Abstract
The functional amyloid state of proteins has in recent years garnered much attention for its role in serving crucial and diverse biological roles. Amyloid is a protein fold characterised by fibrillar morphology, binding of the amyloid-specific dyes Thioflavin T and Congo Red, insolubility and underlying cross-β structure. Amyloids were initially characterised as an aberrant protein fold associated with mammalian disease. However, in the last two decades, functional amyloids have been described in almost all biological systems, from viruses, to bacteria and archaea, to humans. Understanding the structure and role of these amyloids elucidates novel and potentially ancient mechanisms of protein function throughout nature. Many of these microbial functional amyloids are utilised by pathogens for invasion and maintenance of infection. As such, they offer novel avenues for therapies. This review examines the structure and mechanism of known microbial functional amyloids, with a particular focus on the pathogenicity conferred by the production of these structures and the strategies utilised by microbes to interfere with host amyloid structures. The biological importance of microbial amyloid assemblies is highlighted by their ubiquity and diverse functionality.
Keywords: Functional amyloid, Fibrils, Biofilm, Curli, RHIM, Hydrophobin
Introduction
In 2000, the term ‘functional amyloid’ was coined to describe amyloid structures with normal biological purposes within organisms, in contrast to amyloid fibrils that are associated with disease (Wosten and de Vocht 2000). Amyloids are fibrillar protein assemblies with a unique quaternary structure comprised of extended β-sheets formed from intermolecular hydrogen bonding (Fowler et al. 2005; Fowler et al. 2007). The constituent β-strands of the β-sheets run perpendicular to the axis of the fibril, resulting in a conformation known as the ‘cross-β sheet’ (Sunde and Blake 1997). Amyloid fibrils can bind to dyes such as Thioflavin T (ThT) and Congo Red, generating characteristic spectral changes (Chiti and Dobson 2006). Amyloid fibrils also exhibit a common X-ray fibre diffraction pattern, with strong meridional reflections at approximately 4.7 Å, and more diffuse equatorial reflections at approximately 10 Å, arising from inter-strand and inter-sheet spacings, respectively (Sunde and Blake 1997). Amyloid also displays several other characteristics, including aqueous insolubility, and resistance to cleavage by endogenous and exogenous proteases (Chiti and Dobson 2017).
The amyloid fold has been particularly associated with insidious, fatal neurodegenerative diseases, including Alzheimer’s and Parkinson’s, as well as diverse systemic amyloidoses (Blancas-Mejia and Ramirez-Alvarado 2013; Chiti and Dobson 2017). The proteins involved in these diseases are different for each condition, with a wide range of monomer sequence, structure and function. In pathophysiological states, however, these proteins misfold to adopt a common underlying amyloid structure (Booth et al. 1997; Sunde et al. 1997).
The first functional amyloid identified in humans was described in 2005, by Kelly and colleagues (Fowler et al. 2005). They demonstrated that the Pmel17 protein forms amyloid-like structures within melanosomes, the organelles where melanin is synthesised and stored, in order to sequester toxic biosynthetic intermediates that are produced within this environment. More recently, a heteromeric functional amyloid signalling complex comprised of the human proteins receptor interacting serine/threonine protein kinases 1 (RIPK1) and 3 (RIPK3) has been shown to be central in the inflammatory programmed cell death pathway necroptosis (Li et al. 2012; Mompean et al. 2018). Amyloid deposits are also a feature of regenerating muscle in human myocytes (Vogler et al. 2018). Even amyloids traditionally thought to be pathogenic may have some underlying functional role, for example, the Aβ42 peptide associated with Alzheimer’s disease. Recent studies have suggested that Aβ42 may have developed as a mechanism of containing microbial infection (Eimer et al. 2018; Kumar et al. 2016; Readhead et al. 2018; Soscia et al. 2010).
Functional amyloids are widespread in microbes including bacteria, archaea, fungi, protozoa and viruses (Chapman et al. 2002; Dueholm et al. 2015; Low et al. 2007; Pham et al. 2014; Sunde et al. 2008; Pham et al. 2019). Functional amyloids with diverse sequence and function play several roles which promote survival, and in particular, aid directly in pathogenicity. The ubiquity of functional amyloid as a mechanism of pathogenesis implies that it provides a strong competitive advantage. The effects of microbial functional amyloids include surface adherence, dissemination of virulence factors (Van Gerven et al. 2018), propagation (Fowler et al. 2007), structural support (Chapman et al. 2002) and evasion of the host immune system (Pham et al. 2019).
This review will detail the way in which amyloid functions as a direct mechanism for pathogenesis by microbes. It will also examine indirect use of amyloid in microbe-host interactions, wherein the pathogen uses carefully controlled mechanisms to alter host functional amyloid activity. By comparing and analysing the presence of amyloid in pathogens and their natural host, it is possible develop an understanding of the importance of this stable fold across nature.
Bacterial applications of functional amyloids
The characterisation of functional amyloid in bacteria initially centred on gram-negative bacteria such as Escherichia coli, which produce curli amyloid fibrils (Chapman et al. 2002; Jordal et al. 2009). Since this first identification, bacterial functional amyloid has been described across various phyla, including both gram-negative and gram-positive bacteria (Van Gerven et al. 2018). All bacterial amyloid proteins described here, unless otherwise stated, display similar physiochemical characteristics to other amyloids. These include binding to ThT and Congo Red with resultant increased fluorescence emission at 485 nm and green birefringence, respectively, fibril-like morphology under transmission electron microscopy (TEM), and a β-sheet rich structure as demonstrated by biophysical investigations including X-ray fibre diffraction (XRFD), Fourier-transform infrared spectroscopy (FT-IR) and circular dichroism (CD).
Functional amyloids within bacterial biofilms provide structural stability and adherence properties
Bacteria utilise diverse community-based mechanisms to promote the growth and survival of colonies, in which mutual benefit is derived by the individuals that form the colony. One form of bacterial community is termed a biofilm, wherein bacteria are contained within an extracellular matrix constructed by the bacteria. This matrix is composed of proteins, saccharides and other organic molecules. Important structural components of the biofilm matrix have been demonstrated to have an amyloid structure (Barnhart and Chapman 2006; Chapman et al. 2002; Flemming et al. 2016).
Curli fibrils from gram-negative proteobacteria
Curli are proteins long known to be crucial for the formation of biofilms in a diverse phylogenetic range of bacteria, both pathogenic and non-pathogenic (Barnhart and Chapman 2006) (Table 1). The curli from E. coli are most well understood. Curli are extracellular matrix proteins that form functional amyloid structures through tightly regulated mechanisms (Chapman et al. 2002). Curli fibrils act as a ‘scaffold’ within the biofilm. The amyloid nature of assembled curli fibrils confers a robustness that allows for the stable display of associated proteins and saccharides on the curli fibrils. The scaffold also means that the curliated bacteria are tightly associated within the biofilm (Blanco et al. 2012). These scaffolding features allow for several functional features to be effected by the colony (Barnhart and Chapman 2006). These include adhesion properties, including cell-to-cell adhesion (Van Houdt and Michiels 2005), cell-to-host adhesion (Kikuchi et al. 2005) and cell-to-abiotic surface adhesion (DeBenedictis et al. 2016). Curli can also play other roles including direct interaction with host proteins for pro-bacterial outcomes (Olsen et al. 1993; Olsen et al. 1998) and direct activation of host defence (Tukel et al. 2005).
Table 1.
Microbial functional amyloids
| Protein | Organism | Reference/s |
|---|---|---|
| Bacterial | ||
| Curli | Primarily studied in E. coli, but also present in genera including Aeromonas, Pseudolalteromonas, Shewanella, Citrobacter, Cronobacter Enterobacter, Shigella, Rahnella, Salmonella, Halomonas, Pseudomonas, Vibrio | Chapman et al. (2002); Dueholm et al. (2012) |
| Fap | Pseudomonas aeruginosa | Dueholm et al. (2010) |
| TasA | Bacillus subtilis | Romero et al. (2010) |
| TasA-like | Bacillus cereus | Romero et al. (2010) |
| Bap | Staphylococcus aureus | Taglialegna et al. (2016) |
| Bap-like | Many Staphylococci | Taglialegna et al. (2016) |
| Sbp | Staphylococcus epidermidis | Wang et al. (2018) |
| P1 adhesins | Streptococcus mutans | Besingi et al. (2017); Tang et al. (2016) |
| MTP | Mycobacterium tuberculosis | Alteri et al. (2007) |
| Microcin E492 | Klebsiella pneumoniae | Bieler et al. (2005); Shahnawaz and Soto (2012) |
| Harpins | Xanthomonas axonopodis pv. glycines | Oh et al. (2007) |
| Phenol-soluble modulins | Staphylococcus aureus | Tayeb-Fligelman et al. (2017); Salinas et al. (2018) |
| Rho | Clostridium botulinum | Yuan and Hochschild (2017) |
| CarD | Mycobacterium tuberculosis | Kaur et al. (2018b) |
| HelD | Bacillus subtilis | Kaur et al. (2018a) |
| Protozoan | ||
| MSP2 | Plasmodium falciparum | Adda et al. (2009); Low et al. (2007) |
| Fungal | ||
| EAS | Neurospora crassa | Morris et al. (2012) |
| RodA and RodB | Aspergillus fumigatus | Aimanianda et al. (2009); Beauvais and Latge (2015); Valsecchi et al. (2017); Zykwinska et al. (2014) |
| MPG1 | Magnaporthe oryzae | Pham et al. (2016); Talbot et al. (1996) |
| Repellents | Ustilago maydis | Teertstra et al. (2006); Teertstra et al. (2009) |
| ALS adhesins | Candida albicans | Alsteens et al. (2012); Garcia-Sherman et al. (2014); Peters et al. (2012); Ramsook et al. (2010) |
| [URE3] | Saccharomyces cerevisiae | Baxa et al. (2005); Wickner (1994) |
| [PSI+] | Saccharomyces cerevisiae | True et al. (2004); Wickner (1994); Shewmaker et al. (2006) |
| HET-s | Podospora anserina | Coustou et al. (1997); Daskalov et al. (2015); Dos Reis et al. (2002) |
| HELLP | Chaetomium globosum | Daskalov et al. (2015) |
| Viral | ||
| M45 | Murine cytomegalovirus | Pham et al. (2019) |
The mechanism of curli fibril formation on the extracellular surface is extremely ordered, with many control steps, both for initiation and maintenance. The details of these biochemical events are outside the scope of this review and have been extensively discussed elsewhere (Evans and Chapman 2014; Van Gerven et al. 2015). However, in brief, the major constituent of scaffold curli on bacterial surfaces is the protein CsgA, which forms the repeating β-structure of curli fibrils (Fig. 1b). The CsgA protein assembles into fibrils based upon the nucleation capabilities of another protein CsgB. CsgB is anchored to the outer bacterial membrane, where it serves as a seed for the extracellular formation of curli. The display of CsgB and the secretion of CsgA are controlled very carefully by an outer membrane secretion apparatus composed of the three proteins CsgE, CsgF and CsgD (Evans and Chapman 2014; Van Gerven et al. 2015).
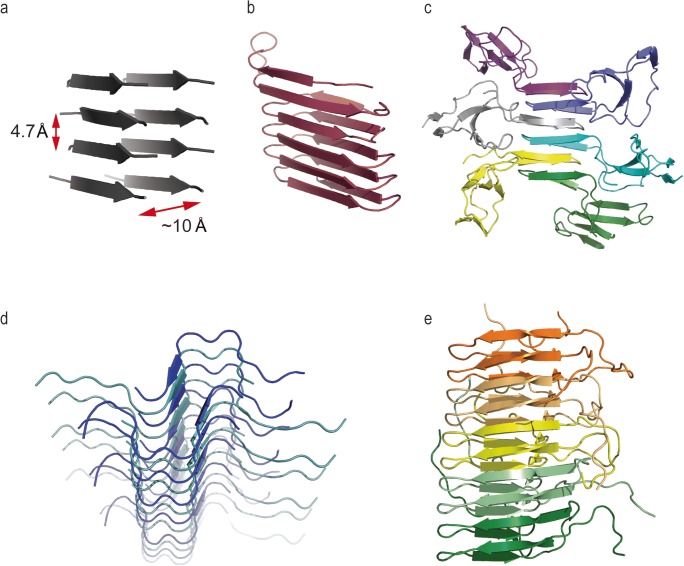
Fig. 1.
Comparison of different amyloid fibril structures. Although all amyloids have a common cross-β structure, they have different backbone conformations dependent on backbone and inter-sheet interactions. It is likely that these different conformers of the amyloid fold confer some differences in functionality. a Generic fibril structure. All amyloid structures are defined by an underlying cross-β structure, wherein two β-sheets are parallel to the fibril axis, and component β-strands lie perpendicular to the same axis. The inter-sheet distance is approximately 10 Å and the inter-strand distance is approximately 4.7 Å. b Model of an E. coli curli fibril. Curli fibrils appear on the surface of certain bacteria and provide adherence properties and structural stability. Curli fibrils are comprised of repeat monomers of the protein CsgA. CsgA is nucleated on the cell surface by accessory proteins. Left-handed β-helix model reproduced with kind permission from K. Lindorff-Larsen, adapted from Tian et al. (2015). c Model of an EASΔ15 hydrophobin rodlet from Neurospora crassa. Hydrophobins undergo a conformational change at air:water interfaces and self-associate into an amyloid-like structure termed a rodlet. Five hydrophobin monomers shown in different colours. EASΔ15 rodlet model reproduced with kind permission from A. Kwan, adapted from Macindoe et al. (2012). d Model structure of a heteromeric amyloid fibril containing M45 from murine cytomegalovirus and human RIPK3. It is hypothesised that the human and viral proteins are integrated into the same fibril by RHIM:RHIM interactions, forming a serpentine fold. The formation of this hybrid amyloid inhibits the signalling capabilities of the host protein by unknown mechanisms. Image produced using a model of RIPK3:M45 reported in (Pham et al. 2019) and based on the RIPK1:RIPK3 hetero-amyloid structure PDB 5V7V (Mompean et al. 2018). e Structure of the Het-s prion amyloid from Podospora anserina. When assembled into an amyloid structure, the Het-s protein adopts a β-solenoid conformation. This amyloid induces programmed cell death upon encountering heterokaryon incompatibility. Five protein monomers shown in different colours. Imaged produced from PDB 2RNM Wasmer et al. (2008)
Sequence analysis of these amyloid-forming proteins reveals several structural similarities among amyloid-forming domains. CsgA and CsgB are defined by a common repeat motif QXGXXN, which appears multiple times throughout each protein, from three copies (in Vibrio species) up to 22 copies (in the Shewanella genus) (Dueholm et al. 2012). Glutamine, glycine and asparagine have all been demonstrated to be highly amyloidogenic residues in other circumstances (Eisenberg and Sawaya 2017; Tzotzos and Doig 2010). This repeat motif is present in CsgA and CsgB from all known bacterial curli. There are some other common trends in terms of sequence across curli fibrils, including multiple residues of glutamine, glycine, serine and alanine, all of which have been implicated in the amyloid-forming domains of other proteins (Eisenberg and Sawaya 2017; Mompean et al. 2018; Tzotzos and Doig 2010).
Functional amyloid in Pseudomonas
The Pseudomonas genus utilises another type of functional amyloid in biofilm formation, termed Fap, for functional amyloid in Pseudomonas. Within Fap fibrils, the protein FapC forms the repeating subunit of the fibril and is part of a gene cluster coding for six proteins, FapA–F (Dueholm et al. 2010). Current understanding suggests that FapB acts as a nucleator protein for the assembly of FapC into fibrils, whilst FapE acts as an accessory protein. FapA and FapD reside in the periplasm where the former is thought to be an accessory protein with a chaperone role to guide FapC monomers out of the periplasm, whilst the later has a proteolytic role. Finally, FapF functions as an outer membrane pore allowing secretion of other Fap protein subunits (Bleem et al. 2018; Dueholm et al. 2013b). Heterologous expression of the Fap operon resulted in an altered phenotype characterised by increased colony adherence and biofilm formation (Dueholm et al. 2013b). This change in phenotype suggests that the Fap system may serve as an adherence factor through biofilm formation (Van Gerven et al. 2018). A study has shown the presence of Fap amyloid fibrils increases the integrity of the biofilm and increases hydrophobicity of the cells (Zeng et al. 2015), making Pseudomonas biofilms mechanically robust and resistant to drying. These features allow the microbes to successfully colonise environments where there is limited contact with water, as well as in aquatic environments (Zeng et al. 2015).
Whilst the proteins encoded by the Fap system are genetically distinct from the curli machinery of E. coli, inhibitors of curli fibril formation also inhibit FapC fibril formation (Taylor et al. 2016). It is hypothesised that Fap subunits, like curli, are maintained as monomers within the periplasm until secretion, which occurs through a membrane pore apparatus (Rouse et al. 2017). These shared characteristics suggest a comparable functionality between curli and Fap systems.
The FapC subunit contains three repeat regions and two linker regions. The repeat regions share a high degree of similarity across Pseudomonas species (Bleem et al. 2018). They are characterised by conserved glutamine, asparagine and serine residues, separated by glycine or alanine residues (Rasmussen et al. 2018). The linker regions vary in length and are hypothesised to be structurally disordered (Rouse et al. 2017). Analogous residues are found in the repeat regions of curli fibrils, albeit with short linkers. This suggests that the linker region of FapC can determine the different characteristics of amyloid fibrils from various homologues, whilst for curli, the repeating units will determine fibril properties due to the small linker regions (Dueholm et al. 2013a).
Apart from facilitating adhesion of bacterial colonies to surfaces, Pseudomonas biofilms may also harbour and modulate the release of signalling molecules such as quorum-sensing (QS) molecules (Seviour et al. 2015). This allows bacterial colonies to modulate gene expression based on their size, and to better adapt to environmental pressures (Dietrich et al. 2006). FapC fibrils have been shown to bind QS molecules such as 2-heptyl-3-hydroxy-4-(1H)-quinolone (PQS) and N-(3-oxododecanoyl)-Lhomoserine lactone (3-oxo-C12-HSL). Amyloid-forming biofilms harbouring QS molecules could be important for bacterial survival in non-laboratory conditions, such as in acute lung infections (Rouse et al. 2018; Seviour et al. 2015; Van Gerven et al. 2018).
Further evidence of a role for Fap in virulence was seen with a FapC deletion mutant strain of P. aeruginosa which was found to be significantly attenuated in killing of Caenorhabditis elegans in a nematode infection model (Wiehlmann et al. 2007). Similarly, in murine models of acute and chronic infections, the transcription of the Fap operon has been found to be highly upregulated (Rouse et al. 2018; Turner et al. 2014; Van Gerven et al. 2018).
TasA from Bacillus
Bacillus is a genus of ubiquitous gram-positive bacteria with both pathogenic and non-pathogenic constituent species (Bottone 2010; Earl et al. 2008). Like curli-expressing bacteria, they form biofilms to aid in their life cycles (Romero et al. 2010). In the case of Bacillus subtilis, their biofilms were initially identified as mechanisms of sporulation (Branda et al. 2001; Serrano et al. 1999; Stover and Driks 1999). The specific protein underlying biofilm formation in B. subtilis is TasA (Branda et al. 2006; Romero et al. 2010). TasA allows the formation of two different biofilm variants. One of these has more traditional hallmarks, allowing cell-cell adhesion and agglutination on the surface of agar plates (Romero et al. 2010). The other forms at air-water interfaces to allow for spore dispersal, motility and matrix formation (Romero et al. 2010; Vlamakis et al. 2008). These air-water bacterial colony biofilms are termed pellicles, and the constituent protein TasA is indispensable for their formation (Romero et al. 2010). Knockout tasA strains of B. subtilis lose the ability to form pellicles (Romero et al. 2010). Recombinant TasA is detected by the A11 antibody, which detects oligomeric amyloid species, confirming its amyloidogenic nature (Romero et al. 2010).
Although B. subtilis is not a pathogenic species, it shares many similarities with the closely related bacterium B. cereus, which is a causative agent of food poisoning (Caro-Astorga et al. 2014). There is a homologue of TasA from B. subtilis present in B. cereus, also called TasA. TasA from B. cereus is coded by the same chromosomal region as TasA from B. subtilis, indicating that they share common ancestry. There is limited information about the biogenesis and detailed function of TasA from B. cereus. However, its importance for Bacillus survival in general is inferred from the observation that TasA from B. cereus can functionally substitute for TasA from B. subtilis in tasA knockout colonies, rescuing pellicle formation and aiding adhesion and biofilm formation (Caro-Astorga et al. 2014).
Biofilm-associated proteins from Staphylococcus
The gram-positive bacteria, Staphylococcus aureus, is a commensal member of the microbiome in most circumstances, but it can also play major roles in disseminated disease (Krismer et al. 2017; Liu 2009). S. aureus has developed widespread antibiotic resistance, particularly in hospital settings, with some strains now resistant to most available antibiotics, including last-resort drugs (Rasmussen et al. 2011). Like many other human pathogenic bacteria, S. aureus forms biofilms in order to scaffold interactions of colonies of individual cocci, as well as facilitating interactions between these colonies and host cells or surfaces. The formation of S. aureus biofilms is dependent on biofilm-associated proteins (Baps) (Taglialegna et al. 2016). Structurally, Baps are multi-domain proteins with a repetitive structure, located at the cell surface (Lasa and Penades 2006). The formation of Bap amyloid is tightly controlled by the environment—Bap aggregation is promoted under acidic conditions. In a physiological context, this is indicative of replication that occurs in a high glucose environment, from which acidic by-products are produced in abundance (Taglialegna et al. 2016).
Within S. aureus infections, Bap proteins are functionally crucial. Knockout of bap from bacterial cultures reduces the adhesive properties of the bacteria to cultured bovine epithelial cells, and also reduces the total bacterial titre 10 days after infection (Taglialegna et al. 2016). Interestingly, when Lasa and colleagues (Taglialegna et al. 2016) expressed homologous putative amyloid-forming Bap domains from related bacteria from the Staphylococcus genus in E. coli, the domains from S. straprophyticus, S. simiae, S. xylosis, S. epidermidis and S. simulans bound Congo red. It can be extrapolated from this result that the production of Bap and its integral role in biofilm genesis may be a generalised Stacphylococcus mechanism.
Small basic protein from Staphylococcus epidermidis
Staphylococcus epidermidis is well-known for its commensal role as part of normal human skin flora (Otto 2009). In addition to this long-held non-pathogenic role, S. epidermidis is increasingly recognised as a major hospital-based pathogen, due to its ability to colonise medical devices within patients, especially in immunocompromised patients (Otto 2009). Like other bacteria, S. epidermidis utilises biofilms which facilitate adhesion to, and colonisation of, both biotic and abiotic surfaces (Otto 2009). There is also some evidence to suggest that Staphylococcal biofilms also serve to promote resistance to antibiotics and some components of the immune system (Otto 2009; Paharik and Horswill 2016; Wang et al. 2018). Small basic protein (Sbp) is a secreted surface protein that plays a scaffolding role in S. epidiermidis biofilms. In vivo, knockout of sbp reduces Thioflavin S binding to individual bacteria and also reduces cell-cell adhesion, indicating that Sbp is crucial for amyloid formation, and that this amyloid is required for cell-cell adhesion into biofilms (Wang et al. 2018).
Amyloid forming proteins from Streptococcus mutans biofilms
Another bacterium that forms biofilms is Streptococcus mutans. These bacteria are mainly found in the oral cavity and are associated with tooth decay in humans. In vitro studies have identified three candidate proteins responsible for amyloid formation in the S. mutans biofilms: P1 adhesin (also known as antigen I/II), the recently identified wall-associated protein A (WapA) and the secreted protein SMU_63c (Besingi et al. 2017). All three proteins are located within the extracellular matrix of the biofilm and are important for the structure and formation of the biofilm. Single deletion mutations of either the P1 adhesins or WapA genes (spa and wapA respectively) reduce biofilm production. Conversely, deletion of smu_63c results in a small enhancement in biofilm formation. Additionally, double deletion of spa and wapA and the triple deletion of all three genes drastically inhibit biofilm formation by S. mutans (Besingi et al. 2017). The C-terminal region of the well-characterised adhesin P1, known as the C123 domain, has been shown to be the amyloid-forming moiety in this protein in in vitro studies (Besingi et al. 2017; Heim et al. 2015; Tang et al. 2016). Earlier studies suggested that P1 induces pro-inflammatory effects in various cell lines including monocytes (Soell et al. 1994), endothelial cells (Vernier et al. 1996) and synoviocytes (Gourieux et al. 2001). Similarly, mutant strains of P. mutans lacking P1 adhesin were found to be less virulent in a rat model (Crowley et al. 1999). However, the functional role of amyloid in these events is currently unknown.
Functional amyloid also confers adhesive properties independent of bacterial biofilms
Mycobacterium tuberculosis pili
Mycobacterium tuberculosis is a respiratory pathogen of humans that is the key bacterial causative agent in tuberculosis (Blanco et al. 2012; Fogel 2015). Approximately ten million people per year are newly infected with tuberculosis, and in 2017 there were 1.6 million tuberculosis-related deaths worldwide (Global Tuberculosis Report, WHO). One mechanism of M. tuberculosis pathogenicity is the expression of organelles termed pili on their outer cellular surface (Alteri et al. 2007). The pilus is an organelle with a polymeric substructure that has adhesive properties, which allows for colonisation and attachment to host eukaryotic cells (Finlay and Falkow 1997). The importance of M. tuberculosis pili (MTP) for Mycobacterium pathogenicity is evidenced by the fact that only pathogenic members of the genus express pili on their surface (Alteri et al. 2007; Blanco et al. 2012). MTP present on the surface of individual bacteria have similar morphology to curli fibres on the surface of curliated E. coli (Alteri et al. 2007; Blanco et al. 2012). Alteri et al. (2007) also indicate that MTP bind the amyloid-specific dye Congo Red but other amyloid-determination experiments are yet to be reported. The specific mechanism by which MTP adhere to host cells is through direct binding to the protein laminin which is expressed on the cell surface of host cells and is a constituent of the human extracellular matrix (Sasaki et al. 2004). Interestingly, although they have the same morphology and similar functionality, the underlying sequence of MTP is distinct from both fap and curli (Alteri et al. 2007). The underlying monomeric sequence that forms the repeat unit of MTP (termed a pilin) is defined by a preponderance of glycine, alanine and proline residues.
Amyloid-forming bacterial proteins with unique functions
Microcin E492 from Klebsiella pneumoniae
Amyloid formation as a means of protein storage is found in Klebsiella pneumoniae. K. pneuomoniae sequesters the self-produced bacterial toxin microcin E492 (mccE492) into an amyloid form that is non-toxic (Bieler et al. 2005). The monomeric form is a pore-forming bacteriotoxin that is utilised for killing other competing Enterobacteria (Shahnawaz and Soto 2012). The amyloid-like fibres can be dissociated into active monomers with changes to environmental conditions such as pH (Shahnawaz and Soto 2012). Apart from its bactericidal properties, mccE492 has also been shown to cause apoptosis in human cell lines (Hetz et al. 2002). Whilst no direct involvement of mccE492 with pathogenesis is found, large scale genome-wide analysis studies have shown that it plays a role in virulence in various K. pneumoniae isolates (Marcoleta et al. 2016). The internal region of mccE492 shares similarity with the central domain of the prion protein PrP, and synthetic peptides spanning these sequences can convert active monomers into amyloid aggregates (Shahnawaz et al. 2017).
Harpins from Xanthomonas
Harpins are proteins produced by various species of the plant pathogenic bacterium Xanthomonas. The most studied harpin, HpaG, is secreted by type III secretion systems (Zimaro et al. 2014). Secretion of HpaG initiates a process termed the hypersensitive response (HR) in plants. The HR functions as a defence mechanism to contain the growth of plant pathogens by causing cell death (Oh et al. 2007). It has been found that HpaG forms amyloid-like fibrils in vitro, and that mutation of a key residue (L50P) prevents amyloid formation (Oh et al. 2007). Nevertheless, the exact role of amyloid in inducing the HR response is not known.
Interestingly, an HpaG homologue in X. campestris, known as XopA, does not induce HR reaction in plants nor forms amyloid structures. However, a gain-of-function mutant F48L/M52L, can form curvilinear protofibrils and fibrils, which suggests a positive correlation as the L50 residue in HpaG is needed for amyloid formation (Oh et al. 2007). Overall, the induction of plant HR by the amyloid-forming harpins suggests another role for bacterial amyloid that may be explored in the future.
Phenol-soluble modulins from Staphylococcus aureus
Phenol-soluble modulins (PSMs) are peptides secreted by S. aureus that form aggregated structures which have a variety of roles in bacterial survival. Like other bacterial functional amyloids, they perform roles in biofilm assembly and maintenance, and also act as a lytic agent, killing host cells. They also induce an inflammatory immune response, implicating them indirectly in pathogenesis (Cheung et al. 2014; Schwartz et al. 2012; Tayeb-Fligelman et al. 2017). Initially, PSMs were thought to be a traditional cross-β functional amyloid (Schwartz et al. 2012). The most lytic and cytotoxic member of this peptide family, the 22-residue PSMα3, forms a repeating subunit fibril structure. However, instead of β-strands perpendicular to the fibre axis forming the ‘rungs’ of the amyloid ladder, the fibril is instead composed of stacked alpha helices in place of the β-strands, forming what is termed a cross-α structure (Chiti and Dobson 2017; Tayeb-Fligelman et al. 2017). In this sense, the polymerised form of PSMα3 cannot strictly be categorised as an amyloid, despite the fact that it is still able to bind ThT (Tayeb-Fligelman et al. 2017). This ‘cross-α’ structure is directly linked to the function of this peptide, because non-fibrillar mutants and detergent-based reduction in fibril formation reduce toxicity in human T cells significantly (Tayeb-Fligelman et al. 2017).
Other members of the PSM peptide family secreted by S. aureus do indeed have traditional amyloid substructure when assembled (Salinas et al. 2018). These include PSMα1 and PSMα4, which function largely as biofilm-forming agents in vivo (Schwartz et al. 2012). This indicates a complicated heterogeneity of fibrils produced by S. aureus for differing purposes. Interestingly, truncation of the 22-residue PSMα3 causes a change in the fibril-forming characteristics of this peptide, as there is a polymorphic return to a nominal cross-β substructure, but with an atypical packing arrangement (Salinas et al. 2018). Truncations of PSMs occur in nature, and often increase the bactericidal activity of these peptides towards other bacterial species, whilst the secreting organism is protected from harm (Gonzalez et al. 2012; Salinas et al. 2018). A six-residue truncated version of PSMα3, which displays atypical cross-β packing, demonstrates bactericidal activity against Micrococcus luteus and Staphylococcus hominis (Salinas et al. 2018).
Protozoan applications of functional amyloid
Protozoa are eukaryotic single cell microbes and functional amyloid has been associated with a pathogenic member of this kingdom.
Merozoite surface protein 2 (MSP2) from Plasmodium falciparum
The merozoite surface protein 2 (MSP2) from the malaria-causing Plasmodium falciparum was initially identified as a potential candidate vaccine target for malaria (Low et al. 2007). It is a surface protein on the membranes of merozoites, a form of the parasite present in the bloodstream that is implicated in infection of erythrocytes (Low et al. 2007). In vitro studies have shown that MSP2 is disordered in solution but can form amyloid-like fibrils under physiological conditions (Low et al. 2007). The N- and C-terminal domains are conserved but the variable middle region can be divided into two families: 3D7 and FC27. Fibril formation by the N-terminal 25-residue region (MSP21–25) can be seeded and follows a nucleation dependent pathway (Adda et al. 2009).
Interestingly, fibrils formed by the 3D7 variant can be stained by Congo Red but not ThT whereas the FC27 fibril variant binds to both Congo Red and ThT, suggesting that the forms are structurally different (Adda et al. 2009). Glutaraldehyde cross-linking experiments show higher molecular weight bands corresponding to oligomers of various sizes of MSP2 from both recombinant and purified 3D7 parasitic strains (Adda et al. 2009).
Further evidence for the association of MSP2 amyloid with virulence comes from lipid membrane studies (Lu et al. 2019). MSP21–25 undergoes conformational change to β-structure and interacts with lipid membrane mimetics. The self-assembly of MSP21–25 and its interaction with membranes are associated with disruption of membrane integrity (Lu et al. 2019).
Fungal applications of functional amyloid
Fungi use amyloid to aid their passage through their life cycle and to increase their pathogenicity. This use of amyloid has been shown in both unicellular/yeast and multicellular/filamentous fungi. Fungal amyloid has diverse roles between fungal species and can be utilised for either commensal or pathogenic interactions between the fungus and the host.
Hydrophobins from filamentous fungi serve diverse functions
Hydrophobins are small surface active proteins produced by filamentous/multicellular fungi (Wessels 1994). Two classes of hydrophobins exist, with only class I shown to exhibit fibrillar amyloid structure (Bayry et al. 2012). When exposed to a hydrophobic:hydrophilic interface the class I hydrophobin monomers undergo a conformational change to form amyloid fibrils known as rodlets (Morris et al. 2011). These rodlets pack together to form an amphipathic monolayer that has multiple functions for the fungus (Pham et al. 2018). Aerial hyphae are coated in the hydrophobin monolayer, reversing their wettability and therefore allowing growth into the air (Wosten et al. 1999). In addition, hydrophobins can form a robust coat on spores that resists wetting and therefore facilitates spore dispersal (Wösten and Wessels 1997). Currently, there is no solved structure for a hydrophobin rodlet. However, a model exists for the hydrophobin EAS, from Neurospora crassa, a fungus known for causing bread mould (Macindoe et al. 2012) (Fig. 1c). In this model, the EAS monomers undergo a conformational change and bind to one another to form long, non-twisted amyloid rodlets that can further assemble laterally, to form an amphipathic monolayer (Macindoe et al. 2012).
Hydrophobins are found in pathogenic and commensal filamentous fungi and are therefore not an inherently pathogenic feature (Gravagnuolo et al. 2016). However, in addition to the generic roles of hydrophobins mentioned previously, specific functions of the expressed hydrophobins may increase the pathogenicity of the fungus. In humans, Aspergillus fumigatus can cause invasive aspergillosis in immunocompromised patients (Zykwinska et al. 2014). The hydrophobin monolayer on spores prevents activation of the host innate immune response, subsequently allowing infection which can ultimately spread from the lung to the brain and kidneys (Aimanianda et al. 2009). A. fumigatus contains at least six hydrophobins, however, only one, RodA is characterised. RodA is responsible for rodlet formation and the prevention of the activation of the immune response (Valsecchi et al. 2017; Valsecchi et al. 2019). In plants, Magnaporthe oryzae causes rice blast, a fungal disease responsible for the loss of one-third of the global annual rice crop (Pham et al. 2016). M. oryzae contains one class I hydrophobin, MPG1 (Talbot et al. 1996). MPG1 has a critical role in the formation of the appressorium, the tool used by the fungus to puncture the host surface (Talbot et al. 1996). Deletion of mpg1 results in a reduced ability of the fungus to infect, therefore directly linking the amyloid formation to increased pathogenicity (Talbot et al. 1996). More recently, MPG1 has been shown to also direct the actions of the enzyme cutinase 2, involved in the penetration of the host (Pham et al. 2016).
Another plant pathogen, Ustilago maydis, utilises surface active proteins known as repellents instead of hydrophobins, for attachment to surfaces and to aid the formation of aerial hyphae (Teertstra et al. 2009). Like hydrophobins, repellents are amyloidogenic and form amphipathic fibrillar layers (Teertstra et al. 2009). U. maydis does contain two hydrophobin genes but they do not have a critical role in aerial hyphae formation (Teertstra et al. 2006). In contrast to M. oryzae where the knockout of mpg1 significantly reduces pathogenicity, the repellents are not vital for pathogenicity of U. maydis (Teertstra et al. 2006). Class I hydrophobins show a low degree of sequence conservation with the exception of eight cysteine residues (Sunde et al. 2008). These cysteines form four disulphide bonds that are critical for stabilising the hydrophobin structure, which has a large, exposed hydrophobic surface. Hydrophobins specifically self-assemble at hydrophobic:hydrophilic interfaces (Sunde et al. 2008). Repellents in U. maydis, however, do not display any sequence homology with hydrophobins, and the mechanism that controls the self-assembly of repellents is unknown (Teertstra et al. 2009).
Role of functional amyloid in fungal biofilms
Unicellular and multicellular fungi use biofilms to adhere to surfaces, e.g. other fungal cells or host tissue, and for protection. Different forms of functional amyloid are present in these biofilms. Unicellular yeast biofilms contain amyloid-forming adhesins whilst filamentous multicellular fungi have been shown to contain amyloidogenic hydrophobins in their biofilms.
Yeast biofilms are comprised of closely packed cells bound together by adhesins and an extracellular matrix (Nobile and Johnson 2015). Yeast adhesins are glycoproteins localised on the surface of the cell wall. They function to protect fungal cells, bind cells together and aid adherence to the infection surface (Garcia et al. 2013; Lipke et al. 2018). Candida albicans is a fungal member of the human microbiome that can become pathogenic in immunocompromised patients and is a well-studied example of a fungus that utilises amyloid-forming adhesins to increase its pathogenicity (Garcia-Sherman et al. 2014). The ALS family of adhesin proteins are critical in anchoring the C. albicans fungal cells to each other and allowing attachment to the host (Ramsook et al. 2010). A section of the Als5p adhesin forms an amyloid structure with neighbouring adhesins (Ramsook et al. 2010). This aggregation increases local adhesin concentration, as the adhesins are bound to one another, and therefore increases the strength of the binding between cells (Alsteens et al. 2012). Another member of the ALS family, Als3p, mediates the co-adherence of C. albicans to the bacterium S. aureus and has also been shown to facilitate infection by inducing endocytosis into host cells (Peters et al. 2012). Adhesins within the C. albicans ALS protein family contain a highly conserved amyloid core β-aggregation sequence (Otoo et al. 2008). A non-pathogenic yeast, Saccharomyces cerevisiae contains similar adhesin amyloids to C. albicans, highlighting that fungal adhesin amyloids do not necessarily increase pathogenicity (Lipke et al. 2018; Ramsook et al. 2010).
Certain multicellular filamentous fungi demonstrate expression of amyloidogenic hydrophobins in their biofilms. A. fumigatus has been shown to express at least two different hydrophobins in its biofilm but gene deletion experiments have failed to identify any role for these hydrophobins in the formation, structure or hydrophobicity of the biofilm (Beauvais and Latge 2015; Valsecchi et al. 2017). However, it was recently shown that a polyphenolic compound could downregulate expression of the hydrophobin genes in A. fumigatus, and this downregulation resulted in a weakened extracellular matrix and therefore increased the susceptibility of the fungi to antifungal drugs (Luo et al. 2018). Therefore, the exact role of hydrophobins in the fungal biofilm of A. fumigatus, and potentially other filamentous fungi, remains to be elucidated.
Viral applications of functional amyloid
Viruses are ubiquitous pathogens capable of interfering with host cell signalling for a range of purposes to maximise virulence (Baker et al. 2018). Herpesviruses have been demonstrated to interfere with host functional amyloids, in order to inhibit the programmed cell death pathway necroptosis (Pham et al. 2019).
Herpesviruses form hybrid amyloid structures to prevent programmed necrosis
Necroptosis itself requires the formation of signalling complexes, known as necrosomes (Li et al. 2012). These consist of the kinase RIPK3, and one of three host effector proteins, RIPK1, TIR-domain-containing adapter-inducing interferon-β (TRIF) or Z-DNA binding protein 1 (ZBP1). When RIPK1 or ZBP1 interact with RIPK3, they form into an amyloid structure with signalling capability (Li et al. 2012; Mompean et al. 2018; Pham et al. 2019). It is likely that TRIF:RIPK3 interactions also form an amyloid structure (Gentle et al. 2017). The formation of these amyloid signalling platforms, in response to inflammation or microbial infection, leads to activation of RIPK3, subsequent downstream phosphorylation of the pseudokinase mixed lineage kinase domain-like protein (MLKL) and lytic cell death. The RIP homotypic interaction motif (RHIM) within RIPK1, RIPK3, TRIF and ZBP1 is the amyloidogenic sequence within these proteins and it forms the β-sheet core of the necrosome amyloid (Mompean et al. 2018). The RHIM is ~ 18 amino acids in length, with a core sequence of I/V-Q-I/V-G (Baker et al. 2018).
Murine cytomegalovirus expresses a RHIM-containing protein known as M45 (Lembo et al. 2004), which leads to inhibition of necroptosis in murine (Upton et al. 2010; Upton et al. 2012) and human cells (Guo et al. 2015). An intact RHIM within M45 is essential for the virus to propagate and cause disease within mice (Upton et al. 2010). Without the function of the M45 RHIM, virally infected cells rapidly undergo necroptosis following activation of ZBP1 and subsequently RIPK3 and MLKL (Upton et al. 2012). In vitro studies have shown that the M45 RHIM, which has an IQIG core sequence, can interact with the RHIMs of human RIPK1, RIPK3 and ZBP1 proteins to form heteromeric fibrils containing both virus and host proteins (Pham et al. 2019). M45 preferentially interacts with RIPK3 and ZBP1, over RIPK1, suggesting that the virus inhibits necroptosis by sequestering RIPK3 and ZBP1 in alternative hetero-amyloid structures that prevent the activation of RIPK3 (Fig. 1d).
In addition to M45, two other RHIM-containing viral proteins have been shown to modulate necroptosis, ICP6 from Herpes Simplex Virus 1 and ICP10 from Herpes Simplex Virus 2 (Guo et al. 2018; Guo et al. 2015; Huang et al. 2015; Wang et al. 2014). However, unlike the RHIM from M45, ICP6 does not abrogate signalling for necroptotic cell death in all circumstances. ICP6 was first identified as a direct activator of RIPK3 activation, leading to necroptosis in both murine cells in vitro and in an in vivo mouse model (Huang et al. 2015; Wang et al. 2014). However, when overexpressed in human cell lines, the natural host of HSV infections, the presence of the ICP6 protein is sufficient to inhibit necroptosis (Guo et al. 2018; Guo et al. 2015), and a full virus model of infection demonstrates the same outcome (Guo et al. 2018). These studies show that the activity of ICP6 towards host necroptosis is dependent on the presence of an intact RHIM in ICP6, which has a VQCG core sequence.
The functional and sequence similarities between the RHIMs of M45 and ICP6 make it likely that ICP6 also makes amyloid-based interactions with the host proteins involved in necroptosis (Baker et al. 2018). However, it appears that subtle sequence differences can alter the structures of heteromeric amyloid fibrils and change signalling outcomes. This is highlighted by the work of Huang et al. (2015), who have demonstrated that a chimeric ICP6 construct containing the 18-amino acid M45 RHIM instead of the wildtype ICP6 RHIM prevents necroptosis in murine cells, instead of the activating role of wildtype ICP6 in murine cells previously established (Wang et al. 2014). Molecular dynamic simulations suggest that RIPK1:RIPK3 heteromeric fibrils are more stable than RIPK1:RIPK1 or RIPK3:RIPK3 homomeric fibrils (Mompean et al. 2018). These data collectively indicate that small changes in amino acid sequence can have marked effects on the nature and function of the heteromeric fibrils formed by RHIM-containing proteins. Amyloid formation by the viral protein M45 is a unique example of a microbial functional amyloid that targets a host mammalian functional amyloid, in order to increase virulence.
Interference with host amyloid as an invasive strategy
Enteropathogenic E. coli (EPEC) have diverse mechanisms to initiate and maintain their pathogenic invasion of hosts (Clements et al. 2012), including evasion of host cell death programmes, which normally act to limit the spread of infection within the host (Giogha et al. 2014; Pearson et al. 2017; Pearson et al. 2013). As described above, the human necroptosis cell death cascade requires the formation of a functional amyloid signalling complex known as a necrosome (Li et al. 2012). The strategy employed by EPEC to undermine host functional amyloid is different to the viral mechanism described above. EPEC can prevent the formation of the necrosome by the secretion of a cysteine protease known as EspL, which selectively cleaves within the RHIMs of the host RIPK1, RIPK3, TRIF and ZBP1 proteins to prevent their amyloid assembly (Pearson et al. 2017). EspL is unable to cleave the amyloid forms of these proteins, indicating a clear need for the bacterium to prevent formation of this structure, and again highlighting the utility provided by the stable amyloid structure.
Microbial prions: a special category of functional amyloids
Fungal prions
Prions are infectious proteins found in many organisms including yeast and mammals (Wickner et al. 2018). Although transmissible, in contrast to other prions, e.g. the mammalian prion PrP, yeast prions have not been shown to contribute to or cause disease. In their amyloid forms, yeast prions are cytoplasmically inherited and able to convert soluble protein to amyloid after cell division (Wickner et al. 2018). The capacity to be transferred from mother to daughter cells has resulted in yeast prions being classified as infectious. S. cerevisiae is a non-pathogenic yeast that contains two prions, [URE3] and [PSI+] (Fowler et al. 2007). Both prions display loss-of-function phenotypes (Fowler et al. 2007). After [URE3] forms amyloid, it causes inappropriate derepression of genes involved in utilising nitrogen-poor environments (Fowler et al. 2007). [PSI+] in its native soluble form is a translational termination factor; however, upon forming amyloid, protein synthesis continues past the stop codon, resulting in diversity in the proteome (Otzen and Nielsen 2008; True et al. 2004). These prion-based protein assemblies are non-pathogenic to yeast, but their exact role is still debated (Lipke et al. 2018).
In Podospora anserina, a non-pathogenic filamentous fungus, an amyloid-forming prion, HET is involved in programmed cell death (Dos Reis et al. 2002) (Fig. 1e). HET exists in three forms, soluble HET-s*, insoluble amyloid HET-s and soluble HET-S (Coustou et al. 1997). HET-s can convert HET-s* of a merging colony into amyloid HET-s, exhibiting infectious prion behaviour (Coustou et al. 1997). However, HET-s and HET-S are incompatible, and therefore when two colonies join to form a heterokaryon and contain these two HETs, the HET-s amyloid is the trigger to initiate programmed cell death to prevent the colonies fusing (Daskalov et al. 2015). HET-s displays a biological activity as opposed to the loss-of-function phenotypes shown in [URE3] and [PSI+] of S. cerevisiae, therefore, highlighting the diversity of function even within prion forms of amyloid. Prion domains involved in fungal cell death are not limited to P. anserina, as a cell-death associated amyloid-forming prion protein called HELLP was recently identified in Chaetomium globosum, and this shows homology to the MLKL protein that polymerises to induce lytic cell death in mammalian necroptosis (Daskalov et al. 2015).
Yeast prions have been shown to contain an amyloid-forming ‘prion domain’ that varies in length between prions (Fernandez et al. 2017). This domain is characterised by the presence of uncharged residues and in contrast to hydrophobins and adhesins, often has another role in the protein aside from forming amyloid (Wickner et al. 2018).
Bacterial prions
Prion domains serving functional roles in cells have historically been associated largely with fungal species. Recently, however Yuan and Hochschild (2017) demonstrated that bacterial RNA-associated protein machinery also contains prion domains. Within pathogenic species of bacteria, the best-characterised prion is found in Clostridium botulinum, within the Rho protein. Rho is a conserved helicase involved in termination of translation. The C-terminal domain of this protein contains a prion domain that forms amyloid (Yuan and Hochschild 2017). When converted from monomer to a prion amyloid, Rho loses ability to terminate translation. This leads to transmitted changes in the transcriptome, and presumably the proteome, of the Rho-amyloid-converted colonies (Yuan and Hochschild 2017).
Since this initial identification of a bacterial prion domain, other transcription-associated proteins have also been shown to form amyloid assemblies. Within the pathogenic M. tuberculosis, the transcription regulator CarD has been shown to form amyloid fibrils both in vitro and in vivo (Kaur et al. 2018b). Similarly, the RNA polymerase-binding protein HeiD from B. subtilis can form amyloid fibrils in living cells (Kaur et al. 2018a). These recent discoveries about bacterial prions indicate that prion amyloid assemblies may be a common mechanism for genetic control in bacteria, as well as yeast. However, like yeast prions, the specific functional role of bacterial prions has yet to be fully established.
Conclusion
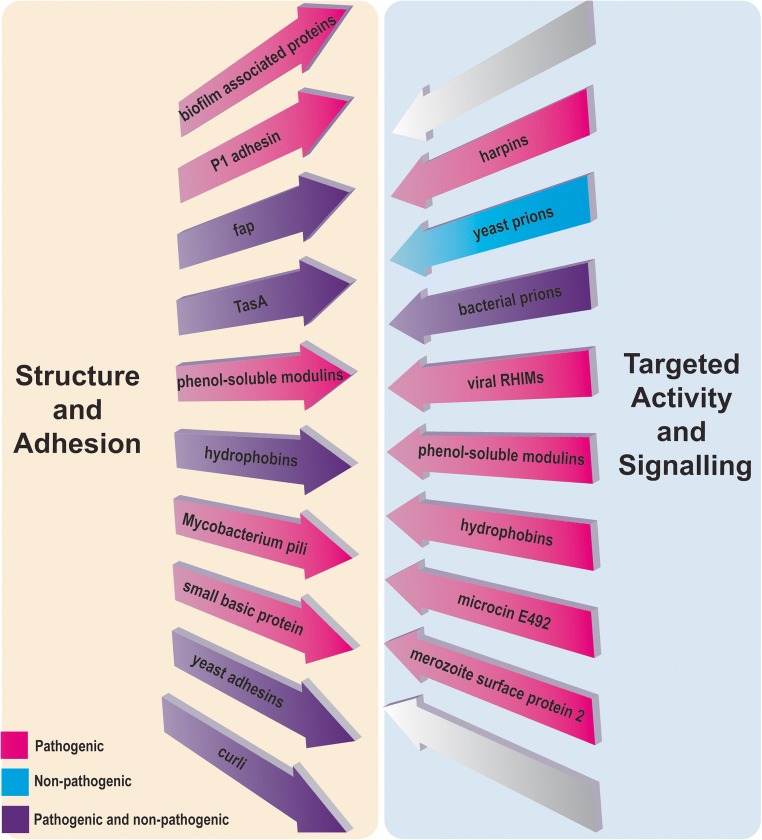
Within nature, different microbes have evolved to utilise amyloid to their own specific advantage, leading to roles for microbial functional amyloids in transitions between different stages of the life cycle, colonisation, infection and evasion of host defence mechanisms. The increase in the number of functional amyloids reported in the scientific literature over the last 20 years suggests that it is likely that a substantial number of microbial amyloids remain to be discovered and investigated. This review highlights the very wide range of structurally and genetically distinct proteins that form functional amyloids in microbial organisms. Although many different proteins form amyloid fibrils that contribute similar structural support and/or adhesive properties, other proteins form amyloids with unique functions that are related to the nature or biological role of the component protein (Fig. 2).
Fig. 2.
Classification of functional amyloids utilised by bacteria, fungi, protozoa and viruses, according to their roles
Proteins that contribute structural amyloids or amyloids involved in adhesion, e.g. hydrophobins or curli, exist in both non-pathogenic and pathogenic organisms, but the majority of the signalling or targeted activity amyloids, e.g. viral RHIM proteins or harpins, are found in pathogenic microbes. Certain amyloids, e.g. those formed by hydrophobins and PSMs, operate as both a structural support and have targeted actions. Overall, this classification illustrates the diversity within microbial amyloid and also the similarities of function in distantly related biological systems.
Whereas amyloid fibrils were initially described as a detrimental outcome resulting from misfolding of proteins, amyloid formation is now known to underpin many significant functional roles in mammals and microbes. Indeed, the comparison of functional microbial amyloids and pathogenic mammalian amyloids may provide an opportunity to identify some of the deleterious features of disease-associated amyloid fibrils.
Compliance with ethical standards
Funding information
MS is funded by the Australian Research Council (DP180101275). NS, MODGB and SRB are supported by the Research Training Program of the Australian Government.
Conflict of interest
Nirukshan Shanmugam declares that he has no conflict of interest. Max O. D. G. Baker declares that he has no conflict of interest. Sarah R. Ball declares that she has no conflict of interest. Megan Steain declares that she has no conflict of interest. Chi L. L. Pham declares that she has no conflict of interest. Margaret Sunde declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nirukshan Shanmugam, Max O. D. G. Baker and Sarah R. Ball contributed equally to this work.
References
- Adda CG, et al. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol Biochem Parasitol. 2009;166:159–171. doi: 10.1016/j.molbiopara.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimanianda V, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- Alsteens D, Ramsook CB, Lipke PN, Dufrene YF. Unzipping a functional microbial amyloid. ACS Nano. 2012;6:7703–7711. doi: 10.1021/nn3025699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alteri CJ, Xicohtencatl-Cortes J, Hess S, Caballero-Olin G, Giron JA, Friedman RL. Mycobacterium tuberculosis produces pili during human infection. Proc Natl Acad Sci U S A. 2007;104:5145–5150. doi: 10.1073/pnas.0602304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Shanmugam N, Pham CLL, Strange M, Steain M, Sunde M (2018) RHIM-based protein:protein interactions in microbial defence against programmed cell death by necroptosis. Semin Cell Dev Biol. 10.1016/j.semcdb.2018.05.004 [DOI] [PubMed]
- Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxa U, Cheng N, Winkler DC, Chiu TK, Davies DR, Sharma D, Inouye H, Kirschner DA, Wickner RB, Steven AC (2005) Filaments of the Ure2p prion protein have a cross-beta core structure. J Struct Biol 150(2):170–179 [DOI] [PubMed]
- Bayry J, Aimanianda V, Guijarro JI, Sunde M, Latge JP. Hydrophobins--unique fungal proteins. PLoS Pathog. 2012;8:e1002700. doi: 10.1371/journal.ppat.1002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais A, Latge JP (2015) Aspergillus biofilm in vitro and in vivo Microbiol Spectr 3 doi:10.1128/microbiolspec.MB-0017-2015 [DOI] [PubMed]
- Besingi RN, Wenderska IB, Senadheera DB, Cvitkovitch DG, Long JR, Wen ZT, Brady LJ. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology. 2017;163:488–501. doi: 10.1099/mic.0.000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieler S, Estrada L, Lagos R, Baeza M, Castilla J, Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J Biol Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- Blancas-Mejia LM, Ramirez-Alvarado M. Systemic amyloidoses. Annu Rev Biochem. 2013;82:745–774. doi: 10.1146/annurev-biochem-072611-130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleem A, et al. Protein engineering reveals mechanisms of functional amyloid formation in Pseudomonas aeruginosa biofilms. J Mol Biol. 2018;430:3751–3763. doi: 10.1016/j.jmb.2018.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DR, et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature. 1997;385:787–793. doi: 10.1038/385787a0. [DOI] [PubMed] [Google Scholar]
- Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Caro-Astorga J, Perez-Garcia A, de Vicente A, Romero D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front Microbiol. 2014;5:745. doi: 10.3389/fmicb.2014.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MR, et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Joo HS, Chatterjee SS, Otto M. Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. 2012;3:71–87. doi: 10.4161/gmic.19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci U S A. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley PJ, Brady LJ, Michalek SM, Bleiweis AS. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect Immun. 1999;67:1201–1206. doi: 10.1128/iai.67.3.1201-1206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalov A, Dyrka W, Saupe SJ (2015) Theme and variations: evolutionary diversification of the HET-s functional amyloid motif Sci Rep 5. doi:10.1038/srep12494 [DOI] [PMC free article] [PubMed]
- DeBenedictis EP, Liu J, Keten S. Adhesion mechanisms of curli subunit CsgA to abiotic surfaces. Sci Adv. 2016;2:e1600998. doi: 10.1126/sciadv.1600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- Dos Reis S, Coulary-Salin B, Forge V, Lascu I, Begueret J, Saupe SJ. The HET-s prion protein of the filamentous fungus Podospora anserina aggregates in vitro into amyloid-like fibrils. J Biol Chem. 2002;277:5703–5706. doi: 10.1074/jbc.M110183200. [DOI] [PubMed] [Google Scholar]
- Dueholm MS, et al. Functional amyloid in Pseudomonas. Mol Microbiol. 2010;77:1009–1020. doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- Dueholm Morten S., Albertsen Mads, Otzen Daniel, Nielsen Per Halkjær. Curli Functional Amyloid Systems Are Phylogenetically Widespread and Display Large Diversity in Operon and Protein Structure. PLoS ONE. 2012;7(12):e51274. doi: 10.1371/journal.pone.0051274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueholm MS, Otzen D, Nielsen PH. Evolutionary insight into the functional amyloids of the pseudomonads. PLoS One. 2013;8:e76630–e76630. doi: 10.1371/journal.pone.0076630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueholm MS, et al. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. MicrobiologyOpen. 2013;2:365–382. doi: 10.1002/mbo3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueholm MS, et al. The tubular sheaths encasing Methanosaeta thermophila filaments are functional amyloids. J Biol Chem. 2015;290:20590–20600. doi: 10.1074/jbc.M115.654780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl AM, Losick R, Kolter R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008;16:269–275. doi: 10.1016/j.tim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer WA, et al. Alzheimer’s disease-associated beta-amyloid is rapidly seeded by Herpesviridae to protect against brain infection. Neuron. 2018;100:1527–1532. doi: 10.1016/j.neuron.2018.11.043. [DOI] [PubMed] [Google Scholar]
- Eisenberg DS, Sawaya MR. Structural studies of amyloid proteins at the molecular level. Annu Rev Biochem. 2017;86:69–95. doi: 10.1146/annurev-biochem-061516-045104. [DOI] [PubMed] [Google Scholar]
- Evans ML, Chapman MR. Curli biogenesis: order out of disorder. Biochim Biophys Acta. 2014;1843:1551–1558. doi: 10.1016/j.bbamcr.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MR, Batlle C, Gil-Garcia M, Ventura S. Amyloid cores in prion domains: key regulators for prion conformational conversion. Prion. 2017;11:31–39. doi: 10.1080/19336896.2017.1282020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB, Falkow S. Common themes in microbial pathogenicity revisited microbiology and molecular biology reviews. MMBR. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Fogel N. Tuberculosis: a disease without boundaries. Tuberculosis (Edinb) 2015;95:527–531. doi: 10.1016/j.tube.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Koulov A, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation in mammalian tissue. Biophys J. 2005;88:199A–199A. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid - from bacteria to humans. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Bois M, Lundberg TM, Sobonya RE, Klotz SA, Lipke PN (2013) Functional amyloids in host-fungal interactions. Mol Biol Cell 24
- Garcia-Sherman Melissa C., Lysak Nataliya, Filonenko Alexandra, Richards Hazel, Sobonya Richard E., Klotz Stephen A., Lipke Peter N. Peptide Detection of Fungal Functional Amyloids in Infected Tissue. PLoS ONE. 2014;9(1):e86067. doi: 10.1371/journal.pone.0086067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle IE, McHenry KT, Weber A, Metz A, Kretz O, Porter D, Hacker G. TIR-domain-containing adapter-inducing interferon-beta (TRIF) forms filamentous structures, whose pro-apoptotic signalling is terminated by autophagy. FEBS J. 2017;284:1987–2003. doi: 10.1111/febs.14091. [DOI] [PubMed] [Google Scholar]
- Giogha C, Lung TW, Pearson JS, Hartland EL. Inhibition of death receptor signaling by bacterial gut pathogens. Cytokine Growth Factor Rev. 2014;25:235–243. doi: 10.1016/j.cytogfr.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Gonzalez DJ, et al. Novel phenol-soluble modulin derivatives in community-associated methicillin-resistant Staphylococcus aureus identified through imaging mass spectrometry. J Biol Chem. 2012;287:13889–13898. doi: 10.1074/jbc.M112.349860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourieux B, Al-Okla S, Scholler-Guinard M, Klein J, Sibilia J, Wachsmann D. Pro-inflammatory cytokine production by synoviocytes following exposure to protein I/II, a modulin from oral streptococci. FEMS Immunol Med Microbiol. 2001;30:13–19. doi: 10.1111/j.1574-695X.2001.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Gravagnuolo AM, et al. Class I Hydrophobin Vmh2 adopts atypical mechanisms to self-assemble into functional amyloid fibrils. Biomacromolecules. 2016;17:954–964. doi: 10.1021/acs.biomac.5b01632. [DOI] [PubMed] [Google Scholar]
- Guo H, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, et al. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 2018;9:816. doi: 10.1038/s41419-018-0868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim KP, et al. Identification of a supramolecular functional architecture of Streptococcus mutans adhesin P1 on the bacterial cell surface. J Biol Chem. 2015;290:9002–9019. doi: 10.1074/jbc.M114.626663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Bono MR, Barros LF, Lagos R. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci U S A. 2002;99:2696–2701. doi: 10.1073/pnas.052709699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Jordal PB, et al. Widespread abundance of functional bacterial amyloid in Mycolata and other gram-positive bacteria. Appl Environ Microbiol. 2009;75:4101–4110. doi: 10.1128/aem.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Kapoor S, Thakur KG. Bacillus subtilis HelD, an RNA polymerase interacting helicase, forms amyloid-like fibrils. Front Microbiol. 2018;9:1934. doi: 10.3389/fmicb.2018.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Kaundal S, Kapoor S, Grimes JM, Huiskonen JT, Thakur KG. Mycobacterium tuberculosis CarD, an essential global transcriptional regulator forms amyloid-like fibrils. Sci Rep. 2018;8:10124. doi: 10.1038/s41598-018-28290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Mizunoe Y, Takade A, Naito S, Yoshida S. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol Immunol. 2005;49:875–884. doi: 10.1111/j.1348-0421.2005.tb03678.x. [DOI] [PubMed] [Google Scholar]
- Krismer B, Weidenmaier C, Zipperer A, Peschel A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol. 2017;15:675–687. doi: 10.1038/nrmicro.2017.104. [DOI] [PubMed] [Google Scholar]
- Kumar DK, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra372. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa I, Penades JR. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol. 2006;157:99–107. doi: 10.1016/j.resmic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lembo D, et al. The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol. 2004;78:4278–4288. doi: 10.1128/JVI.78.8.4278-4288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke PN, Klotz SA, Dufrene YF, Jackson DN, Garcia-Sherman MC (2018) Amyloid-like beta-aggregates as force-sensitive switches in fungal biofilms and infections Microbiol Mol Biol Rev 82. doi:10.1128/mmbr.00035-17 [DOI] [PMC free article] [PubMed]
- Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009;65:71R–77R. doi: 10.1203/PDR.0b013e31819dc44d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A, et al. Merozoite surface protein 2 of Plasmodium falciparum: expression, structure, dynamics, and fibril formation of the conserved N-terminal domain. Biopolymers. 2007;87:12–22. doi: 10.1002/bip.20764. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. Interaction of merozoite surface protein 2 with lipid membranes. FEBS Lett. 2019;593:288–295. doi: 10.1002/1873-3468.13320. [DOI] [PubMed] [Google Scholar]
- Luo J, et al. 3,5-Dicaffeoylquinic acid disperses Aspergillus fumigatus biofilm and enhances fungicidal efficacy of Voriconazole and Amphotericin B. Med Sci Monit. 2018;24:427–437. doi: 10.12659/MSM.908068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macindoe I, Kwan AH, Ren Q, Morris VK, Yang WR, Mackay JP, Sunde M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin. Proc Natl Acad Sci U S A. 2012;109:E804–E811. doi: 10.1073/pnas.1114052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoleta AE, Berrios-Pasten C, Nunez G, Monasterio O, Lagos R. Klebsiella pneumoniae asparagine tDNAs are integration hotspots for different Genomic Islands encoding Microcin E492 production determinants and other putative virulence factors present in hypervirulent strains. Front Microbiol. 2016;7:849. doi: 10.3389/fmicb.2016.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompean M, et al. The structure of the Necrosome RIPK1-RIPK3 Core, a human hetero-Amyloid Signaling Complex. Cell. 2018;173:1244–1253 e1210. doi: 10.1016/j.cell.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VK, Ren Q, Macindoe I, Kwan AH, Byrne N, Sunde M. Recruitment of class I hydrophobins to the air: water interface initiates a multi-step process of functional amyloid formation. J Biol Chem. 2011;286:15955–15963. doi: 10.1074/jbc.M110.214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Vanessa K., Linser Rasmus, Wilde Karyn L., Duff Anthony P., Sunde Margaret, Kwan Ann H. Solid-State NMR Spectroscopy of Functional Amyloid from a Fungal Hydrophobin: A Well-Ordered β-Sheet Core Amidst Structural Heterogeneity. Angewandte Chemie International Edition. 2012;51(50):12621–12625. doi: 10.1002/anie.201205625. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu Rev Microbiol. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Kim JG, Jeon E, Yoo CH, Moon JS, Rhee S, Hwang I. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J Biol Chem. 2007;282:13601–13609. doi: 10.1074/jbc.M602576200. [DOI] [PubMed] [Google Scholar]
- Olsen A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993;7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Olsen A, Wick MJ, Morgelin M, Bjorck L. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect Immun. 1998;66:944–949. doi: 10.1128/iai.66.3.944-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoo HN, Lee KG, Qiu W, Lipke PN. Candida albicans Als adhesins have conserved amyloid-forming sequences. Eukaryot Cell. 2008;7:776–782. doi: 10.1128/ec.00309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otzen D, Nielsen PH. We find them here, we find them there: functional bacterial amyloid. Cell Mol Life Sci. 2008;65:910–927. doi: 10.1007/s00018-007-7404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paharik AE, Horswill AR (2016) The staphylococcal biofilm: adhesins, regulation, and Host Response. Microbiol Spectr 4. doi:10.1128/microbiolspec.VMBF-0022-2015 [DOI] [PMC free article] [PubMed]
- Pearson JS, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JS, et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, et al. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology. 2012;158:2975–2986. doi: 10.1099/mic.0.062109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham Chi L.L., Kwan Ann H., Sunde Margaret. Functional amyloid: widespread in Nature, diverse in purpose. Essays in Biochemistry. 2014;56:207–219. doi: 10.1042/bse0560207. [DOI] [PubMed] [Google Scholar]
- Pham CLL et al (2016) Self-assembly of MPG1, a hydrophobin protein from the rice blast fungus that forms functional amyloid coatings, occurs by a surface-driven mechanism. Sci Rep 6. 10.1038/srep25288 [DOI] [PMC free article] [PubMed]
- Pham CLL, et al. Probing structural changes during self assembly of surface-active hydrophobin proteins that form functional amyloids in fungi. J Mol Biol. 2018;430:3784–3801. doi: 10.1016/j.jmb.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Pham CL et al. (2019) Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep 20. doi:10.15252/embr.201846518 [DOI] [PMC free article] [PubMed]
- Ramsook CB, et al. Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot Cell. 2010;9:393–404. doi: 10.1128/ec.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RV, Fowler VG, Jr, Skov R, Bruun NE. Future challenges and treatment of Staphylococcus aureus bacteremia with emphasis on MRSA. Future Microbiol. 2011;6:43–56. doi: 10.2217/fmb.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen Casper B., Christiansen Gunna, Vad Brian S., Lynggaard Carina, Enghild Jan J., Andreasen Maria, Otzen Daniel. Imperfect repeats in the functional amyloid protein FapC reduce the tendency to fragment during fibrillation. Protein Science. 2019;28(3):633–642. doi: 10.1002/pro.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readhead B, et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64–82 e67. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse SL, et al. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat Commun. 2017;8:263. doi: 10.1038/s41467-017-00361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse SL, Matthews SJ, Dueholm MS. Ecology and biogenesis of functional amyloids in Pseudomonas. J Mol Biol. 2018;430:3685–3695. doi: 10.1016/j.jmb.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas N, Colletier JP, Moshe A, Landau M. Extreme amyloid polymorphism in Staphylococcus aureus virulent PSMalpha peptides. Nat Commun. 2018;9:3512. doi: 10.1038/s41467-018-05490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol. 2004;164:959–963. doi: 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Zilhao R, Ricca E, Ozin AJ, Moran CP, Jr, Henriques AO. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J Bacteriol. 1999;181:3632–3643. doi: 10.1128/jb.181.12.3632-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seviour T, et al. Functional amyloids keep quorum-sensing molecules in check. J Biol Chem. 2015;290:6457–6469. doi: 10.1074/jbc.M114.613810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz M, Soto C. Microcin amyloid fibrils A are reservoir of toxic oligomeric species. J Biol Chem. 2012;287:11665–11676. doi: 10.1074/jbc.M111.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz M, Park KW, Mukherjee A, Diaz-Espinoza R, Soto C. Prion-like characteristics of the bacterial protein Microcin E492. Sci Rep. 2017;7:45720. doi: 10.1038/srep45720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker F., Wickner R. B., Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel beta-sheet structure. Proceedings of the National Academy of Sciences. 2006;103(52):19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soell M, Holveck F, Scholler M, Wachsmann RD, Klein JP. Binding of Streptococcus mutans SR protein to human monocytes: production of tumor necrosis factor, interleukin 1, and interleukin 6. Infect Immun. 1994;62:1805–1812. doi: 10.1128/iai.62.5.1805-1812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia SJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover AG, Driks A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J Bacteriol. 1999;181:1664–1672. doi: 10.1128/jb.181.5.1664-1672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde M, Blake C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem. 1997;50:123–159. doi: 10.1016/S0065-3233(08)60320-4. [DOI] [PubMed] [Google Scholar]
- Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273:729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- Sunde M, Kwan AH, Templeton MD, Beever RE, Mackay JP. Structural analysis of hydrophobins. Micron. 2008;39:773–784. doi: 10.1016/j.micron.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Taglialegna A, et al. Staphylococcal bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016;12:e1005711. doi: 10.1371/journal.ppat.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot NJ, Kershaw MJ, Wakley GE, De Vries O, Wessels J, Hamer JE. MPG1 encodes a fungal hydrophobin involved in surface interactions during infection-related development of magnaporthe grisea. Plant Cell. 1996;8:985–999. doi: 10.1105/tpc.8.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Bhatt A, Smith AN, Crowley PJ, Brady LJ, Long JR. Specific binding of a naturally occurring amyloidogenic fragment of Streptococcus mutans adhesin P1 to intact P1 on the cell surface characterized by solid state NMR spectroscopy. J Biomol NMR. 2016;64:153–164. doi: 10.1007/s10858-016-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb-Fligelman E, et al. The cytotoxic Staphylococcus aureus PSMalpha3 reveals a cross-alpha amyloid-like fibril. Science. 2017;355:831–833. doi: 10.1126/science.aaf4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JD, et al. Electrostatically-guided inhibition of Curli amyloid nucleation by the CsgC-like family of chaperones. Sci Rep. 2016;6:24656. doi: 10.1038/srep24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teertstra WR, Deelstra HJ, Vranes M, Bohlmann R, Kahmann R, Kamper J, Wosten HA. Repellents have functionally replaced hydrophobins in mediating attachment to a hydrophobic surface and in formation of hydrophobic aerial hyphae in Ustilago maydis. Microbiology. 2006;152:3607–3612. doi: 10.1099/mic.0.29034-0. [DOI] [PubMed] [Google Scholar]
- Teertstra WR, et al. The filament-specific Rep1-1 repellent of the phytopathogen Ustilago maydis forms functional surface-active amyloid-like fibrils. J Biol Chem. 2009;284:9153–9159. doi: 10.1074/jbc.M900095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Boomsma W, Wang Y, Otzen DE, Jensen MH, Lindorff-Larsen K. Structure of a functional amyloid protein subunit computed using sequence variation. J Am Chem Soc. 2015;137:22–25. doi: 10.1021/ja5093634. [DOI] [PubMed] [Google Scholar]
- True HL, Berlin I, Lindquist SL. Epigenetic regulation of translation reveals hidden genetic variation to produce complex traits. Nature. 2004;431:184–187. doi: 10.1038/nature02885. [DOI] [PubMed] [Google Scholar]
- Tukel C, et al. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol. 2005;58:289–304. doi: 10.1111/j.1365-2958.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014;10:e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzotzos S, Doig AJ. Amyloidogenic sequences in native protein structures. Protein Sci. 2010;19:327–348. doi: 10.1002/pro.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi Isabel, Dupres Vincent, Stephen-Victor Emmanuel, Guijarro J., Gibbons John, Beau Rémi, Bayry Jagadeesh, Coppee Jean-Yves, Lafont Frank, Latgé Jean-Paul, Beauvais Anne. Role of Hydrophobins in Aspergillus fumigatus. Journal of Fungi. 2017;4(1):2. doi: 10.3390/jof4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi Isabel, Lai Jennifer I., Stephen-Victor Emmanuel, Pillé Ariane, Beaussart Audrey, Lo Victor, Pham Chi L.L., Aimanianda Vishukumar, Kwan Ann H., Duchateau Magalie, Gianetto Quentin Giai, Matondo Mariette, Lehoux Melanie, Sheppard Donald C., Dufrene Yves F., Bayry Jagadeesh, Guijarro J. Iñaki, Sunde Margaret, Latgé Jean-Paul. Assembly and disassembly of Aspergillus fumigatus conidial rodlets. The Cell Surface. 2019;5:100023. doi: 10.1016/j.tcsw.2019.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven N, Klein RD, Hultgren SJ, Remaut H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 2015;23:693–706. doi: 10.1016/j.tim.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven N, Van der Verren SE, Reiter DM, Remaut H. The role of functional amyloids in bacterial virulence. J Mol Biol. 2018;430:3657–3684. doi: 10.1016/j.jmb.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt R, Michiels CW. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res Microbiol. 2005;156:626–633. doi: 10.1016/j.resmic.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Vernier A, Diab M, Soell M, Haan-Archipoff G, Beretz A, Wachsmann D, Klein JP. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 1996;64:3016–3022. doi: 10.1128/iai.64.8.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamakis H, Aguilar C, Losick R, Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler TO, et al. TDP-43 and RNA form amyloid-like myo-granules in regenerating muscle. Nature. 2018;563:508–513. doi: 10.1038/s41586-018-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. Staphylococcus epidermidis small basic protein (Sbp) forms amyloid fibrils, consistent with its function as a scaffolding protein in biofilms. J Biol Chem. 2018;293:14296–14311. doi: 10.1074/jbc.RA118.002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Amyloid fibrils of the HET-s(218-289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- Wessels J. Developmental regulation of fungal cell wall formation. Annu Rev Phytopathol. 1994;32:413–437. doi: 10.1146/annurev.py.32.090194.002213. [DOI] [Google Scholar]
- Wickner R. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264(5158):566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Son M, Bersonov EE, DeWilde M, Ducatez M. Yeast prions compared to functional prions and amyloids. J Mol Biol. 2018;430:3707–3719. doi: 10.1016/j.jmb.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Wiehlmann L, Munder A, Adams T, Juhas M, Kolmar H, Salunkhe P, Tummler B. Functional genomics of Pseudomonas aeruginosa to identify habitat-specific determinants of pathogenicity. Int J Med Microbiol. 2007;297:615–623. doi: 10.1016/j.ijmm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Wosten HA, de Vocht ML. Hydrophobins, the fungal coat unravelled. Biochim Biophys Acta. 2000;1469:79–86. doi: 10.1016/S0304-4157(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Wösten HAB, Wessels JGH. Hydrophobins, from molecular structure to multiple functions in fungal development. Mycoscience. 1997;38:363–374. doi: 10.1007/bf02464099. [DOI] [Google Scholar]
- Wosten HA, van Wetter MA, Lugones LG, van der Mei HC, Busscher HJ, Wessels JG. How a fungus escapes the water to grow into the air. Curr Biol. 1999;9:85–88. doi: 10.1016/S0960-9822(99)80019-0. [DOI] [PubMed] [Google Scholar]
- Yuan AH, Hochschild A. A bacterial global regulator forms a prion. Science. 2017;355:198–201. doi: 10.1126/science.aai7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, et al. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front Microbiol. 2015;6:1099. doi: 10.3389/fmicb.2015.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimaro T, et al. The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation. BMC Microbiol. 2014;14:96. doi: 10.1186/1471-2180-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykwinska A, Pihet M, Radji S, Bouchara JP, Cuenot S. Self-assembly of proteins into a three-dimensional multilayer system: investigation of the surface of the human fungal pathogen Aspergillus fumigatus. Biochim Biophys Acta. 2014;1844:1137–1144. doi: 10.1016/j.bbapap.2014.03.001. [DOI] [PubMed] [Google Scholar]