DNA or RNA strands that are composed stretches of guanines (G-tracts) divided by other bases are able to form G-quadruplexes, a tetra-helical structure with stacked G-tetrad planes connected by Hoogsteen hydrogen bonds and stabilized by cations such as Na+ and K+ (Largy et al. 2016; Neidle et al. 2006; Neidle and Parkinson 2003). Inter- or intramolecular G-quadruplex structures are polymorphic and can form parallel or antiparallel structures based on the orientation of the strands in a G-quadruplex. G-quadruplexes have been revealed in human cells using an antibody and small molecules (Biffi et al. 2013; Hansel-Hertsch et al. 2017; Henderson et al. 2014) and shown to play important roles in numerous processes such as DNA replication, recombination, transcription, translation, and telomere maintenance (Blackburn 2001; Cahoon and Seifert 2009; de Lange 2002; Jiang et al. 2018; Kumari et al. 2007; Lei et al. 2004; Lopes et al. 2011; Paeschke et al. 2011; Siddiqui-Jain et al. 2002). In addition, G-quadruplexes have been implicated in neurological diseases such as amyotrophic lateral sclerosis (ALS), ataxia, and fragile X syndrome (Simone et al. 2015). And the presence of putative sequences forming G-quadruplexes has also been reported in various viral genomes (Metifiot et al. 2014).
Therefore, these findings made G-quadruplexes attractive therapeutic targets for anti-disease/virus drug design (Hansel-Hertsch et al. 2017; Neidle 2012; Raffa et al. 2018). To date, thousands of small compounds have been reported to bind with G-quadruplexes through stacking with the terminal G-tetrad layer typified by the structure of the bimolecular human telomeric quadruplex, d[TAGGGTTAGGGT]2, in complex with the anti-cancer drug BRACO-19 (Campbell et al. 2008). However, the inherent structural polymorphism of G-quadruplex implies that a unique G-quadruplex conformation could be recognized by a specific ligand. In this light, knowledge of the precise 3D-structure is important and required to design highly specific ligands capable of discriminating the diverse G-quadruplex topologies/structures.
Intramolecular human telomere G-quadruplex structures formed in K+ solution
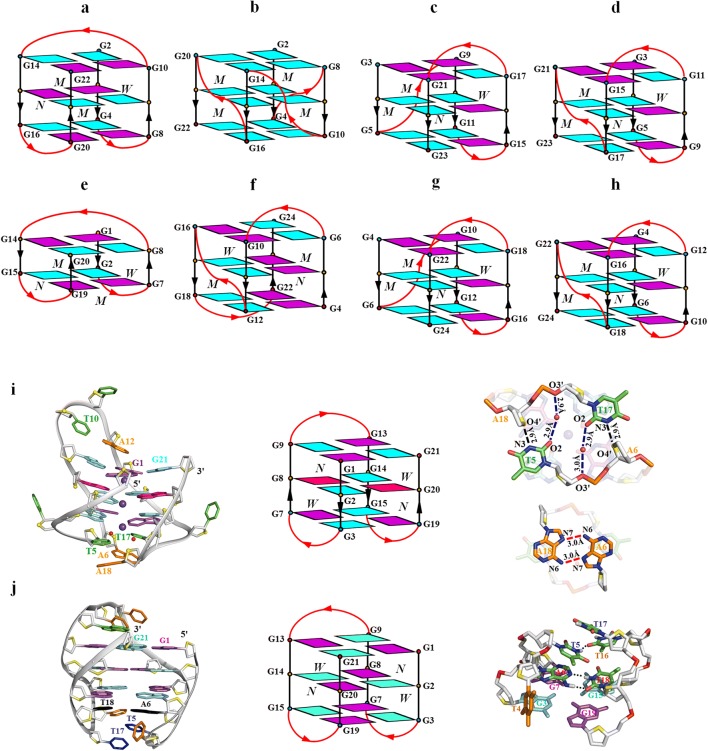
Over the past 30 years, eight distinct intramolecular G-quadruplex structures were observed for human telomeric d[GGGTTA]n sequence including parallel, hybrid, antiparallel basket, and (2 + 2) form (Ambrus et al. 2006; Dai et al. 2007a; Dai et al. 2007b; Lim et al. 2009; Lim et al. 2013; Parkinson et al. 2002; Phan et al. 2007; Wang and Patel 1993; Zhang et al. 2010) (Fig. 1a–h). All of these reported structures contain the central d[(GGGTTA)3GGG] core, named htel21, and the final folding depends on the flanking sequences at the 5′ and 3′ ends.
Fig. 1.
Schematic representation of known intramolecular G-quadruplexes formed by four-repeat human telomeric sequences and its variant: a the basket-type form observed for d[A(GGGTTA)3GGG] in Na+ solution, b the propeller-type form observed for d[A(GGGTTA)3GGG] in a K+-containing crystal, c the (3 + 1) form 1 observed for d[TA(GGGTTA)3GGG] in K+ solution, d the (3 + 1) form 2 observed for d[TA(GGGTTA)3GGGTT] in K+ solution, e the basket-type form with two G-tetrad layers observed for the modified d[(GGGTTA)3GGGT] sequence in K+ solution, f the antiparallel (2 + 2) form observed for d[(TTAGGGTTA)4TTA] G-quadruplex in Na+ solution, g the (3 + 1) form 1 observed for the end-modified sequence d[AAA(GGGTTA)3GGGAA] in K+ solution, h the (3 + 1) form 2 observed for d[TTA(GGGTTA)3GGGTT] sequence in K+ solution, i the chair-type G-quadruplex structure formed by htel21_Br-8,20 in K+ solution and the detailed loop structures of T4-T5-A6 and T16-T17-A18 in which the hydrogen bonds are shown in dashed lines, and j the chair-type G-quadruplex structure formed by htel21T18 in K+ solution and the detailed loop structures of T4-T5-A6 and T16-T17-T18 in which the hydrogen bond of the A6•T18 base pair and T5(H3)…T16(O4) is shown in dash line. anti and syn guanines are colored in cyan and magenta respectively. 8-Bromosubstituted guanine bases are in hot pink (Geng et al. 2019; Liu et al. 2019)
However, accumulating studies imply the existence of unreported antiparallel chair-type G-quadruplex structures (Aznauryan et al. 2016; Gray et al. 2014; Hou et al. 2017; Mashimo et al. 2010). Recently, a novel G-quadruplex folding has been observed for htel21_Br-8,20, two 8Br-dG substitutions at 8 and 20 positions of htel21 (Geng et al. 2019). It adopts a unique chair-type G-quadruplex fold with three G-tetrad layers (Fig. 1i). Particularly, T5 and T17 are coplanar with two water molecules stacking on the G-tetrad layer in a sandwich-like mode through a coordinating K+ ion and an A6•A18 base pair and a twisted Hoogsteen A12•T10 base pair caps on the G-tetrad core. Importantly, the stacking interaction through this sandwich-like stacking mode plays a key role in stabilizing the unique chair-type structure and driving conformational equilibrium toward this novel three-layer chair-type fold.
Interestingly, an antiparallel chair-type G-quadruplex has also been found to be formed by a native sequence located in subtelomeric regions of human chromosomes 8, 11, 17, and 19 as well as in the DNase hypersensitive region and in the subcentromeric region of chromosome 5, named as htel21T18 with a T substitution at A18 of htel21 (Liu et al. 2019) (Fig. 1j). htel21T18 also adopts a chair-type G-quadruplex fold with three G-tetrad layers. However, the loop-loop interactions in the structure of htel21T18 are mediated by reverse Watson-Crick A6•T18 base pair and an additional hydrogen bond between T5 and T16. Although both htel21_Br-8,20 and htel21T18 adopt the same topology, interestingly they are different in the content of the sequence, the donor-acceptor directionalities in each individual G-quartet, and the conformations of the lateral loops.
The discovery of novel G-quadruplex forms expands our knowledge of the diversity of the human telomeric G-quadruplex and the surge in the studies of the folding pathways including the relevant intermediates of G-quadruplex formation for which structural characterization has remained elusive. Especially, these novel structures are invaluable for the improvement of current G-quadruplex predictive tools as well as for computer-aided anti-cancer drug screening and design.
G-quadruplex formed by ALS and FTD related GGGGCC sequence
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are fatal degenerative neurological diseases which have a singular genetic and pathological background (Lillo and Hodges 2009; Rademakers et al. 2012; Rowland and Shneider 2001). It has been demonstrated that the abnormal expansion of a GGGGCC (G4C2) hexanucleotide repeat (HRE) in the first noncoding region of C9orf72 gene was the most important genetic cause of ALS and FTD (DeJesus-Hernandez et al. 2011; Majounie et al. 2012; Renton et al. 2011). And C9orf72 HRE DNA and RNA were found to be able to form noncanonical secondary structures, such as DNA or RNA G-quadruplexes and DNA/RNA hybrids, which may pause and abort transcription resulting in the loss of full-length RNA transcripts and accumulation of abortive RNA transcripts (Cammas and Millevoi 2017; Fratta et al. 2012; Haeusler et al. 2014; Kumar et al. 2016; Reddy et al. 2013). This could be the basis for a mechanistic model for the neurodegenerative disease of ALS/FTD.
Great efforts have been devoted to investigate C9orf72 HRE G-quadruplexes (Brcic and Plavec 2015; Haeusler et al. 2014; Sket et al. 2015; Zamiri et al. 2015; Zhou et al. 2015). The C9orf72 HRE DNA with different lengths was shown to adopt different G-quadruplex topologies by circular dichroism (CD) and NMR approaches (Fig. 2a–b). The d(G4C2)G4 formed an unreported dimeric parallel G-quadruplex in which all guanines adopt syn conformation indicated by CD spectroscopy (Fig. 2a and c). The d(G4C2)4 formed an intramolecular antiparallel G-quadruplex (Fig. 2d). However, d(G4C2)2, d(G4C2)2, d(G4C2)3, and d(G4C2)5 formed mixed G-quadruplexes topologies potentially including parallel, antiparallel, and other forms based on CD and NMR spectrum (Haeusler et al. 2014; Zhou et al. 2018; Zhou et al. 2015). Especially, the topology of d(G4C2)4 (Zhou et al. 2015) and the solution structure of a bromo-substituted (G4C2)3G4 DNA, d[(G4C2)3GG-BrGG] (Brcic and Plavec 2015; Brcic and Plavec 2018) were solved by NMR spectroscopy. Both of the two sequences formed the antiparallel four-layer chair-type G-quadruplex (Fig. 2d). In summary, the C9orf72 HRE DNA G-quadruplexes present variation in the sequence with different lengths which can adopt different G-quadruplex folds including parallel, antiparallel, and even hybrid (3 + 1) form.
Fig. 2.
C9orf72 HRE G4C2 DNAs form complicated G-quadruplex structures. a CD spectra of d(G4C2)G4, d(G4C2)2, d(G4C2)3, d(G4C2)4, and d(G4C2)5. b The imino region of 1D 1H spectra of various DNA in (a). c The proposed parallel dimeric G-quadruplex formed by C9orf72 HRE d(G4C2)G4 DNA in which all guanines adopt syn conformation and two separated strands are colored by red and green. d The antiparallel G-quadruplex formed by C9orf72 HRE d(G4C2)4 DNAs. anti and syn guanines are colored in cyan and magenta respectively (Zhou et al. 2018; Zhou et al. 2015)
Previous findings suggested that the parallel topology is adopted by most C9orf72 (G4C2)n RNAs (Haeusler et al. 2014; Sket et al. 2015; Zhou et al. 2015). However, the structure of the parallel G-quadruplex formed by RNAs still remains elusive.
One general approach taking advantage of the stabilization of G-quadruplex structures by small molecules has been proposed for therapeutic purposes (Simone et al. 2015). Therefore, the structural details of these C9orf72 (G4C2)n RNA/DNA G-quadruplexes are crucial to develop ligands which can achieve sufficient specificity to recognize unique G-quadruplex fold conformations for ALS/FTD and individual therapeutic purposes.
Conclusion
The canonical double helix structure of DNA was regarded as the only structure that allowed normal physiological function. Now, it is well known that G-quadruplexes are normal structures in human cells with convincing evidence supporting their regulatory roles and implications for disease. Although a multitude of novel G-quadruplex structures implicated with diseases are still being elucidated, the emerging role of G-quadruplexes in neurodegenerative diseases promises that it will be an exciting future area of investigation which should represent a change in pharmacotherapy of G-quadruplex related diseases. These works were supported by GRF (16103714, 16104315, 16118416, and 16103717), VPRGO17SC07PG, 1419-281-0091-41000, and AoE/M-403-16.
Conflict of interest
Changdong Liu declares that he has no conflict of interest. Yanyan Geng declares that she has no conflict of interest. Haitao Miao declares that he has no conflict of interest. Xiao Shi declares that he has no conflict of interest. Yingying You declares that she has no conflict of interest. Naining Xu declares that she has no conflict of interest. Bo Zhou declares that he has no conflict of interest. Guang Zhu declares that he has no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006;34:2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznauryan M, Sondergaard S, Noer SL, Schiott B, Birkedal V. A direct view of the complex multi-pathway folding of telomeric G-quadruplexes. Nucleic Acids Res. 2016;44:11024–11032. doi: 10.1093/nar/gkw1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn Elizabeth H. Switching and Signaling at the Telomere. Cell. 2001;106(6):661–673. doi: 10.1016/S0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Brcic J, Plavec J. Solution structure of a DNA quadruplex containing ALS and FTD related GGGGCC repeat stabilized by 8-bromodeoxyguanosine substitution. Nucleic Acids Res. 2015;43:8590–8600. doi: 10.1093/nar/gkv815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brcic J, Plavec J. NMR structure of a G-quadruplex formed by four d(G4C2) repeats: insights into structural polymorphism. Nucleic Acids Res. 2018;46:11605–11617. doi: 10.1093/nar/gky886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon LA, Seifert HS. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science. 2009;325:764–767. doi: 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammas A, Millevoi S. RNA G-quadruplexes: emerging mechanisms in disease. Nucleic Acids Res. 2017;45:1584–1595. doi: 10.1093/nar/gkw1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NH, Parkinson GN, Reszka AP, Neidle S. Structural basis of DNA quadruplex recognition by an acridine drug. J Am Chem Soc. 2008;130:6722–6724. doi: 10.1021/ja8016973. [DOI] [PubMed] [Google Scholar]
- Dai JX, Carver M, Punchihewa C, Jones RA, Yang DZ. Structure of the Hybrid-2 type intramolecular human telomeric G-quadruplex in K+ solution: insights into structure polymorphism of the human telomeric sequence. Nucleic Acids Res. 2007;35:4927–4940. doi: 10.1093/nar/gkm522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JX, Punchihewa C, Ambrus A, Chen D, Jones RA, Yang DZ. Structure of the intramolecular human telomeric G-quadruplex in potassium solution: a novel adenine triple formation. Nucleic Acids Res. 2007;35:2440–2450. doi: 10.1093/nar/gkm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta P, Mizielinska S, Nicoll AJ, Zloh M, Fisher EMC, Parkinson G, Isaacs AM (2012) C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep 2 [DOI] [PMC free article] [PubMed]
- Geng Yanyan, Liu Changdong, Zhou Bo, Cai Qixu, Miao Haitao, Shi Xiao, Xu Naining, You Yingying, Fung Chun Po, Din Rahman Ud, Zhu Guang. The crystal structure of an antiparallel chair-type G-quadruplex formed by Bromo-substituted human telomeric DNA. Nucleic Acids Research. 2019;47(10):5395–5404. doi: 10.1093/nar/gkz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RD, Trent JO, Chaires JB. Folding and unfolding pathways of the human telomeric G-quadruplex. J Mol Biol. 2014;426:1629–1650. doi: 10.1016/j.jmb.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler AR, et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507:195. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel-Hertsch R, Di Antonio M, Balasubramanian S. DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat Rev Mol Cell Biol. 2017;18:279–284. doi: 10.1038/nrm.2017.3. [DOI] [PubMed] [Google Scholar]
- Henderson A, et al. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014;42:860–869. doi: 10.1093/nar/gkt957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XM, et al. Involvement of G-triplex and G-hairpin in the multi-pathway folding of human telomeric G-quadruplex. Nucleic Acids Res. 2017;45:11401–11412. doi: 10.1093/nar/gkx766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Jiansen, Wang Yaqiang, Sušac Lukas, Chan Henry, Basu Ritwika, Zhou Z. Hong, Feigon Juli. Structure of Telomerase with Telomeric DNA. Cell. 2018;173(5):1179-1190.e13. doi: 10.1016/j.cell.2018.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Kashav T, Islam A, Ahmad F, Hassan MI. Structural insight into C9orf72 hexanucleotide repeat expansions: towards new therapeutic targets in FTD-ALS. Neurochem Int. 2016;100:11–20. doi: 10.1016/j.neuint.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5 ' UTR of the NRAS proto-oncogene modulates translation. Nat Chem Biol. 2007;3:218–221. doi: 10.1038/nchembio864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largy E, Mergny JL, Gabelica V. Role of alkali metal ions in G-quadruplex nucleic acid structure and stability. Met Ions Life Sci. 2016;16:203–258. doi: 10.1007/978-3-319-21756-7_7. [DOI] [PubMed] [Google Scholar]
- Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–1229. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- Lillo P, Hodges JR. Frontotemporal dementia and motor neurone disease: overlapping clinic-pathological disorders. Journal of Clinical Neuroscience. 2009;16:1131–1135. doi: 10.1016/j.jocn.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Lim KW, et al. Structure of the human telomere in K+ solution: a stable basket-type G-quadruplex with only two G-tetrad layers. J Am Chem Soc. 2009;131:4301–4309. doi: 10.1021/ja807503g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KW, Ng VCM, Martin-Pintado N, Heddi B, Phan AT. Structure of the human telomere in Na+ solution: an antiparallel (2+2) G-quadruplex scaffold reveals additional diversity. Nucleic Acids Res. 2013;41:10556–10562. doi: 10.1093/nar/gkt771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. A chair-type G-quadruplex structure formed by a human telomeric variant DNA in K(+) solution. Chem Sci. 2019;10:218–226. doi: 10.1039/c8sc03813a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J, et al. G-quadruplex-induced instability during leading-strand replication. EMBO J. 2011;30:4033–4046. doi: 10.1038/emboj.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Yagi H, Sannohe Y, Rajendran A, Sugiyama H. Folding pathways of human telomeric type-1 and type-2 G-quadruplex structures. J Am Chem Soc. 2010;132:14910–14918. doi: 10.1021/ja105806u. [DOI] [PubMed] [Google Scholar]
- Metifiot M, Amrane S, Litvak S, Andreola ML. G-quadruplexes in viruses: function and potential therapeutic applications. Nucleic Acids Res. 2014;42:12352–12366. doi: 10.1093/nar/gku999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle Stephen. Therapeutic Applications of Quadruplex Nucleic Acids. 2012. RNA Quadruplexes; pp. 139–149. [Google Scholar]
- Neidle S, Balasubramanian S, Harrell WA (2006) Quadruplex nucleic acids. RSC Pub
- Neidle S, Parkinson GN. The structure of telomeric DNA. Curr Opin Struct Biol. 2003;13:275–283. doi: 10.1016/S0959-440X(03)00072-1. [DOI] [PubMed] [Google Scholar]
- Paeschke K, Capra JA, Zakian VA. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 2011;145:678–691. doi: 10.1016/j.cell.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson GN, Lee MPH, Neidle S. Crystal structure of parallel quadruplexes from human telomeric. DNA Nature. 2002;417:876–880. doi: 10.1038/Nature755. [DOI] [PubMed] [Google Scholar]
- Phan AT, Kuryavyi V, Luu KN, Patel DJ. Structure of two intramolecular G-quadruplexes formed by natural human telomere sequences in K+ solution. Nucleic Acids Res. 2007;35:6517–6525. doi: 10.1093/nar/gkm706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa Robert B., Pergolizzi Joseph V., Taylor Robert, Ossipov Michael H. Discovery of “folded DNA” structures in human cells: Potential drug targets. Journal of Clinical Pharmacy and Therapeutics. 2018;44(1):125–128. doi: 10.1111/jcpt.12758. [DOI] [PubMed] [Google Scholar]
- Reddy K, Zamiri B, Stanley SYR, Macgregor RB, Pearson CE. The disease-associated r(GGGGCC)(n) repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. 2013;288:9860–9866. doi: 10.1074/jbc.C113.452532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci U S A. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone R, Fratta P, Neidle S, Parkinson GN, Isaacs AM. G-quadruplexes: emerging roles in neurodegenerative diseases and the non-coding transcriptome. FEBS Lett. 2015;589:1653–1668. doi: 10.1016/j.febslet.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Sket P, et al. Characterization of DNA G-quadruplex species forming from C9ORF72 G4C2-expanded repeats associated with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Neurobiol Aging. 2015;36:1091–1096. doi: 10.1016/j.neurobiolaging.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Patel DJ. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure. 1993;1:263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
- Zamiri B, Mirceta M, Bomsztyk K, Macgregor RB, Pearson CE (2015) Quadruplex formation by both G-rich and C-rich DNA strands of the C9orf72(GGGGCC)8 center dot(GGCCCC)8 repeat: effect of CpG methylation Nucleic Acids Res 43:10055-+ 10.1093/nar/gkv1008 [DOI] [PMC free article] [PubMed]
- Zhang ZJ, Dai JX, Veliath E, Jones RA, Yang DZ. Structure of a two-G-tetrad intramolecular G-quadruplex formed by a variant human telomeric sequence in K+ solution: insights into the interconversion of human telomeric G-quadruplex structures. Nucleic Acids Res. 2010;38:1009–1021. doi: 10.1093/nar/gkp1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B et al. (2018) Characterizations of distinct parallel and antiparallel G-quadruplexes formed by two-repeat ALS and FTD related GGGGCC sequence Scientific reports 8 doi:Artn 2366 10.1038/S41598-018-20852-W [DOI] [PMC free article] [PubMed]
- Zhou B, Liu C, Geng Y, Zhu G. Topology of a G-quadruplex DNA formed by C9orf72 hexanucleotide repeats associated with ALS and FTD. Sci Rep. 2015;5:16673. doi: 10.1038/srep16673. [DOI] [PMC free article] [PubMed] [Google Scholar]