physiological changes in the tears of MALT lymphoma patient
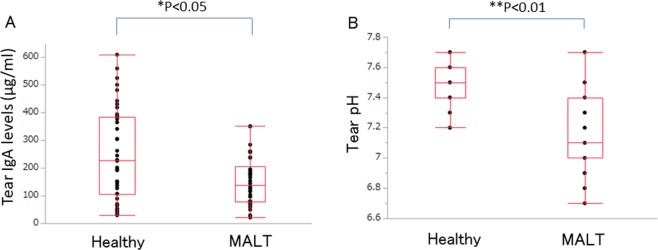
Previous reports describe that Bacteroides and Clostridium maintain ocular surface homeostasis through the production of IgA, which raises a possibility that a decrease of these microorganism could result in lower IgA concentration22,23. To this end, we measured the tear IgA levels of MALT lymphoma patients and the healthy controls. The IgA concentration in tears of conjunctival MALT lymphoma patients (153.4 ± 166.9 μg/mL) showed statistically significant difference compared to that of the control group (252.9 ± 87.1 μg/mL) (Wilcoxon rank sum test, P < 0.05) (Fig. 5A). On the other hand, the serum IgA concentration from conjunctival MALT lymphoma patients (N = 10) was 206.5 ± 86.6 mg/dL and was within normal limits24. A previous report showing that Delftia has the ability to utilize glucose oxidatively via catalase and oxidase activity and may change the conjunctival environment25, this raises the possibility that on increase of Delftia resulted in oxidation of tear pH (normal range, 6.5–7.6)26. The tear pH levels of conjunctival MALT lymphoma patients (7.15 ± 0.23) were statistically lower than those of age-matched healthy controls (7.46 ± 0.14) (Wilcoxon rank sum test, P < 0.01) (Fig. 5B).
Figure 5.
Levels of tear IgA and tear pH in the conjunctiva of healthy controls and conjunctival MALT lymphoma patients. (A) There were statistically significant differences of tear IgA level between healthy controls and conjunctival MALT lymphoma patients (Wilcoxon rank sum test, P < 0.05). (B) There were statistically significant differences of tear pH between healthy controls and conjunctival MALT lymphoma patients (Wilcoxon rank sum test, P < 0.01).
Discussion
Several studies have shown that the commensal microbiota exists in different body locations9–13. In the current study, we showed that the commensal conjunctival microbiota existed against the invasion of foreign microbes, and the conjunctival microbiota of MALT lymphoma patients was compositionally different from that of healthy controls, as highlighted by PCA. A detailed analysis showed that in conjunctival MALT lymphoma patients, the genus Delftia was significantly more abundant and the genera Bacteroides and Clostridium were less abundant than in healthy controls. Delftia might have a pathophysiological role in the development of conjunctival MALT lymphoma, and Bacteroides and Clostridium may have defensive properties in conjunctival MALT lymphoma.
Previous reports showed that a lower abundance of ocular surface microbiota has a great influence on the severity of Sjögren syndrome and dry eye, and the continuous administration of eye drops for glaucoma treatment might affect the microbiota27–29. At the meibomian gland, bacteria-related cytotoxicity or inflammation may interfere with the pathological process of meibomian gland dysfunction30. Therefore, this altered microbiota might be associated with the development of ocular surface diseases. Previous reports also showed, using PCA, that the skin microbiota is distinct31. In each location, the number of conjunctival bacteria was lower than that on the hand and equal to that of the meibomian gland. The conjunctival microbiota differed in composition between conjunctival MALT lymphoma patients and healthy controls. High-diversity skin sites were the palm, finger and foot. On the other hand, the conjunctival microbiota had significantly lower phylogenetic diversity than that of the hand31. Some factors, such as humidity, the existence of sebaceous sites, and the use of systemic treatments, have been reported to interfere with conditions31. For conjunctival MALT lymphoma, several treatments could be performed to improve the disease prognosis. In this study, no significant difference in the microbial composition among the previous therapies was observed because the dysbiosis of the conjunctival microbiota might already exist during the development of MALT lymphoma, even if some interventions were performed. Environmental characteristics may play an important role in forming the conjunctival bacterial microbiota.
In the human body, mucosal organs, including the conjunctiva, are equipped with characteristic immune defense mechanisms, and the same anatomic mechanism, CALT (which contains MALT), exists at the ocular surface32,33. In the stomach, a similar phenomenon could occur in the presence of H. pylori, which changes the microenvironment of the stomach by changing the urease activity34. In our study, the frequency of Delftia was significantly higher in the conjunctiva than in the three other body locations for conjunctival MALT lymphoma patients and healthy controls. Previous reports showed that Delftia was an aerobic, gram-negative motile bacillus with polar or bipolar flagella25. Delftia does not produce urease and is catalase and oxidase positive25. Characteristically, Delftia has the ability to degrade and utilize glucose oxidatively, which may change the conjunctival environment25. Delftia was isolated from the soil and is known only as a rare pathogen that can affect immunocompromised patients35. Clinically, Delftia has resistance to antibiotics, such as β-lactams and aminoglycosides, and can adhere to contact lens cases and form biofilms, resulting in the development of both microbial and infiltrative keratitis at the ocular surface36,37. The glucose levels in the tears are known to be correlated with the levels in the blood, and several corneal abnormalities, such as healing of the corneal epithelium, are caused by changes in glucose levels38,39. Delftia, known as rare pathogens, may predispose individuals to the development of conjunctival MALT lymphoma by changing conjunctival conditions to interfere with CALT25,35.
In our study, in the conjunctiva of MALT lymphoma, there were two decreased microorganisms, Bacteroides and Clostridium, which are known as commensals and an anti-allergic microbiota22. Previous reports have shown that normal gut bacteria, such as Bacteroides, Bifidobacterium and Lactobacillus, play a protective role in preventing the proliferation of pathogens and form gut-associated lymphoid tissue (GALT) to maintain homeostasis40. Similarly, if disruptions of the ocular surface barrier, such as disruptions of CALT and MALT, occur, these changes could trigger ocular inflammation41. The development of immune-mediated diseases such as allergies has been hypothesized to arise as a result of deficiencies in exposure to microbial organisms and their products42. It is known that Bacteroides produces a bacterial polysaccharide, and it directs the cellular and physical maturation of the developing immune system43. In the small intestine, Bacteroides interacts with dendritic cells at intestinal Peyer’s patches and induces the production and maturation of immunoglobulin A as a protection mechanism in the GALT23. Therefore, the existence of Bacteroides from birth is essential to maintain the homeostasis of gut immunity, and likewise, bacteria are helpful to develop focal defensive mechanisms at the ocular surface44. Several reports suggest that mucosa-associated Clostridium populations play an important role in the induction of Tregs and IgA and the suppression of inflammatory and allergic responses14,23,43–45. The lowering of tear IgA concentration and pH in conjunctival MALT lymphoma patients suggest that the change in the microbiota profiles impacts the physiological response of the eye. We have not clarified whether the physiological change indeed resulted from specific bacterium or their compositions in this study because of insufficient data size, but there may be a tendency due to treatment especially in the case of Delftia. Hence, Delftia is thought to play a pathophysiological role in the development of conjunctival MALT lymphoma, and Bacteroides and Clostridium may play protective roles. The current study raises an intriguing hypothesis that the fluctuation of bacterial compositions causes disturbance of innate immunity in ocular MALT, while further validation is needed to determine whether Delftia or other bacteria identified here are responsible for MALT lymphoma.
Methods
Study subjects
Patients
We studied 25 consecutive patients (50 eyes) (7 men, 18 women; mean age, 61.7 ± 15.6 years) who were diagnosed by biopsy with conjunctival MALT lymphoma and were followed at our hospital between 2015 and 2017. The subtype was extranodal marginal zone B-cell lymphoma of MALT-type lymphoma in all cases. At the same time, 25 age-matched healthy volunteers (50 eyes) (7 men, 18 women; mean age, 58.3 ± 13.0 years) participated in this study as healthy controls. Exclusion criteria included obvious ocular surface disease, history of recent contact lens usage, use of systemic/topical antibiotics or prescription eye medications in the past 12 months, ocular surgery in the last 12 months, active ocular infection, dry eye, systemic diseases such as diabetes, or smoking. The current study adhered to the tenets of the Declaration of Helsinki, the local ethics committee of the Osaka University Medical Hospital approved the study, and written informed consent was obtained from all subjects.
Microbacterial sample collection and DNA isolation
Sample collections were performed in a clean ophthalmic treatment room. After instillation of sterile, topical proparacaine, DNA swabs (Osaki Sterilized Cotton Swabs S0475-10, JAPAN) were used to collect samples from the superior and inferior fornixes of the conjunctiva in both eyes. To compare the conjunctiva with other parts of the skin, swabs were taken from the hand, meibomian glands and skin around the eyes. Samples were carefully transferred into DNA LoBind tubes (Eppendorf, Fremont, CA), and all samples were promptly frozen at −80 °C until the time of DNA extraction. DNA was extracted from each sample using a PowerSoil DNA Isolation Kit (MoBio, Carlsbad, CA) according to the manufacturer’s instructions. The extracted genomic DNA was eluted in 100 µl of the kit elution buffer and stored at −20 °C until analysis.
Tear sample collections
To investigate the tear immunoglobulin A (IgA) levels, tear samples were collected from 34 eyes of MALT lymphoma patients and 40 eyes of healthy controls without anesthesia. Schirmer test strips (Ayumi Pharmaceutical Co., Tokyo, Japan) were placed at the outer one-third of the temporal lower conjunctival fornix for 5 minutes to collect tears. The strips were stored at −80 °C in glass vials until further analysis. IgA levels were assayed by enzyme-linked immunosorbent assay (E80-102, Bethyl Laboratories, Montgomery, AL, USA). Additionally, the serum from 10 out of 25 patients were collected to measure the IgA levels.
To investigate the tear pH, tears were collected from 30 eyes of MALT lymphoma patients and 26 eyes of healthy controls. Tear fluid were collected from the inferior meniscus of unanesthetized eyes after instillation of 30 μl sterile water by micropipette onto the ocular surface, followed by movement of the eyes to mix the tear fluid content. All the collected tear volumes were over 10 μl and were carefully transferred into DNA LoBind tubes (Eppendorf, Fremont, CA). For the measurement, all samples were measured immediately after sample collection by an ion-selective electrode handheld meter (LAQUA twin B-731; Horiba).
16S rRNA sequencing and data processing
Each library was prepared according to the “Illumina 16S Metagenomic Sequencing Library Preparation Guide” with a primer set (27Fmod: 5′-AGR GTT TGA TCM TGG CTC AG-3′ and 338R: 5ʹ-TGC TGC CTC CCG TAG GAG T-3ʹ) targeting the V1–V2 region of the 1S rRNA gene. Then, 251 bp paired end sequencing of the amplicon was performed on a MiSeq (Illumina) using a MiSeq v2 500 cycle kit. Paired end sequences were merged using PEAR (http://sco.h-its.org/exelixis/web/software/pear/). Merged reads were quality-trimmed with BBtrim (bbmap.sourceforge.net). Twenty thousand reads per sample were randomly selected using random_sequence_sample.pl (ualberta.ca/~stothard/software.html) for further analysis. The processed sequences were clustered into OTUs defined by a 97% similarity cutoff using UCLUST version 1.2.22q. Representative sequences for each OTU were then classified taxonomically by using RDP Classifier version 2.2 and the Greengenes 13_8 database. The bioinformatics pipeline QIIME, version 1.9.1, was used as the informatics environment for all relevant processing of raw sequencing data.
Statistical analysis
Data are shown as the mean ± SE. Statistical analyses were carried out using JMP software version 9.0 (SAS Inc, Cary, North Carolina, USA) and the R software environment (in the public domain, http://cran.r-project.org/), version 3.1.3. To discover different features of the microbiota in healthy and MALT subjects, the classified data were analyzed by linear discriminant analysis effect size (LefSe). A P < 0.05 was considered statistically significant.
Supplementary information
Stability of bacterial compositions in four locations of healthy controls.
Acknowledgements
We are grateful to Masakazu Hirota (Osaka University) for his technical assistance. This work was supported by Integrated Frontier Research for Medical Science Division, Institute for Open and Transdisciplinary Research Initiatives, Osaka University.
Author Contributions
K. Asao, N.H., S.A., K.M., S.K. and K. Nishida planned the experiments; K. Asao, N.H., S.A., D.M. and S.N. performed the research and acquired the data; K. Asao, N.H., S.A., H.K. and T.Y. analyzed the data and wrote the respective methods and results; K.M., S.K., T.I. and K. Nishida supervised the project; and K. Asao, N.H., S.A., D.Y. and K. Nishida wrote and edited the paper. All authors contributed to critical revision of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44861-5.
References
- 1.Wotherspoon AC, et al. Primary low-grade B-cell lymphoma of the conjunctiva: a mucosa-associated lymphoid tissue type lymphoma. Histopathology. 1993;23:417–424. doi: 10.1111/j.1365-2559.1993.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 2.Lachapelle KR, Rathee R, Kratky V, Dexter DF. Treatment of conjunctival mucosa-associated lymphoid tissue lymphoma with intralesional injection of interferon alfa-2b. Arch. Ophthalmol. 2000;118:284–285. [PubMed] [Google Scholar]
- 3.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::AID-CNCR2820290138>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Verdijk RM. Lymphoproliferative tumors of the ocular adnexa. Asia Pac. J. Ophthalmol. 2017;6:132–142. doi: 10.22608/APO.2016209. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, et al. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes—the 2017 J. Donald M. Gass lecture. Asia Pac. J. Ophthalmol. 2017;6:109–120. doi: 10.22608/APO.201710. [DOI] [PubMed] [Google Scholar]
- 6.Zullo A, et al. Gastric MALT lymphoma: old and new insights. Ann. Gastroenterol. 2014;27:27–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Collina F, et al. Chlamydia psittaci in ocular adnexa MALT lymphoma: a possible role in lymphomagenesis and a different geographical distribution. Infect. Agent Cancer. 2012;7:8. doi: 10.1186/1750-9378-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CC, et al. Helicobacter pylori (H. pylori) molecular signature in conjunctival mucosa-associated lymphoid tissue (MALT) lymphoma. Histol. Histopathol. 2004;19:1219–1226. doi: 10.14670/hh-19.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohland CL, Jobin C. Microbial activities and intestinal homeostasis: a delicate balance between health and disease. Cell. Mol. Gastroenterol. Hepatol. 2015;1:28–40. doi: 10.1016/j.jcmgh.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J. Gastrointest. Pathophysiol. 2012;3:27–43. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gevers D, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostic AD, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smeekens SP, et al. Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J. Innate Immun. 2014;6:253–262. doi: 10.1159/000351912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid Y, Hand T. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 17.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of Lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 2017;7:318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Kim EK, Kim HY, Kim TI. Effects of exposure to ozone on the ocular surface in an experimental model of allergic conjunctivitis. PLoS One. 2017;12:e0169209. doi: 10.1371/journal.pone.0169209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babizhayev MA. Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin. 2016;6:49–68. doi: 10.1016/j.bbacli.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandhyala SM, et al. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulange CL. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood S, Taylor KE, Overman TL, McCormick MI. Acute infective endocarditis caused by Delftia acidovorans, a rare pathogen complicating intravenous drug use. J. Clin. Microbiol. 2012;50:3799–3800. doi: 10.1128/JCM.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abelson MB, Udell IJ, Weston JH. Normal human tear pH by direct measurement. Arch Ophthalmol. 1981;99:301. doi: 10.1001/archopht.1981.03930010303017. [DOI] [PubMed] [Google Scholar]
- 27.de Paiva CS, et al. Altered mucosal microbiome diversity and disease severity in sjogren syndrome. Sci. Rep. 2016;6:23561. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Dry eye management: targeting the ocular surface microenvironment. Int. J. Mol. Sci. 2017;18:E1398. doi: 10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtani S, et al. Conjunctival bacteria flora of glaucoma patients during long-term administration of prostaglandin analog drops. Invest. Ophthalmol. Vis. Sci. 2017;58:3991–3996. doi: 10.1167/iovs.16-20853. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SD, et al. Bacteriological profile of ocular surface flora in meibomian gland dysfunction. Ocul. Surf. 2017;15:242–247. doi: 10.1016/j.jtos.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knop E, Knop N. Eye-associated lymphoid tissue (EALT) is continuously spread throughout the ocular surface from the lacrimal gland to the lacrimal drainage system. Ophthalmologe. 2003;100:929–942. doi: 10.1007/s00347-003-0936-6. [DOI] [PubMed] [Google Scholar]
- 34.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun J, et al. Delftia acidovorans isolated from the drainage in an immunocompetent patient with empyema. Tuberc. Respir. Dis. 2009;67:239–243. doi: 10.4046/trd.2009.67.3.239. [DOI] [Google Scholar]
- 36.Ravaoarinoro M, Therrien C. Beta-lactamases and outer membrane investigations in beta-lactam-resistant Comamonas acidovorans strains. Int. J. Antimicrob. Agents. 1999;12:27–31. doi: 10.1016/S0924-8579(98)00095-8. [DOI] [PubMed] [Google Scholar]
- 37.Kam S-K, Lee W-S, Ou T-Y, Teng S-O, Chen F-L. Delftia acidovorans bacteremia associated with ascending urinary tract infections proved by molecular method. J. Exp. Clin. Med. 2012;4:180–182. doi: 10.1016/j.jecm.2012.04.010. [DOI] [Google Scholar]
- 38.Zagon IS, Sassani JW, Immonen JA, McLaughlin PJ. Ocular surface abnormalities related to type 2 diabetes are reversed by the opioid antagonist naltrexone. Clin. Exp. Ophthalmol. 2014;42:159–168. doi: 10.1111/ceo.12144. [DOI] [PubMed] [Google Scholar]
- 39.Badugu R, Lakowicz JR, Geddes CD. Noninvasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal. Chem. 2004;76:610–618. doi: 10.1021/ac0303721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howarth GS, Wang H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients. 2013;5:58–81. doi: 10.3390/nu5010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantelli F, Mauris J, Argueso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol. 2013;13:563–568. doi: 10.1097/ACI.0b013e3283645899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laforest-Lapointe I, Arrieta MC. Patterns of early-life gut microbial colonization during human immune development: an ecological perspective. Front. Immunol. 2017;8:788. doi: 10.3389/fimmu.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stability of bacterial compositions in four locations of healthy controls.