Abstract
Background
Treatment and control of schistosomiasis, one of the most insidious and serious parasitic diseases, depend almost entirely on a single drug, praziquantel. Since the funding for drug development for poverty-associated diseases is very limited, drug repurposing is a promising strategy. In this study, 73 nonsteroidal anti-inflammatory drugs (NSAIDs) commonly used in medical and veterinary fields were evaluated for their anti-schistosomal properties.
Methods
The efficacy of NSAIDs was first tested against adult Schistosoma mansoni ex vivo using phenotypic screening strategy, effective drugs were further tested in a murine model of schistosomiasis. The disease parameters measured were worm and egg burden, hepato- and splenomegaly.
Findings
From 73 NSAIDs, five (mefenamic acid, tolfenamic acid, meclofenamic acid, celecoxib, and diclofenac) were identified to effectively kill schistosomes. These results were further supported by scanning electron microscopy analysis. In addition, the octanol-water partition coefficient, both for neutral and ionized species, revealed to be a critical property for the ex vivo activity profile. Compounds were then tested in vivo using both patent and a prepatent S. mansoni infection in a mouse model. The most effective NSAID was mefenamic acid, which highly reduced worm burden, egg production, and hepato- and splenomegaly.
Interpretation
The treatment regimen used in this study is within the range for which mefenamic acid has been used in clinical practice, thus, it is demonstrated the capacity of mefenamic acid to act as a potent anti-schistosomal agent suitable for clinical repurposing in the treatment of schistosomiasis.
Keywords: Schistosomiasis, Nonsteroidal anti-inflammatory drugs, Mefenamic acid, Anthelmintic, Schistosomicidal activity
Research in context.
Evidence before this study
In recent years, a variety of drugs have been identified via a drug repurposing screen initiative. However, very few drugs have been approved for neglected diseases in the last decades. Currently, phenotypic screening strategies using approved drug collections have been considered more relevant for drug repurposing. Non-steroidal anti-inflammatory drugs (NSAIDs) are widely-used therapeutic agents that have anti-inflammatory, analgesic, and antipyretic activities. However, the therapeutic potential of NSAIDs against schistosomiasis has not been investigated.
Added value of this study
From a phenotypic screening of 73 NSAIDs, we identified that the most effective NSAID was mefenamic acid. In vitro assays demonstrated that mefenamic acid affected parasite motility, viability, and it induced severe tegumental damage in schistosomes. In addition, various parasitological criteria indicated the in vivo antischistosomal effects of mefenamic acid: it caused significant reductions in worm burden, egg production, and hepato- and splenomegaly.
Implications of all the available evidence
In both in vitro and in animal preclinical studies, the anti-helminthic activity of mefenamic acid surpasses criteria established by the World Health Organization for potential compounds for schistosomiasis. First and foremost, the treatment regimen used in this study is within the range for which mefenamic acid has been used in clinical practice. Considering the importance of the repositioning of drugs in infectious diseases, especially those related to poverty, in this study we provide strong evidence for the potential of mefenamic acid as an oral drug for the treatment of schistosomiasis.
Alt-text: Unlabelled Box
1. Introduction
Schistosomiasis remains one of the most insidious and serious parasitic diseases of clinical and public health significance. Caused by trematode flatworms of the genus Schistosoma, schistosomiasis is highly linked to poverty, it affects hundreds of millions globally, with nearly one billion people at risk [1]. The clinically relevant species in humans are S. mansoni, S. japonicum and S. haematobium. Once Schistosoma infection is established, mature worms can reside for decades in the definitive host's organism until provided adequate treatment. Disease morbidity, as a result of inflammation and fibrosis associated with the parasite's eggs, is typically chronic, can be painful and debilitating affecting both personal productivity and community development [2].
In the absence of a vaccine, disease treatment and control depend almost entirely on a single drug, praziquantel, which has been in clinical use for nearly 40 years [3]. Although effective against all species of human schistosomes, praziquantel presents significant limitations, since it is not active against juvenile forms of the parasite and frequent re-treatment of patients is required in order to target all helminth stages. In addition, reports of praziquantel resistance both in the field and experimentally-induced are emerging, leading to concerns regarding treatment-resistant parasites [4]. It should be noted that the World Health Organization (WHO) recommends preventive chemotherapy consisting of periodic administration of praziquantel as a short-term measure for the control of morbidity associated with schistosomiasis. In 2017, 98·7 million people (81·8 million school-aged children and 16·9 million adults) received preventive chemotherapy for schistosomiasis, it is estimated that at least 220 million people worldwide need treatment, yearly [1]. Praziquantel has also been extensively used to treat other parasitic flatworm infections in domestic and livestock animals [5]. Although often underestimated, these helminths infections affect hundreds of millions of animals, and they are often a cause of serious animal mortality and morbidity, with several species involved [5,6]. In this regard, considering the limitations of current treatment and control options for parasitic worms, it is important to identify new drugs to treat helminthiasis. However, efforts to develop safer, more effective anti-parasitic drugs have not been successful in producing alternative therapies [3,7].
Drug development is onerous and a time-consuming process [8]. It is estimated to exceed billions of dollars to bring a single new drug to the market, also, the average number of new drugs approved by regulatory agencies has declined in the last decades, while the total number of new molecular entities has remained about the same [9]. Within the neglected disease field, drug development has been historically hampered resulting from lack of market incentives due to the fact that many consumers of anti-helminthic drugs are from poor regions. In this regard, drug repositioning, the process of finding new indications within already available medications in the market has been considered a highly promising approach [10]. Indeed, it is estimated that the total cost for FDA approval through the repurposing route does not exceed a few million, compared to the billion dollar figure it takes to develop a drug starting from hit selection in vitro [9,10]. During recent years a variety of drugs have been identified via a drug repurposing screen initiative with particular attention to infectious diseases [[11], [12], [13], [14]]. However, very few drugs have been approved for neglected diseases [15].
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely-used therapeutic agents that have anti-inflammatory, analgesic, and antipyretic activities. Although they differ from one another in their chemical class, they all inhibit the cyclooxygenase enzymes [16]. Carvalho et al. [17] recently reported the anti-schistosomal properties of diclofenac in vitro. In the same study, it is also highlighted that diclofenac has a high structural similarity with praziquantel (approximately 70%), even though the drugs belong to different classes. In this study, 73 NSAIDs commonly used in medical and veterinary fields were evaluated for their anti-schistosomal properties through phenotypic drug screening. The NSAIDs were first tested against adult S. mansoni ex vivo. Drugs that showed to effectively kill adult stages of the worm were then tested in vivo using a patent and a prepatent S. mansoni infection in a mouse model. The most effective NSAID was mefenamic acid, which highly reduced worm burden, egg production, hepato-, and splenomegaly. More importantly, the treatment regimen used in this study is within the range for which mefenamic acid has been used in clinical practice, emphasizing its potential for repositioning.
2. Materials and methods
2.1. Drug and reagents
All NSAIDs were purchased from Sigma-Aldrich (St. Louis, MO, USA), Cayman Chemical (Ann Arbor, MI, USA) and Toronto Research Chemicals (Toronto, Ontario, Canada). Praziquantel was purchased from Merck (São Paulo, SP, Brazil). Stock solutions for use in adult bioassays in vitro were prepared in Dimethyl sulfoxide (DMSO) at a concentration of 10 mM. All drug tested (n = 73) are in Supplementary Table 1.
Roswell Park Memorial Institute (RPMI 1640) culture medium containing phenol red and l-glutamine, M199 medium, inactivated fetal bovine serum (FBS), penicillin G/streptomycin sulfate, and HEPES buffer were obtained from Vitrocell (Campinas, SP, Brazil). DMSO and glutaraldehyde solution were obtained from Sigma-Aldrich.
2.2. Animals
Animal studies were reported in compliance with the ARRIVE guidelines. All experimental protocols were approved and adhered to the guidelines of the Animal Welfare Committee of the Guarulhos University (Approval number 17/2017), in accordance with the ethical and safety rules and guidelines for the use of animals in biomedical research provided by the Conselho Nacional de Controle de Experimentação Animal (CONCEA), under The Brazilian Federal Law on Animal Experimentation (Law 11·794/2008).
Three-week-old female Swiss mice were obtained from Laboratory Animals Anilab (São Paulo, Brazil). All animals were housed in a 12 h light–dark cycle in a temperature-controlled room and had unrestricted access to food and water. All animals were randomized into different groups and experiments were carried out in a blinded manner.
2.3. Parasite maintenance
Schistosoma mansoni (BH strain) life cycle was maintained using Biomphalaria glabrata snails and mice following the standard procedures of our laboratory [18,19]. Female Swiss mice, three weeks old, were sub-cutaneously injected a suspension containing approximately 120 cercariae collected from S. mansoni-infected intermediate host snails Biomphalaria glabrata. All animals were maintained under controlled conditions (temperature, 22 °C; humidity, 50%; 12/12-h light/dark cycle), with unrestricted access to rodent diet and water.
2.4. Adult schistosomes drug assay in vitro
In vitro anti-schistosomal assay using adult parasites was performed as previously described [20,21]. Concisely, mice were dissected and adult schistosomes (seven-week-old) were collected from the mesenteric veins (parasite ex vivo). Drugs were tested at a concentration of 50 μM in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, arranged in a 24-well culture plate (TPP, St. Louis, MO) with one pair of worms per well and with 5 replicate wells per treatment condition. Parasites were kept for 72 h (37 °C, 5% CO2) and their viability was monitored twice daily via microscopic evaluation (Leica Microsystems, Wetzlar, Germany). Drugs highly active (effect ≥ 90%) after 72 h at 50 μM, were subsequently tested at different concentrations ranging from 50 to 0·78 μM using a two-fold dilution series for half maximum lethal concentration (LC50) determination [22,23]. Each concentration was tested in triplicate, and experiments were repeated once.
2.5. Scanning electron microscopy analysis
For scanning electron microscopy, control and treated adult parasites were fixed for at least three hours in 2·5% glutaraldehyde at room temperature. Experimental protocols for scanning electron microscopy were published previously [24,25]. Samples were metalized with gold using a Desk II sputter coater (Denton Vacuum LLC, Moorestown, NJ, USA) and then observed using a JEOL SM -6460LV high -resolution scanning electron microscopy (JEOL Ltd., Tokyo, Japan).
2.6. In vivo studies in a mouse model of schistosomiasis
In vivo anti-schistosomal assay was performed in a mouse model of schistosomiasis according to the operating procedures for experimental chemotherapy for anti-schistosomal drugs [26,27]. Three-week-old Swiss female mice were used for in vivo drug efficacy studies. In all treatments, mice were subcutaneously injected a suspension containing 80 S. mansoni cercariae.
Drugs were administered 21 days (prepatent infection) or 42 days (patent infection) post-infection by oral gavage to groups of five mice. For treatment, drugs were dissolved in 2% ethanol in water (v/v), and tested at a 400 mg/kg single dose or a 100 mg/kg daily for five consecutive days, depending on the pharmacological and toxicological information of the drug such as maximal plasma concentration (Cmax), plasma half-life (t1/2) and toxicity (lethal single oral dose LD50). Groups of S. mansoni-infected control were given a corresponding amount of vehicle on the same timetable. All mice were euthanized and dissected at 56 days post-infection. The worms were then collected, separated by sex, and counted [28]. Assessment of therapeutic efficacy was further based on the technique of quantitative and qualitative oograms using a fragment (10 mm) of the ascending colon [29] as well as the Kato-Katz method for quantitative faecal examination [30]. All experiments were repeated once.
2.7. Randomization and blinding
For the assessment of the in vivo testing, the animals were randomly assigned to their experimental groups, and pharmacological treatments. Animals were euthanized in a random manner inside a group. All results were acquired and analyzed by investigators who were blind to the group conditions. All parameters, i.e. (i) worm count, (ii) analysis of the egg stages in the intestine (oogram), (iii) faecal egg count, and (iv) measurement of organ mass were conducted by two different investigators.
2.8. Chemoinformatics
Molecular properties and heat maps were calculated using the quantitative-structure property relationship (QSPR) models embedded in the ADME/QSAR module of StarDrop version 6·5. The similarity map was built by applying a principal component analysis (PCA) on the entire set of compounds. UNITY fingerprints were used as molecular descriptors to determine the structural similarity between the tested drugs. First, the principal components were extracted by the PCA algorithm and used as initial coordinates to construct the map. Next, Tanimoto distances between the UNITY molecular descriptors were calculated to plot all points. The map was built using an outlier radius of 30% and an accuracy horizon of 70% [31].
2.9. Statistical analysis
Statistical analyses were performed by Prism 7·0 (GraphPad Software, La Jolla, CA). All data from the in vitro antischistosomal assay are presented as means ± SD of at least three independent experiments. IC50 values were calculated using sigmoid dose-response curves [32,33]. For in vivo experimental analysis, a parametric Dunnett's multiple-comparison test was applied to compare the vehicle group versus the treated group. In vivo experimental graphics represent data from individual mice and are expressed as scatterplots [34]. A value of P < 0·05 was considered statistically significant.
3. Results
3.1. Five drugs exhibited anti-helminthic effect against S. mansoni ex vivo
Male and female schistosomes are permanently paired while they reside in the bloodstream of their mammalian hosts. These adult worms can survive for decades and, hence, they are the target for an antischistosomal drug. Since in vitro cultivation of schistosomes throughout the entire life cycle is not possible, adult S. mansoni were obtained by portal perfusion of mice 42 days post-infection, i.e., parasites are considered “ex vivo”.
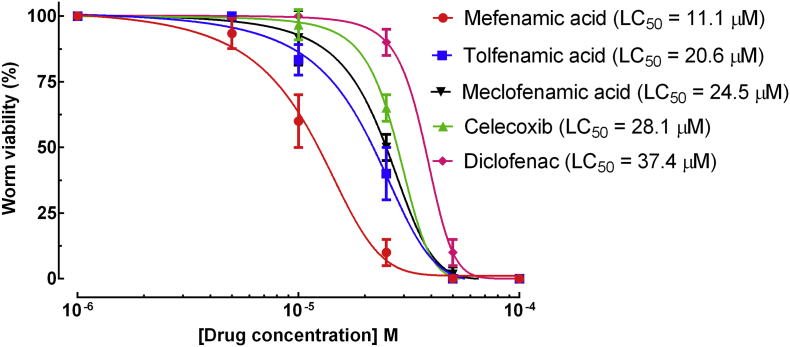
In the first step, adopting a phenotypic drug screening strategy, 73 NSAIDs of different classes were screened against adult schistosomes ex vivo (Supplementary Table 1). Of these, we identified five drugs that exhibited schistosomicidal activity against adult worms at 50 μM, mefenamic acid, tolfenamic acid, meclofenamic acid, celecoxib, and diclofenac. Accordingly, we tested the drugs at a range of concentrations and 50% lethal concentration (LC50) values were determined. The most active drugs were the fenamates, mefenamic acid, tolfenamic acid, and meclofenamic acid, with LC50 of 11·01, 20·6, and 24·5 μM, respectively, whereas celecoxib, and diclofenac had a moderate schistosomicidal activity (LC50 > 25 μM). In greater details, all five drugs had concentration-dependent effects on survival of the worms. Comparison of LC50 values showed that the order of potency was mefenamic acid > tolfenamic acid > meclofenamic acid>celecoxib>diclofenac (Fig. 1). For the remaining compounds tested, no LC50 could be calculated due to lack of activity at 50 μM. In addition to the physicochemical and anthelmintic properties, we evaluated the effect of the five drugs on the tegument of schistosomes by scanning electron microscopy (Supplementary Fig. 1). All drugs exhibited profound alterations on the morphology of the helminths, and rupture of the tegument was observed along the whole dorsal body surface on all worms examined. Compared to control group (Supplementary Fig. 1A), male worms exposed to mefenamic acid (Supplementary Fig. 1B), tolfenamic acid (Supplementary Fig. 1C), meclofenamic acid (Supplementary Fig. 1D), celecoxib (Supplementary Fig. 1E), and diclofenac (Supplementary Fig. 1F) presented changes on the tubercles and loss of spines on the surface.
Fig. 1.
NSAIDs action on adult S. mansoni parasites ex vivo. Adult parasites were collected from the hepatic portal and mesenteric veins of mice and placed on plates containing the indicated concentrations of the drugs, and worms were scored at 72 h for survival analysis. Calculated LC50 values are shown. Pairwise comparisons of LC50 values for mortality showed significant differences between all four drugs (p < 0·05). Worm survival data reflect mean ± SD of the mean for at least three replicates.
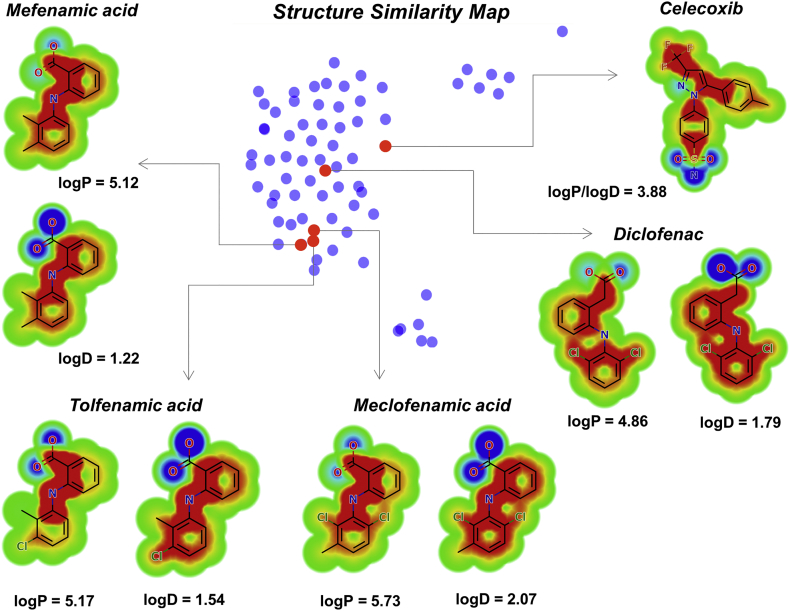
Calculation of physicochemical properties for all tested compounds (Supplementary Table 1) revealed that the ex vivo activity is correlated with the lipophilicity of the drugs. The logarithm of the octanol/water partition coefficient for neutral (logP) and ionized compounds at pH 7·4 (logD) were significantly higher for the active drugs than for the inactive molecules. The set of active compounds showed a mean logP of 5·12, whereas the inactive set exhibited the much lower value of 2·74. As for logD, a mean value of 1·79 was obtained for the group of active drugs, whereas a value of 0·37 was calculated for the set of inactive compounds. Heat maps calculated for the active drugs, shown in Fig. 2 along with a structure similarity map for the entire dataset, highlight the key role played by the aromatic moieties (red contours) in the lipophilic character of the compounds. Otherwise, the ionized carboxylate groups contribute negatively to the lipophilicity of the drugs, as illustrated by the blue contours.
Fig. 2.
Heat maps for logP and logD and structure similarity map. The closer the points are to each other the higher the structural similarity between them. Active drugs are highlighted as red dots. The red regions in the heat maps contribute positively to the property, the blue regions contribute negatively, and the green regions have no influence. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Efficacy in a murine model of schistosomiasis
On the basis of their in vitro activity against adult schistosomes ex vivo, data on toxicity, other accessible information of interest were consulted for the five drugs (mefenamic acid, tolfenamic acid, meclofenamic acid, celecoxib, and diclofenac) characterized by an LC50 ≤ 50 μM against adult schistosomes in order to select good in vivo candidates. The dosing regimen used for the treatment with NSAIDs was based on the protocol recommended for experimental schistosomiasis, i.e., 400 mg/kg in a single oral administration [3]. Since meclofenamic acid and diclofenac have a lethal dose of LD50 < 400 mg/kg in mice, and its serious side effects on animals are well described in literature (e.g. gastrointestinal complications) [35,36], they were not tested in vivo. Therefore, the efficacy of NSAIDs mefenamic acid, tolfenamic acid, and celecoxib at a single oral dose of 400 mg/kg were assessed in a murine model of schistosomiasis.
3.2.1. Treatment using a single oral dose of mefenamic acid, tolfenamic acid and celecoxib
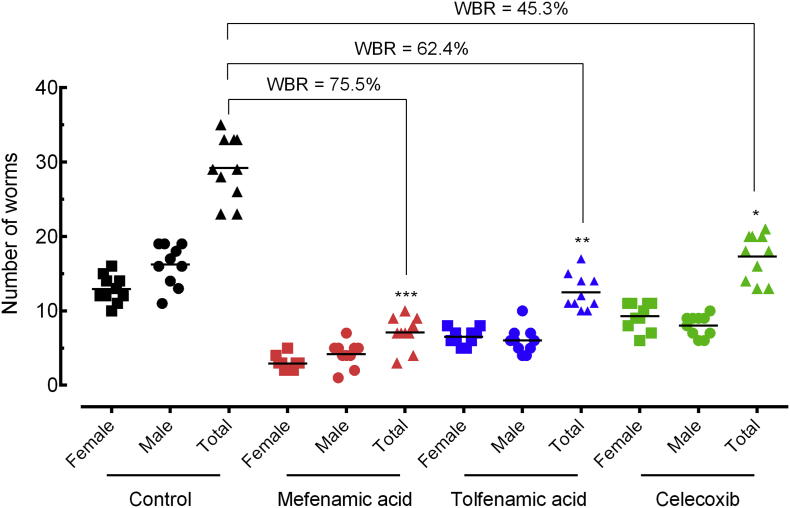
Groups of mice harbouring adult S. mansoni (patent infection) were orally treated with a single dose of 400 mg/kg body weight 42 days post-infection. Moreover, 56 days post-infection, animals were euthanized, and we evaluated worm burden, egg production, and hepato- and splenomegaly in all experimental treatments compared to the control S. mansoni-infected mice. All drugs were well tolerated at this dose, with no mice displaying any overt signs of toxicity.
As shown in Fig. 3, mefenamic acid has the highest schistosomicidal properties, with worm burden reduction of 75·5% (p < 0.001). The NSAIDs tolfenamic acid and celecoxib demonstrated a moderate effect, with worm burden reduction of 62·4% and 45·3%, respectively (p < 0.01 and p < 0.05).
Fig. 3.
Effect of mefenamic acid, tolfenamic acid, and celecoxib on the parasite burden in mice with patent infection. Drugs were administered orally using a single dose (400 mg/kg, 42 days after infection) to mice harbouring adult S. mansoni. On day 56 post-infection, all animals were humanely euthanized and parasite burdens were determined by sex (male and female schistosomes). Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0·001 compared with untreated groups by parametric Dunnett's multiple-comparison test. WBR: Worm burden reduction.
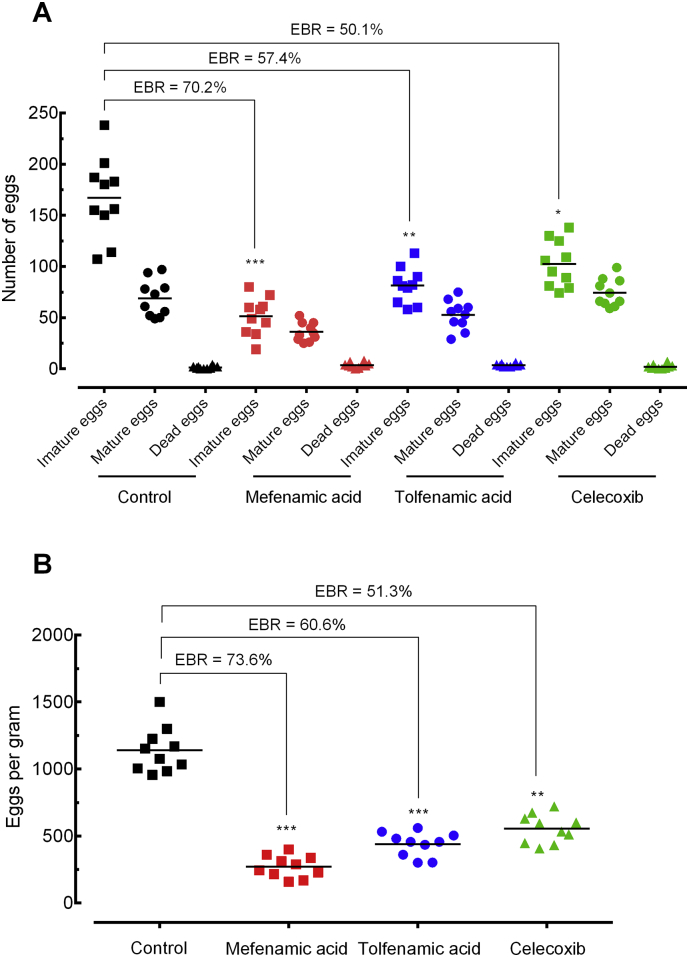
Egg production is important for both, pathogenesis and disease transmission. Therefore, it is important to evaluate the egg stages on the intestinal wall (oogram), and to analyze the number of eggs in faecal samples (Kato–Katz method). In all experimental treatments, a significant reduction in egg burden was observed, mainly with mefenamic acid (Fig. 4). On the wall of the intestine, eggs at all developmental stages were seen in the treated group, but the frequency of immature eggs was significantly lower compared with infected untreated controls. Indeed, the oogram showed that mefenamic led to a reduction of 70·2% (p < 0·001) in the number of immature eggs (Fig. 4A), whereas tolfenamic acid celecoxib induced a reduction of 57·4% and 50·1%, respectively. In faecal samples collected from mice, NSAIDs mefenamic, tolfenamic acid and celecoxib significantly (p < 0·05 to p < 0·001) reduced the number of eggs, with a reduction of 73·6%, 60·6%, 51·3%, respectively (Fig. 4B).
Fig. 4.
Effect of mefenamic acid, tolfenamic acid, and celecoxib on the egg burden of mice with patent infection. Egg development stages, i.e. oogram (A). Stool egg load (B). Drugs were administered orally using a single dose (400 mg/kg, 42 days after infection) to mice harbouring adult S. mansoni. On day 56 post-infection, all animals were humanely euthanized and egg burdens were determined by counting eggs in the intestine (oogram analysis) and in the faeces (Kato-Katz technique). Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0·001 compared with untreated groups by parametric Dunnett's multiple-comparison test. EBR: Egg burden reduction.
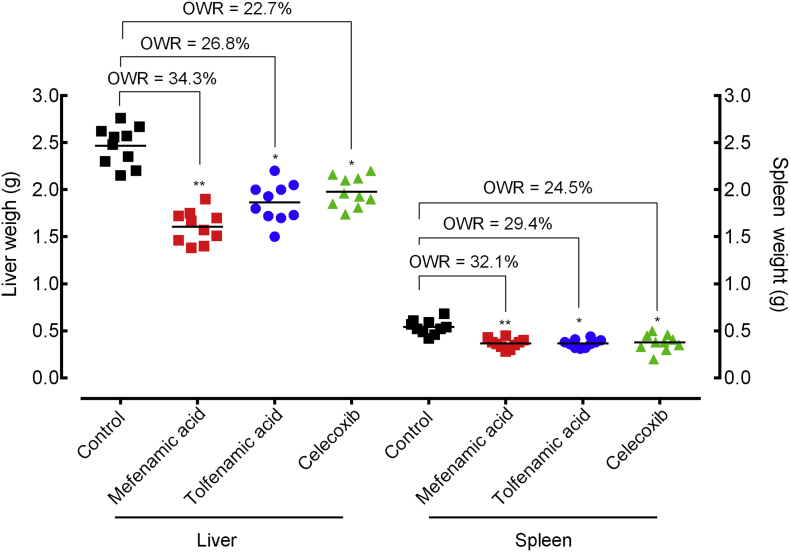
The pathology of chronic schistosomiasis mansoni is due to granulomatous inflammation in response to eggs that are trapped in host tissue, particularly the liver and spleen. As a consequence, gross hepato- and splenomegaly is evident in S. mansoni- infected animals. Treatment with NSAIDs significantly decreased liver and spleen weight (p < 0·05 to p < 0·001) relative to untreated controls. In mice treated with mefenamic acid, liver and spleen weight were significantly decreased by 34·3% and 32·1% (p < 0.01), respectively. Treatment with meclofenamic acid or celecoxib led to 22·7% to 34·3% decrease in the organ weight (Fig. 5).
Fig. 5.
Effect of mefenamic acid, tolfenamic acid and celecoxib on the liver and spleen pathology of mice with patent infection. Drugs were administered orally using a single dose (400 mg/kg, 42 days after infection) to mice harbouring adult S. mansoni (patent infection). On day 56 post-infection, all animals were humanely euthanized and organ pathology was determined by liver and spleen weights. Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0·001 compared with untreated groups by parametric Dunnett's multiple-comparison test. OWR: Organ weight reduction.
3.2.2. Treatment using multiple oral doses of mefenamic acid
As aforementioned, a single oral dose of 400 mg/kg is the recommended treatment for experimental murine schistosomiasis. However, mefenamic acid is rapidly absorbed after oral administration and it has a short half-life (two hours), which we considered a disadvantage feature for killing parasites that reside in the bloodstream. We then decided to administer mefenamic acid once a day for five consecutive days (100 mg/kg/day), which is also used in drug discovery programs for murine model of schistosomiasis [34,37].
Based on the remarkable in vivo effects of mefenamic acid in mice infected with adult parasites, we tested whether mefenamic acid displayed efficacy as a treatment against immature infections, where praziquantel is ineffective. Hence, we investigated the anti-schistosomal properties of mefenamic acid (100 mg/kg/day) in mice harbouring either adult (42 days post-infection, i.e., patent infection) or juvenile (21 days post-infection, i.e., prepatent infection) stages of S. mansoni.
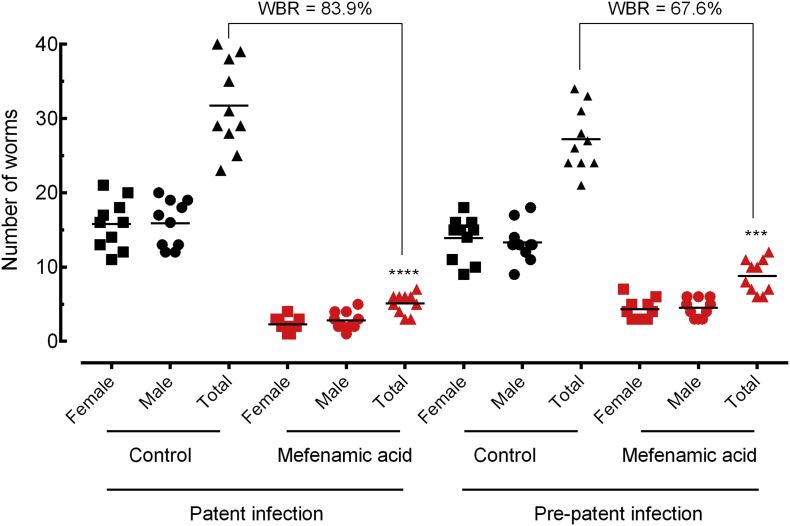
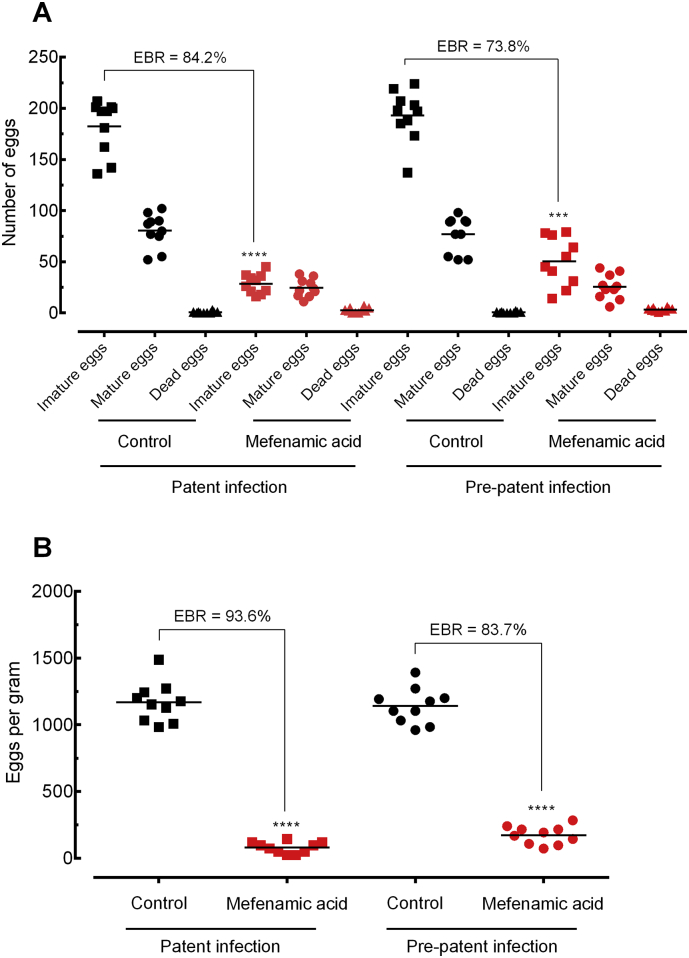
Interestingly, results from two independent trials showed that mefenamic acid dramatically decreased the number of parasites and eggs as well as ameliorates pathology in the liver and spleen in treated mice compared to untreated controls, more pronounced in patent infection than in prepatent infection. In more details, as shown in Fig. 6, treatment with mefenamic acid achieved a high total worm burden reduction of 82·1% and 67·2% in mice harbouring adult and juvenile schistosomes, respectively. With respect to egg burden, the use of mefenamic acid resulted in a decrease of 92% (P < .0001) and 78% (P < 0·001) in immature eggs in patent and prepatent infection, respectively (Fig. 7A). Similarly, the frequency of the eggs in faecal samples was dramatically reduced, with a reduction of 94·3% and 71·2% in adult e juvenile stages, respectively (Fig. 7B).
Fig. 6.
Effect of mefenamic acid on the parasite burden of mice with either patent or prepatent infections. Mefenamic acid was administered orally once daily for five consecutive days (100 mg/kg/day) 21 days (prepatent infection) or 42 days (patent infection) postinfection with S. mansoni. On day 56 post-infection, all animals were humanely euthanized and parasite burdens were determined by sex (male and female schistosomes). Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0·001 compared with untreated groups by parametric Dunnett's multiple-comparison test. WBR: Worm burden reduction.
Fig. 7.
Effect of mefenamic acid on the egg burden of mice with either patent or prepatent infections. Egg development stages, i.e. oogram (A). Stool egg load (B). Mefenamic acid was administered orally once daily for five consecutive days (100 mg/kg/day) 21 days (prepatent infection) or 42 days (patent infection) postinfection with S. mansoni. On day 56 post-infection, all animals were humanely euthanized and egg burdens were determined by counting eggs in the intestine (oogram analysis) and in the faeces (Kato-Katz technique). Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0.001 compared with untreated groups by parametric Dunnett's multiple-comparison test. EBR: Egg burden reduction.
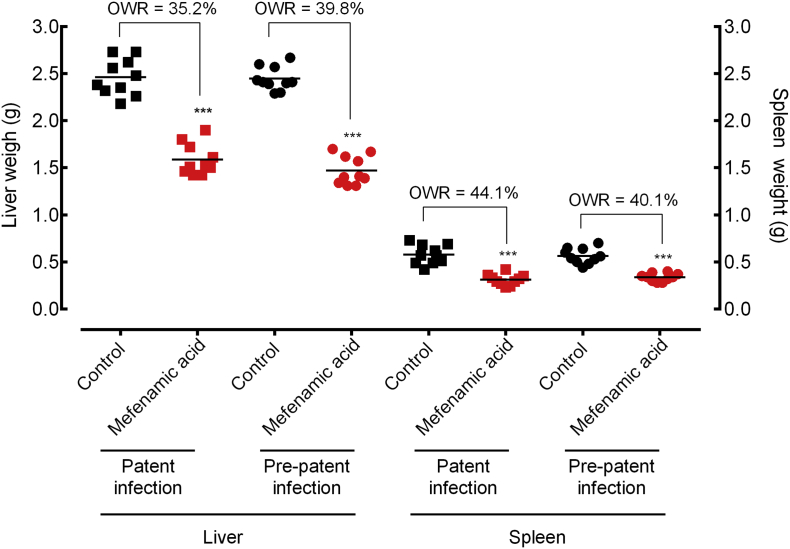
Regarding liver and spleen pathologies, mefenamic acid reduced the infection-promoted increase in liver mass by 42·1% and spleen mass by 48·8% in adult S. mansoni-infected mice, in comparison to vehicle treated controls. Hepato- and splenomegaly were reduced more markedly in juvenile S. mansoni-infected mice, with a reduction of 52·3% and 56·7% in liver and spleen mass, respectively (Fig. 8).
Fig. 8.
Effect of mefenamic acid on the liver and spleen pathology of mice with either patent or prepatent infections. Mefenamic acid was administered orally once daily for five consecutive days (100 mg/kg/day) 21 days (prepatent infection) or 42 days (patent infection) postinfection with S. mansoni. On day 56 post-infection, all animals were humanely euthanized and organ pathology was determined by liver and spleen weights. Points represent data from individual mice and are the combination of two independent experiments (n = 5 per group). Horizontal bars represent median values. * P < 0·05, ** P < 0·01, *** P < 0·001 compared with untreated groups by parametric Dunnett's multiple-comparison test. OWR: Organ weight reduction.
4. Discussion
Drug repurposing of already approved drugs provide an alternative method for rapid identification of new therapeutic agents. This approach can offer a better risk-versus-reward trade-off as compared with other drug development strategies. Currently, phenotypic screening strategies using approved drug collections has been considered more relevant for drug repurposing, exceeding those discovered through the molecular target-based approach [9,38,39]. In this study, from a phenotypic screening of 73 NSAIDs, we identified five NSAIDs (mefenamic acid, tolfenamic acid, meclofenamic acid, celecoxib, and diclofenac) able to impair the viability of adult S. mansoni. We next investigated the effect of three NSAIDs (mefenamic acid, meclofenamic acid, and celecoxib) in a mouse model of schistosomiasis.
Taken together, the most potent compound investigated in this study was mefenamic acid (lowest LC50 and greatest worm burden reduction). In vitro assays demonstrated that mefenamic acid affected parasite motility, viability, and it induced severe tegumental damage in schistosomes. In addition, various parasitological criteria indicated the in vivo antischistosomal effects of mefenamic acid: it caused significant reductions in worm burden, egg production, and hepato- and splenomegaly. Interestingly, in both in vitro and in the murine model of S. mansoni infection, the anti-helminthic activity of mefenamic acid surpasses criteria established by the World Health Organization (WHO) for schistosomiasis potential compounds. Active compounds should alter the viability of the adult parasites at 5 μg/ml and highly active compounds are defined as those with 80% reduction in worm burdens [40]. At 10 μM (~3 μg/ml) mefenamic acid caused mortality of adult parasites. All trials in mice resulted in at least an 82% reduction in worm burdens when mefenamic acid was administered in five doses at 100 mg/kg. In schistosomiasis, reduction in worm burden is associated with reduced pathology, and there is no concern about relapse because worms in this stage do not multiply in the mammalian host [41].
In the animal model, an initial test at the recommended dose for anti-helminthic agents (single dose of 400 mg/kg at day 42 post-infection) established mefenamic acid as a potent anti-schistosomal agent. Next, the significant amelioration of disease parameters in the daily dose (100 mg/kg for five days) was more dramatic than that seen with treatment using a single dose. Taken together, adult schistosomes appeared more sensitive to mefenamic acid (patent infection) than juvenile stages (prepatent infection). A high total worm burden reduction was achieved with mefenamic acid in patent infections (effect > 80%), whereas a moderate total worm burden reduction was observed in prepatent infections (effect ~ 65%). Unlike praziquantel, which is characterized by low activity against juvenile schistosomes, producing only a 25 to 30% reduction in worm burden [42], our results show that mefenamic acid has significant activity against the juvenile stage of the parasite. Concerning adult worm infection, the anti-schistosomal effect of mefenamic is similar to that of praziquantel, which is known to reduce ~90% of the worm burden [42].
Similar to other anti-schistosomal agents, the exact mechanism by which mefenamic acid and others NSAIDs exert their effect on S. mansoni is still not clear. Structurally, NSAIDs constitute of an acidic moiety (carboxylic acid or enols) attached to a planar, aromatic group. Three of the NSAIDs which we considered most effective against schistosomes, mefenamic acid tolfenamic acid, and meclofenamic acid are anthranilic acid derivatives, members of the fenamate group. Diclofenac is closely related as well, having methylene (CH2) separating the diphenylamine substructure from the acid moiety. Interestingly, all five drugs exerted marked effects on the tegument of the schistosomes. Comparing the lipophilicity among the tested NSAIDs, the active compounds have higher values of logP, and more importantly, higher values of logD. The latter property is critical in this context given that four out of the five active drugs have readily ionizable carboxylate groups at physiological pH. The tegumental damages in adult worms could be due to the inherent lipophilicity of the five NSAIDs, which allows these drugs to cross the tegument of the parasite and reach their molecular target(s). Furthermore, although structurally different, the mechanism of action of fenamates and other NSAIDs is related to cyclooxygenase inhibition in humans. As schistosome species do not appear to have cyclooxygenase, the mechanism of action of these drugs on the worms remains to be discovered. In addition to the lipophilic profile, the lack of activity of the remaining 68 compounds can be related to their intermolecular interaction with their respective, so far unknown, molecular target(s).
In addition to analgesic, anti-inflammatory and antipyretic properties, mefenamic acid has often been used for the treatment of dysmenorrhea in adolescents and adults [43,44]. As an advantage, mefenamic acid is rapidly absorbed after oral administration, has a short half-life, and it is more tolerant than other NSAIDs [35]. Mefenamic acid showed relatively high peak plasma level (Cmax: 20 μg/mL or 83 μM) [45,46]. In the present work, although the exposure profile is very important for anti-parasitic activity, we found that mefenamic acid exhibited schistosomicidal activity with LC50 values from ~ 10 μM, which are far below the clinically achievable plasma concentrations in vivo. It also exhibited potent anti-schistosomal properties in mice with no apparent toxicity. Using a dose translation formula [47], an effective daily dose of 100 mg/kg in our in vivo mouse model equates to a human equivalent dose of 8 mg/kg, in keeping with the current treatment regimen for oral mefenamic acid (25 mg/kg/day for seven days). It is important to emphasize that, ideally, clinical administration of anthelmintic drugs is limited to a single or very few doses, which is a treatment protocol recommended for mefenamic acid. Therefore, these results suggest that mefenamic acid should have anthelmintic activity under its current anti-inflammatory drug regimen.
Furthermore, when comparing the cost between both drugs, praziquantel and mefenamic acid, a substantial variation is noticeable. It should be noted that the available data demonstrate that drug prices vary across countries, classes of consumers, and whether the drugs acquired are generic or from a brand name. Regarding praziquantel, the cost of a 600-mg tablet can range between US$ 0.08 and US$ 6.60. However, access to praziquantel has been facilitated over the years by bilateral agencies, and it can also be found free of charge in high-disease burden countries in sub-Saharan Africa through donation programs offered by WHO. On the other hand, the cost of a single mefenamic acid 500-mg tablet can range between US$ 0.39 and US$ 13.00, and a specific donation program for this drug is currently unavailable. As previously mentioned, although generally efficient against schistosomiasis, there is a reduced efficacy of praziquantel treatment, and drug resistance remains a concern. In this sense, mefenamic acid may be a promising alternative for schistosomiasis control.
In conclusion, in vitro and in vivo studies established mefenamic acid as a potent anti-schistosomal agent by reducing both egg and worm burden. In addition, the pathology normally associated with the deposition of schistosome eggs in the spleen and liver was clearly ameliorated. Considering the importance of the repositioning of drugs in infectious diseases, especially those related to poverty, in this study we provide strong evidence for the potential of mefenamic acid as an oral drug for the treatment of schistosomiasis.
Acknowledgments
Acknowledgments
We thank Silvia G. Chiodelli, Anderson B. Gonçalves, and Yone C. Xavier for technical assistance with S. mansoni life cycle (Núcleo de Enteroparasitas, Instituto Adolfo Lutz, São Paulo, SP, Brazil). We thank Ana C. Mengarda, Bianca C. Silva, and Daniel B. Roquini for support during in vivo studies (Núcleo de Pesquisa em Doenças Negligenciadas, Universidade Guarulhos, Guarulhos, SP, Brazil). We are also grateful to Dr. Onofre de Paula Lima for providing drug data.
Funding sources
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number 2016/22488-3), Brazil. T.G.Q. was supported by a fellowship from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (PIBIC/CNPq, Brazil). J.M. received an established investigator fellowship from CNPq. A.D.A was supported by FAPESP-CIBFar, grant number 2013/07600-3. J.A.R. was supported by a fellowship from Fundação de Amparo à Pesquisa do Estado do Maranhão (FAPEMA, Brazil). The funding institutions had not any role in study design, data collection, data analysis, interpretation or writing of the report in this study.
Declaration of interests
The authors declare no conflicts of interest.
Author contributions
E.M.L. and J.M. designed the study. J.M. obtained the funding. E.M.L., M.P.S., T.G.Q., S.F.M and V.C.R. performed the in vitro and in vivo experiments. P.U.C., P.L.P. J.A.R. and J.M. provided methodological support. L.L.G.F. and A.D.A. performed the chemoinformatic studies. E.M.L. and J.M. analyzed the data. E.M.L., S.F.M. and J.M. wrote the paper. All authors approved the final version of the manuscript.
Data and materials availability
All the data are included in the main text or in the Supplementary Materials.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.04.029.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.World Health Organization Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2017. Wkly Epidemiol Rec. 2018;50:681–692. [Google Scholar]
- 2.McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.N. Schistosomiasis. Nat Rev Dis Primers. 2018;9:4–13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 3.Lago E.M., Xavier R.P., Teixeira T.R., Silva L.M., da Silva Filho A.A., de Moraes J. Antischistosomal agents: state of art and perspectives. Fut Med Chem. 2018;10:89–120. doi: 10.4155/fmc-2017-0112. [DOI] [PubMed] [Google Scholar]
- 4.Vale N., Gouveia M.J., Rinaldi G., Brindley P.J., Gärtner F., da Costa J.M.C. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother. 2017;5:24–61. doi: 10.1128/AAC.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You H., Cai P., Tebeje B.M., Li Y., McManus D.P. Schistosome vaccines for domestic animals. Trop Med Infect Dis. 2018;3 doi: 10.3390/tropicalmed3020068. [pii: E68] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlier J., van der Voort M., Kenyon F., Skuce P., Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Mafud A.C., Ferreira L.G., Mascarenhas Y.P., Andricopulo A.D., de Moraes J. Discovery of novel antischistosomal agents by molecular modeling approaches. Trends Parasitol. 2016;32:874–886. doi: 10.1016/j.pt.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Jin G., Wong S.T. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discov Today. 2014;19:637–644. doi: 10.1016/j.drudis.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W., Sun W., Simeonov A. Drug repurposing screens and synergistic drug combinations for infectious diseases. Br J Pharmacol. 2018;175:181–191. doi: 10.1111/bph.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papapetropoulos A., Szabo C. Inventing new therapies without reinventing the wheel: the power of drug repurposing. Br J Pharmacol. 2018;175:165–167. doi: 10.1111/bph.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debnath A., Personage D., Andrade R.M. A high-throughput drug screen for Entamoeba histolytica indentifies a new lead and target. Nat Med. 2012;18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbut M.B., Vilchèze C., Luo X. Auranofin exerts broad-spectrum bactericidal activitiesby targeting thiol-redox homeostasis. Proc Natl Acad Sci. 2015;112:4453–4458. doi: 10.1073/pnas.1504022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S., Lin B., Chu V. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci Transl Med. 2015;7:282ra49. doi: 10.1126/scitranslmed.3010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M., Lee E.M., Wen Z. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira L.L.G., Andricopulo A.D. Drugs and vaccines in the 21st century for neglected diseases. Lancet Infect Dis. 2019;19:125–127. doi: 10.1016/S1473-3099(19)30005-2. [DOI] [PubMed] [Google Scholar]
- 16.Rao P.P.N., Kabir S.N., Mohamed T. Nonsteroidal anti-inflammatory drugs (NSAIDs): progress in small molecule drug development. Pharmaceuticals (Basel) 2010;3:1530–1549. doi: 10.3390/ph3051530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho A.A., Mafud A.C., Pinto P.L., Mascarenhas Y.P., de Moraes J. Schistosomicidal effect of the anti-inflammatory drug diclofenac and its structural correlation with praziquantel. Int J Antimicrob Agents. 2014;44:372–374. doi: 10.1016/j.ijantimicag.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes J. Antischistosomal natural compounds: present challenges for new drug screens. In: Rodriguez-Morales A.J., editor. Current topics in tropical medicine. 2012. pp. 333–358. [Google Scholar]
- 19.Silva A.P., Silva M.P., Oliveira C.G. Garcinielliptone FC: antiparasitic activity without cytotoxicity to mammalian cells. Toxicol In Vitro. 2015;29:681–687. doi: 10.1016/j.tiv.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 20.de Moraes J., Dario B.S., Couto R.A., Pinto P.L., da Costa Ferreira A.M. Antischistosomal activity of oxindolimine-metal complexes. Antimicrob Agents Chemother. 2015;59:6648–6652. doi: 10.1128/AAC.01371-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Castro C.C., Costa P.S., Laktin G.T. Cardamonin, a schistosomicidal chalcone from Piper aduncum L. (Piperaceae) that inhibits Schistosoma mansoni ATP diphosphohydrolase. Phytomedicine. 2015;22:921–928. doi: 10.1016/j.phymed.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Mafud A.C., Silva M.P.N., Nunes G.B.L. Antiparasitic, structural, pharmacokinetic, and toxicological properties of riparin derivatives. Toxicol In Vitro. 2018;50:1–10. doi: 10.1016/j.tiv.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Mafud A.C., Silva M.P., Monteiro D.C. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma mansoni parasite. Chem Biol Interact. 2016;244:129–139. doi: 10.1016/j.cbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Guimarães M.A., de Oliveira R.N., Véras L.M. Anthelmintic activity in vivo of epiisopiloturine against juvenile and adult worms of Schistosoma mansoni. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guimarães M.A., Oliveira R.N., Almeida R.L. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. Plos One. 2018;13:e0196667. doi: 10.1371/journal.pone.0196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva M.P., de Oliveira R.N., Mengarda A.C. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int J Antimicrob Agents. 2017;50:467–472. doi: 10.1016/j.ijantimicag.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Lima L.I., Py-Daniel K.R., Guimarães M.A. Self-nanoemulsifying drug-delivery systems improve oral absorption and antischistosomal activity of epiisopiloturine. Nanomedicine. 2018;13:689–702. doi: 10.2217/nnm-2017-0308. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino J., Oliveira C.A., Faria J., Cunha A.S. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1962;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- 29.Katz N., Chaves A., Pellegrino J. A simple device for quantitative stool thick-smear 366 technique in Schistosoma mansoni. Rev Inst Med Trop. 1972;14:397–400. [PubMed] [Google Scholar]
- 30.de Moraes J., de Oliveira R.N., Costa J.P. A diterpene alcohol from chlorophyll, as a drug against neglected tropical disease schistosomiasis mansoni. PLoS Negl Trop Dis. 2014;8:e2617. doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willett P. Similarity-based virtual screening using 2D fingerprints. Drug Discov Today. 2006;11:1046–1053. doi: 10.1016/j.drudis.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Campelo Y.D.M., Mafud A.C., Véras L.M.C. Synergistic effects of in vitro combinations of piplartine, epiisopiloturine and praziquantel against Schistosoma mansoni. Biomed Pharmacother. 2017;88:488–499. doi: 10.1016/j.biopha.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 33.de Brito M.R.M., Peláez W.J., Faillace M.S. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicol In Vitro. 2017;44:273–279. doi: 10.1016/j.tiv.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Guerra A.R., Silva M.P., Silva T.C. Spironolactone as an antischistosomal drug capable of clinical repurposing: in vitro and in vivo studies. Antimicrob Agents Chemother. 2019;63:e01722-18. doi: 10.1128/AAC.01722-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winder C.V., Kaump D.H., Glazko A.J., Holmes E.L. Experimental observations on flufenamic, mefenamic, and meclofenamic acids. Rheumatology. 1966;VIII:7–49. doi: 10.1093/rheumatology/viii.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 36.Scholer D.W., Ku E.C., Boettcher I., Schweizer A. Pharmacology of diclofenac sodium. Am J Med. 1986;80:34–38. doi: 10.1016/0002-9343(86)90077-x. [DOI] [PubMed] [Google Scholar]
- 37.Botros S., William S., Ebeid F. Lack of evidence for an antischistosomal activity of myrrh in experimental animals. Am J Trop Med Hyg. 2004;71:206–210. [PubMed] [Google Scholar]
- 38.Zheng W., Thorne N., McKew J.C. Phenotypic screens as a renewed approach for drug discovery. Drug Discov Today. 2013;18:1067–1073. doi: 10.1016/j.drudis.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eder J., Sedrani R., Wiesmann C. The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov. 2014;13:577–587. doi: 10.1038/nrd4336. [DOI] [PubMed] [Google Scholar]
- 40.Nwaka S., Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 41.Sayed A.A., Simeonov A., Thomas C.J., Inglese J., Austin C.P., Williams D.L. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med. 2008;14:407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergquist R., Elmorshedy H. Artemether and praziquantel: origin, mode of action, impact, and suggested application for effective control of human schistosomiasis. Trop Med Infect Dis. 2018;1:118–125. doi: 10.3390/tropicalmed3040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cimolai N. The potential and promise of mefenamic acid. Expert Rev Clin Phamacol. 2013;6:289–305. doi: 10.1586/ecp.13.15. [DOI] [PubMed] [Google Scholar]
- 44.Feng X., Wang X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: a network meta-analysis. Mol Pain. 2018;14:1–14. doi: 10.1177/1744806918770320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winder C.V. Experimental observations on flufenamic, mefenamic, and meclofenamic acids. I. Pharmacology. Ann Phys Med. 1966;(7–16):1966. doi: 10.1093/rheumatology/viii.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 46.Glazko A.J. Experimental observations on flufenamic, mefenamic and meclofenamic acids. 3. Metabolic disposition. Ann Phys Med. 1966;(Suppl):23–36. [PubMed] [Google Scholar]
- 47.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2