Abstract
Depending on the purpose and use, bioprocesses are carried out in order to reduce, maintain or increase the molar O/C ratio of biomass as the initial substrate. Cascade use considers the holistic and efficient use of biomass. In the current debate of biomass use, however, one may admit that an efficient use of biomass can further be based on the maintenance of initially present molar O/C ratio and functionality. In this regard, what compound should be formed that possesses highest functionality and similar molar O/C ratio as the substrate? How much energy should be spent on bioprocesses for the conversion of biomass under aerobic or anaerobic conditions? This study discusses and contributes to the efficiency assessment of aerobic and anaerobic bioprocesses based on chemical functionality and molar O/C ratio and their scale-depended energy need for creating appropriate environmental conditions for biological agents.
Keywords: Chemical functionality, Efficiency, Molar O/C ratio, Cascade use
Introduction
Fossil resources are by 90% energetically and by 10% materially used (Keim 2010). The resulting need to replace fossil energy by renewable energy is used as argument for an energetic use of biomass. However, the energy need for biomass production may exceed the amount of energy obtainable (Ptasinski 2016). A number of energetic biomass utilization processes are based on the microbial conversion of biomass constituents. Typical processes are anaerobic digestion to biogas, fermentative production of bioethanol and microbial fatty acid formation and esterification of fatty acids to obtain biodiesel. Depending on the type of substrate and process design, fermentative bioethanol production, for instance, can have a positive or negative net-energy value, meaning that more or less energy is created than initially spent on production (Bentsen et al. 2008; Lopes et al. 2019; Pimentel and Patzek 2005; Rempel et al. 2019). Thus, as generally recommended, a cascade use of biomass should have priority over a material or energetic use. First, biomass constituents are to be converted into valued materials and chemicals, and later the remaining fraction into energy. This approach allows the production of various products (Mair and Stern 2017), economic flexibility as well as, to a certain extent, independency from market prices of single products.
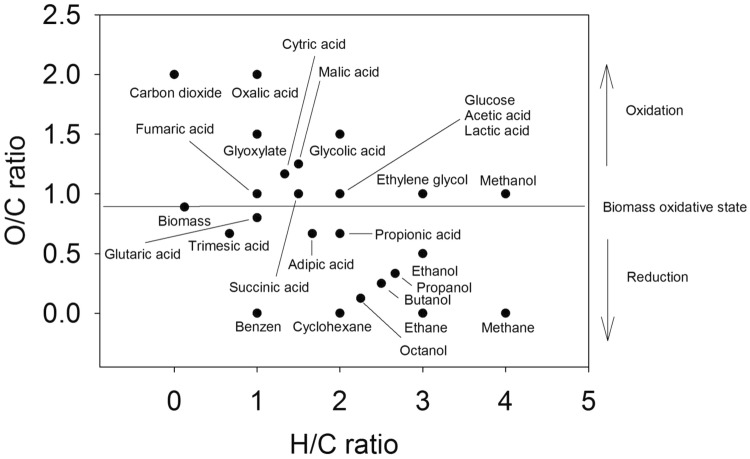
The potential of biomass is its high molar O/C ratio and chemical functionality (Clark et al. 2015) (Fig. 1). A cascade use of biomass can generate various compounds with higher, similar or lower molar O/C ratio (Clark et al. 2015). For example, compounds with lower molar O/C ratio result from an energetic use of biomass, such as from the production of alcohols or hydrocarbons, while material use of biomass results in compounds with higher or similar molar O/C ratio. A couple of compounds obtainable from bioprocesses using biomass as substrate are shown in Fig. 1.
Fig. 1.
Molar O/C and H/C ratios of biomass and representative organic compounds. The ratios were calculated from the data of 52 types of biomass published earlier (Shen et al. 2010)
Maintaining and reducing molar O/C ratio rely on anaerobic processes, while increasing relies on aerobic processes. Bioprocesses rely on biological agents, involved in the form of enzymes (Gurung et al. 2013; Zhang et al. 2018), single cultures or microbial consortia (de Souza et al. 2019; Koutinas et al. 2014). The activity of biological agents relies on proper environmental conditions, such as pH, temperature and O2-partial pressure. Creating and maintaining proper environmental conditions are energy intensive and the energy need is scale dependent. The upscaling of bioprocesses can lead to mixing, mass and heat transfer problems (Sweere et al. 1987). Those problems can result in the restricted production of compounds of interest due to reduced metabolic activity. Consequently, a higher energy input might be necessary to overcome a restricted production and to maintain productivity. Nevertheless, upscaling is necessary when bio-based compounds, irrespective of whether rich in energy or chemical functionality, are to be produced at larger amounts.
The assessment of the efficiency of bioprocesses is associated with the question: How much energy should be spent on bioprocesses for the conversion of biomass into compounds with a certain chemical functionality and molar O/C ratio? In order to focus on this question, first a deeper introduction to factors affecting energy need of bioprocesses and impact on productivity will be given. Energy efficiency will be explained on the example of energetic use of biomass. Furthermore, the importance of maintaining chemical functionality and molar O/C ratio as a measure of energy saving will be discussed. This study contributes to the assessment of upstream bio-processing regarding the efficient conversion of biomass into compounds. The novelty presented is the establishment of a link between chemical functionality, energy need as well as scale of bioprocesses.
Results and discussion
Factors affecting the energy need of bioprocesses
The energy need of bioprocesses (, kW Eq. (1) depends on the energy needed for agitation (, kW), aeration (, kW), heating (, kW) and cooling (, kW). Particularly, temperature control is of importance as heat is introduced by agitation and aeration as well as metabolic activity.
| 1 |
The net-energy need for external heating or cooling results from the equilibrium of heat generation and heat consumption (e.g., heat loss by evaporation). Furthermore, measuring and control devices require energy as well as (). The need, however, is scale independent (Knoll et al. 2005).
Contrarily, the energy need for agitation, aeration and heating/cooling is dependent on turbine design and dimension, medium viscosity, gas velocity and vessel geometry (Oldshue 1966; Rushton et al. 1950). Generally, the amount of energy that must be expended for the transport of oxygen and/or carbon dioxide, and heat to match the needs of biological agents increases with reactor volume (Sweere et al. 1987). For technically similar processes and similar fluid properties, it is assumed here that the calculation of (Eq. 2) of a bioprocess can be simplified by considering liquid working volume (V, m3) and volumetric power input (a, kW m−3).
| 2 |
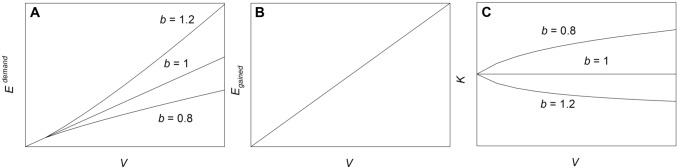
The sum of energy needed for aeration, agitation and heating/cooling attributed to a certain liquid working volume is considered by the volumetric energy input a (Kreyenschulte et al. 2016). Exponent b in Eq. (2) indicates how the energy need changes with increasing liquid working volume. A value of b = 1 means that the energy need increases linearly. However, it is more likely that exponent b has a value of ≥ 1 or ≤ 1, which results in a totally different pattern of energy need with increasing liquid working volume (Fig. 2a).
Fig. 2.
Energy need (Eneed, kW), amount of energy gained (Egained, kW) and energy efficiency (K, –) as a function of liquid volume (V, m3). To illustrate the impact of exponent b, values of 0.8, 1 and 1.2 have been used to calculate energy need and energy efficiency
The assessment of a process for energy production using the net-energy gain is rather simple. The net-energy gain (, kW) is the difference of energy gained (, kW) and .
| 3 |
The amount of can be calculated by multiplying the volumetric productivity (c, kW m−3) with the liquid working volume (V, m3) (Eq. (4), Fig. 2b). The volumetric productivity is thereby dependent on the activity of microorganisms at certain environmental conditions and is expected to decrease if, for instance, the energy input to agitation is insufficient as the conditions are negatively affected. To linearly increase the energy gain by linearly increasing scale, the energy need needs to be covered to keep biological agents active.
| 4 |
As outlined above, energy supplied is used for several operations, such as pH regulation, heating, cooling, pumping, agitation and aeration, and can be compared to . The energy efficiency (Eq. (5), K, –) thereby reflects the ratio of to .
| 5 |
With b = 1, energy efficiency remains constant with increasing liquid volume when volumetric energy input and volumetric productivity do not change (K = const., Fig. 2c). With b ≥ 1, energy efficiency is decreasing and at a certain liquid working volume the amount of energy spent may exceed the amount of energy gained (K ≤ 1). However, when b is ≤ 1, energy efficiency is increasing, which means that at a certain volume more energy might be gained than initially supplied (K ≥ 1). It should be admitted that the energy of energy-rich compounds comes from biomass and a criterion for an energetic use of biomass is K ≥ 1.
Aerobic and anaerobic processes
Aerobic processes
Aeration and agitation are important factors to provide oxygen, to strip carbon dioxide, to enhance heat and mass transfer to the medium and to suspend biological agents as well as substrates in aerobic processes. Particularly oxygen is important for the metabolic activity of cells and eventually to reach high productivities, but oxygen is sparingly soluble. Oxygen transfer is influenced by several variables, such as physical properties of the fluid, operational conditions, filling volume and geometry of the reactor. Oxygen transfer rate, representing the transfer of oxygen from gaseous to liquid phase, can be increased by altering agitation speed and gas flow, which eventually affects energy input. Furthermore, when carbon dioxide reaches the critical partial pressure, it can limit the maximum oxygen transfer capacity and, thus, agitation speed needs appropriate alteration to strip carbon dioxide.
Predictions about the rate of absorption of a gaseous species like oxygen in a stirred tank are usually based on correlations of volumetric mass transfer coefficient (, h−1) with mechanical agitation energy per unit liquid volume (, kW m−3) and superficial gas velocity (, m h−1) (Eq. 6). and are the major correlation coefficients for (Van’t Riet 1979) and α, β as well as γ empirical coefficients. The superficial gas velocity is dependent on the volumetric gas flow (, m3 h−1) and cross-sectional area (, m2, Eq. (7), d = diameter of the reactor).
| 6 |
| 7 |
Montes et al. (1999) proposed an empirical correlation for the determination of the volumetric mass transfer coefficient (Montes et al. 1999). They investigated the cultivation of the yeast Trigonopsis variabilis over a wide range of impeller speeds and gas velocities in three different sized mechanically stirred and baffled reactors of 2, 5 and 15 L, and found following empirical relationship Eq. (8).
| 8 |
Another example is the biotransformation of benzaldehyde to l-phenyl acetyl carbinol at scales from 0.1 L shake flasks to 5 L bioreactor using resting cells of Saccharomyces cerevisiae Eq. (9) (Shukla et al. 2001).
| 9 |
Equations (8) and (9) illustrate that the coefficients α, β and γ can vary considerably (Marques et al. 2010). The coefficients and consequently the volumetric mass transfer coefficient depend on the geometry of the reactor (type of reactor, distributor or stirrer design, etc.), the liquid properties (viscosity, superficial tension, etc.) and the dissipated energy in the fluid, which depends on the airflow rate, the stirrer speed, etc. Therefore, composition and rheological properties of the medium changing with time have an important effect on mass transfer rate (Sivaprakasam et al. 2008), which explain the differences in Eqs. (8) and (9).
Equation (6) reveals that the volumetric mass transfer coefficient decreases with increasing liquid working volume when the gassed energy input is kept constant. At a certain liquid working volume, a negative impact on volumetric productivity by insufficient oxygen supply and/or carbon dioxide removal is expected. However, when the energy input is increased by agitation at higher rates and increased gas flow, the volumetric mass transfer coefficient can be kept constant or even increased.
Ward (1989) provided an example where the energy input for agitation of a cultivation medium with a volume of 100 L was 2 kW, 2500 L required 15 kW and in order to stir 50,000 L 100 kW was needed (Ward 1989). The volumetric energy input is 0.02, 0.006 and 0.002 kW m−3, respectively. Thus, with increasing volume the volumetric energy input for agitation decreases. Banks (1979) published decreasing β-coefficients with increasing fermentation scale (Banks 1979). At 8 L scale, a coefficient of 0.95 was found at 400 L scale 0.67 and at 23,000–46,000 L scale 0.5. Coefficient γ was 0.67 at 8–400 L scales, and 0.5 at 23,000–46,000 L scale. A β-coefficient of 0.1 and 0.11 was found for 1.94% (w/w) carboxymethylcellulose and 1.80% (w/w) xanthan fluids, respectively (de Jesus et al. 2015). It should be admitted here that a β-coefficient is only applicable for a given process setting, conditions and medium properties. Nevertheless, β-coefficient can be taken as indicators to estimate whether the volume-specific energy input to keep the volumetric mass transfer coefficient constant increases or decreases with improving scale.
Scale-up strategies are commonly based on constant parameters of volumetric mass transfer coefficient, volume-specific energy input, volumetric energy consumption or impeller tip speed (Marques et al. 2010). However, to efficiently produce energy-rich compounds, scale-up should be based on maximum productivity. When the volumetric mass transfer coefficient is reduced for instance by inefficient agitation as a consequence of scale-up, then the energy input for agitation needs to be increased to supply sufficient oxygen, to keep biological agents active and to achieve maximum yields. Thus, the volume-specific energy input has a considerable impact on growth rate, productivity and yield. An experiment carried out with Escherichia coli revealed the importance of an appropriate adjustment of volumetric energy input for agitation when the fermentation scale increased from 0.1 to 2 L (Gill et al. 2008). Their results revealed that the volumetric energy input had to be increased by a factor of 5 to achieve similar final biomass concentration. This finding might be explained by the mass balance for dissolved oxygen Eq. (10) (Marques et al. 2010):
| 10 |
where OTR is the oxygen transfer rate defined as:
| 11 |
and OUR (mol m−3 s−1) is the oxygen uptake rate which stands for the metabolic activity:
| 12 |
where µ (s−1) is the specific growth rate, X (g m−3) is the biomass concentration and YX/O2 (g mol−1) is the yield of biomass on oxygen. With insufficient oxygen supply, metabolic activity is restricted.
Other energy needing processes are the transfer of heat and compression of gases. In geometrically similar reactors, the amount of heat transferred per volume increases with d3 while the cooling surface area increases with d2. Hence at very large scale, cooling, in order to compensate the input of heat in the form of mechanical energy and metabolic activity, is energy intensive (Hewitt and Nienow 2007). The energy consumption of a compressor for aeration depends on its degree of efficiency, pressure and pressure loss from piping.
As outlined above, a number of studies are available focusing on the impact of volume-specific energy input on the volumetric mass transfer coefficient; however, no comprehensive studies are available taking the efficiency of heating/cooling, compression of gases and aeration as well as agitation into account and use findings to assess the energy needed for changing the molar O/C ratio.
Anaerobic processes
Anaerobic digestion and anaerobic ethanol fermentation do not rely on aeration and, thus, the total energy need is predominantly formed by the energy needed for heating/cooling as well as agitation. In anaerobic bioprocesses, kLa does not reflect the transport of oxygen, but the transport of other gaseous species, such as carbon dioxide and methane, from the liquid to the gas phase. Agitation is, therefore, of importance for the removal of gaseous metabolic products from the liquid phase to avoid effects on biological agents, but agitation speed is much slower and heat introduction is negligible compared with aerobic bioprocesses.
When aeration is skipped, temperature inside the reactor can change by the heat loss in form of radiation, heat gain by higher temperatures at the outside or by differently tempered substrate (Lindorfer et al. 2006).
Anaerobic digestion, for instance, usually has a positive net-energy balance, meaning that more energy is produced as needed to cover the need of heating/cooling, stirring and other process operations (Lindorfer et al. 2006). But this depends on the temperature of the surrounding. Generally, the greater the temperature difference the more reduced the energy efficiency as more energy is required for tempering the substrate. However, the energy gain from anaerobic digestion makes it a feasible process all year (Lindorfer et al. 2006).
Suggestion of assessment
There are different criteria to assess a reaction, a product or a process regarding efficiency. Atom economy, for instance, can be used to describe on atom level the fraction of a substrate that can be found in a product (Trost 1991). Another criterion is the environmental factor (E-factor) describing the amount of waste or side stream produced associated with the formation of a wanted product (Sheldon 2007).
So far the focus of this study was on the energy need to create appropriate environmental conditions in order to stimulate biological agents to form wanted products. Factors affecting energy need of aerobic and anaerobic processes have been explained. The separation and purification of products, the so-called downstream processing, however, have been excluded. Downstream processing is essential to obtain ready-to-market products, but can be more intensive in terms of energy need than the actual bioprocess. Furthermore, the amount of waste streams, predominantly in form of wastewater, can be high, which results in an unfavorable E-factor. Despite the importance of the downstream processing the assessment here shall first be focused on the bioprocess.
All bioprocesses for the oxidative conversion of substrate rely on aeration and agitation to overcome the restriction by a changing volumetric mass transfer coefficient with increasing scale in accordance with Eq. (6). The supply of oxygen to cells in stirred vessel is cost intensive (Humbird et al. 2017). Atom economy would be a preferred criterion when oxygen is part of the reaction (Table 1). In bioprocesses, however, oxygen is not only part of a product, but also released as carbon dioxide (Table 1).
Table 1.
Examples of aerobic and anaerobic processes including respiratory quotient (RQ) for the production of organic compounds from glucose
| Compound | Chemical formula | Reaction equation | RQ |
|---|---|---|---|
| Aerobic | |||
| Acetic acid | C2H4O2 | C6H12O6 + 2O2 => 2C2H4O2 + 2CO2 + 2H2O | 1 |
| Glycolic acid | C2H4O3 | C6H12O6 + 3O2 => 2C2H4O3 + 2CO2 + 2H2O | 2/3 |
| Oxalic acid | C2H2O4 | C6H12O6 + 5O2 => 2C2H2O4 + 2CO2 + 4H2O | 2/5 |
| Anaerobic | |||
| Lactic acid | C3H6O3 | C6H12O6 => 2C3H6O3 | – |
| Succinic acid | C4H6O4 | C6H12O6 + 6/7CO2 => 12/7C4H6O4 | – |
| Malic acid | C4H6O5 | C6H12O6 + 2CO2 => 2C4H6O5 | – |
| Methane | CH4 | C6H12O6 => 3CH4 + 3CO2 | – |
Respiratory quotient (, Eq. (13)) can be used to describe the part of oxygen that is released as carbon dioxide by substrate oxidation.
| 13 |
The lower RQ, the fewer moles carbon dioxide are formed per mole oxygen. For instance, the oxidation of glucose to acetic acid results in an RQ of 2/2, while the oxidation of glucose to glycolic acid or oxalic acid results in an RQ of 2/3 or 2/5, respectively (Table 1).
Alternatively, RQ can also be written as:
| 14 |
where and are the yields stating the amount of substrate consumed per mole carbon dioxide (YSC) or oxygen (YSO).
Considering that aeration and substrate are cost-intensive, one may consider a product that is formed under minimum oxygen need and carbon dioxide formation, and consequently gives a high RQ. Of course the oxygen need of cells in order to be viable should be ensured. Taking the compounds listed in Table 1, formed under aerobic conditions, as an example, acetic acid should have priority over glycolic and oxalic acids. Furthermore, the formation of acetic acid from glucose does not change the molar O/C and H/C ratios (Fig. 1).
Maintenance of chemical functionality and molar O/C ratio is considered here as a measure to save energy and this might be used as a quantitative criterion for finding the most efficient conversion scenario. The most efficient conversion scenario, however, may not cover the need of certain products. As stated at the beginning of this work, 90% of crude oil is used energetically. This makes sense due to its reduced state and high energy content. Biomass, however, has a high O/C ratio (Fig. 1) and contains chemical functionality. The high chemical functionality of biomass is not associated with a single compound, but to a mixture of different classes, such as sugars, amino acids, carboxylic acids and long-chain fatty acids as well as to polysaccharides, proteins, lipids. Instead of energetically using the whole biomass, a separation of compounds with high O/C ratio and chemical functionality from compounds with low chemical functionality should be carried out and those compounds with low chemical functionality energetically used. This approach allows use of the whole biomass and goes behind the recommended cascade use, where chemical functionality can be destroyed while converting biomass into food and feed, materials, chemicals and energy. A separation and use of highly functionalized compounds can save energy. As illustrated above, it is energy intensive to create chemical functionality and to increase the O/C ratio and due to limited oxygen transfer coefficients, aerobic processes are restricted in scale. Conclusively, maintenance of chemical functionality is energy efficient.
A straight forward criterion for the energetic use of biomass is the energy efficiency Eq. (5). Energy efficiency expresses the ratio of to and shows that the production of energy-rich compounds is only feasible when the ratio is larger than 1. However, is it possible to assess the efficiency of processes for the material use of biomass in a similar way? Assuming is changed to product gained (, Eq. (15)), what is the criterion for an efficient conversion of a certain substrate into product? How much energy can be spent on the formation of product?
| 15 |
The delivery of 1 mol oxygen, under consideration of an OUR of 100 mmol L−1 h−1, to a 1000 m3 vessel requires 0.057 kWh energy (Humbird et al. 2017). Neglecting the energy for agitation and heating/cooling, the energy need to produce 1 mol of acetic, glycolic or oxalic acid is 0.057, 0.086 or 0.143 kWh. Thus, the more the substrate is oxidized, the more the energy is needed to provide the required oxygen. It should also be noted here that Humbird et al. also considered a changing energy need for aeration with increasing scale and calculated at an OUR of 100 mmol L−1 h−1, an energy need of 0.070, 0.061 and 0.057 kWh for 200, 500 or 1000 m3 scale (Humbird et al. 2017).
Needless to write that anaerobic process will show due to the missing aeration a much beneficial product-to-energy efficiency and a consequence might be to favor anaerobic over aerobic processes (Table 1, products from anaerobic processes). This, however, is not necessarily efficient as anaerobic processes do not make always use of the potential in the form of diverse chemical functionality and high molar O/C ratio of substrates. The fermentative production of lactic acid, malic acid or succinic acid results still in chemical functionality-rich compounds and the O/C ratio is similar to that of glucose or even higher. Contrarily, the complete reduction of glucose to methane (Table 1 and Fig. 1) clearly indicates a loss in chemical functionality and O/C ratio and should be avoided, and this is even clearer if one considers the energy and harsh conditions needed to introduce chemical functionality in the form of an alcohol group in methane to obtain methanol (Zakaria and Kamarudin 2016).
Conclusions
It seems rather challenging to find one single criterion for the assessment of bioprocesses. Alternatively, it is recommended to assess bioprocesses regarding their energy need, atom efficiency and waste production including carbon dioxide production as well as regarding their maintenance of chemical functionality and O/C ratio. The bioconversion under anaerobic conditions seems superior to aerobic conditions due to reduced energy need. One should, however, consider that an anaerobic process is only recommended when chemical functionality and molar O/C ratio are maintained and the complete reduction of substrates to, for instance, methane is avoided.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest in the publication.
References
- Banks G. Scale-up of fermentation processes. In: Wiseman A, editor. Topics in enzyme and fermentation biotechnology. New York: Wiley; 1979. pp. 170–266. [Google Scholar]
- Bentsen NS, Felby C, Ipsen KH (2008) Energy balance of 2nd generation bioethanol production in Denmark. Royal Veterinary and Agricultural University Danish Centre for Forest, Landscape and Planning, Copenhagen, Denmark. http://www.tekno.dk/pdf/projekter/p09_2gbio/ClausFelby/p09_2gbio%20Bentsen%20et%20al%20(2006).pdf
- Clark JH, Farmer TJ, Hunt AJ, Sherwood J. Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources. Int J Mol Sci. 2015;16:17101. doi: 10.3390/ijms160817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus SS, Moreira Neto J, Santana A, Maciel Filho R. Influence of impeller type on hydrodynamics and gas-liquid mass-transfer in stirred airlift bioreactor. AIChE J. 2015;61:3159–3171. doi: 10.1002/aic.14871. [DOI] [Google Scholar]
- de Souza Moraes B, Mary dos Santos G, Palladino Delforno T, Tadeu Fuess L, José da Silva A. Enriched microbial consortia for dark fermentation of sugarcane vinasse towards value-added short-chain organic acids and alcohol production. J Biosci Bioeng. 2019;127:594–601. doi: 10.1016/j.jbiosc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Gill NK, Appleton M, Baganz F, Lye GJ. Quantification of power consumption and oxygen transfer characteristics of a stirred miniature bioreactor for predictive fermentation scale-up. Biotechnol Bioeng. 2008;100:1144–1155. doi: 10.1002/bit.21852. [DOI] [PubMed] [Google Scholar]
- Gurung N, Ray S, Bose S, Rai V. A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int. 2013;2013:18. doi: 10.1155/2013/329121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt CJ, Nienow AW. The scale-up of microbial batch and fed-batch fermentation processes. Adv Appl Microbiol. 2007;62:105–135. doi: 10.1016/S0065-2164(07)62005-X. [DOI] [PubMed] [Google Scholar]
- Humbird D, Davis R, McMillan JD. Aeration costs in stirred-tank and bubble column bioreactors. Biochem Eng J. 2017;127:161–166. doi: 10.1016/j.bej.2017.08.006. [DOI] [Google Scholar]
- Keim W. Petrochemicals: raw material change from fossil to biomass? Pet Chem. 2010;50:298–304. doi: 10.1134/S0965544110040079. [DOI] [Google Scholar]
- Knoll A, Maier B, Tscherrig H, Büchs J. The oxygen mass transfer, carbon dioxide inhibition, heat removal, and the energy and cost efficiencies of high pressure fermentation. In: Kragl U, editor. Technology transfer in biotechnology: from lab to industry to production. Berlin Heidelberg: Springer; 2005. pp. 77–99. [DOI] [PubMed] [Google Scholar]
- Koutinas AA, et al. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev. 2014;43:2587–2627. doi: 10.1039/c3cs60293a. [DOI] [PubMed] [Google Scholar]
- Kreyenschulte D, Emde F, Regestein L, Büchs J. Computational minimization of the specific energy demand of large-scale aerobic fermentation processes based on small-scale data. Chem Eng Sci. 2016;153:270–283. doi: 10.1016/j.ces.2016.07.016. [DOI] [Google Scholar]
- Lindorfer H, Braun R, Kirchmayr R. Self-heating of anaerobic digesters using energy crops. Water Sci Technol. 2006;53:159–166. doi: 10.2166/wst.2006.246. [DOI] [PubMed] [Google Scholar]
- Lopes TF, et al. Process simulation and techno-economic assessment for direct production of advanced bioethanol using a genetically modified Synechocystis sp. Bioresour Technol Rep. 2019;6:113–122. doi: 10.1016/j.biteb.2019.02.010. [DOI] [Google Scholar]
- Mair C, Stern T. Cascading utilization of wood: a matter of circular economy? Curr For Rep. 2017;3:281–295. [Google Scholar]
- Marques MPC, Cabral JMS, Fernandes P. Bioprocess scale-up: quest for the parameters to be used as criterion to move from microreactors to lab-scale. J Chem Technol Biotechnol. 2010;85:1184–1198. doi: 10.1002/jctb.2387. [DOI] [Google Scholar]
- Montes FJ, Catalán J, Galán MA. Prediction of kLa in yeast broths. Process Biochem. 1999;34:549–555. doi: 10.1016/S0032-9592(98)00125-3. [DOI] [Google Scholar]
- Oldshue JY. Fermentation mixing scale-up techniques. Biotechnol Bioeng. 1966;8:3–24. doi: 10.1002/bit.260080103. [DOI] [Google Scholar]
- Pimentel D, Patzek TW. Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat Resour Res. 2005;14:65–76. doi: 10.1007/s11053-005-4679-8. [DOI] [Google Scholar]
- Ptasinski J. Efficiency of biomass energy: an exergy approach to biofuels, power, and biorefineries. New York: Wiley; 2016. [Google Scholar]
- Rempel A, et al. Bioethanol from Spirulina platensis biomass and the use of residuals to produce biomethane: an energy efficient approach. Bioresour Technol. 2019;288:121588. doi: 10.1016/j.biortech.2019.121588. [DOI] [PubMed] [Google Scholar]
- Rushton J, Costich E, Everett H. Power characteristics of mixing impellers - Part II. Chem Eng Prog. 1950;46:467–476. [Google Scholar]
- Sheldon RA. The E factor: fifteen years on. Green Chem. 2007;9:1273–1283. doi: 10.1039/b713736m. [DOI] [Google Scholar]
- Shen J, Zhu S, Liu X, Zhang H, Tan J. The prediction of elemental composition of biomass based on proximate analysis. Energy Convers Manag. 2010;51:983–987. doi: 10.1016/j.enconman.2009.11.039. [DOI] [Google Scholar]
- Shukla VB, Parasu Veera U, Kulkarni PR, Pandit AB. Scale-up of biotransformation process in stirred tank reactor using dual impeller bioreactor. Biochem Eng J. 2001;8:19–29. doi: 10.1016/S1369-703X(00)00130-3. [DOI] [PubMed] [Google Scholar]
- Sivaprakasam S, Mahadevan S, Gopalaraman S. Oxygen mass transfer studies on batch cultivation of P. aeruginosa in a biocalorimeter. Electron J Biotechnol. 2008 doi: 10.2225/vol11-issue1-fulltext-15. [DOI] [Google Scholar]
- Sweere APJ, Luyben KCAM, Kossen NWF. Regime analysis and scale-down: tools to investigate the performance of bioreactors. Enzyme Microb Technol. 1987;9:386–398. doi: 10.1016/0141-0229(87)90133-5. [DOI] [Google Scholar]
- Trost BM. The atom economy—a search for synthetic efficiency. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- Van’t Riet K. Review of measuring methods and results in nonviscous gas-liquid mass transfer in stirred vessels. Ind Eng Chem Process Des Dev. 1979;18:357–364. doi: 10.1021/i260071a001. [DOI] [Google Scholar]
- Ward O. Cultivation biotechnology. London: Open University Press; 1989. [Google Scholar]
- Zakaria Z, Kamarudin SK. Direct conversion technologies of methane to methanol: an overview. Renew Sust Energ Rev. 2016;65:250–261. doi: 10.1016/j.rser.2016.05.082. [DOI] [Google Scholar]
- Zhang Y, He S, Simpson BK. Enzymes in food bioprocessing—novel food enzymes, applications, and related techniques. Curr Opin Food Sci. 2018;19:30–35. doi: 10.1016/j.cofs.2017.12.007. [DOI] [Google Scholar]