Abstract
During the last few decades, nanotechnology has established many essential applications in the biomedical field and in particular for cancer therapy. Not only can nanodelivery systems address the shortcomings of conventional chemotherapy such as limited stability, non-specific biodistribution and targeting, poor water solubility, low therapeutic indices, and severe toxic side effects, but some of them can also provide simultaneous combination of therapies and diagnostics. Among the various therapies, the combination of chemo- and photothermal therapy (CT-PTT) has demonstrated synergistic therapeutic efficacies with minimal side effects in several preclinical studies. In this regard, inorganic nanostructures have been of special interest for CT-PTT, owing to their high thermal conversion efficiency, application in bio-imaging, versatility, and ease of synthesis and surface modification. In addition to being used as the first type of CT-PTT agents, they also include the most novel CT-PTT systems as the potentials of new inorganic nanomaterials are being more and more discovered. Considering the variety of inorganic nanostructures introduced for CT-PTT applications, enormous effort is needed to perform translational research on the most promising nanomaterials and to comprehensively evaluate the potentials of newly introduced ones in preclinical studies. This review provides an overview of most novel strategies used to employ inorganic nanostructures for cancer CT-PTT as well as cancer imaging and discusses current challenges and future perspectives in this area.
Keywords: Chemo/Photothermal therapy, Gold-based nanomaterials, Iron- based nanomaterials, Metal Sulfiedbased nanomaterials
Introduction
Cancer is the second leading global cause of death that inflicts more people every year as the lifestyle changes (Caricati-neto et al. 2017; Li et al. 2017a) Current main clinical cancer treatment methods, namely chemotherapy, radiotherapy, and surgery, do not provide satisfactory outcomes and are associated with some limitations as well as inconvenience for the patients as a result of their far-reaching side effects (Zhang et al. 2015d; Wang et al. 2016a, 2017a; Ren et al. 2017). Thus, in order to improve the full treatment chance, chemotherapy is usually supplemented by other methods (Jing et al. 2018). However, not only metastasis is still a serious problem but also the side effects could be exhausting for patients as the drug dose should be progressively increased to overcome the drug resistance of cancer cell (Zhang et al. 2015d; Chen et al. 2017a). For these reasons, the need for development of new generations of cancer treatment methods with enhanced therapeutic efficacy, high selectivity and sensitivity, and very low side effects is well felt.
In this regard, a major share of research for cancer diagnostic and therapy (theranostic) is dedicated to nanotechnology. Applications of nanoparticle for drug delivery, bioimaging, and thermal therapy have been extensively studied. Nanocarriers can significantly increase the drug accumulation in tumor tissue through enhanced permeability and retention (EPR) effect or by targeting and also may overcome the drug resistance by entering the cancer cells resulting in an improved therapeutic effect in comparison to free drug (Guo et al. 2017; Tian et al. 2017b; Xu et al. 2017; Girma et al. 2018; Wang et al. 2018b; Sun et al. 2019).
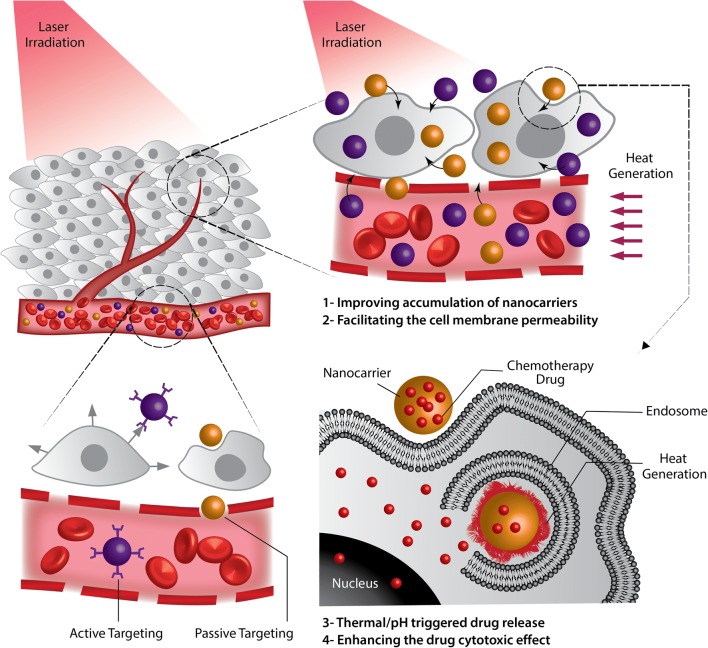
Considering the more sensitivity of cancer cells to high temperatures, nanoparticle-mediated thermal ablation with high precision at tumor tissue serves as a suitable treatment mean (Liu et al. 2014b; Oh et al. 2017). Various nanomaterials have been introduced to possess promising application as remotely controlled thermal therapy agents. Among the two thermal therapies namely magnetic hyperthermia and photothermal therapy (PTT), the latter which benefits from the advantage of high precision, i.e., it can generate heat at the exact tumor site by radiating a near infrared (NIR) laser beam from an external source, is more favorable for clinical application (Oh et al. 2017). This non-invasive method offers different advantages including high selectivity and precision while it is harmless to normal tissues (Khafaji et al. 2016; Gautam et al. 2018; Girma et al. 2018; Zhang et al. 2018b). As presented schematically in Fig. 1, when coupled with targeted drug delivery, PTT can synergically enhance the therapeutic index via four different mechanisms: (i) improving the accumulation of the nanocarriers in the tumor tissue, (ii) facilitating the cell membrane permeability, (iii) enhancing the drug cytotoxic effect, and (iv) triggering the drug release at the target site (Li et al. 2017a; Cao et al. 2018). Overall, in comparison to regular sequential treatments, the simultaneous combination of chemo- and photothermal therapy (CT-PTT) significantly enhances the treatment efficacy so that the complete eradication of cancer with minimal side effects and no metastasis has been reported by only two cycles of combination therapy (Ma et al. 2013). As a result of this, quite ample studies have been aimed to develop advanced nanostructures for CT-PTT.
Fig. 1.
Photothermal therapy (PTT) in combination with targeted drug delivery. The required heat at the exact tumor site is generated by radiating a near infrared (NIR) laser beam from an external source. This method can synergically enhance the therapeutic index via improving the accumulation of the nanocarriers in the tumor tissue, facilitating the cell membrane permeability, enhancing the drug cytotoxic effect, and triggering the drug release at the target site
Due to their bioimaging ability, high thermal conversion efficiency, versatility, ease of synthesis, and surface modification, inorganic nanoparticles are of special interest for CT-PTT (Zhang et al. 2015a; Li et al. 2016c; Gautam et al. 2018). Gold and iron-based nanoparticles, as well as transition metal sulfides, are among the most prevalent types of photosensitive inorganic nanomaterials for this application.
Gold-based nanoparticles are among the first generations of nanomaterials introduced for combination therapy due to their deep-seated application as PTT agent (Huang et al. 2006). By altering the size or shape of nanoparticles, their surface plasmon resonance can be adjusted to the wavelength in the NIR region to achieve the maximum thermal conversion for PTT application (Kim et al. 2017; Li Volsi et al. 2017; Ji et al. 2018). In an optimized condition, a temperature of above 42 is achievable in a short time with a low laser power density. Nevertheless, in order to achieve effective gold-based nanocarriers with improved biocompatibility, surface modification is necessary, yet it may hinder their surface plasmon resonance for PTT. Huge efforts have been dedicated to investigating new gold-based nanoparticles with different structures and modifications to overcome this challenge (Zhang et al. 2015e; Deng et al. 2016; Zhou et al. 2017; Hernández Montoto et al. 2018).
Iron-based nanoparticles, unlike gold-based ones, are considerably less expensive to produce and are more biocompatible so that some types of them have the approval from the Food and Drug Administration (FDA) for clinical usage (Dacarro et al. 2018). Some types of iron-based nanomaterials offer a comparable molar excitation coefficient with gold nanoparticles (Xue et al. 2015a), while superparamagnetic iron oxide nanoparticles (SPIONs), which have weaker NIR absorption, enjoy the advantage of magnetic targetability (Guo et al. 2017). Although it is less than a decade that the application of these nanoparticles for CT-PTT is being investigated, considerable publications with various designs and modifications are available in this field some of which are really promising. In addition, these nanoparticles can serve as magnetic resonance imaging (MRI) contrast agents making them good candidates also for theranostic applications (Ansari et al. 2018; Sun et al. 2019).
Transition metal sulfides are another group of inorganic nanomaterials which thanks to their high photothermal conversion potency in a broad range of NIR wavelengths, bioimaging ability, low toxicity, and low production cost have been the subject of several studies for cancer therapy in recent years (Liu et al. 2014a; Zhang et al. 2018a). The NIR absorption in these nanomaterials is derived from d–d energy band transition; therefore, unlike gold nanoparticles, it is not a function of particles geometry or dielectric coefficient of the media (Yang et al. 2015). Additionally, due to the low surface functionality, they are not facile drug carriers; hence, their application for CT-PTT has not still been widely studied and, in comparison to gold and iron NPs, they are at their early stages of development.
The aim of this paper is to give an overview of recent publications and advances in the use of photosensitive inorganic-based nanocarriers for combination cancer therapy. We develop examples of the methods used for drug loading and surface modifications of common inorganic photothermal agents and evaluate their behavior in vitro and in vivo. Finally, we critically investigate their cons and pros as a new generation of cancer therapy agents.
Gold-based nanostructures
Due to their unique property of “surface plasmon resonance,” gold-based nanostructures were the first nanomaterials that were studied for cancer photothermal therapy in 2006 (Huang et al. 2006). The foremost advantage of gold-based nanostructures over other nanomaterials is their surface plasmon resonance which can be adjusted to the desired wavelength by changing the particles size and shape to achieve the maximum NIR absorption and thermal conversion efficiency (Huang et al. 2006; Xu et al. 2017). In noble metals, the coherent collective oscillation of electrons in the conduction band induces large surface electric fields which greatly enhance the radiative properties of gold and silver nanoparticles when they interact with resonant electromagnetic radiation. In addition, it is realized that the strongly absorbed radiation is converted efficiently into heat on a picosecond time domain due to electron-phonon and phonon-phonon processes (Huang et al. 2006). However, the photothermal conversion efficiency of some gold-based nanostructure such as gold nanorods and branched nanostructure is higher than other ones, but it strongly affected by the plasmonic resonance wavelength, nanostructure volume, coating shell, and assembly state (Wang et al. 2014a). The other special advantage of these nanomaterials is the possibility of direct surface functionalization through self-assembly of sulfur ended molecules, thanks to the intrinsic affinity between gold and sulfur atoms. Gold-based nanostructures have been widely used as drug carriers. Drug molecules can be attached to the surface of polymer/silica-coated solid gold nanostructures or be encapsulated into the hollow ones. Surface modification also enhances the biodistribution and biocompatibility of the gold-based nanostructures and has a significant role in controllability of drug release. Given the mentioned capabilities, gold-based nanostructures have become excellent candidates for CT-PTT. As far as the morphology is considered, gold-based nanostructures can be classified to five different groups: (a) gold nanorods, (b) gold nanoparticles, (c) hollow gold nanostructures, (d) gold shells, and (e) branched gold nanostructures, which are elaborated upon in detail hereunder.
Gold Nanorods
Due to their anisotropic shape, tunable aspect ratio, strong optical absorption at NIR region with almost 100% light-to-heat conversion efficiency, prolonged circulation time, enhanced accumulation in tumors, and well-established synthesis and surface modification procedures, gold nanorods (GNRs) are the mostly investigated nanostructures in PTT (Zhang et al. 2015e; Wang et al. 2017c; Xu et al. 2017; Sun et al. 2018). However, there are some drawbacks that severely limit their applications in CT-PTT including poor stability in the physiological condition, low drug loading capacity, and potential toxicity from cetyltrimethylammonium bromide—the capping agent that used during their synthesis (Li et al. 2014; Xu et al. 2017; Zhou et al. 2017; Sun et al. 2018). In order to overcome these shortcomings, various modifications were presented in recent years.
Coating GNRs with a mesoporous silica shell seems to be the simplest way to improve their drug loading capacity, biocompatibility, and in vivo colloidal stability, but it does not provide a proper control over the drug release. Zhou et al. used hyaluronic acid (HA) as the second coating material which not only prevented the early drug leakage, but it also targeted the GNRs to the cancer cells which have CD44 receptor overexpressed on them. In this nanosystem, the drug released in the tumor microenvironment through degradation of HA shell by hyaluronidase enzyme (Zhou et al. 2017). Zhang et al. grew polyamidoamine (PAMAM) dendrimers onto the surface of silica-coated GNRs which enabled the attachment of siRNAs to GNRs for chemo/photothermal/gene therapy application (Wang et al. 2017c). Because of the high loading capacity of mesoporous silica, Xu et al. loaded 89Zr radioisotope alongside with the chemotherapy drug on PEGylated silica-coated GNRs to achieve a single nanoplatform for combination therapy and photoacoustic tomography applications (Sun et al. 2018).
Polymers are also good coating candidates to increase the biocompatibility and drug loading capacity of GNRs. There are several articles reporting a successful targeting and enhanced combination therapy by coating GNRs with various polymers such as inulin, α,β-poly (N-2-hydroxyethyl)-dl-aspartamide, and PAMAM dendrimer (Li et al. 2014; Li Volsi et al. 2017). Liao et al. encapsulate GNRs and doxorubicin (DOX) molecules in mPEG-PCL polymersome achieved a more drug loading content, less cytotoxicity, and controlled release. Under local hyperthermic condition, the polymersome melt or destroyed and the anticancer drug released (Zhang et al. 2015e). To reduce the immune responses and to prolong the blood circulation time, natural biopolymers and proteins are better coatings. Xu et al. used HA decorated GNRs for combination therapy. HA acts as an active targeting agent and improves cellular uptake. Also, DOX molecules were effectively loaded via acid-labile hydrazine linkage and released in response to acidic pH and photothermal conditions (Xu et al. 2017). Li et al. applied albumin modification to promote the GNRs stability, biocompatibility, and cellular uptake via albumin binding protein pathway. By this surface, modification hydrophobic drugs like paclitaxel could easily be loaded in the albumin lipophilic domain (Chen et al. 2017d).
To have both the therapeutic and diagnostic functions with collective physicochemical properties, multicomponent nanostructures have been raised as attractive multifunctional nanosystems. In this regard, Wang et al. synthesized aptamer targeted GNRs coated with a carbon layer which resulted in an excellent fluorescent emission. In addition, the aromatic anticancer drugs were efficiently loaded on the carbon shell via π–π interaction. Therefore, the nanoparticles could be used for bioimaging in addition to the combination therapy (Wang et al. 2016b). Because of their high drug loading capacity and special physicochemical properties, metal organic frameworks (MOFs) are among the best candidates for modification of GNRs. Zeng et al. were proposed porphyrinic zirconium-based MOF as a nanoshell which demonstrated excellent fluorescence emission as well as photodynamic therapy ability. Thus, these hybrid MOF–GNR nanoplatforms could be used simultaneously for bioimaging in addition to photodynamic/photothermal/chemotherapy (Zeng et al. 2018). Although many of these studies are still in the early stage of drug development, their remarkable results represent their promise as new generations of multifunctional anticancer drugs.
Gold nanoparticles
Ease of synthesis, inherent biocompatibility, versatile surface functionalization, and fine-tunable localized surface plasmon resonance (LSPR) are the properties that make gold nanoparticles (GNPs) to be considered for CT-PTT application (Zheng et al. 2016). Synthetic polymers are generally used to tune the plasmonic wavelength and to enhance the biocompatibility and stability of GNPs. However, there are obvious drawbacks that hinder the biomedical applications of these nanoparticles which must be addressed including limited LSPR in the visible range, low drug loading capacity, complicated fabrication procedures, and presence of toxic reducing agents and non-biodegradable polymeric templates. Employing natural, biocompatible, and biodegradable polymers is an effective way to overcome these problems. To this aim, GNPs were coated with polymers such as fucoidan, a natural polysaccharide and polypeptide which resulted in enhanced combination therapy (Kim et al. 2017; Nam et al. 2018; Yang et al. 2019). To increase the drug loading capacity, porous nanoparticles were synthesized through the intraparticle alloying and dealloying process. DOX molecules were loaded onto surfaces of gold-nanosponges through electrostatic interactions. The pH- and thermal dual-stimulus-responsive capability was provided by the poly (N-isopropylacrylamide–methacrylic acid–1,4-dioxane, octadecyl acrylate) (p (NIPAM-MAA-ODA)) copolymer, which was further incorporated with liposome to serve as a gate-keeping shell. By conjugating amine-functionalized cell-targeting aptamers (EpDT3–NH2), targeted delivery of DOX was achieved. As a result of the high drug loading content and active targeting, combined CT-PTT killed 98% of the cancer cells, which is a promising outcome (Zheng et al. 2016).
Hollow gold nanostructure
In view of their high drug loading capacity, strong and tunable surface plasmon resonance in NIR region, and high photothermal conversion efficiency, hollow nanostructures are proposed as the promising gold-based agents for combination therapy (Deng et al. 2016; Sun et al. 2017). Among the several hollow nanostructures, gold hollow nanospheres (GHNSs) and gold nanocages (GNCs) have been strongly investigated owing to their well-established synthesis process. As for Au nanocages, they were prepared using a galvanic replacement reaction between Ag nanocubes and HAuCl4. However, these nanostructures may suffer from drug leakage because of their hollow interior and wall pores. Also, the inadequate stability, quick aggregation in physiological conditions, and poor immune-escape capacity limit their biological applications (Li et al. 2018b; Zhu et al. 2018). To overcome these deficiencies, coating of drug loaded GHNSs and GNCs has been mostly studied. Polyethylene glycol is the biocompatible polymer that has been widely used for this goal. Although the PEG coating prolongs the blood circulation time of nanoparticles and enhances their biodistribution, it is not effective enough in controlling the drug release (You et al. 2012). Using high-molecular weight HA alongside with PEG can improve the biocompatibility and limit the release of encapsulated drugs in undesired sites (Wang et al. 2014b; Sun et al. 2017). In order to stimulate the drug release in the target tissue during the photothermal treatment, thermosensitive polymers are excellent coating materials (Deng et al. 2016; Ji et al. 2018). As Ji et al. reported, almost complete controlled drug release was achieved by coating the GNCs with thermally responsive P (NIPAM-co-Am) copolymer as a NIR stimuli gatekeeper (Ji et al. 2018). Although coating the nanoparticle with a variety of polymers provides controlled drug delivery, the early removal of nanoparticles by the immune system needs to be addressed. Therefore, new approaches to ensure more effective tumor targeting with a reduced macrophage cell uptake are highly desirable (Li et al. 2018b; Zhu et al. 2018). To this aim, encapsulation of GHNSs in DOX-loaded thermo-sensitive liposomes was reported by Li et al. By this method, several GHNSs were encapsulated in a single liposome, which resulted in an enhanced aggregation of photothermal agents into cancer cells which led to an improved photothermal treatment. During the photothermal treatment, the thermo-sensitive liposomal membrane disrupted and the drug released at the tumor site (Li et al. 2018b). As another encapsulation method, nanoparticles cloaked with natural cell membranes. The biomimetic cell membrane coatings significantly increase nanoparticles stability, blood circulation time, ability of immune escape, and limit the drugs leakage in physiological conditions. Zhu et al. reported the anti-EpCam antibody modified RBC vesicles that were capped with paclitaxel loaded GNCs. Upon NIR laser irradiation at 808 nm, this biomimetic nanoplatform can release paclitaxel and generate hyperthermia to realize combination of photothermal and chemotherapy (Zhu et al. 2018).
Gold nanoshells
Gold nanoshells (GNSs) are another group of the gold-based nanostructures which were investigated for CT-PTT. GNSs are more biocompatible comparing to other gold nanostructures. As an example, pilot clinical studies with PEG-coated GNSs with a diameter of about 150 nm have been approved by FDA and given intravenously to patients for the treatment of head and neck cancer, as well as primary and/or metastatic lung tumors (Wang et al. 2013). The LSPR of gold nanoshells exhibits a redshift from visible to NIR region in comparison to gold nanoparticles. By changing the core size, surface plasmon resonance of GNSs can also be easily tuned to the desired wavelength (Gobin et al. 2008; Luo et al. 2016a). Although there are several reports on the application of GNSs with solid cores for photothermal therapy, the hollow and porous cores are usually preferred as they enable the encapsulation of nearly all kind of drugs for efficient CT-PTT (Huang et al. 2018). Liu et al. studied silica nanorattles as mesoporous biocompatible cores which were exhibited higher drug loading capacity and sustained release (Liu et al. 2011). Luo et al. were synthesized gold-coated oleic acid/chitosan/soya lecithin liposomes for combination therapy (Luo et al. 2016a). Liu et al. used a liposome core/branched GNSs. The rough shells have large absorption cross section, higher molar extinction coefficient, and exhibit stronger electromagnetic field, so they can demonstrate higher photothermal efficiency. Although the drug loading efficiency was high in liposomal-based systems, almost 50% of encapsulated drug molecules could escape through the gold shell openness in physiological conditions (Liu et al. 2018). To have a better control on the drug release, Huang et al. investigated a gold-coated DOX-loaded poly (aspartic acid-butanediamine)-poly (2-(diisopropylamino)ethyl methacrylate) diblock copolymer micelles. This copolymer is pH-sensitive, so the drug was released in the acidic environment of lysosome (Huang et al. 2018).
Branched gold nanostructures
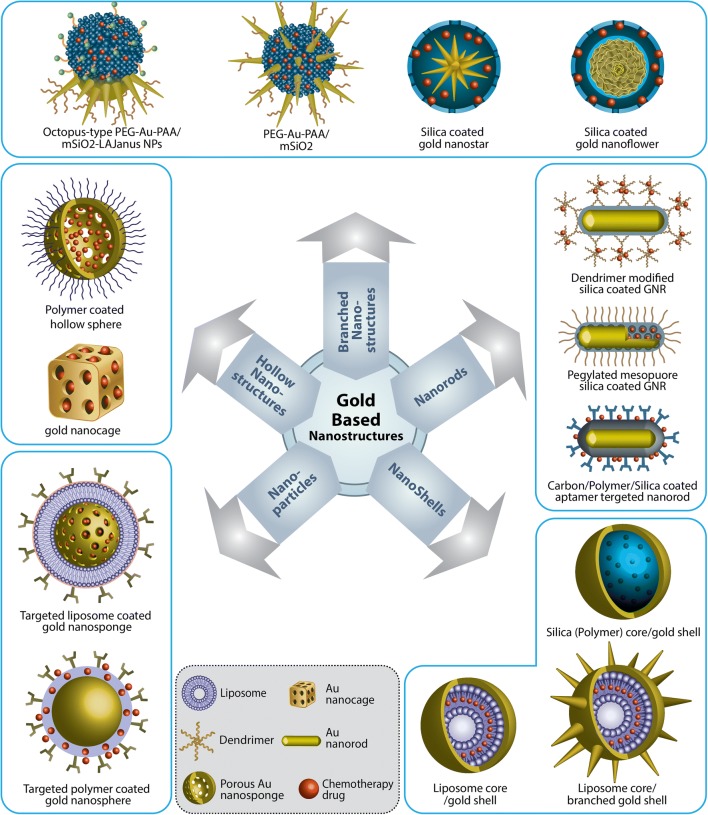
Among gold-based nanostructures, anisotropic multibranched nanoparticles such as gold nanostars and nanoflowers have attracted more attention in recent years. These nanostructures have several sharp branches which can act as hot spots and amplify the electromagnetic field and result in a high light-to-heat conversion efficiency. In addition, ease of synthesis for large-scale production and LSPR in the NIR region makes them excellent candidates for photothermal therapy (Ma et al. 2013; Hernández Montoto et al. 2018; Nam et al. 2018). The most notable drawbacks of these nanoparticles are their limited stability, reshaping or intraparticle ripening, and partial oxidation of tips (Song et al. 2016; Hernández Montoto et al. 2018). To overcome these issues, Song et al. proposed silica coating. They synthesized gold nanoflowers and coated them with a thin layer of silica to protect them from intraparticle ripening. To improve drug loading capacity, the resulted nanoparticles coated by a thick layer of mesoporous silica (Song et al. 2016). Zhang et al. synthesized octopus-type Janus nanoparticles with enhanced biocompatibility, stability, blood circulation time, and targeting. They used polyacrylic acid nanoparticles as a template to specifically grow a mesoporous silica shell on one side of particles and gold branches separately modified with methoxy-poly(ethylene glycol)-thiol on the other side and targeted them by attaching lactobionic acid on the silica surface. As a result of this smart design, the targeted hybrid nanostructure showed improved CT-PTT (Zhang et al. 2016). Li et al. were utilized calcium phosphate as a biodegradable, pH-responsive, and nontoxic platform to grew GNR assemblies in a single nanoparticle with an enhanced photothermal efficiency (Li et al. 2017b). Nam et al. were proposed polydopamine-coated spiky gold nanoparticles as a new photothermal agent with extensive photothermal stability and efficiency. This new nanoplatform could elicit robust antitumor immune responses and eliminated local as well as untreated distant tumors in > 85% of animals (Nam et al. 2018). In order to have a better control over the drug release and to increase the nanostructures biocompatibility and targeting, Chen et al. synthesized glutathione (GSH)-modified gold nanostars by a green method and linked the tripeptide Arg-Gly-Asp (RGD) as a targeting agent and DOX molecules covalently to the surface of nanoparticles. In this system, DOX released as a result of the replacement of GSH on the surface of nanoparticles by the abundant intracellular GSH (Ma et al. 2013). Complete controlling on the drug release from mesoporous silica-coated gold nanostars was achieved by capping the drug-loaded nanostructure with paraffin gates which can melt during the photothermal therapy (Hernández Montoto et al. 2018). It can be concluded that although the branched gold nanostructures demonstrate an excellent light to heat conversion, to overcome their instability, it is necessary to protect them with a biocompatible shell like silica before biological use. In Fig. 2, novel candidates of gold-based nanostructures for CT-PTT are schematically illustrated.
Fig. 2.
Five different classes of gold-based nanostructures: gold nanorods, nanoparticles, hollow nanostructures, shells, and branched nanostructures which are introduced as candidates for CT-PTT
Iron-based nanomaterials
Iron-based nanomaterials have demonstrated numerous therapeutic and diagnostic applications including for iron deficiency treatment, MRI, drug delivery, photodynamic therapy, and recently for photothermal therapy (Li et al. 2017a, 2019). Today, a few types of these nanomaterials are approved by the FDA while there are still many high-potential ones which their applications have been limited only to academic publications. Although iron-based nanomaterials have not a long age history in photothermal therapy, considerable efforts have been dedicated to using them for the combination of photothermal and chemotherapy under two major categories: (a) iron oxide-based nanoparticles and (b) metal–organic frameworks.
Iron oxide-based nanoparticles
Due to their excellent biocompatibility, high stability in the physiological environment, and biodegradability, iron oxide nanoparticles (IONs) have been used for both diagnostic (imaging) and therapeutic purposes (Shen et al. 2017; Zhang et al. 2018b). Recently, the NIR thermal conversion properties of various shapes of IONs have been studied both in vitro and in vivo which demonstrate the great promise of IONs for PTT (Wu et al. 2017; Shen et al. 2017; Hu et al. 2018). In order to achieve a remotely controlled drug release system, the drug molecules can be attached to the ION surface through a thermosensitive bond (Chen et al. 2016; Wu et al. 2017). During the PTT, by increasing the temperature, the thermal-cleavable linker could decompose and result in a burst release in the tumor site. Nevertheless, this method may not be a perfect option as the drug should be camouflaged for the drug-resistant cancer cells.
One of the most straightforward choices to address this issue, while preserving other advantages (i.e., magnetic targetability, MRI, and controlled release), is to use a biocompatible and biodegradable polymer to entrap both SPIONs and the drug molecules in individual nanospheres. These nanoparticles can be actively targeted by simply choosing a polymer that has a specific interaction with receptors which are overexpressed on cancer cells (Luo et al. 2016b; Zheng et al. 2018) or by conjugating specific targeting ligands on their polymeric surface (Wang et al. 2018c). Guo et al. prepared a nanotheranostic agent composed of carboxyl-modified PEGylated poly (lactic-co-glycolic acid) (PLGA-PEG-COOH) as the base material to entrap SPIONs, drug, and perfluorohexane (PFH) and actively targeted them by conjugating the antibody Herceptin on their polymeric surface (Guo et al. 2017). Upon exposure to NIR, SPIONs generate heat for PTT which also trigger the optical droplet vaporization of PFH and generate PFH gas microbubbles. This phenomenon not only provides ultrasound-imaging ability to monitor the treatment procedure but also synergically improves the tumor therapeutic efficacy through three different mechanisms: (1) enhancing the drug release from nanoparticles, (2) enhancing the cell permeability by cavitation effect, and (3) physically intoxicating cancer cells by rapid increase in the volume of PFH. In an attempt to combine the PDT with CT-PTT, Zhu et al. synthesized CuS-coated SPIONs and co-loaded them with a drug in gelatin nanoparticles (Zhu et al. 2017). Nevertheless, although this formulation could generate reactive oxygen species (ROS) in cancer cells under laser irradiation and resulted in higher apoptosis, they could not outperform the free drug in cancer cell killing.
In another approach, in order to make most of the intracellular drug delivery and cell internalization, single SPIONs are encapsulated in a drug-loaded polymeric shell which results in smaller particle size in comparison to the aforementioned method (Luo et al. 2016c; Oh et al. 2017; Shen et al. 2017). However, due to the smaller polymeric portion, these nanoplatforms lack the sufficient capacity for drug loading. In order to enhance the drug loading capacity of these systems, different methods are studied to produce a hollow space underneath the external polymeric shell. As a first attempt, a mesoporous silica layer was designed as a mid-layer which offers more space for drug loading (Luo et al. 2016b). In another study, Shen et al. used such silica layer as a sacrificial material to achieve a yolk–shell structure with a hollow space between the SPION and a thermosensitive polymeric shell (Shen et al. 2017). In this method, the drug loading capacity increased by 2.5-fold after etching the silica layer. Recently, Zhang et al. introduced a virus-like particle by embedding the drug-conjugated SPION in a hepatitis B virus core antigen (HBc) (Zhang et al. 2018b). As a natural nanocarrier, HBc presented empty interior space that can enhance the stability and biocompatibility of the nanodrugs. As a result of the viral nature of the surface proteins and the smaller particle size, these nanoparticles can easily be internalized by the cancer cells.
It has been shown that although smaller particle sizes are more suitable for cell internalization and intracellular drug delivery, the advantages of larger ION-based nanoparticles (up to the cutoff size for extravasation from the tumor vasculature) excel those of the smaller ones (Guo et al. 2017). Larger magnetic particles offer higher thermal conversion efficiency and are more magnetically targetable so it can be more effectively accumulated in the tumor tissue and result in a more therapeutic effect (Guo et al. 2017). Taking all of this into account, magnetic nanoclusters which offer better PTT (in comparison to singular SPIONs) and considerable space for drug loading could be one of the best options for CT-PTT (Zhang et al. 2015d; Hu et al. 2018). Very recently, Sun et al. introduced a new CT-PTT-PDT platform by encapsulating the chlorin e6 (Ce6)-loaded ION clusters in a drug-loaded thermosensitive polymer (Sun et al. 2019). In this system, the SPIONs generate heat for PTT and melt the coated polymer by NIR laser irradiation with a low power density (230 mW/cm2), resulting in the release of encapsulated DOX. Simultaneously, the exposed Ce6 can also generate cytotoxic ROS under the same NIR light irradiation, thereby resulting in an enhanced combined therapy triggered by a single light.
Another method to overcome the rather poor PTT efficacy of SPIONs is to supplement them by another well-established photothermal agent. To this end, PTT agents can be co-loaded in a polymeric sheath alongside with SPIONs and drug (Chen et al. 2017a; Wang et al. 2018b), or a photosensitive polymer can be employed to load the drug and encapsulate the SPIONs (Chen et al. 2018). In a study conducted by Yang et al., a magnetic mesoporous CoFe2O4@PDA@ZIF-8 sandwich NP was introduced as a theranostic agent for MRI, multidrug chemotherapy, and photothermal synergistic therapy with pH and NIR-triggered release behavior (Yang et al. 2017). In this system, the mesoporous CoFe2O4 core which acts as MRI probe, PTT agent, and loading platform of hydrophilic DOX is coated by a photosensitive polydopamine layer to avoid the early leakage of DOX before arriving at the tumor site and to enhance the PTT efficiency. The ZIF-8, a metal–organic framework, shell serves to encapsulate hydrophobic camptothecin and as the switch for the pH and NIR stimulation-responsive release of the two drugs. By embedding each drug in a separate region, this formulation provided a sequential release profile for the two drugs in the acidic microenvironment of the tumor with an interval of approximately 12 h which has a positive effect on the therapeutic efficiency (Yang et al. 2017).
Another approach to achieve a controlled drug delivery system is attaching IONs to various transmembrane protein channels. Using molecular dynamic (MD) simulation and patch fluorometry, it has been proposed that liposomes in which mechanosensitive channels are reconstituted can be used as drug delivery systems for targeted release of cancer therapy agents (Nakayama et al. 2015; Bavi et al. 2017; Martinac et al. 2017). For this reason, the channels should be either attached to a magnetic particle or be genetically manipulated such that it is, for example, pH or light sensitive (Koçer et al. 2005, 2006). Moreover, there several reports on magnetically mediated activation of different ion channels in vivo that are fused with molecules such as ferritin. It is however proposed that the mechanism of action of these channels fused with ferritin is through magnetic heating of ferritin, hence elevating the entropy of the system after being exposed to a magnetic field (Duret et al. 2019). If true, such effect may be potentially induced through shining laser at channel proteins that are attached to nanoparticles and are reconstituted into liposomes. This way, one can activate the channel which is acting as “nanovalve” and subsequently release the drug exactly at the targeted tissues. This is certainly a potential path for further development in this area.
MOF-based nanoparticles
Metal–organic frameworks (MOFs) are crystalline, porous, and hybrid nanostructures consisting of metal nodes and organic linkers (Gautam et al. 2018). They have shown great potential in biomedical applications and also in cancer therapy as theranostic agents for synergistic combination therapy. Prussian blue (PB), a dark blue pigment with a cubic structure in which Fe(II) and Fe(III) atoms occupy the corners of the cube and cyanide groups positioned on the sides, has been extensively studied for CT-PTT. PB offers numerous features including biocompatibility supported by the FDA approval, long-term stability in a wide range of conditions, biodegradability, controllable shape and size, low production cost, excellent PTT conversion in a broad range of NIR region, magnetic properties, and ease of synthesis (Dacarro et al. 2018; Gautam et al. 2018). PB is reported to be coated or decorated on other nanoparticles to enhance their therapeutic efficiency by PTT effect (Xue et al. 2015a; Santha Moorthy et al. 2018; Li et al. 2019). In a same strategy, PB has been used as a core for different drug-containing shells including polymeric, silica-, and MOF-based materials to supplement them with PTT (Xue et al. 2015b; Su et al. 2016; Wang et al. 2016b, 2017a; Tian et al. 2017a). These hybridizations can add various functions to the CT-PTT such as pH triggered drug release (Wang et al. 2017a), enzyme triggered drug release (Xue et al. 2015b), MRI contrast enhancement (Xue et al. 2017; Li et al. 2019), magnetic targetability (Xue et al. 2017), or just a porous layer for higher drug loading capacity (Su et al. 2016).
Hollow PB nanoparticles have been reported to have an extraordinary drug loading capacity as well as PTT and imaging (Cai et al. 2015). Chen et al. have designed a red blood cell (RBC) membrane camouflaged hollow mesoporous PB nanoparticles (HMPB@RBC NPs) with 130% DOX loading capacity for CT-PTT (Wang et al. 2017d). This nanoagent demonstrated suitable cyto- and hemocompatibility with low immune response. Nonetheless, although the RBC membrane coating enhanced the accumulation in tumor tissue and reduced its accumulation in other tissues, the majority of nanoparticles were still accumulated in the spleen and lung. In order to overcome this issue, routine targeting strategies can be applied to PB-based nanostructures. Jing et al. have employed a HA grafting PEG coating as a capping agent for prolonged blood circulation time and CD44 receptor-mediated tumor targeting into one single agent (Jing et al. 2018). This coating method significantly increased the drug concentration in the tumor in comparison to other tissues. Additionally, in comparison to those nanoparticles coated with only PEG, the drug accumulation in tumor increased to more than two times which is attributed to its suitable size, passive targeting by EPR effect, and the active ligand of HA on the surface, which binds CD44 receptor which is overexpressed in cancer cells.
In order to improve the poor MRI contrast of PB, Cai et al. have incorporated manganese, a well-defined positive contrast enhancer, to hollow mesoporous PB nanoparticles (Zhang et al. 2015a). They coated a Mn-containing PB analogue onto both the outer surface and the inner mesoporous channels of HMPB to form a core–shell hollow structure. A pH-triggered release system was achieved which concurrently releases both Mn2+ ions and DOX molecules; thereby, the dynamic drug release can be monitored by MRI. Some of the distinct nanostructured candidates of iron oxide- and PB-based nanoparticles for CT-PTT are schematically presented in Fig. 3.
Fig. 3.
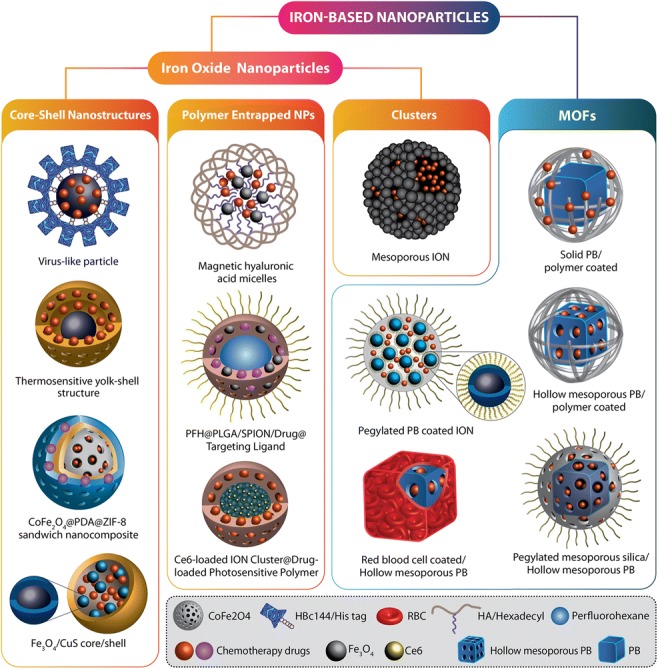
Two major categories of iron-based nanomaterials: iron oxide-based nanoparticles and metal–organic frameworks that are used for the combination of photothermal and chemotherapy
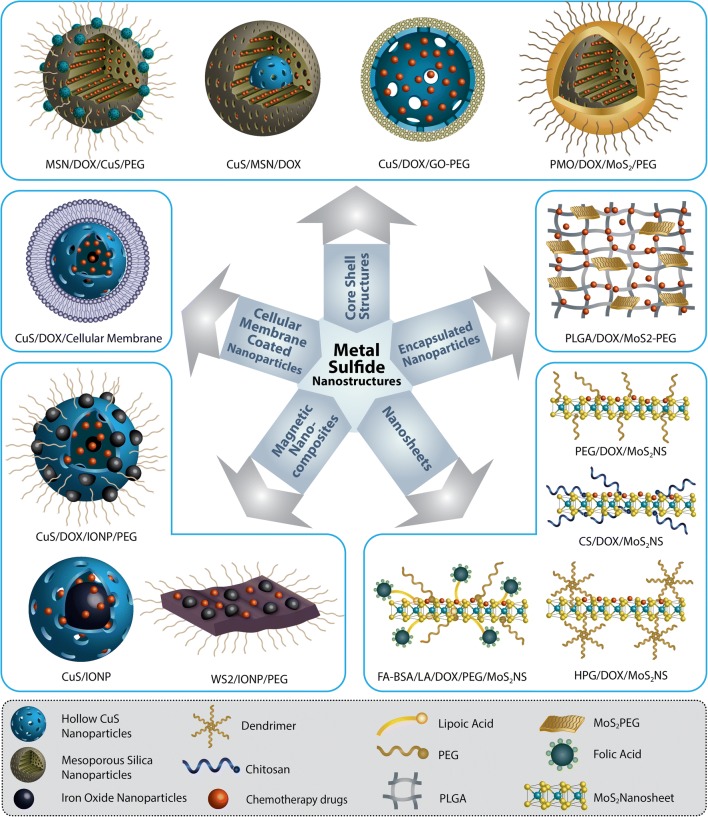
Metal sulfide-based nanomaterials
Recently, metal sulfides have gained more attention due to their low production cost, high photothermal conversion efficiency, and good photothermal stability as photothermal agents. Integrated CT-PTT systems based on metal sulfides have been shown to have potential to increase the efficiency of cancer therapy. These nanomaterials can be classified in two distinct groups: two-dimensional (2D) and three-dimensional (3D) metal sulfide nanostructures.
Two-dimensional metal sulfides
Among the diverse group of 2D nanomaterials, 2D transition metal sulfides have become the focus of various research studies and clinical applications. 2D transition metal sulfides are typically consisting of a layer of transition metal atoms (such as Mo, W, Bi, Ti, Zr, and so on) sandwiched between two layers of sulfur atoms (Zhang et al. 2018a). Molybdenum disulfide (MoS2) is one of the mostly explored 2D metal sulfide nanosheets used as a drug nanocarriers for CT-PTT due to its high specific surface area and efficient photothermal conversion (Gong et al. 2017). However, application of MoS2 was limited by its poor stability and dispersibility in aqueous environments. These disadvantages of MoS2 nanosheets can be overcome by surface modification with various biopolymers including bovine serum albumin (Chen et al. 2017c), PEG (Wang et al. 2015b), chitosan (Yin et al. 2014), glutathione (Liu et al. 2016), silk fibroin (Li et al. 2017c), poly (acrylic acid) (Chen et al. 2017b), and soybean phospholipid (Li et al. 2016a). For the first time, Liu et al. reported the great potential of using MoS2 nanocarriers as a promising candidate for CT-PTT (Liu et al. 2014a). MoS2 nanosheets were functionalized with lipoic acid modified PEG (LA-PEG) and a specific cancer cell targeting ability was promoted by incorporation of folic acid to the surface which binds the folate receptors on cancer cells. The highest DOX loading ratios (weight ratios between the DOX and MoS2) reached ~ 239%, which was much higher than DOX loading ratios on PEGylated graphene-based nanocomposites which were found to reach ∼ 150% (Sun et al. 2008). After either intratumoral or intravenous administration, drug-loaded MoS2 nanocarriers exhibited remarkable in vivo synergistic anticancer effects. It is also reported that branched polymers are more effective in enhancement of blood circulation time in comparison to linear ones. For this reason, hyperbranched polyglycidyl (HPG)-modified MoS2 was developed as a desired formulation to prolong the blood circulation time of nanocarriers (Wang et al. 2017b). In order to enhance the biocompatibility of MoS2 nanocomposites, Zhang et al. constructed a smart folic acid-grafted bovine serum albumin functionalized MoS2 nanosheets to permit targeting of human breast cancer cells with an extra PEGylation to enhance their dispersibility and colloidal stability (Zhang et al. 2019). In an effort to find a simple, low-cost, and high-throughput method to synthesis mono layered MoS2 for combined CT-PTT, Yin et al. fabricated chitosan-functionalized MoS2 nanosheets as a highly effective NIR stimuli-responsive drug delivery system with appropriate photothermal conversion efficiency (Yin et al. 2014). The controlled release of the non-covalently loaded DOX in this system could completely eradicate the tumor cells in vitro and in vivo showing the synergistic efficacy of CT-PTT.
In another approach, surface modification using silica has been investigated in order to overcome problems like poor colloidal dispersity after drug loading and structural limitations to the types of compatible drugs with these nanocarriers. Mesoporous silica nanostructures are widely used in drug delivery due to good biocompatibility, large surface and cavity volumes, controllable porosity, good stability, and facile modification. The DOX loading content in a mono layered MoS2 nanosheets coated with a porous silica shell was reported to be as high as 230 mg/g (Lee et al. 2016). In another work, a NIR-light-triggered MoS2 nanoplatform wrapped DOX-loaded periodic mesoporous organosilica (PMO) was synthesized and then decorated with PEG which demonstrated excellent synergistic efficiency in mice (Shao et al. 2016).
Unlike “dispersed-in-suspension” drug/carrier manner, Wang et al. encapsulated MoS2 nanosheets along with DOX molecules in a PLGA matrix based on the phase transforming behavior of PLGA polymer (Wang et al. 2015a). This formulation exhibited numerous advantages including CT-PTT with a very low dosage of photothermal agents and drug molecules, photoacoustic imaging, and no leakage of MoS2 nanosheets and DOX into bloodstream. In vivo studies indicated that release of encapsulated DOX molecules was triggered by NIR laser irradiation and the synergistic CT-PTT caused significant coagulation necrosis of tumor tissues.
Tungsten disulfide (WS2) nanosheets are another metal sulfide nanoparticles evaluated for CT-PTT applications. Cheng et al. reported the in vivo tumor ablation potential of WS2 nanosheets as photothermal agents for the first time (Cheng et al. 2014). WS2 nanoplatforms with high surface area are ideal for physical or chemical drug loading (Yong et al. 2014). WS2 quantum dots (WQDs) with smaller lateral size layered structure (≤ 5 nm) have great potential for medical application due to their excellent size for evading the reticuloendothelial system absorption and effective excretion through kidney. Liao et. al. introduced a WQD-coated DOX-loaded PMO drug delivery system for light triggered drug release (Liao et al. 2018). They indicated that the fabricated nanoplatform possesses a great potential for combined chemo-photothermal cancer therapy. WS2 nanosheets can also act as a good imaging contrast agents. Cheng et al. described coating of IONs self-assembled WS2 with porous silica and PEG for multimodal imaging and chemo/photothermal therapy (Cheng et al. 2015).
Three-dimensional metal sulfides
Recently, CuxS (x = 1–2) nanoparticles as a new type of nanomaterials with adjustable photothermal conversion efficiency emerged as promising agents in cancer treatment (Feng et al. 2015). Among these materials, copper sulfide (CuS) nanostructures not only have comparable NIR absorption as gold but also have some advantages over gold such as ease of synthesis, low toxicity, low production cost, and constant NIR light absorption (Li et al. 2010). The most common type of CuS nanostructures studied for CT-PTT is their mesoporous silica-coated ones (Liu et al. 2015; Yang et al. 2015; Wu et al. 2015; Peng et al. 2017). These nanocomposites can produce lethal heat upon NIR light irradiation for photothermal cancer treatment and release the payload from the mesoporous silica shell in a pH and NIR light–responsive manner for chemotherapy. CuS nanoparticles were also reported to be coated on the outer surface of mesoporous silica and carbon nanoparticles as another approach to achieve a dual-modal therapeutic agents for cancer therapy (Liu et al. 2014a). Lu et al. reported an in situ expansion of CuS nanoparticles on PMOs by growing them on their thiol bonds (Zhang et al. 2017). CuS nanoparticles can also be adsorbed by an electrostatic interaction on the surface of porous Se@SiO2 nanospheres (Wang et al. 2018d). CuS-covered mesoporous carbon nanosphere (MCN) was investigated by Zhang et al. as an improved drug carrier for effective CT-PTT (Zhang et al. 2015b). Intracellular drug release can be facilitated by appropriate MCN carriers because of their high surface area and large and uniform porous structure. Moreover, MCNs and DOX-specific interactions allow pH-dependent DOX loading (pH ≥ 7.4) and release (pH ≤ 5.5).
Hollow mesoporous CuS nanoparticles (HMCuS NPs) are other common nanoparticles in this group which thanks to their high surface area and homogeneous pore structure are also good drug carriers. Nevertheless, similar to other porous nanomaterials, early drug leakage in the blood stream is a serious challenge for these structures which could be resolved by an extra surface coating. Different materials have been studied as the coating for the outer surface of HMCuS NPs such as multifunctional hyaluronic acid (Feng et al. 2016), iron-dependent artesunate (Hou et al. 2017), and PEGylated graphene oxide nanosheet (Han et al. 2017). Cellular membrane-coated hollow CuS nanoparticles were reported by Wang et al. which in comparison to the bare CuS nanoparticles exhibited higher in vitro source cell line recognition and high in vivo source cells targeting ability with an almost 100% efficiency for melanoma tumor growth inhibition through CT-PTT (Wang et al. 2018a).
Enzyme-responsive CuS nanoparticles are another structure that reported for combined cancer therapy (Zha et al. 2013; Zhu et al. 2017). Gelatin, a natural macromolecule with excellent properties, can be employed as a surface coating for CuS nanoparticles to release the payload at the tumor site where the overexpressed gelatinase can hydrolyze the coating and trigger the drug release.
In order to improve the photothermal and photodynamic property of CuS nanoparticles, integration of plasmonic noble metals and CuS nanoparticles has been taken into consideration. Yolk–shell nanoparticles with Au core@void@CuS shell structure were prepared by Chang et al. for potential CT-PTT (Chang et al. 2018).
For the purpose of smart tumor targeting, Zhang et al. incorporated two complementary DNA sequences to mesoporous silica conjugated CuS nanoparticles (Zhang et al. 2015c). The de-hybridization of DNA duplex was happened by laser generated heat and subsequently by DOX released. Another smart chemo/photothermal agent for cancer therapy was developed based on aptamers (Meng et al. 2017). Cu1.8S nanoparticles covered by aptamer/PEG and conjugated with MoS2 nanosheets were developed to enable effective detection of cancer-related miRNA. Efficient cancer cell treatment was obtained by NIR-triggered DOX release from this smart nanocomposite.
Bismuth sulfide (Bi2S3) nanoparticles are other 3D metal sulfides which due to their high X-ray attenuation coefficient, cost-effectiveness, biological tolerance, and long circulation half-lives can be used in the biomedical field. Li et al. demonstrated highly porous PEGylated Bi2S3 as a simple but powerful multimodal imaging-guided CT-PTT nanoagent for precise and efficient cancer treatments (Li et al. 2016a). In another approach, polyvinylpyrrolidone-decorated Bi2S3 nanorodes were encapsulated with a mesoporous silica layer and loaded with DOX. Trastuzumab as a monoclonal antibody was also conjugated to nanostructure for smart targeting breast cancer cells. The multifunctional Bi2S3 nanoplatform shows excellent cancer-targeted bioimaging and cancer metastasis prevention (Li et al. 2018a). In Fig. 4, different candidates of transition metal sulfides for CT-PTT are schematically illustrated.
Fig. 4.
Five different classes of transition metal sulfides: core–shell structures, encapsulated nanoparticles, magnetic nanocomposites, nanosheets, and cellular membrane–coated nanoparticles which are reported as theranostic agents for CT-PTT
Other nanostructures
Besides the above-discussed nanomaterials, some other inorganic nanomaterials have been demonstrated to have the potential for CT-PTT which can be categorized into two groups based on their light to heat conversion mechanisms. The first group is the nanomaterials that generate heat through the surface plasmon resonance including noble metal nanostructures. In these nanostructures, the conduction band electrons absorb light and produce heat by vibration. Similar to gold nanostructures which were discussed earlier, palladium and cupper belong to this group that have been studied in various 2D and 3D shapes and with several modifications which showed promising results both in vitro and in vivo. The ability to adjust the surface plasmon by variation in nanostructure geometry and drug loading through coordination bonding is the major advantage of these nanomaterials (Tang et al. 2014; Lin et al. 2015; Shi et al. 2016; Song et al. 2018).
The second group consists of nanomaterials that produce heat by intervalence electron transition between molecular/atomic orbital energy levels where the energy gap matches the light energy in the NIR region, including selenides, oxides, and tellurides of transition metals. Selenium (Se) is a vital trace element which can reduce the occurrence and fatality of liver, prostate, and lung cancers. Among transition metal chalcogenides, selenides of Ni, Mo, Bi, and Co have been of higher interest due to their high photothermal conversion efficiency (above 30%) and photoacoustic imaging ability. Still, surface coating is necessary for drug loading and environmental stability of these nanostructures (Chen et al. 2015; Li et al. 2016b, c, 2017b; Wang et al. 2016a, 2017e).
Transition metal oxides are other chalcogenides that are of particular interest due to their adjustable NIR absorption. Due to their structural diversity and significant NIR absorption, oxides of molybdenum and tungsten have been the mostly investigated transition metal oxides. The application of RbxWO3 nanorods, MoO3−x hollow nanospheres, and polymolybdate clusters as theranostic agents for simultaneous imaging and CT-PTT has been studied so far. It seems plausible to see abundant publications using transition metal oxides in this field in the upcoming years (Tian et al. 2014; Bao et al. 2016; Vimala et al. 2017).
In comparison to other chalcogenides, transition metal tellurides, such as MoTe2 and Cu2Te, though demonstrated numerous advantages including high thermal conversion coefficient, ease of synthesis, biocompatibility, high drug loading capacity, and biodegradability (for MoTe2), have been studied very limitedly only in recent years (Wang et al. 2017f; Ma et al. 2018). Therefore, further studies are required in order to comment on their potentials for cancer therapy application.
Conclusions and perspectives
The foremost aim of combination therapies using nanocarriers is to target the tumor tissue, reduce the undesired side effects, and synergically enhance the therapeutic efficiency. An ideal cancer therapy should cover different aspects such as tumor identification, tumor imaging, and drug delivery for effective cancer eradication. Inorganic nanomaterials include a diverse group of nanoparticles which thanks to their special properties and versatility have been extensively investigated as theranostic agents for combination cancer therapy. Besides being suitable drug carriers and having superb photothermal properties, some types of these nanomaterials also serve as mediators for PDT and magnetic hyperthermia. Nevertheless, although some of these nanomaterials are approved by the FDA for some clinical applications, different inorganic nanostructures exhibited variations in efficacy and toxicity which should be precisely optimized. For instance, in some of the reviewed studies, very high and unsafe laser powers were employed to achieve a desirable cytotoxicity and in a similar manner laser wavelengths rather than 808 nm were employed in some studies which not only may damage the normal flesh but also may not penetrate the body and reach the tumors in the depth. In order to improve their biosafety, dispersibility, environmental stability, and functionality various coating materials have been incorporated in inorganic nanostructures which plays not only a passive role (for example for stealthiness) but also an active and stimuli-responsive role to target the tumor and control the drug release. Furthermore, incorporation of some organic and inorganic compounds of these nanostructures is reported to enhance their therapeutic efficiency by PDT, PTT, or radiotherapy and to enable them for extra imaging applications.
Several novel inorganic theranostic nanostructures have already demonstrated exciting results in the past few years in this rapidly growing field. However, translational efforts are lacking and all these studies are still in the prototype stage and the very few types of these nanostructures that are approved by the FDA are in their simplest form without any functionalization. It has been suggested (Li et al. 2017a) that this issue could be partly due to academic policies in these area which mostly support development of new compounds and high-quality publications rather than clinical development and improvement of existing formulations. Hence, more support is required for validation and clinical improvement of both novel and existing theranostics.
For the clinical and translational applications of nanomaterials for CT-PTT as well as successful bench-to-bedside transition, further studies are required to carefully examine the potential toxic side effects of hybrid nanomaterials both in short and long term in vivo; evaluate the feasibility of producing these nanostructures on a large scale with a facile, reproducible, and inexpensive method; precisely optimize the drug loading and release behaviors to be compatible with the in vivo use, based on the time needed to reach the targeted tumor to minimize the drug dissemination in organs and optimize the accumulated drug dose at the tumor site; and realize the exertion pathways and retention time in tumor tissue for bioimaging applications, to name but a handful.
Inorganic nanostructures have a bright future as potential theranostic agents due to their established unique properties. Nevertheless, extensive interdisciplinary studies, characterization, and modifications are required before it can lead to routine processes and marketing authorizations. Moreover, basic science has taken baby steps towards developing more advanced, yet completely biocompatible, liposomal drug delivery systems which seem promising particularly for cancer therapy. One of the examples is the use of mechanosensitive channel proteins as nanovalves in liposomal drug delivery systems. The idea is to have more control over the drug release process during the treatment and reduce the side effects of the chemotherapy drugs by activating these channels exactly at the targeted tissues. Although still far from being implemented, this is certainly another potential path for further research and development in nanobased drug delivery and cancer therapy field.
Compliance with ethical standards
Conflict of interest
Mona Khafaji declares that he has no conflict of interest. Masoud Zamani declares that he has no conflict of interest. Mortaza Golizadeh declares that he has no conflict of interest. Omid Bavi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mona Khafaji, Email: mona.khafaji@gmail.com.
Omid Bavi, Email: o.bavi@sutech.ac.ir.
References
- Ansari MO, Ahmad MF, Shadab GGHA, Siddique HR. Superparamagnetic iron oxide nanoparticles based cancer theranostics: a double edge sword to fight against cancer. J Drug Deliv Sci Technol. 2018;45:177–183. doi: 10.1016/J.JDDST.2018.03.017. [DOI] [Google Scholar]
- Bao T, Yin W, Zheng X, et al. One-pot synthesis of PEGylated plasmonic MoO3-x hollow nanospheres for photoacoustic imaging guided chemo-photothermal combinational therapy of cancer. Biomaterials. 2016;76:11–24. doi: 10.1016/j.biomaterials.2015.10.048. [DOI] [PubMed] [Google Scholar]
- Bavi N, Nikolaev YA, Bavi O, et al. The biophysics of cell membranes. Singapore: Springer; 2017. Principles of mechanosensing at the membrane interface; pp. 85–119. [Google Scholar]
- Cai X, Jia X, Gao W, et al. A versatile nanotheranostic agent for efficient dual-mode imaging guided synergistic chemo-thermal tumor therapy. Adv Funct Mater. 2015;25:2520–2529. doi: 10.1002/adfm.201403991. [DOI] [Google Scholar]
- Cao J, Chen Z, Chi J, et al. Recent progress in synergistic chemotherapy and phototherapy by targeted drug delivery systems for cancer treatment. Artif Cells, Nanomedicine, Biotechnol. 2018;46:817–830. doi: 10.1080/21691401.2018.1436553. [DOI] [PubMed] [Google Scholar]
- Caricati-neto A, Errante PR, Menezes-rodrigues FS. Emerging a new strategy for the antitumor immunotherapy: pharmacological modulation of the Ca2+/Camp signaling. Interaction. 2017;1:89–97. doi: 10.26502/ami.93650012. [DOI] [Google Scholar]
- Chang Y, Cheng Y, Feng Y, et al. Resonance energy transfer-promoted photothermal and photodynamic performance of gold-copper sulfide yolk-shell nanoparticles for chemophototherapy of cancer. Nano Lett. 2018;18:886–897. doi: 10.1021/acs.nanolett.7b04162. [DOI] [PubMed] [Google Scholar]
- Chen X, Li S-H, Liu G, et al. Co9Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv Mater. 2015;27:3285–3291. doi: 10.1002/adma.201405634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wu L, Liu F, et al. Azo-functionalized Fe3O4 nanoparticles: a near-infrared light triggered drug delivery system for combined therapy of cancer with low toxicity. J Mater Chem B. 2016;4:3660–3669. doi: 10.1039/C5TB02704G. [DOI] [PubMed] [Google Scholar]
- Chen CW, Syu WJ, Huang TC, et al. Encapsulation of Au/Fe3O4 nanoparticles into a polymer nanoarchitecture with combined near infrared-triggered chemo-photothermal therapy based on intracellular secondary protein understanding. J Mater Chem B. 2017;5:5774–5782. doi: 10.1039/c7tb00944e. [DOI] [PubMed] [Google Scholar]
- Chen L, Feng Y, Zhou X, et al. One-pot synthesis of MoS2 nanoflakes with desirable degradability for photothermal cancer therapy. ACS Appl Mater Interfaces. 2017;9:17347–17358. doi: 10.1021/acsami.7b02657. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhou X, Nie W, et al. Marriage of albumin-gadolinium complexes and MoS2 nanoflakes as cancer theranostics for dual-modality magnetic resonance/photoacoustic imaging and photothermal therapy. ACS Appl Mater Interfaces. 2017;9:17786–17798. doi: 10.1021/acsami.7b04488. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen B, Huang Y, et al. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm Sin B. 2017;8:74–84. doi: 10.1016/j.apsb.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang F, Wang Q, et al. The synthesis of LA-Fe3O4@PDA-PEG-DOX for photothermal therapy-chemotherapy. Dalton Trans. 2018;47:2435–2443. doi: 10.1039/c7dt04080f. [DOI] [PubMed] [Google Scholar]
- Cheng L, Liu J, Gu X, et al. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv Mater. 2014;26:1886–1893. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]
- Cheng L, Liu T, Liu Z, et al. Two-dimensional magnetic WS2@Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials. 2015;60:62–71. doi: 10.1016/j.biomaterials.2015.04.053. [DOI] [PubMed] [Google Scholar]
- Dacarro G, Taglietti A, Pallavicini P. Prussian blue nanoparticles as a versatile photothermal tool. Molecules. 2018;23:1–20. doi: 10.3390/molecules23061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Chen Y, Cheng Z, et al. Rational design of a comprehensive cancer therapy platform using temperature-sensitive polymer grafted hollow gold nanospheres: simultaneous chemo/photothermal/photodynamic therapy triggered by a 650 nm laser with enhanced anti-tumor efficacy. Nanoscale. 2016;8:6837–6850. doi: 10.1039/c5nr08253f. [DOI] [PubMed] [Google Scholar]
- Duret G, Polali S, Anderson ED, et al. Magnetic entropy as a proposed gating mechanism for magnetogenetic ion channels. Biophys J. 2019;116:454–468. doi: 10.1016/j.bpj.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Nie W, Cheng Y, et al. In vitro and in vivo toxicity studies of copper sulfide nanoplates for potential photothermal applications. Nanomed Nanotechnol Biol Med. 2015;11:901–912. doi: 10.1016/j.nano.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Feng Q, Zhang Y, Zhang W, et al. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016;38:129–142. doi: 10.1016/j.actbio.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Gautam M, Poudel K, Yong CS, Kim JO. Prussian blue nanoparticles: synthesis, surface modification, and application in cancer treatment. Int J Pharm. 2018;549:31–49. doi: 10.1016/j.ijpharm.2018.07.055. [DOI] [PubMed] [Google Scholar]
- Girma WM, Tzing SH, Tseng PJ, et al. Synthesis of cisplatin(IV) prodrug-tethered CuFeS2 nanoparticles in tumor-targeted chemotherapy and photothermal therapy. ACS Appl Mater Interfaces. 2018;10:4590–4602. doi: 10.1021/acsami.7b19640. [DOI] [PubMed] [Google Scholar]
- Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3:351–358. [PMC free article] [PubMed] [Google Scholar]
- Gong L, Yan L, Zhou R, et al. Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy. J Mater Chem B. 2017;5:1873–1895. doi: 10.1039/C7TB00195A. [DOI] [PubMed] [Google Scholar]
- Guo X, Li W, Luo L, et al. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl Mater Interfaces. 2017;9:16581–16593. doi: 10.1021/acsami.6b16513. [DOI] [PubMed] [Google Scholar]
- Han L, Hao YN, Wei X, et al. Hollow copper sulfide nanosphere-doxorubicin/graphene oxide core-shell nanocomposite for photothermo-chemotherapy. ACS Biomater Sci Eng. 2017;3:3230–3235. doi: 10.1021/acsbiomaterials.7b00643. [DOI] [PubMed] [Google Scholar]
- Hernández Montoto Andy, Montes Roberto, Samadi Akbar, Gorbe Mónica, Terrés José Manuel, Cao-Milán Roberto, Aznar Elena, Ibañez Javier, Masot Rafael, Marcos María Dolores, Orzáez Mar, Sancenón Félix, Oddershede Lene B., Martínez-Máñez Ramón. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Applied Materials & Interfaces. 2018;10(33):27644–27656. doi: 10.1021/acsami.8b08395. [DOI] [PubMed] [Google Scholar]
- Hou Lin, Shan Xiaoning, Hao Lisha, Feng Qianhua, Zhang Zhenzhong. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomaterialia. 2017;54:307–320. doi: 10.1016/j.actbio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Hu Y, Hu H, Yan J, et al. Multifunctional porous iron oxide nanoagents for MRI and photothermal/chemo synergistic therapy. Bioconjug Chem. 2018;29:1283–1290. doi: 10.1021/acs.bioconjchem.8b00052. [DOI] [PubMed] [Google Scholar]
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li XX, Zhang L, et al. Multifunctional nanoplatform based on pH-responsive micelle coated with discontinuous gold shell for cancer photothermo-chemotherapy and photoacoustic tomography. Chin J Polym Sci. 2018;36:1139–1149. doi: 10.1007/s10118-018-2141-8. [DOI] [Google Scholar]
- Ji MF, Qiu XJ, Hou L, et al. Construction and application of a liver cancer-targeting drug delivery system based on core-shell gold nanocages. Int J Nanomedicine. 2018;13:1773–1789. doi: 10.2147/IJN.S151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Zhi Z, Wang D, et al. Multifunctional nanoflowers for simultaneous multimodal imaging and high-sensitivity chemo-photothermal treatment. Bioconjug Chem. 2018;29:559–570. doi: 10.1021/acs.bioconjchem.8b00053. [DOI] [PubMed] [Google Scholar]
- Khafaji M, Vossoughi M, Hormozi-Nezhad MR, et al. A new bifunctional hybrid nanostructure as an active platform for photothermal therapy and MR imaging. Sci Rep. 2016;6:27847. doi: 10.1038/srep27847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Kang HW, Oh J, et al. Doxorubicin-fucoidan-gold nanoparticles composite for dual-chemo-photothermal treatment on eye tumors. Oncotarget. 2017;8:113719–113733. doi: 10.18632/oncotarget.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koçer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science. 2005;309(80):755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- Koçer A, Walko M, Bulten E, et al. Rationally designed chemical modulators convert a bacterial channel protein into a pH-sensory valve. Angew Chem Int Ed. 2006;45:3126–3130. doi: 10.1002/anie.200503403. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim J, Kim WJ. Photothermally controllable cytosolic drug delivery based on core-shell MoS2-porous silica nanoplates. Chem Mater. 2016;28:6417–6424. doi: 10.1021/acs.chemmater.6b02944. [DOI] [Google Scholar]
- Li Volsi A, Scialabba C, Vetri V, et al. Near-infrared light responsive folate targeted gold nanorods for combined photothermal-chemotherapy of osteosarcoma. ACS Appl Mater Interfaces. 2017;9:14453–14469. doi: 10.1021/acsami.7b03711. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, Huang Q, et al. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine. 2010;5:1161–1171. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- Li X, Takashima M, Yuba E, et al. PEGylated PAMAM dendrimer-doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials. 2014;35:6576–6584. doi: 10.1016/j.biomaterials.2014.04.043. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu Y, Chang M, et al. Highly porous PEGylated Bi2S3nano-urchins as a versatile platform for: in vivo triple-modal imaging, photothermal therapy and drug delivery. Nanoscale. 2016;8:16005–16016. doi: 10.1039/c6nr03398a. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu Y, Howard KA, et al. Multifunctional bismuth selenide nanocomposites for antitumor thermo-chemotherapy and imaging. ACS Nano. 2016;10:984–997. doi: 10.1021/acsnano.5b06259. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Hu Y, et al. Multimodal imaging-guided antitumor photothermal therapy and drug delivery using bismuth selenide spherical sponge. ACS Nano. 2016;10:9646–9658. doi: 10.1021/acsnano.6b05427. [DOI] [PubMed] [Google Scholar]
- Li K, Nejadnik H, Daldrup-Link HE. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov Today. 2017;22:1421–1429. doi: 10.1016/j.drudis.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang L, Zhang H, et al. Rationally designed calcium phosphate/small gold nanorod assemblies using poly(acrylic acid calcium salt) nanospheres as templates for chemo-photothermal combined cancer therapy. ACS Biomater Sci Eng. 2017;3:3215–3221. doi: 10.1021/acsbiomaterials.7b00612. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang Y, Yao J, et al. A facile fabrication of silk/MoS2 hybrids for photothermal therapy. Mater Sci Eng C. 2017;79:123–129. doi: 10.1016/j.msec.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Li L, Lu Y, Jiang C, et al. Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core-shell nanoparticles. Adv Funct Mater. 2018;28:1704623. doi: 10.1002/adfm.201704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He D, Tu J, et al. The comparative effect of wrapping solid gold nanoparticles and hollow gold nanoparticles with doxorubicin-loaded thermosensitive liposomes for cancer thermo-chemotherapy. Nanoscale. 2018;10:8628–8641. doi: 10.1039/c7nr09083h. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang W, Ding L, et al. Prussian blue-modified ferritin nanoparticles for effective tumor chemo-photothermal combination therapy via enhancing reactive oxygen species production. J Biomater Appl. 2019;33:1202–1213. doi: 10.1177/0885328218825175. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang L, Zhong Y, et al. Fabrication of ultrasmall WS2quantum dots-coated periodic mesoporous organosilica nanoparticles for intracellular drug delivery and synergistic chemo-photothermal therapy. Onco Targets Ther. 2018;11:1949–1960. doi: 10.2147/OTT.S160748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Wang D, Liu S, et al. Cupreous complex-loaded chitosan nanoparticles for photothermal therapy and chemotherapy of oral epithelial carcinoma. ACS Appl Mater Interfaces. 2015;7:20801–20812. doi: 10.1021/acsami.5b05866. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen D, Li L, et al. Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed. 2011;50:891–895. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- Liu Teng, Wang Chao, Gu Xing, Gong Hua, Cheng Liang, Shi Xiaoze, Feng Liangzhu, Sun Baoquan, Liu Zhuang. Drug Delivery with PEGylated MoS2Nano-sheets for Combined Photothermal and Chemotherapy of Cancer. Advanced Materials. 2014;26(21):3433–3440. doi: 10.1002/adma.201305256. [DOI] [PubMed] [Google Scholar]
- Liu Xijian, Wang Qian, Li Chun, Zou Rujia, Li Bo, Song Guosheng, Xu Kaibing, Zheng Yun, Hu Junqing. Cu2−xSe@mSiO2–PEG core–shell nanoparticles: a low-toxic and efficient difunctional nanoplatform for chemo-photothermal therapy under near infrared light radiation with a safe power density. Nanoscale. 2014;6(8):4361–4370. doi: 10.1039/C3NR06160D. [DOI] [PubMed] [Google Scholar]
- Liu X, Ren Q, Fu F, et al. CuS@mSiO2-PEG core–shell nanoparticles as a NIR light responsive drug delivery nanoplatform for efficient chemo-photothermal therapy. Dalton Trans. 2015;44:10343–10351. doi: 10.1039/C5DT00198F. [DOI] [PubMed] [Google Scholar]
- Liu T, Chao Y, Gao M, et al. Ultra-small MoS 2 nanodots with rapid body clearance for photothermal cancer therapy. Nano Res. 2016;9:3003–3017. doi: 10.1007/s12274-016-1183-x. [DOI] [Google Scholar]
- Liu Yanping, Zhang Xuwu, Luo Liyao, Li Lei, Zhu Rui Yan, Li Anshuo, He Yuchu, Cao Weiwei, Niu Kang, Liu Huan, Yang Jingyue, Gao Dawei. Gold-nanobranched-shell based drug vehicles with ultrahigh photothermal efficiency for chemo-photothermal therapy. Nanomedicine: Nanotechnology, Biology and Medicine. 2019;18:303–314. doi: 10.1016/j.nano.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Luo L, Bian Y, Liu Y, et al. Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect. Small. 2016;12:4103–4112. doi: 10.1002/smll.201503961. [DOI] [PubMed] [Google Scholar]
- Luo X, Wang Y, Lin H, Qu F. DOX-Fe3O4@mSiO2-PO-FA nanocomposite for synergistic chemo- and photothermal therapy. RSC Adv. 2016;6:112232–112240. doi: 10.1039/C6RA23292B. [DOI] [Google Scholar]
- Luo X, Wang Y, Lin H, Qu F. DOX-Fe3O4@mSiO2-PO-FA nanocomposite for synergistic chemo- and photothermal therapy. RSC Adv. 2016;6:112232–112240. doi: 10.1039/C6RA23292B. [DOI] [Google Scholar]
- Ma Y, Cui S, Zhang X, et al. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics. 2013;3:633–649. doi: 10.7150/thno.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Ning, Zhang Ming-Kang, Wang Xiao-Shuang, Zhang Lu, Feng Jun, Zhang Xian-Zheng. NIR Light-Triggered Degradable MoTe2 Nanosheets for Combined Photothermal and Chemotherapy of Cancer. Advanced Functional Materials. 2018;28(31):1801139. doi: 10.1002/adfm.201801139. [DOI] [Google Scholar]
- Martinac AD, Bavi N, Bavi O, Martinac B. Pulling MscL open via N-terminal and TM1 helices: a computational study towards engineering an MscL nanovalve. PLoS One. 2017;12:e0183822. doi: 10.1371/journal.pone.0183822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Liu Z, Cao Y, et al. Fabricating aptamer-conjugated PEGylated-MoS2/Cu1.8S theranostic nanoplatform for multiplexed imaging diagnosis and chemo-photothermal therapy of cancer. Adv Funct Mater. 2017;27:1–10. doi: 10.1002/adfm.201605592. [DOI] [Google Scholar]
- Nakayama Y, Mustapić M, Ebrahimian H, et al. Magnetic nanoparticles for “smart liposomes”. Eur Biophys J. 2015;44:647–654. doi: 10.1007/s00249-015-1059-0. [DOI] [PubMed] [Google Scholar]
- Nam J, Son S, Ochyl LJ, et al. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9:1074. doi: 10.1038/s41467-018-03473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Je JY, Moorthy MS, et al. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int J Pharm. 2017;531:1–13. doi: 10.1016/j.ijpharm.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Peng S, He Y, Er M, et al. Biocompatible CuS-based nanoplatforms for efficient photothermal therapy and chemotherapy: in vivo. Biomater Sci. 2017;5:475–484. doi: 10.1039/c6bm00626d. [DOI] [PubMed] [Google Scholar]
- Ren L, Liu X, Wang Q, et al. Facile fabrication of a magnetically smart PTX-loaded Cys–Fe3O4/CuS@BSA nano-drug for imaging-guided chemo-photothermal therapy. Dalton Trans. 2017;46:2204–2213. doi: 10.1039/C6DT04308A. [DOI] [PubMed] [Google Scholar]
- Santha Moorthy M, Hoang G, Subramanian B, et al. Prussian blue decorated mesoporous silica hybrid nanocarriers for photoacoustic imaging-guided synergistic chemo-photothermal combination therapy. J Mater Chem B. 2018;6:5220–5233. doi: 10.1039/c8tb01214h. [DOI] [PubMed] [Google Scholar]
- Shao T, Wen J, Zhang Q, et al. NIR photoresponsive drug delivery and synergistic chemo-photothermal therapy by monodispersed-MoS 2-nanosheets wrapped periodic mesoporous organosilicas. J Mater Chem B. 2016;4:7708–7717. doi: 10.1039/C6TB02724E. [DOI] [PubMed] [Google Scholar]
- Shen S, Ding B, Zhang S, et al. Near-infrared light-responsive nanoparticles with thermosensitive yolk-shell structure for multimodal imaging and chemo-photothermal therapy of tumor. Nanomedicine Nanotechnology, Biol Med. 2017;13:1607–1616. doi: 10.1016/J.NANO.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Shi S, Chen X, Wei J, et al. Platinum(IV) prodrug conjugated Pd@Au nanoplates for chemotherapy and photothermal therapy. Nanoscale. 2016;8:5706–5713. doi: 10.1039/c5nr09120a. [DOI] [PubMed] [Google Scholar]
- Song W, Gong J, Wang Y, et al. Gold nanoflowers with mesoporous silica as “nanocarriers” for drug release and photothermal therapy in the treatment of oral cancer using near-infrared (NIR) laser light. J Nanopart Res. 2016;18:101. doi: 10.1007/s11051-016-3377-2. [DOI] [Google Scholar]
- Song Menglin, Liu Nian, He Le, Liu Gang, Ling Daishun, Su Xinhui, Sun Xiaolian. Porous hollow palladium nanoplatform for imaging-guided trimodal chemo-, photothermal-, and radiotherapy. Nano Research. 2018;11(5):2796–2808. doi: 10.1007/s12274-017-1910-y. [DOI] [Google Scholar]
- Su YY, Teng Z, Yao H, et al. A multifunctional PB@mSiO2-PEG/DOX nanoplatform for combined photothermal-chemotherapy of tumor. ACS Appl Mater Interfaces. 2016;8:17038–17046. doi: 10.1021/acsami.6b01147. [DOI] [PubMed] [Google Scholar]
- Sun X, Liu Z, Welsher K, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Song C, Li C, et al. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int J Nanomedicine. 2017;12:5163–5176. doi: 10.2147/ijn.s131089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Jiang D, Cai W, et al. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials. 2018;165:56–65. doi: 10.1016/j.biomaterials.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, He F, Bi H, et al. An intelligent nanoplatform for simultaneously controlled chemo-, photothermal, and photodynamic therapies mediated by a single NIR light. Chem Eng J. 2019;362:679–691. doi: 10.1016/J.CEJ.2019.01.095. [DOI] [Google Scholar]
- Tang S, Chen M, Zheng N. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 2014;8:165–174. doi: 10.1007/s12274-014-0605-x. [DOI] [Google Scholar]
- Tian G, Zhang X, Zheng X, et al. Multifunctional Rbx WO3 nanorods for simultaneous combined chemo-photothermal therapy and photoacoustic/CT imaging. Small. 2014;10:4160–4170. doi: 10.1002/smll.201401237. [DOI] [PubMed] [Google Scholar]
- Tian W, Su Y, Tian Y, et al. Periodic mesoporous organosilica coated Prussian blue for MR/PA dual-modal imaging-guided photothermal-chemotherapy of triple negative breast cancer. Adv Sci. 2017;4:1–10. doi: 10.1002/advs.201600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Teng Z, et al. pH-dependent transmembrane activity of peptide-functionalized gold nanostars for computed tomography/photoacoustic imaging and photothermal therapy. ACS Appl Mater Interfaces. 2017;9:2114–2122. doi: 10.1021/acsami.6b13237. [DOI] [PubMed] [Google Scholar]
- Vimala K, Shanthi K, Sundarraj S, Kannan S. Synergistic effect of chemo-photothermal for breast cancer therapy using folic acid (FA) modified zinc oxide nanosheet. J Colloid Interface Sci. 2017;488:92–108. doi: 10.1016/j.jcis.2016.10.067. [DOI] [PubMed] [Google Scholar]
- Wang Y, Black KCL, Luehmann H, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7:2068–2077. doi: 10.1021/nn304332s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li G, Ding Y, Sun S. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. 2014;4:30375–30383. doi: 10.1039/C4RA02978J. [DOI] [Google Scholar]
- Wang Z, Chen Z, Liu Z, et al. A multi-stimuli responsive gold nanocage–hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials. 2014;35:9678–9688. doi: 10.1016/J.BIOMATERIALS.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Wang S, Chen Y, Li X, et al. Injectable 2D MoS2-integrated drug delivering implant for highly efficient NIR-triggered synergistic tumor hyperthermia. Adv Mater. 2015;27:7117–7122. doi: 10.1002/adma.201503869. [DOI] [PubMed] [Google Scholar]
- Wang S, Li K, Chen Y, et al. Biocompatible PEGylated MoS2 nanosheets: controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials. 2015;39:206–217. doi: 10.1016/j.biomaterials.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Wang C, Bai J, Liu Y, et al. Polydopamine coated selenide molybdenum: a new photothermal nanocarrier for highly effective chemo-photothermal synergistic therapy. ACS Biomater Sci Eng. 2016;2:2011–2017. doi: 10.1021/acsbiomaterials.6b00416. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhou J, Chen R, et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials. 2016;100:27–40. doi: 10.1016/J.BIOMATERIALS.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhou J, Shi R, et al. Biodegradable core-shell dual-metal-organic-frameworks nanotheranostic agent for multiple imaging guided combination cancer therapy. Theranostics. 2017;7:4605–4617. doi: 10.7150/thno.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen Q, Xue W, et al. Combined chemo-photothermal antitumor therapy using molybdenum disulfide modified with hyperbranched polyglycidyl. ACS Biomater Sci Eng. 2017;3:2325–2335. doi: 10.1021/acsbiomaterials.7b00499. [DOI] [PubMed] [Google Scholar]
- Wang L, Gao W, Yang X, et al. Gold nanorods with silica shell and PAMAM dendrimers for efficient photothermal therapy and low toxic codelivery of anticancer drug and siRNA. Adv Mater Interfaces. 2017;4:1701166. doi: 10.1002/admi.201701166. [DOI] [Google Scholar]
- Wang L, Liu Y, Liu Y-N, et al. Cell membrane camouflaged hollow Prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv Funct Mater. 2017;27:1605795. doi: 10.1002/adfm.201605795. [DOI] [Google Scholar]
- Wang X, Li F, Yan X, et al. Ambient aqueous synthesis of ultrasmall Ni0.85Se nanoparticles for noninvasive photoacoustic imaging and combined photothermal-chemotherapy of cancer. ACS Appl Mater Interfaces. 2017;9:41782–41793. doi: 10.1021/acsami.7b15780. [DOI] [PubMed] [Google Scholar]
- Wang X, Ma Y, Chen H, et al. Novel doxorubicin loaded PEGylated cuprous telluride nanocrystals for combined photothermal-chemo cancer treatment. Colloids Surf B: Biointerfaces. 2017;152:449–458. doi: 10.1016/j.colsurfb.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Wang D, Dong H, Li M, et al. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12:5241–5252. doi: 10.1021/acsnano.7b08355. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen S, Zhu Y, et al. Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl Mater Interfaces. 2018;10:42102–42114. doi: 10.1021/acsami.8b16323. [DOI] [PubMed] [Google Scholar]
- Wang X-Y, Ao M, Chen Y-L, et al. A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy. Acta Biomater. 2018;80:308–326. doi: 10.1016/j.actbio.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Deng G, et al. Se@SiO2-FA-CuS nanocomposites for targeted delivery of DOX and nano selenium in synergistic combination of chemo-photothermal therapy. Nanoscale. 2018;10:2866–2875. doi: 10.1039/c7nr09237g. [DOI] [PubMed] [Google Scholar]
- Wu L, Wu M, Zeng Y, et al. Multifunctional PEG modified DOX loaded mesoporous silica nanoparticle@CuS nanohybrids as photo-thermal agent and thermal-triggered drug release vehicle for hepatocellular carcinoma treatment. Nanotechnology. 2015;26:025102. doi: 10.1088/0957-4484/26/2/025102. [DOI] [PubMed] [Google Scholar]
- Wu Lin, Chen Ling, Liu Fei, Qi Xueyong, Ge Yanru, Shen Song. Remotely controlled drug release based on iron oxide nanoparticles for specific therapy of cancer. Colloids and Surfaces B: Biointerfaces. 2017;152:440–448. doi: 10.1016/j.colsurfb.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Xu W, Qian J, Hou G, et al. Hyaluronic acid-functionalized gold nanorods with pH/NIR dual-responsive drug release for synergetic targeted photothermal chemotherapy of breast cancer. ACS Appl Mater Interfaces. 2017;9:36533–36547. doi: 10.1021/acsami.7b08700. [DOI] [PubMed] [Google Scholar]
- Xue P, Bao J, Wu Y, et al. Magnetic Prussian blue nanoparticles for combined enzyme-responsive drug release and photothermal therapy. RSC Adv. 2015;5:28401–28409. doi: 10.1039/c5ra01616a. [DOI] [Google Scholar]
- Xue P, Cheong KKY, Wu Y, Kang Y. An in-vitro study of enzyme-responsive Prussian blue nanoparticles for combined tumor chemotherapy and photothermal therapy. Colloids Surf B: Biointerfaces. 2015;125:277–283. doi: 10.1016/j.colsurfb.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Xue P, Zhang L, Sun L, et al. PEGylated magnetic Prussian blue nanoparticles as a multifunctional therapeutic agent for combined targeted photothermal ablation and pH-triggered chemotherapy of tumour cells. J Colloid Interface Sci. 2017;509:384–394. doi: 10.1016/j.jcis.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Yang G, Lv R, He F, et al. A core/shell/satellite anticancer platform for 808 NIR light-driven multimodal imaging and combined chemo-/photothermal therapy. Nanoscale. 2015;7:13747–13758. doi: 10.1039/C5NR03085D. [DOI] [PubMed] [Google Scholar]
- Yang JC, Chen Y, Li YH, Yin XB. Magnetic resonance imaging-guided multi-drug chemotherapy and photothermal synergistic therapy with pH and NIR-stimulation release. ACS Appl Mater Interfaces. 2017;9:22278–22288. doi: 10.1021/acsami.7b06105. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhou L, Su Y, et al. One-pot photoreduction to prepare NIR-absorbing plasmonic gold nanoparticles tethered by amphiphilic polypeptide copolymer for synergistic photothermal-chemotherapy. Chin Chem Lett. 2019;30:187–191. doi: 10.1016/j.cclet.2018.02.015. [DOI] [Google Scholar]
- Yin W, Yan L, Yu J, et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano. 2014;8:6922–6933. doi: 10.1021/nn501647j. [DOI] [PubMed] [Google Scholar]
- Yong Y, Zhou L, Gu Z, et al. WS 2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells. Nanoscale. 2014;6:10394–10403. doi: 10.1039/C4NR02453B. [DOI] [PubMed] [Google Scholar]
- You J, Zhang R, Zhang G, et al. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J Control Release. 2012;158:319–328. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zhang M, Peng M, et al. Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic/photothermal/chemotherapy of tumor. Adv Funct Mater. 2018;28:1705451. doi: 10.1002/adfm.201705451. [DOI] [Google Scholar]
- Zha Z, Zhang S, Deng Z, et al. Enzyme-responsive copper sulphide nanoparticles for combined photoacoustic imaging, tumor-selective chemotherapy and photothermal therapy. Chem Commun. 2013;49:3455–3457. doi: 10.1039/c3cc40608c. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen H, Shi J, et al. A Prussian blue-based core-shell hollow-structured mesoporous nanoparticle as a smart theranostic agent with ultrahigh pH-responsive longitudinal relaxivity. Adv Mater. 2015;27:6382–6389. doi: 10.1002/adma.201503381. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Jin Z, et al. Mesoporous carbon/CuS nanocomposites for pH-dependent drug delivery and near-infrared chemo-photothermal therapy. RSC Adv. 2015;5:93226–93233. doi: 10.1039/c5ra19458j. [DOI] [Google Scholar]
- Zhang L, Li Y, Jin Z, et al. An NIR-triggered and thermally responsive drug delivery platform through DNA/copper sulfide gates. Nanoscale. 2015;7:12614–12624. doi: 10.1039/c5nr02767e. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zheng X, Shen S, Wang X. Doxorubicin-loaded magnetic nanoparticle clusters for chemo-photothermal treatment of the prostate cancer cell line PC3. Biochem Biophys Res Commun. 2015;466:278–282. doi: 10.1016/j.bbrc.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liao J, Yang Q, et al. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics. 2015;5:345–356. doi: 10.7150/thno.10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen Y, Li Z, et al. Tailored synthesis of octopus-type Janus nanoparticles for synergistic actively-targeted and chemo-photothermal therapy. Angew Chem Int Ed. 2016;55:2118–2121. doi: 10.1002/anie.201510409. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu W, Wang Z, et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials. 2017;126:39–48. doi: 10.1016/j.biomaterials.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Zhang Aitang, Li Aihua, Zhao Wei, Liu Jingquan. Recent Advances in Functional Polymer Decorated Two-Dimensional Transition-Metal Dichalcogenides Nanomaterials for Chemo-Photothermal Therapy. Chemistry - A European Journal. 2017;24(17):4215–4227. doi: 10.1002/chem.201704197. [DOI] [PubMed] [Google Scholar]
- Zhang Qiang, Shan Wenjun, Ai Chaochao, Chen Zhiwei, Zhou Tiantian, Lv Xiaolin, Zhou Xi, Ye Shefang, Ren Lei, Wang Xiumin. Construction of Multifunctional Fe3O4-MTX@HBc Nanoparticles for MR Imaging and Photothermal Therapy/Chemotherapy. Nanotheranostics. 2018;2(1):87–95. doi: 10.7150/ntno.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Williams GR, et al. Functionalized MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy. Colloids Surf B: Biointerfaces. 2019;173:101–108. doi: 10.1016/j.colsurfb.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Zheng T, Li GG, Zhou F, et al. Gold-nanosponge-based multistimuli-responsive drug vehicles for targeted chemo-photothermal therapy. Adv Mater. 2016;28:8218–8226. doi: 10.1002/adma.201602486. [DOI] [PubMed] [Google Scholar]
- Zheng S, Han J, Jin Z, et al. Dual tumor-targeted multifunctional magnetic hyaluronic acid micelles for enhanced MR imaging and combined photothermal-chemotherapy. Colloids Surf B: Biointerfaces. 2018;164:424–435. doi: 10.1016/J.COLSURFB.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu H, Li X, et al. Dual targeting hyaluronic acid - RGD mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater Sci Eng C. 2017;81:261–270. doi: 10.1016/j.msec.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Zhu X, Huang H, Zhang Y, et al. Cit/CuS@Fe3O4-based and enzyme-responsive magnetic nanoparticles for tumor chemotherapy, photothermal, and photodynamic therapy. J Biomater Appl. 2017;31:1010–1025. doi: 10.1177/0885328216676159. [DOI] [PubMed] [Google Scholar]
- Zhu D-M, Xie W, Xiao Y-S, et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology. 2018;29:84002. doi: 10.1088/1361-6528/aa9ca1. [DOI] [PubMed] [Google Scholar]