Abstract
Since the mechanosensitive channel MscCG has been identified as the major glutamate efflux system in Corynebacterium glutamicum, studies of mechanotransduction processes in this bacterium have helped to unpuzzle a long-unresolved mystery of glutamate efflux that has been utilised for industrial monosodium glutamate production. The patch clamp recording from C. glutamicum giant spheroplasts revealed the existence of three types of mechanosensitive (MS) channels in the cell membrane of this bacterium. The experiments demonstrated that the MS channels could be activated by membrane tension, indicating that the channel gating by mechanical force followed the “Force-From-Lipids (FFL)” principle characteristic of ion channels inherently sensitive to transbilayer pressure profile changes in the mechanically stressed membrane bilayer. Mechanical properties of the C. glutamicum membrane are characteristics of very soft membranes, which in the C. glutamicum membrane are due to negatively charged lipids as its exclusive constituents. Given that membrane lipids are significantly altered during the fermentation process in the monosodium glutamate production, MS channels seem to respond to changes in force transmission through the membrane bilayer due to membrane lipid dynamics. In this review, we describe the recent results describing corynebacterial FFL-dependent mechanosensation originating from the particular lipid composition of the C. glutamicum membrane and unique structure of MscCG-type channels.
Keywords: NCgl1221, MscS, Mechanosensation, Glutamate efflux, Fatty acid synthesis
Introduction
Glutamate efflux mechanism through the Corynebacterium glutamicum cell envelope has presented an unresolved mystery since the discovery of this bacterium as a workhorse for industrial monosodium glutamate (MSG) production (Kinoshita et al. 1957; Marshavina and Gazaryan 1975; Shiio et al. 1962; Udaka 1960). The serendipitous discovery of the mechanosensitive channel MscCG as a major glutamate exporter is expected to reveal the mechanism of how glutamate efflux is triggered by inhibition of fatty acid synthesis using specific treatments, such as biotin limitation and supplementation of tween 40, in the fermentative process (Becker et al. 2013; Boerngen et al. 2010; Hashimoto et al. 2012; Hashimoto et al. 2010; Nakamura et al. 2007). By using the patch clamp from giant spheroplasts of C. glutamicum, activities of three types of mechanosensitive (MS) channels, MscCG, MscCG2, and MscL, have been characterised (Nakayama et al. 2018b). Consequently, the role of MS channels in glutamate production has been modelled as follows: 1) biotin limitation and supplementation of tween 40 inhibit fatty acid synthesis and change the metabolic flow to produce l-glutamate, 2) inhibition of fatty acid synthesis increases membrane tension, 3) only MscCG is activated by this increased membrane tension, and 4) l-glutamate is excreted by passive diffusion through the open pore of MscCG (Fig. 1a). In this scenario, C. glutamicum mechanosensation by MscCG is a key determinant of the glutamate efflux; however, the activation mechanism of C. glutamicum mechanosensitive channels has remained poorly understood. Notably, how do specific treatments triggering glutamate production increase membrane tension to activate mechanosensitive channels? What membrane changes lead exclusively to the opening of MscCG?
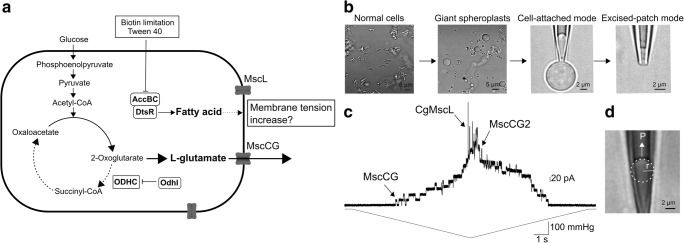
Fig. 1.
C. glutamicum glutamate production by mechanosensitive channels. a Mechanosensitive channel model for glutamate efflux. Biotin limitation and supplementation of tween 40 inhibit fatty acid synthesis and reduce the total amount of membrane lipids. Increased membrane tension activates exclusively the mechanosensitive channel MscCG and the glutamate is excreted by passive diffusion through the open pore of MscCG. bC. glutamicum giant spheroplasts preparation and application of patch clamp technique. c Current recording of all C. glutamicum endogenous mechanosensitive channels by mechanical stimuli in direct patch clamp. Upper and lower traces show channel currents and pressure applied to the membrane, respectively. d Measurement of membrane tension by Laplace’s law. P is pressure applied to the patch membrane, and r is radius of membrane curvature
C. glutamicum mechanosensitive channels, MscS-like and MscL-like channels
Bacterial mechanosensitive channels are known to function as osmotic nanovalves (Booth and Blount 2012; Levina et al. 1999). They release osmolytes rapidly in response to increased membrane tension upon hypoosmotic shock (Boer et al. 2011). Two types of bacterial mechanosensitive channels, MscS and MscL, have been employed as models for studies of the gating mechanism of most known types of mechanosensitive channels (Edwards et al. 2004; Hurst et al. 2008; Sukharev et al. 1996). Interestingly, only MscS homologues are found in all cell-walled organisms from bacteria to land plants (Kloda and Martinac 2002; Wilson et al. 2013). These MscS-like channels are highly diverse in their structure and function (Malcolm and Maurer 2012; Cox et al. 2015). MscCG-type mechanosensitive channels are found only in Corynebacterineae suborder, including C. glutamicum, and are classified into one of MscS-like channel subfamilies according to the structural feature of the large C-terminal extension characteristic of these channels (Boerngen et al. 2010). In the C. glutamicum genome, two MscS-like channels (MscCG and MscCG2) and one MscL-like channel (CgMscL) exist (Nakamura et al. 2007; Nakayama et al. 2018b; Wang et al. 2018). However, only MscCG functions as a major glutamate efflux system among these mechanosensitive channels. In fact, the most laboratory-used Corynebacterium strain ATCC13032 lacks MscCG2. In order to establish whether glutamate efflux is caused by activity of mechanosensitive channels in the C. glutamicum native membrane, direct application of the patch clamp methods to C. glutamicum giant spheroplasts was developed (Fig. 1b) (Nakayama et al. 2018b). This study demonstrated that all three types of C. glutamicum mechanosensitive channels were mechanically activated by applying negative pressure to the spheroplast membrane. When ramp pressure was applied to the membrane, MscCG, major glutamate exporter, was activated first exhibiting a conductance of 0.3 nS, and was followed by activities of MscCG2 and CgMscL, which were activated at higher pressure exhibiting larger conductance of 1 and 3 nS, respectively (Fig. 1c). Moreover, mechanical properties of the giant spheroplast membrane were evaluated by micropipette aspiration method (Nakayama et al. 2018b) showing that elasticity modulus of the C. glutamicum membrane was much smaller compared with the E. coli membrane. This result indicates that Corynebacterial membrane is very soft and expandable. When the membrane is stretched by applying negative pressure (suction) to a patch pipette, the membrane curvature observed inside the pipette indicates that membrane tension is increased because tension can be calculated using Laplace’s law (T = Pr/2, T tension, P pressure, r radius of membrane curvature) (Fig. 1d).
“Force-From-Lipids” principle: lessons from mechanosensitive channels activated by amphipaths
Since the discovery of E. coli mechanosensitive channels as pressure-sensitive channels in giant spheroplasts (Martinac et al. 1987), there have been several breakthroughs reported in the studies of the gating mechanism of mechanosensitive channels. By applying Laplace’s law, mechanosensitive channels have been clearly demonstrated to sense membrane tension rather than pressure (Gustin et al. 1988; Nomura et al. 2012; Sokabe et al. 1991; Sukharev et al. 1999). However, how does increased membrane tension activate mechanosensitive channels? Integral membrane proteins, such as ion channels, are surrounded by annular lipids, and thus it is believed that the mechanical gating force is transferred directly from the lipid bilayer via the annular lipids to the channels. In lipid bilayers, hydrophilic head and hydrophobic tails of phospholipids generate pressure profile called “transbilayer pressure profile” (Cantor 1999; Martinac et al. 2018) and integral membrane proteins are exposed to this force distribution across the membrane bilayer. For mechanosensitive channels, mechanical stimuli change the lateral pressure profile, which leads to a conformational change of the protein. This gating mechanism has been proposed as “Force-From-Lipids (FFL)” principle (Martinac et al. 1990; Cox et al. 2018; Ridone et al. 2018; Teng et al. 2015). Although the FFL gating mechanism has not been demonstrated for the MscCG channel, this principle was completely proven by the experiments investigating the effect of amphipathic molecules including conical lipids, lysophosphatidylcholine, that create membrane local curvature and asymmetric transbilayer pressure profile (Martinac et al. 1990; Perozo et al. 2002). MscS and MscL are activated without applying any pressure to membranes when amphipaths are added in the patch clamp experiments. In this situation, the patch membrane was not globally curved. Although local membrane tension cannot be calculated by Laplace’s law, it has been suggested that mechanosensitive channels are activated by changes of the transbilayer pressure profile created by local curvature (Bavi et al. 2016). Thus, sensing changes in the transbilayer pressure profile across membranes are the essence of the channel mechanosensitivity (Martinac et al. 1990), and this mechanism will also be investigated for MscCG channels in the future.
Membrane alteration of C. glutamicum cell envelope in glutamate production
C. glutamicum glutamate efflux triggered by biotin limitation and supplementation of tween 40 are known to cause membrane alteration of both inner and outer membranes in the cell envelope (Eggeling et al. 2001; Gutmann et al. 1992; Marshavina and Gazaryan 1975; Hashimoto et al. 2006). The cell envelope has a unique cell surface structure evolutionally specialised in bacterial cells of the Corynebacteria-Mycobacteria-Nocardia group (Chiaradia et al. 2017), and major functions of their cell envelope are mechanical resistance and permeability barriers. The innermost layer of the cell envelope is the plasma membrane (inner membrane), and the cell-wall skeleton consists of peptidoglycan covalently linked to arabinogalactan, which is overlaid on the plasma membrane. On top of the cell-wall skeleton, a mycolic acid layer, so-called “mycomembrane” (outer membrane), exists as a permeation barrier. Recently, lipid separation methods between inner and outer membranes by reverse micelle extraction have been established (Bansal-Mutalik and Nikaido 2011), and their lipid components were dissected with high-resolution mass spectrometry (Klatt et al. 2018). C. glutamicum cell membrane does not contain any phosphatidylethanolamine (PE) as the main lipid component since this bacterium lacks PE synthesis pathway (Nampoothiri et al. 2002). Instead, it comprises mainly phosphatidylglycerol (PG), cardiolipin (CL), phosphatidylinositol (PI), phosphatidylinositol mannosides (PIMs), and glucuronic acid diacylglycerol (GI), resulting in the highly negatively charged plasma membrane. On the other hand, the outer membrane mainly consists of specific types of mycolic acids (MA) called trehalose corynomycolate (TCMC) with chain length ranging from MA22:0–MA38:3, relatively short length of carbon chain compared with mycobacterial counterparts. In normal culture condition, the MAs 32:0, 34:1, and 36:2 are the major components of the outer membrane (Hashimoto et al. 2006). Note that MA34:1 is made of two parallel C16:0–C18:1 chains; thus, it is possible that corynomycolates and phospholipids participate together in the formation of lipid bilayers (Fig. 2a). In fact, the outer membrane is known to contain not only corynomycolates, but also phospholipids, such as PG, CL, and GI (Klatt et al. 2018), suggesting that outer membrane lipids are transported from inner membrane and that corynomycolates are inserted into the plasma membrane during the lipid transport. All membrane lipid biosynthesis takes place initially in the inner membrane, and some lipids are recruited into the inner leaflet of the outer membrane. Therefore, C. glutamicum membrane lipids move dynamically in the vertical direction from the inner membrane to outer membrane to create highly asymmetric membranes (Fig. 2b).
Fig. 2.
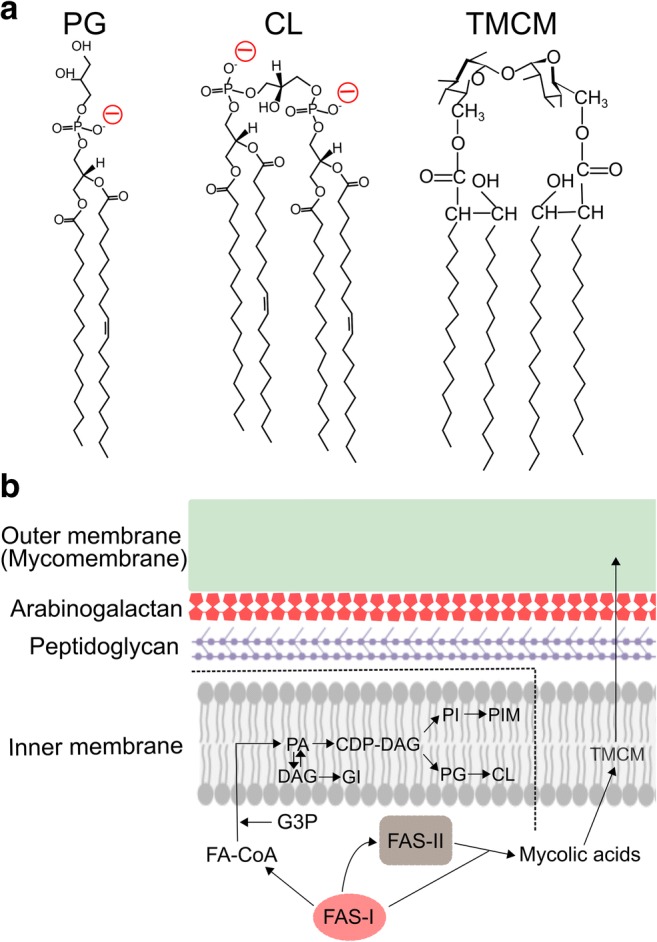
C. glutamicum membrane lipids and the cell envelope structure. a Structural membrane lipids of inner and outer membranes, phosphatidylglycerol (PG), cardiolipin (CL), and trehalose corynomycolate (TCMC). The negatively charged head groups of PG and CL are shown in red. b The structure of the C. glutamicum cell envelope and membrane lipid synthesis. As a branch point, phosphatidyl acid (PA) is biosynthesised by transferring an acyl chain from fatty acyl-CoA (FA-CoA) to glycerol-3-phosphate (G3P), and then forms cytidine diphosphate-diacylglycerol (CDP-DAG), the precursor for the synthesis of PI, PIM, PG, and CL. Thus, all phospholipids are synthesised in the inner membrane. PA is also dephosphorylated to become DAG and GI is synthesised from DAG. Mycolic acids are synthesised by FAS-I and FAS-II separately from phospholipids
Bilayer alteration in the plasma membrane to trigger glutamate efflux can be classified into three factors: total membrane lipid amount, palmitic acid (C16:0) vs oleic acid (C18:1) ratio, and cardiolipin amount. Total membrane lipid amount is reduced almost by half by reducing the expression level of DtsR, a subunit of acetyl-CoA carboxylase (Kimura et al. 1997; Kimura et al. 1999), and the ratio of palmitic acid (C16:0) vs oleic acid (C18:1) increases (Hoischen and Kramer 1990). Since membrane lipid saturation has been suggested to change mechanical properties of the membrane and the activation threshold of MscS (Ridone et al. 2018), it may also have a similar effect on the activation of the MscCG channels. The amount of cardiolipin increases significantly after reduction of total membrane lipid amount. The overexpression of phosphatidyl glycerophosphate synthetase pgsA or cardiolipin synthetase cls causes spontaneous glutamate efflux in C. glutamicum without any treatments (Nampoothiri et al. 2002), suggesting that increased amount of cardiolipin in the membrane induces glutamate efflux. Cardiolipin is a negatively charged non-bilayer phospholipid, and its structure resembles two phosphatidylglycerols joined together via the head groups. Due to its inversed conical shape, cardiolipin contributes to creating membrane curvature and localises at the curved poles and septa of rod-shaped bacteria (Oliver et al. 2014). Recently, computational simulations have suggested that cardiolipin shows a strong preference to negative membrane curvature, thus accumulates in a leaflet of negatively curved side of bilayers and creates highly asymmetric “buckled” membrane (Boyd et al. 2017; Elias-Wolff et al. 2019).
Mechanical activation vs lipid modulation activation of mechanosensitive channels
Global curvature (μm scale) by applying negative pressure seen in the patch clamp experiments increases membrane tension and activates all C. glutamicum mechanosensitive channels, MscCG, MscCG2, and CgMscL. On the other hand, specific treatments to trigger glutamate efflux activate exclusively MscCG although other mechanosensitive channels can be activated “mechanically.” Despite that the cell shape is not deformed, how does lipid modulation by specific treatments activates C. glutamicum mechanosensitive channels without applying mechanical stimuli, especially MscCG? The activation mechanism of mechanosensitive channels by amphipaths suggests the importance of asymmetry of transbilayer pressure profile generated by local curvature (Bavi et al. 2016; Martinac et al. 2018). Only high local membrane curvature corresponding to a radius of < 50 nm, which is comparable with the size of mechanosensitive channels (nm scale), can generate enough force to activate mechanosensitive channels (Bavi et al. 2016). Unlike ideal symmetric lipid bilayers, bacterial membranes have wrinkled or buckled areas, and these curved membranes contribute to generating asymmetry of transbilayer pressure profile. Membrane lipid modulation in C. glutamicum glutamate production causes significantly different transbilayer pressure profile in the membranes compared with normal condition (Hoischen and Kramer 1990; Klatt et al. 2018; Nakayama et al. 2018a; Nampoothiri et al. 2002), suggesting that the force distribution change in the membrane bilayers due to insertion of cardiolipin, for example, could activate the mechanosensitive channel MscCG. Although to date the functional reconstitution of MscCG into liposomes has not been successful, this approach remains to be applied in studies of inherent mechanosensitivity of this channel in the future.
Size and shape matter for structurally diverse MscS-like channels
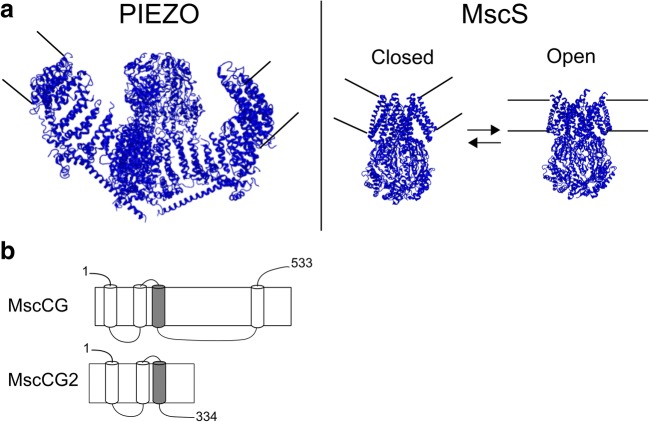
Local membrane curvature is not only generated by amphipaths and membrane lipid components, but also by the incorporation of mechanosensitive channels themselves (Clausen et al. 2017; Bavi et al. 2016). The eukaryotic mechanosensitive channel PIEZO1 and the prokaryotic mechanosensitive channel MscS are known to produce inward membrane bending around themselves deforming surrounding lipids when they are incorporated into membrane bilayers. It has been suggested that during the channel opening, the membrane flattens out (Fig. 3a) (Liang and Howard 2018; Phillips et al. 2009; Guo and MacKinnon 2017). The effects of local curvature generated by these mechanosensitive channels are of great interest to understand the FFL activation mechanism. Indeed, MscS mechanosensitivity is strongly dependent on its size and shape (Cox et al. 2016; Nomura et al. 2012; Shaikh et al. 2014). MscS-like channels including MscCG-type channels are the most diverse family with regard to their structure and function among all mechanosensitive channels (Malcolm and Maurer 2012; Cox et al. 2015). It should be noted that MscL has not been shown to curve membranes although this channel is activated by FFL principle. This is because FFL principle does not only include membrane stretching (tension) but also membrane curvature as a mechanical stimulus sufficient for activation of MS channels, including MscL (Perozo et al. 2002). The channel-induced membrane bending may be required for the activation of MscS-like channels, but this hypothesis needs still to be demonstrated. The structural feature of MscCG-type mechanosensitive channels is the large C-terminal extension including an additional fourth transmembrane helix, whereas the other MscS-like channel MscCG2 does not have this characteristic extension (Fig. 3b). Thus, an interesting question is whether the structure of MscCG channels with the additional helix creates membrane deformation such as inward bending sufficiently greater than MscCG2 to change the transbilayer pressure profile for mechanosensitivity.
Fig. 3.
Local membrane curvature generated by mechanosensitive channels. a PIEZO (left) and closed and open MscS (right). b Secondary structure of C. glutamicum mechanosensitive channel MscCG and MscCG2. Pore-forming helix is shown in grey
Prospects
C. glutamicum glutamate efflux through MscCG channel has been utilised for industrial MSG production. To understand this process, Corynebacterial membranes and three-dimensional structures of MscS-like channels are of great interest because they contribute to creating local membrane curvature resulting in a change of the asymmetry of transbilayer pressure profile necessary to activate mechanosensitive channels. Given that the size as well as the shape of mechanosensitive channels plays a significant role in changing this asymmetry, the unique structure of the MscCG channel needs to be elucidated to understand how this channel itself creates membrane curvature required for its gating by mechanical force. Consequently, understanding corynebacterial FFL mechanosensation will provide insights not only for improvement of MSG production, but also for further understanding of the gating mechanism of mechanosensitive channels.
Acknowledgements
We acknowledge the Japanese Society for Promotion of Science (JSPS) for a fellowship to YN and the National Health and Medical Research Council of Australia for a Principal Research Fellowship to BM.
Author contributions
YN and BM wrote the manuscript. YN, HK, KH, and BM contributed to editing.
Compliance with ethical standards
Conflict of interest
Yoshitaka Nakayama declares that he has no conflict of interest. Ken-ichi Hashimoto declares that he has no conflict of interest. Hisashi Kawasaki declares that he has no conflict of interest. Boris Martinac declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bansal-Mutalik R, Nikaido H. Quantitative lipid composition of cell envelopes of Corynebacterium glutamicum elucidated through reverse micelle extraction. Proc Natl Acad Sci U S A. 2011;108:15360–15365. doi: 10.1073/pnas.1112572108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavi Omid, Cox Charles, Vossoughi Manouchehr, Naghdabadi Reza, Jamali Yousef, Martinac Boris. Influence of Global and Local Membrane Curvature on Mechanosensitive Ion Channels: A Finite Element Approach. Membranes. 2016;6(1):14. doi: 10.3390/membranes6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Boerngen K, Nomura T, Battle AR, Marin K, Martinac B, Kramer R. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828:1230–1240. doi: 10.1016/j.bbamem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Boer M, Anishkin A, Sukharev S. Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time. Biochemistry. 2011;50:4087–4096. doi: 10.1021/bi1019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR, Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol. 2012;194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerngen K, Battle AR, Moker N, Morbach S, Marin K, Martinac B, Kramer R. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta. 2010;1798:2141–2149. doi: 10.1016/j.bbamem.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Boyd KJ, Alder NN, May ER. Buckling under pressure: curvature-based lipid segregation and stability modulation in cardiolipin-containing bilayers. Langmuir. 2017;33:6937–6946. doi: 10.1021/acs.langmuir.7b01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor RS. Lipid composition and the lateral pressure profile in bilayers. Biophys J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaradia L, et al. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci Rep. 2017;7:12807. doi: 10.1038/s41598-017-12718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MV, Jarerattanachat V, Carpenter EP, Sansom MSP, Tucker SJ. Asymmetric mechanosensitivity in a eukaryotic ion channel. Proc Natl Acad Sci U S A. 2017;114:E8343–E8351. doi: 10.1073/pnas.1708990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Nakayama Y, Nomura T, Martinac B. The evolutionary ‘tinkering’ of MscS-like channels: generation of structural and functional diversity. Pflügers Arch – Eur J Physiol. 2015;467(1):3–13. doi: 10.1007/s00424-014-1522-2. [DOI] [PubMed] [Google Scholar]
- Cox CD, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B. Bacterial mechanosensors. Annu Rev Physiol. 2018;80:71–93. doi: 10.1146/annurev-physiol-021317-121351. [DOI] [PubMed] [Google Scholar]
- Edwards MD, Booth IR, Miller S. Gating the bacterial mechanosensitive channels: MscS a new paradigm? Curr Opin Microbiol. 2004;7:163–167. doi: 10.1016/j.mib.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Eggeling L, Krumbach K, Sahm H. l-Glutamate efflux with Corynebacterium glutamicum: why is penicillin treatment or Tween addition doing the same? J Mol Microbiol Biotechnol. 2001;3:67–68. [PubMed] [Google Scholar]
- Elias-Wolff F, Linden M, Lyubartsev AP, Brandt EG. Curvature sensing by cardiolipin in simulated buckled membranes. Soft Matter. 2019;15:792–802. doi: 10.1039/c8sm02133c. [DOI] [PubMed] [Google Scholar]
- Guo YR, MacKinnon R (2017) Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 6. 10.7554/eLife.33660 [DOI] [PMC free article] [PubMed]
- Gustin MC, Zhou X-L, Martinac B, Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- Gutmann M, Hoischen C, Kramer R. Carrier-mediated glutamate secretion by Corynebacterium glutamicum under biotin limitation. Biochim Biophys Acta. 1992;1112:115–123. doi: 10.1016/0005-2736(92)90261-J. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, et al. Changes in composition and content of mycolic acids in glutamate-overproducing Corynebacterium glutamicum. Biosci Biotechnol Biochem. 2006;70:22–30. doi: 10.1271/bbb.70.22. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Nakamura K, Kuroda T, Yabe I, Nakamatsu T, Kawasaki H. The protein encoded by NCgl1221 in Corynebacterium glutamicum functions as a mechanosensitive channel. Biosci Biotechnol Biochem. 2010;74:2546–2549. doi: 10.1271/bbb.100636. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Murata J, Konishi T, Yabe I, Nakamatsu T, Kawasaki H. Glutamate is excreted across the cytoplasmic membrane through the NCgl1221 channel of Corynebacterium glutamicum by passive diffusion. Biosci Biotechnol Biochem. 2012;76:1422–1424. doi: 10.1271/bbb.120366. [DOI] [PubMed] [Google Scholar]
- Hoischen C, Kramer R. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol. 1990;172:3409–3416. doi: 10.1128/jb.172.6.3409-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst AC, Petrov E, Kloda A, Nguyen T, Hool L, Martinac B. MscS, the bacterial mechanosensitive channel of small conductance. Int J Biochem Cell Biol. 2008;40:581–585. doi: 10.1016/j.biocel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Kimura E, Abe C, Kawahara Y, Nakamatsu T, Tokuda H. A dtsR gene-disrupted mutant of Brevibacterium lactofermentum requires fatty acids for growth and efficiently produces l-glutamate in the presence of an excess of biotin. Biochem Biophys Res Commun. 1997;234:157–161. doi: 10.1006/bbrc.1997.6613. [DOI] [PubMed] [Google Scholar]
- Kimura E, Yagoshi C, Kawahara Y, Ohsumi T, Nakamatsu T, Tokuda H. Glutamate overproduction in Corynebacterium glutamicum triggered by a decrease in the level of a complex comprising DtsR and a biotin-containing subunit. Biosci Biotechnol Biochem. 1999;63:1274–1278. doi: 10.1271/bbb.63.1274. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Udaka S, Shimono M. Studies on the amino acid fermentation part I. Production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol. 1957;3:193–205. doi: 10.2323/jgam.3.193. [DOI] [PubMed] [Google Scholar]
- Klatt S, et al. Identification of novel lipid modifications and intermembrane dynamics in Corynebacterium glutamicum using high-resolution mass spectrometry. J Lipid Res. 2018;59:1190–1204. doi: 10.1194/jlr.M082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda A, Martinac B. Common evolutionary origins of mechanosensitive ion channels in Archaea, Bacteria and cell-walled Eukarya. Archaea. 2002;1:35–44. doi: 10.1155/2002/419261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Howard J. Structural biology: Piezo senses tension through curvature. Curr Biol. 2018;28:R357–R359. doi: 10.1016/j.cub.2018.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm HR, Maurer JA. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem. 2012;13:2037–2043. doi: 10.1002/cbic.201200410. [DOI] [PubMed] [Google Scholar]
- Marshavina ZV, Gazaryan VA. Effect of oleic acid and Tween-80 on lysine synthesis by the culture Corynebacterium glutamicum. Prikl Biokhim Mikrobiol. 1975;11:356–361. [PubMed] [Google Scholar]
- Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B, et al. Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile. Biophys Rev. 2018;10:1377–1384. doi: 10.1007/s12551-018-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J, Hirano S, Ito H, Wachi M. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl Environ Microbiol. 2007;73:4491–4498. doi: 10.1128/AEM.02446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Hashimoto KI, Sawada Y, Sokabe M, Kawasaki H, Martinac B. Corynebacterium glutamicum mechanosensitive channels: towards unpuzzling “glutamate efflux” for amino acid production. Biophys Rev. 2018;10:1359–1369. doi: 10.1007/s12551-018-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Komazawa K, Bavi N, Hashimoto KI, Kawasaki H, Martinac B. Evolutionary specialization of MscCG, an MscS-like mechanosensitive channel, in amino acid transport in Corynebacterium glutamicum. Sci Rep. 2018;8:12893. doi: 10.1038/s41598-018-31219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri KM, et al. Expression of genes of lipid synthesis and altered lipid composition modulates l-glutamate efflux of Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2002;58:89–96. doi: 10.1007/s00253-001-0861-z. [DOI] [PubMed] [Google Scholar]
- Nomura T, et al. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci U S A. 2012;109:8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, Crooks JA, Leidl M, Yoon EJ, Saghatelian A, Weibel DB. Localization of anionic phospholipids in Escherichia coli cells. J Bacteriol. 2014;196:3386–3398. doi: 10.1128/JB.01877-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9(9):696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridone P, Grage SL, Patkunarajah A, Battle AR, Ulrich AS, Martinac B. “Force-from-lipids” gating of mechanosensitive channels modulated by PUFAs. J Mech Behav Biomed Mater. 2018;79:158–167. doi: 10.1016/j.jmbbm.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Cox CD, Nomura T, Martinac B. Energetics of gating MscS by membrane tension in azolectin liposomes and giant spheroplasts. Channels. 2014;8(4):321–326. doi: 10.4161/chan.28366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio I, Otsuka SI, Takahashi M. Effect of biotin on the bacterial formation of glutamic acid. I. Glutamate formation and cellular premeability of amino acids. J Biochem. 1962;51:56–62. doi: 10.1093/oxfordjournals.jbchem.a127500. [DOI] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing ZQ. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Guy HR, Kung C. MscL: a mechanosensitive channel in Escherichia coli. Soc Gen Physiol Ser. 1996;51:133–141. [PubMed] [Google Scholar]
- Sukharev SI, Sigurdson WJ, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J Gen Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka S. Screening method for microorganisms accumulating metabolites and its use in the isolation of Micrococcus glutamicus. J Bacteriol. 1960;79:754–755. doi: 10.1128/jb.79.5.754-755.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y et al (2018) A novel Corynebacterium glutamicum l-glutamate exporter. Appl Environ Microbiol 84. 10.1128/AEM.02691-17 [DOI] [PMC free article] [PubMed]
- Wilson ME, Maksaev G, Haswell ES. MscS-like mechanosensitive channels in plants and microbes. Biochemistry. 2013;52:5708–5722. doi: 10.1021/bi400804z. [DOI] [PMC free article] [PubMed] [Google Scholar]