Abstract
This study was designed to assess risk factors for neurocognitive impairment in patients with benign intracranial lesions including tumors and vascular lesions. 74 patients (29 m, 51 f, mean age 54.4 years) with surgery for benign intracranial lesions were included in this prospective single-center study. Extensive neuropsychological testing was performed preoperatively, including tests for attention, memory and executive functions. Furthermore, headache and depression were assessed using the german version of the HDI (IBK) and the BDI-II. Multiple linear regression analyses of the percentile ranks (adjusted for age, sex and education) including the parameters age, Karnofsky Performance Status Scale (KPS), mood, pain and lesion size were performed to identify risk factors for cognitive impairment. Using the Mann-Whitney U test, the influence of hemisphere and type of lesion (tumor/vascular) was assessed. Posthoc Bonferroni correction was performed. Poorer neurocognitive functions were observed only in the category attention in patients with higher age (divided attention, WMS) and reduced KPS (WMS). Lesion volume, mood, pain, hemisphere or the type of the lesion (tumor, vascular) were not identified as risk factors for poorer neurocognitive functions in patients with benign intracranial lesions. Age and KPS are the main risk factors for poorer neurocognitive functions in the category attention in patients with benign intracranial lesions. Knowledge of these risk factors might be important to find appropriate therapy regimes to improve cognitive functions and quality of life.
Subject terms: Neurology, Oncology
Introduction
For patients with intracranial lesions, the most important recorded parameters are age, neurological status and functional independence, as measured by the Karnofsky Performance Status Scale (KPS)1. Recent studies analyzed the role of neurocognitive impairment for glioma patients and showed that cognitive function is a predictor for survival2–5. The most common test to evaluate cognitive function is the mini-mental state examination (MMSE)6. Extensive neurocognitive testing is time-consuming and therefore not routinely used. Tumor location and size, age, KPS and tumor grade as risk factors for neurocognitive impairment of glioma patients were identified3,7. Most studies about neurocognitive functions in brain tumor patients focus on fast growing tumors such as gliomas and/or metastases3,7–12. Benign lesions grow slowly, and therefore, less cognitive impairment was shown due to the plasticity of the brain13–15. Few studies have reported neurocognitive functions in meningioma patients or in patients with incidental meningiomas16,17; most of them, however, have focused on pre- and postoperative comparisons14,18–23. Risk factors for neurocognitive impairment, such as tumor size and location, were identified20. A few studies also analyzed cognitive functions in patients with pituitary adenomas and unruptured intracranial aneurysms24–26. Patients with pituitary adenomas with suprasellar extension showed preoperative cognitive dysfunction that resolved two months after surgery25. For patients with unruptered aneurysms a slight cognitive dysfunction was observed after surgery26.
Very little is known about neurocognitive functions in patients with intracranial vascular lesions (except for intracranial aneurysms26,27) or rare intracranial tumors. Furthermore, many previous studies showed the significant impact of pain on patients’ cognitive functions28. However, only a few studies assessed the relationship between trigeminal neuralgia and cognitive impairment29,30.
There are—to our knowledge—no studies about neurocognitive functions of patients with benign intracranial lesions that include benign tumors and vascular lesions in one cohort.
The aim of this study was to assess preoperative neurocognitive functions in patients with benign intracranial lesions, to identify risk factors for cognitive impairment.
Methods
This prospective non-randomized single-center study was approved by the local ethics committee (Clinical Trial Registration Number: 3094/11) and conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments31. Written informed consent was obtained from all study participants.
Patient population
From September 2012 to December 2014, patients with surgery for a benign intracranial process were enrolled.
Inclusion criteria comprised informed consent, age ≥ 18 years, surgery for a benign intracranial process, preoperative magnetic resonance imaging (MRI) and sufficient knowledge of the German language. Exclusion criteria were: age < 18 years, pregnancy, missing or retrieved informed consent, missing surgery or malign intracranial processes. As the extended test battery comprises many partially complex tests, only patients with a preoperative mini-mental status examination (MMSE) ≥ 18 were included in the study.
Study design
Preoperative tests were performed after informed consent and detailed information of the patient. Patients who met the inclusion criteria performed the basic test battery and the extended test battery as described below before surgery.
Age, preoperative KPS (ordinal scale 0–100 [%]) and tumor location (lobe, hemisphere) were recorded by qualified neurosurgeons for all patients.
Basic test battery
This test battery includes the well-known MMSE6, which measures the patients’ basic cognitive functions.
Extended test battery
Raw scores were adjusted for age, sex and education to a normative population (percentile ranks). Analyses were performed for the percentile ranks. For a few subtests, no percentile ranks were available: visual field subtests and Stroop’s failure. These data are not analyzed.
Attention
This study uses the test battery of attentional performance (TAP), a computer-based test battery described by Zimmermann et al.32. This battery was used to assess a broad range of cognitive deficits in the category attention and was selected by a neuropsychologist. No standardized test protocol is known for the measurement of impairment of attention in brain tumor patients, the TAP test battery was used in a previous study about gliomas3.
Alertness
The subtest Alertness examines the reaction time. Visual stimuli are provided either with (TAP alertness W_sound) or without an acoustic notification (TAP alertness W_O_sound). Data are shown in milliseconds (ms) for delay of the patients’ reaction for tests with/without acoustic notification.
Divided attention
This subtest analyzes the simultaneous reaction to visual and acoustic stimuli (divided attention visual/auditory). Patients are challenged to react to both, visual stimuli (moving crosses, visual task) and acoustic stimuli (two consecutive sounds, auditory task). Data are shown as delay (ms) and mistakes/omissions.
Visual field
This subtest examines the patients’ visual field. The patients are requested to fix on one central point. Visual stimuli are provided; data are recorded for the right/left and central field of view and are shown as delay (ms) and omissions. As only raw data, and no percentile ranks were available for these subtests, no further analyses were performed.
Trail-Making-Test A (TMT-A)
This is a well-known test for visual attention. Patients are asked to connect numbers (1–25) in the right order with a pencil. The test measures the time needed to correctly join all numbers33. Trail-Making-Tests assess the relations between speed and fluid intelligence, the TMT-A uses simple tasks34.
Wechsler Memory Scale (WMS)
For this study, two subtests of the Wechsler Memory Scale revised was used35. The test consists of multiple subtests and analyzes verbal and non-verbal short-term memory. Patients are asked to perform tasks directly in the two subtests - the block-span and the digit-span subtest - as described previously3. Memory span (ms) and work memory (wm) are analyzed for verbal (v) and non-verbal (nv) short-term memory.
Both tests analyze patients’ immediate memory. As only subtests of short-term memory are used in this study, the results are presented in the attention category.
-
(b)
Memory
Verbal Learning and Memory Test (VLMT)
VLMT - the German version of the well-known Rey Auditory-Verbal Learning Test - analyzes patients’ episodic memory function and consists of a learning and interference list with fifteen words each36. The learning list is read out five times (Dg1–5), followed by the interference list. Patients are asked to recite the words from the learning list directly (Dg6) and after 30 minutes (Dg7).
Rey Osterrieth complex figure test (ROCF)
Visual memory function is analyzed by this test37. A geometrical figure is shown to the patients, and then they are asked to draw the figure immediately (ROCF copy) and after 30 minutes (ROCF delay).
-
(c)
Executive functions
Trail-Making-Test B (TMT-B)
In this subtest, patients are asked to connect letters and numbers with a pencil in the appropriate order (1-A-2-B-3-C)33. In contrast to the TMT-A, the TMT-B assesses patients’ ability to switch between tasks and therefore measures also fluid abilities34.
Regensburg Word Fluency Test (RWT)
This test examines lexical and semantic fluency38. Patients are asked to say words with a specific letter (e.g., apple, auto, …) for one minute (lexical fluency). Semantic fluency is analyzed by naming words within a specific category (e.g., food). Furthermore, the ability to change between specific letters (turning lexical) and between specific categories (turning semantic) is tested.
Stroop Word Color Test
This test is also known as “color-word-interference-test” and examines selective attention and patients’ ability to inhibit cognitive interference. First, patients read the names of different colors (blue, green, yellow and red) written in black (word reading). Secondly, the patients are asked to name colored lines (line naming). Thirdly, there are differences between the written color and the color of the word (e.g., the word red is written in blue; interference).
Assessment of mood and pain
As mood and pain are known to influence neurocognition28,39, further measurements and tests were performed. To assess patients’ mood, the well-known Beck-Depression-Inventary II (BDI-II) was used in 61/80 patients40. The BDI-II score ranges from 0–63 (higher scores stand for higher extent of depression), the median of the normative population is 7.4 (population of n = 582 depressive patients and n = 260 healthy controls, manual of Hautziger et al.41).
Headache was assessed using the IBK, the german version of the Headache Disability Inventory (HDI) in 49/80 patients42. Headache is divided into four scales: no headache, slight headache, moderate headache, severe headache.
Volumetric measurement
Pre- and postoperative volumetric measurement of the intracranial lesion was performed by a neuroradiologist by semi-automatic manual segmentation (IPlannet Cranial 3.0, Fa. Brainlab, Munich, Germany). T1-weighted images after contrast agent were used for contrast-enhancing processes.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 23.0 and 25.0 (SPSS Inc., IBM Corp., Armonk, NY, USA). Data are either shown as mean/standard deviation (normally-distributed data) or as median/interquartile range (IR; non-normally-distributed data). A multiple linear regression model was used to analyze the influence of different metric parameters (age, preoperative KPS, preoperative contrast enhancing tumor volume, mood, pain) on neurocognitive functions (attention, memory and executive functions). The correlation matrix shows linear/non-linear relationships between dependent and independent variables. Only linear relationships were included for regression analyses. Correlations between independent variables were assessed by Spearman correlations. Independent variables did not show strong correlations (r < 0.8)43, therefore all three variables were assessed in the regression analysis (Table 1). Scatter plots for residuals of multiple linear regression analyses are recorded. Bonferroni correction was performed for the number of multiple linear regression analyses (n = 29) To assess the influence of nominal parameters (hemisphere, tumor vs. vascular) on neurocognitive functions, Mann-Whitney U tests with posthoc Bonferroni correction were performed. Analyses were performed for percentile ranks (values adjusted for age, education and sex). A P-value of <0.05 was defined as significant.
Table 1.
Correlation matrix between independent variables.
| Age | KPS | Tumor volume | Mood | Pain | |
|---|---|---|---|---|---|
| Age | r = 1.0 | P = 0.061, r = −0.219 | P = 0.248, r = 0.144 | P = 0.043, r = −0.271 | P = 0.075, r = −0.271 |
| KPS | P = 0.061, r = −0.219 | r = 1.0 | P = 0.021, r = −0.284 | P = 0.382, r = −0.119 | P = 0.614, r = 0.078 |
| Tumor volume | P = 0.248, r = 0.144 | P = 0.021, r = −0.284 | r = 1.0 | P = 0.560, r = 0.085 | P = 0.910, r = −0.019 |
| Mood | P = 0.043, r = −0.271 | P = 0.382, r = −0.119 | P = 0.560, r = 0.085 | r = 1.0 | P = 0.040, r = 0.314 |
| Pain | P = 0.075, r = −0.271 | P = 0.614, r = 0.078 | P = 0.910, r = −0.019 | P = 0.040, r = 0.314 | r = 1.0 |
Ethical approval and informed consent
The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments and approved by the local ethics committee (Ethics committee technical university munich). Informed consent was signed by all study participants.
Results
Patient population
81 patients met initially the inclusion criteria and were included in the study. One patient did receive neuroradiological intervention for an intracranial aneurysm and was excluded from the study. 6 patients presented with trigeminal nerve neuralgia and were excluded due to a missing intracranial lesion. Therefore, the study population comprises 74 patients with the following benign lesions: meningioma (n = 31), pituitary adenoma (n = 14), vestibular schwannoma (n = 8), cavernoma (n = 6), intracranial aneurysm (n = 4), pineocytoma (n = 2), arterial–venous malformation (AVM; n = 2), hemangiopericytoma (n = 1), clivus chordoma (n = 1), colloidal cyst (n = 1), subependymoma (n = 1) and others (n = 3). For further analysis, lesions are assessed in two groups: tumor (including meningioma, pituitary adenoma, vestibular schwannoma, pineocytoma, hemangiopericytoma, clivus chordoma, colloidal cyst and subependymoma) and vascular syndrome (including cavernoma, AVM and aneurysm). Median preoperative KPS was 100% (range 40–100). The median preoperative (contrast enhancing) volume of the intracranial lesions was 2.5 cm³ (IR 0.8–10.4 cm³) (Table 2).
Table 2.
Patient population.
| Age (mean [range]) Sex, male | 54.6 years [range 24.0–77.0years] 28/74 |
|---|---|
| Tumor histology | |
| - meningioma | 31/74 |
| - pituitary adenoma | 14/74 |
| - vestibular schwannoma | 8/74 |
| - cavernoma | 6/74 |
| - intracranial aneurysm | 4/74 |
| - pineocytoma | 2/74 |
| - arterio-venous malformation | 2/74 |
| - hemangiopericytoma | 1/74 |
| - clivus chordoma | 1/74 |
| - colloidal cyst | 1/74 |
| - subependymoma | 1/74 |
| - others | 3/74 |
| Main tumor location | |
| - frontal lobe | 28/74 |
| - temporal lobe | 9/74 |
| - parietal lobe | 3/74 |
| - mid line | 17/74 |
| - infratentorial | 16/74 |
| - ventricle | 1/74 |
| Hemisphere | |
| - right | 28/74 |
| - left | 24/74 |
| - median | 22/74 |
| Involvement of the skull base | |
| - Anterior | 20/74 |
| - Middle | 46/74 |
| - Posterior | 20/74 |
| Involvement of the insula | 2/74 |
| Intracerebral | 51/74 |
| Education | |
| - elementary school | 32/69 |
| - intermediate education | 20/69 |
| - high school | 17/69 |
| Recurrent disease | 14/74 |
Basic test battery
MMSE
Median preoperative MMSE was 29.0 (IR 27.8–30.0). Patients’ age showed a significant negative correlation to MMSE (r = −0.303, P = 0.009). Volume of the intracranial lesion did not correlate to preoperative MMSE scores (r = 0.131, P = 0.296).
Furthermore, the type of intracranial lesion and location (lobe/hemisphere) were not significantly associated with MMSE.
Extended test battery
Attention
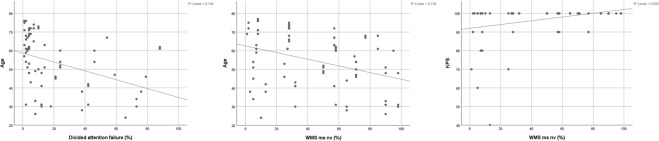
In the analysis of the percentile ranks, higher age predicted poorer functions only in 2/12 subtests (divided attention failure, WMS ms nv), higher KPS predicted better functions in 1/12 subtests (WMS ms nv). Lesion volume, pain and mood did not show significant results in this analysis. (Figs 1 and 2, Table 3).
Figure 1.
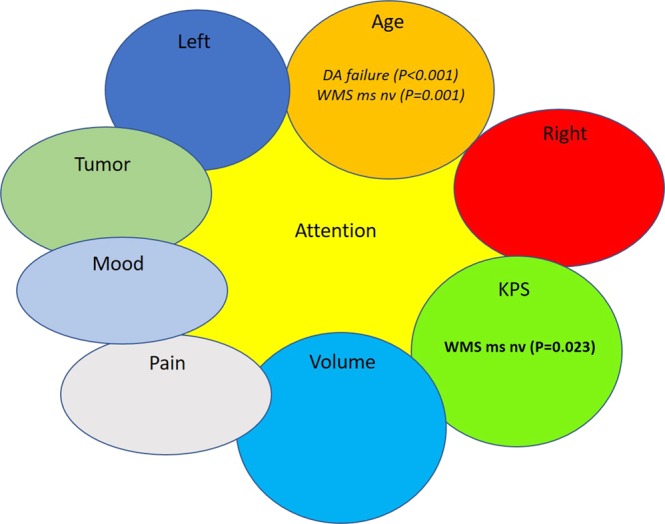
Risk factors for poorer cognitive functions in the attention category. Poorer cognitive functions are shown in Italics, and better neurocognitive functions is shown in Bold. DA: divided attention. WMS ms nv: Wechsler Memory Scale memory span non-verbal.
Figure 2.
Scatter plots for risk factors age and KPS and neurocognitive functions in the subtests DA failure (divided attention failure) and WMS ms nv (Wechsler Memory Scale memory span non-verbal). KPS: Karnofsky Performance Status Scale.
Table 3.
Attention.
| Standardized R² | F | Model significance | Adjusted P-Value | Condition index | Durbin Watson | Independent variables | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | KPS | Tumor volume | Mood | Pain | |||||||||
| Attention | TAP | Alertness W_O_sound | 0.037 | 1.815 | 0.154 | 1.0 | 24.370 | 2.362 | n.s. | n.s. | n.s. | — | — |
| Alertness W_sound | 0.015 | 1.322 | 0.276 | 1.0 | 24.370 | 2.298 | n.s. | n.s. | n.s. | — | — | ||
| Alertness phasic | 0.064 | 3.150 | 0.050 | 20.208 | 2.205 | — | n.s. | n.s. | — | — | |||
| Divided attention visual | 0.032 | 2.028 | 0.140 | 1.0 | 20.205 | 2.088 | — | n.s. | n.s. | — | — | ||
| Divided attention auditive | 0.006 | 1.056 | 0.394 | 1.0 | 21.844 | 2.147 | — | n.s. | n.s. | n.s. | n.s. | ||
| Divided attention failure* | 0.209 | 6.468 | 0.001 | 0.029 | 24.241 | 1.650 | <0.001 | n.s. | n.s. | — | — | ||
| Divided attention selected | 0.107 | 2.110 | 0.102 | 1.0 | 10.039 | 1.991 | n.s. | — | n.s. | n.s. | n.s. | ||
| TMT-A | TMT-A | 0.069 | 3.351 | 0.042 | 22.884 | 1.810 | — | n.s. | n.s. | — | — | ||
| WMS | WMS ms v | 0.032 | 3.435 | 0.068 | 19.237 | 2.012 | — | n.s. | — | — | — | ||
| WMS wm v | 0.022 | 1.722 | 0.187 | 1.0 | 7.874 | 1.923 | n.s. | — | n.s. | — | — | ||
| WMS ms nv* | 0.226 | 7.320 | <0.001 | 0.029 | 24.674 | 2.365 | 0.001 | 0.023 | n.s. | — | — | ||
| WMS wm nv | 0.163 | 2.897 | 0.036 | 1.0 | 22.238 | 2.233 | n.s. | n.s. | n.s. | — | n.s. | ||
| Memory | VLMT | VLMT Dg1 | 0.087 | 1.906 | 0.132 | 1.0 | 9.970 | 2.292 | n.s. | — | n.s. | n.s. | n.s. |
| VLMT Dg5 | 0.187 | 3.238 | 0.023 | 0.667 | 22.238 | 2.154 | n.s. | n.s. | n.s. | — | n.s. | ||
| VLMT Dg1-5 | 0.218 | 3.722 | 0.013 | 0.377 | 22.238 | 2.015 | n.s. | n.s. | n.s. | — | n.s. | ||
| VLMT Dg6 | 0.053 | 1.546 | 0.210 | 1.0 | 22.238 | 2.033 | n.s. | n.s. | n.s. | — | n.s. | ||
| VLMT Dg7 | 0.108 | 2.185 | 0.091 | 1.0 | 22.238 | 2.084 | n.s. | n.s. | n.s. | — | n.s. | ||
| VLMT Dg5-6 | −0.005 | 0.942 | 0.431 | 1.0 | 3.619 | 2.413 | — | — | n.s. | n.s. | n.s. | ||
| VLMT Dg5-7 | −0.015 | 0.806 | 0.499 | 1.0 | 8.809 | 2.120 | n.s. | — | n.s. | — | n.s. | ||
| ROCF | ROCF copy | 0.123 | 3.955 | 0.027 | 0.783 | 8.376 | 1.949 | n.s. | — | — | — | n.s. | |
| ROCF delay | 0.075 | 2.710 | 0.079 | 1.0 | 8.376 | 2.461 | n.s. | — | — | — | n.s. | ||
| Executive functions | Stroop’s | Stroop’s word reading | −0.009 | 0.859 | 0.469 | 1.0 | 9.202 | 1.934 | n.s. | — | n.s. | n.s. | — |
| Stroop’s naming | 0.030 | 1.283 | 0.297 | 1.0 | 26.025 | 1.821 | — | n.s. | n.s. | n.s. | n.s. | ||
| Stroop’s interference | 0.106 | 2.825 | 0.050 | 1.0 | 26.025 | 1.682 | — | n.s. | n.s. | n.s. | — | ||
| RWT | RWT lexical | −0.008 | 0.921 | 0.463 | 1.0 | 22.280 | 1.947 | — | n.s. | n.s. | n.s. | n.s. | |
| RWT semantic | 0.248 | 3.502 | 0.012 | 0.348 | 24.942 | 2.151 | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| RWT turning lexical | 0.139 | 2.577 | 0.054 | 1.0 | 22.238 | 1.317 | n.s. | n.s. | n.s. | — | n.s. | ||
| RWT turning semantic | 0.230 | 4.884 | 0.006 | 0.174 | 19.155 | 1.843 | — | n.s. | n.s. | — | n.s. | ||
| TMT-B | TMT-B | 0.096 | 1.761 | 0.150 | 1.0 | 28.975 | 1.524 | n.s. | n.s. | n.s. | n.s. | n.s. | |
Multiple linear regression analysis for the attention category. Only significant p-values (<0.05) are shown. Better neurocognitive functions are listed in Bold, poorer neurocognitive functions in Italic.
Memory
In the category memory none of the variables (age, KPS, tumor volume, mood, pain) predicted better or poorer neurocognitive functions (Table 3).
Executive functions
In the category executive functions, also none of the parameters predicted better or poorer neurocognitive functions (Table 3).
Tumor location/type of lesion
Prior to these analyses, important patient variables (age, mood, pain, tumor volume) are shown and compared in the different groups (divided in tumor location/hemisphere, and type of the lesion [tumor, vascular lesion]) (Table 4). Patients with tumors were significantly older than patients with vascular lesions (median 56.4 y. vs. 45.9 y, P = 0.031, U = 225, Z = −2.157). Patients with tumors in the left hemisphere showed significantly higher scores of the BDI-II (10.0 vs. 6.0, P = 0.049, U = 252, Z = −1.966). Tumor volume was significantly lower for tumors affecting the left hemisphere (1.6 cm³ vs. 6.2 cm³, P = 0.024, U = 348, Z = −2.264).
Table 4.
Comparison of variables age, mood, pain and tumor volume in the different groups.
| Groups | Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | P-value | Mood | P-value | Pain | P-value | Volume | P-value | |
| Right hemisphere affected | 52.8 y. | 0.093 | 8.5 | 0.704 | 3.0 | 0.475 | 2.7 cm³ | 0.738 |
| Right hemisphere not affected | 58.6 y. | 5.0 | 0.0 | 1.7 cm³ | ||||
| Left hemisphere affected | 54.1 y. | 0.604 | 10.0 | 0.049 | 4.0 | 0.084 | 1.6 cm³ | 0.024 |
| Left hemisphere not affected | 55.6 y. | 6.0 | 0.0 | 6.2 cm³ | ||||
| Tumor | 56.4 y. | 0.031 | 8.5 | 0.36 | 2.0 | 0.627 | 3.0 cm³ | 0.114 |
| Vascular lesion | 45.9 y. | 2.0 | 0.0 | 1.0 cm³ | ||||
Median scores are shown. For mood, the scores of the BDI-II are reported (points), for pain, the scores of the IBK (points). Data of volume are shown as the volume of the contrast enhancing preoperative tumor (cm³); y = years.
Tests for independent samples showed no significant differences of neurocognitive functions in all three categories in patients with tumors or vascular lesions, lesions in the left or right hemisphere.
Mood and pain
The median preoperative BDI-II of the patient cohort was 8.0 (IR: 1.3–15.0), therefore slightly higher than the normal population (median 7.4).
Higher scores of the BDI-II predicted poorer cognitive functions in none of the subtests (Table 3).
17/44 patients did not report preoperative headache, 15/44 patients presented with slight, 7/44 patients with moderate and 5/44 patients with severe headache.
Also pain did not predict poorer or better neurocognitive functions in all three categories (Table 3).
Discussion
This prospective single-center study analyzed neurocognitive functions in 74 patients with benign intracranial lesions including meningiomas, pituitary adenomas and vestibular schwannomas and vascular lesions (aneurysm, cavernoma). Higher age predicted poorer functions only in subtests of the category attention (divided attention, WMS), not in the categories memory and executive functions. Lower KPS predicted poorer functions only in one subtest (WMS) in the category attention (WMS). Higher lesion volume, pain, mood, hemisphere or the type of the lesion (tumor, vascular) did not predict poorer or better neurocognitive functions in any of the three categories.
Analyses were performed for percentile ranks (values adjusted for age, sex and education). Most studies about neurocognitive functions in patients with benign intracranial lesions are either performed with analyses of values adjusted for age, sex and education22,46–48 or with analyses of matched-paired or healthy controls17,21,49. This prospective study only includes patients and no healthy controls, therefore analyses of values adjusted for age, sex and education (percentile ranks) were performed.
The main risk factors for poorer neurocognitive functions identified in this study cohort were higher age and reduced KPS. These results are similar to those of previous studies that included brain tumor patients3,8. Underlining the results of a previous study, age and KPS are more important risk factors than tumor location and size; these results are also confirmed in the patient cohort with benign intracranial lesions3.
For divided attention, significant poorer functions for patients with higher age. Poorer functions in immediate memory (analyzed by subtests of the Wechsler memory scale [WMS]) were observed for patients with higher age and lower KPS. These findings are in common with previous studies as age is a known predictor for cognitive dysfunction44,45.
Pain, especially chronic pain, is a known risk factor for neurocognitive impairment in all three categories28. Previous studies reported impaired working memory and poorer scores in the Wechsler Memory Scale for patients with chronic and induced pain50–52. The exact pathomechanisms are still unknown; studies showed that patients with chronic pain show poorer memory function than patients with induced pain, suggesting also other factors than pain might be attributable for cognitive impairment51. In this patient cohort, pain was not shown as a significant predictor for poorer neurocognitive functions. This might be explained by the fact that patients with tumors or vascular lesions do not present with pain, but with other symptoms like seizures or neurological deficits.
Mood was not shown as significant predictor for cognitive dysfunction in this patient cohort. These results are contrary to previous findings that showed depression as an important predictor for neurocognitive impairment53–56. In the patient cohort of this study the values of the BDI-II were comparable to the values of the normal population. Most studies showed cognitive impairment mostly for patients with major depression, this might explain the conflicting results.
Episodic memory function (analyzed by the Verbal Learning and Memory Test [VLMT]) was poorer in patients with higher age and lower KPS. These results are in common with a previous study that showed poorer episodic memory function in a cognitively normal elderly cohort suggesting dysfunction in the posterior cingulate region as an important factor57.
Lesion volume was not shown as a significant predictor for poorer cognitive. Previous studies reported that verbal fluency was impaired in patients with larger tumor volume3,20. These discrepancies might be explained by the patient cohort of this study. We included only patients with benign, predominantly small lesions. Another explanation for these findings might be neuroplasticity.
Neuroplasticity is a known phenomenon for patients with infiltrating brain tumors, especially slowly growing tumors like diffuse gliomas58. In this cohort we assessed benign and therefore slowly growing lesions, therefore neuroplasticity might influence the results of this study. This might also explain the results that mainly age and KPS affect patients’ cognitive functions.
No significant differences between vascular and tumoral lesions were observed in this patient cohort. These findings might be explained by the fact that only benign tumors were included in this study.
Previous studies reported cognitive functions for patients with predominantly only one tumor/lesion entity, e.g. meningioma, pituitary adenoma, trigeminal neuralgia or aneurysm16,19,24–27,30. In this study, patients with different intracranial lesion were assessed in one cohort. This introduces a large heterogeneity of the cohort, and it is not possible to draw conclusions for one tumor entity. However, cognitive functions for single entities were analyzed in previous studies. This study points out that in a cohort of patients with tumoral and vascular lesions the type of the lesion is no predictor for neurocognitive dysfunction. The main predictors for cognitive dysfunction are the known variables – age and KPS.
This study has limitations. The main limitation is the high variety of diseases and therefore a low number, respectively. However, rare tumors such as chordoma and subependymoma as well as rare vascular lesions like cavernomas were assessed. No conclusions can be drawn for single lesion entities according to the results of this study.
No comparisons between the study population and a normative cohort were performed; this, however, has been shown in previous studies for patients with benign intracranial tumors17,21,22,47,48,59. The results of this study are mainly compared to studies on meningioma patients, also the cited studies with comparisons to the normative cohort were mainly performed on meningioma patients. However, the patient cohort of this study only includes 31/80 meningioma patients which might involve a bias. As described above, only a few studies previously assessed cognitive functions in patients with other benign intracranial tumors and vascular lesions, therefore comparability is low and further studies are necessary to draw conclusions and to assess cognitive functions in comparison to a normative cohort in patients with rare intracranial lesions.
Another limitation might be the manual segmentation of the lesions. Especially for small lesions and for lesions at the skull base this might introduce a bias due to over- or underestimation of lesion volume60.
Conclusions
Main risk factors for poorer neurocognitive functions in the category attention, not in the categories memory and executive functions, are higher age and reduced KPS in patients with benign intracranial lesions. Higher lesion volume, lesion location, type of the lesion (tumor, vascular), mood and pain did not predict poorer or better neurocognitive functions. As neurocognitive impairment influences patients’ quality of life, knowledge of these risk factors is important to perform neuropsychological testing and, if necessary, engineer appropriate therapy regimes.
Supplementary information
Author Contributions
S.B.: data analysis, statistical analysis, interpretation of the data, drafting the manuscript. J.R.: data collection, analysis and interpretation, reviewed the manuscript for intellectual content. B.W.: data interpretation, reviewed the manuscript for intellectual content. M.B.: data collection, reviewed the manuscript for intellectual content. C.Z., B.M. and Y.R. interpretation of the data, reviewed the manuscript for intellectual content. F.R.: study design and supervision, interpretation of the data, reviewed the manuscript for intellectual content. J.G.: study design, study supervision, data collection and analysis, interpretation of the data, reviewed the manuscript for intellectual content.
Competing Interests
C.Z. has served on scientific advisory boards for Philips and Bayer Schering; serves as co-editor on the Advisory Board of Clinical Neuroradiology; has received speaker honoraria from Bayer-Schering and Philips and has received research support and investigator fees for clinical studies from Biogen Idec, Quintiles, MSD Sharp & Dome, Boehringer Ingelheim, Inventive Health Clinical UK Ltd., Advance Cor, Brainsgate, Pfizer, Bayer-Schering, Novartis, Roche, Servier, Penumbra, WCT GmbH, Syngis, SSS Internartional Clinical Research, PPD Germany GmbH, Worldwide Clinical Trials Ltd., Phenox, Covidien, Actelion, Medivation, Medtronic, Harrison Clinical Research, Concentric, Penumbra, Pharmtrace, Reverse Medical Corp., Premier Research Germany Ltd., Surpass Medical Ltd. and GlaxoSmithKline. S.B., J.G., and B.M. work as consultants for Brainlab (Brainlab AG, Munich). All named potential conflicts of interest are unrelated to this study. B.W. received funding from KKF TU Munich. This funding is unrelated to this study. This study received no financial support. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44466-y.
References
- 1.Sacko A, et al. Evolution of the Karnosky Performance Status throughout life in glioblastoma patients. J Neurooncol. 2015;122:567–573. doi: 10.1007/s11060-015-1749-6. [DOI] [PubMed] [Google Scholar]
- 2.Brown PD, et al. Importance of baseline mini-mental state examination as a prognostic factor for patients with low-grade glioma. Int J Radiat Oncol Biol Phys. 2004;59:117–125. doi: 10.1016/j.ijrobp.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Gempt J, et al. Factors influencing neurocognitive function in patients with neuroepithelial tumors. Sci Rep. 2017;7:17764. doi: 10.1038/s41598-017-17833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 5.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 6.Cockrell JRF, Mini-Mental MF. State Examination (MMSE) Psychopharmacol Bull. 1988;24:689–692. [PubMed] [Google Scholar]
- 7.Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. 2015;17:580–587. doi: 10.1093/neuonc/nou233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habets EJ, et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol. 2016;18:435–444. doi: 10.1093/neuonc/nov186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AS, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115:1115–1125. doi: 10.3171/2011.8.JNS11488. [DOI] [PubMed] [Google Scholar]
- 10.van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134:9–18. doi: 10.1007/s11060-017-2503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18:1656–1663. doi: 10.1093/neuonc/now165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang EL, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 13.Hom J, Reitan RM. Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 1984;6:309–324. doi: 10.1080/01688638408401221. [DOI] [PubMed] [Google Scholar]
- 14.Meskal I, Gehring K, Rutten GJ, Sitskoorn MM. Cognitive functioning in meningioma patients: a systematic review. J Neurooncol. 2016;128:195–205. doi: 10.1007/s11060-016-2115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffau H. Brain plasticity and tumors. Adv Tech Stand Neurosurg. 2008;33:3–33. doi: 10.1007/978-3-211-72283-1_1. [DOI] [PubMed] [Google Scholar]
- 16.Butts AM, et al. Neurocognition in individuals with incidentally-identified meningioma. J Neurooncol. 2017;134:125–132. doi: 10.1007/s11060-017-2495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Nieuwenhuizen D, et al. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113:433–440. doi: 10.1007/s11060-013-1132-4. [DOI] [PubMed] [Google Scholar]
- 18.Dijkstra M, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80:910–915. doi: 10.1136/jnnp.2007.138925. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix P, et al. Neurocognitive Function Surrounding the Resection of Frontal WHO Grade I Meningiomas: A Prospective Matched-Control Study. World Neurosurg. 2017;98:203–210. doi: 10.1016/j.wneu.2016.10.095. [DOI] [PubMed] [Google Scholar]
- 20.Liouta E, Koutsarnakis C, Liakos F, Stranjalis G. Effects of intracranial meningioma location, size, and surgery on neurocognitive functions: a 3-year prospective study. J Neurosurg. 2016;124:1578–1584. doi: 10.3171/2015.6.JNS1549. [DOI] [PubMed] [Google Scholar]
- 21.Tucha O, et al. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg. 2003;98:21–31. doi: 10.3171/jns.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 22.Yoshii Yoshihiko, Tominaga Daisuke, Sugimoto Kouichi, Tsuchida Yukihiro, Hyodo Akio, Yonaha Hirokatsu, Kushi Sukemitsu. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surgical Neurology. 2008;69(1):51–61. doi: 10.1016/j.surneu.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 23.Zweckberger K, et al. Prospective analysis of neuropsychological deficits following resection of benign skull base meningiomas. J Neurosurg. 2017;127:1242–1248. doi: 10.3171/2016.10.JNS161936. [DOI] [PubMed] [Google Scholar]
- 24.Bala A, Lojek E, Marchel A. Cognitive functioning of patients with a PRL-secreting pituitary adenoma: A preliminary report. Neurology. 2016;86:731–734. doi: 10.1212/WNL.0000000000002252. [DOI] [PubMed] [Google Scholar]
- 25.Hendrix P, et al. Cognitive function surrounding resection of nonfunctioning pituitary adenomas with suprasellar extension: A prospective matched-control study. J Clin Neurosci. 2017;40:109–114. doi: 10.1016/j.jocn.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Brundl E, et al. Treatment of Unruptured Intracranial Aneurysms and Cognitive Performance: Preliminary Results of a Prospective Clinical Trial. World Neurosurg. 2016;94:145–156. doi: 10.1016/j.wneu.2016.06.112. [DOI] [PubMed] [Google Scholar]
- 27.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 28.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Lin CS. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9:e94300. doi: 10.1371/journal.pone.0094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meskal I, Rutten GJ, Beute GN, Salden ME, Sitskoorn MM. Cognitive deficits in patients with trigeminal neuralgia: opportunities to improve care and quality of life. Acta Neurochir (Wien) 2014;156:1565–1566. doi: 10.1007/s00701-014-2100-2. [DOI] [PubMed] [Google Scholar]
- 31.General Assembly of the World Medical, A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. The Journal of the American College of Dentists. 2014;81:14–18. [PubMed] [Google Scholar]
- 32.Zimmermann, P. F. Testbatterie zur Aufmerksamkeitsprüfung (TAP). Psychologische Testsysteme (2009).
- 33.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 34.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell EW. Factor analysis of the Revised Wechsler Memory Scale tests in a neuropsychological battery. Percept Mot Skills. 1982;54:971–974. doi: 10.2466/pms.1982.54.3.971. [DOI] [PubMed] [Google Scholar]
- 36.Muller H, Hasse-Sander I, Horn R, Helmstaedter C, Elger CE. Rey Auditory-Verbal Learning Test: structure of a modified German version. J Clin Psychol. 1997;53:663–671. doi: 10.1002/(SICI)1097-4679(199711)53:7<663::AID-JCLP4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 2006;1:892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 38.Lange, K.W., Aschenbrenner, S. & Tucha, O. Regensburg Word Fluency Task – a new test for the assessment of verbal fluency. European Archives of Psychiatry and Clinical Neuroscience25 (2000).
- 39.Listunova, L. et al. Cognitive Impairment Along the Course of Depression: Non-Pharmacological Treatment Options. Psychopathology, 1–11 (2018). [DOI] [PubMed]
- 40.Kuhner C, Burger C, Keller F, Hautzinger M. Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt. 2007;78:651–656. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- 41.Hautzinger, M., Keller, F. & Kühner, C. BDI-II Beck Depressions-Inventar Revision Manual. Pearson Assessment and Information GmbH, Frankfurt am Main 2. Auflage (2009).
- 42.Bauer B, Evers S, Gralow I, Husstedt I-W. Psychosoziale Beeinträchtigung durch chronische Kopfschmerzen Evaluation des Inventars zur Beeinträchtigung durch Kopfschmerzen (IBK) Der Nervenarzt. 1999;70:522–529. doi: 10.1007/s001150050475. [DOI] [PubMed] [Google Scholar]
- 43.Backhaus K. Multivariate Analysemethoden, Eine anwendungsorientierte Einführung. Springer13 (2011).
- 44.Zaudig, M. Demenz undleichte kognitive Beeinträchtigung im Alter. Verlag Hans Huber, Bern-Göttingen-Toronto-Seattle (1995).
- 45.Gauthier S, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 46.Meskal I, Gehring K, van der Linden SD, Rutten GJ, Sitskoorn MM. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. J Neurooncol. 2015;121:617–625. doi: 10.1007/s11060-014-1679-8. [DOI] [PubMed] [Google Scholar]
- 47.Koizumi H, et al. Cognitive dysfunction might be improved in association with recovered neuronal viability after intracranial meningioma resection. Brain Res. 2014;1574:50–59. doi: 10.1016/j.brainres.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 48.Steinvorth S, et al. Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: a prospective 1-year follow-up. Radiother Oncol. 2003;69:177–182. doi: 10.1016/S0167-8140(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 49.Tucha O, Smely C, Lange KW. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. Br J Neurosurg. 2001;15:184–188. doi: 10.1080/02688690151127608. [DOI] [PubMed] [Google Scholar]
- 50.Mifflin K, Chorney J, Dick B. Attention and Working Memory in Female Adolescents With Chronic Pain and Pain-free Female Adolescents: A Preliminary Pilot Study. Clin J Pain. 2016;32:609–616. doi: 10.1097/AJP.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 51.Etherton JL, Tapscott BE. Performance on selected visual and auditory subtests of the Wechsler Memory Scale-Fourth Edition during laboratory-induced pain. J Clin Exp Neuropsychol. 2015;37:243–252. doi: 10.1080/13803395.2014.1002756. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Li L, Tang F, Wu S, Hu Y. Memory impairment in chronic pain patients and the related neuropsychological mechanisms: a review. Acta Neuropsychiatr. 2014;26:195–201. doi: 10.1017/neu.2013.47. [DOI] [PubMed] [Google Scholar]
- 53.Dong HS, et al. Characteristics of neurocognitive functions in mild cognitive impairment with depression. Int Psychogeriatr. 2016;28:1181–1190. doi: 10.1017/S1041610216000314. [DOI] [PubMed] [Google Scholar]
- 54.Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: a review. Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- 55.Khan SA, et al. The hippocampus and executive functions in depression. Ind Psychiatry J. 2015;24:18–22. doi: 10.4103/0972-6748.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stordal KI, et al. Impairment across executive functions in recurrent major depression. Nord J Psychiatry. 2004;58:41–47. doi: 10.1080/08039480310000789. [DOI] [PubMed] [Google Scholar]
- 57.Schreiner SJ, et al. Low episodic memory performance in cognitively normal elderly subjects is associated with increased posterior cingulate gray matter N-acetylaspartate: a (1)H MRSI study at 7 Tesla. Neurobiol Aging. 2016;48:195–203. doi: 10.1016/j.neurobiolaging.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Duffau H. Hodotopy, neuroplasticity and diffuse gliomas. Neurochirurgie. 2017;63:259–265. doi: 10.1016/j.neuchi.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Krupp Wolfgang, Klein Christoph, Koschny Ronald, Holland Heidrun, Seifert Volker, Meixensberger Juergen. ASSESSMENT OF NEUROPSYCHOLOGICAL PARAMETERS AND QUALITY OF LIFE TO EVALUATE OUTCOME IN PATIENTS WITH SURGICALLY TREATED SUPRATENTORIAL MENINGIOMAS. Neurosurgery. 2009;64(1):40–47. doi: 10.1227/01.NEU.0000336330.75381.39. [DOI] [PubMed] [Google Scholar]
- 60.Huber T, et al. Reliability of Semi-Automated Segmentations in Glioblastoma. Clin Neuroradiol. 2017;27:153–161. doi: 10.1007/s00062-015-0471-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.