Abstract
Introduction
The aim of this study was to investigate retinal thickness as a biomarker for identifying patients with mild cognitive impairment (MCI) and Alzheimer's disease (AD).
Methods
The retinal thickness, utilizing the spectral domain optical coherence tomography, was compared among 73 patients with AD, 51 patients with MCI, 67 cognitive normal control (NC) subjects.
Results
The retinal thickness of ganglion cell complex and peripapillary retinal nerve fiber layer decreased in both AD and MCI patients, in comparison with NC subjects (AD vs. NC, P < .01; MCI vs. NC, P < .01). The inner retinal layers in macular area in MCI exhibited significant thinning compared with NC (P < .001). Remarkable association was found between the retinal thickness and brain volume (P < .05). Better correlation was seen between the inner perifovea retinal thickness and the hippocampal and entorhinal cortex volume (r: 0.427–0.644, P < .01).
Discussion
The retinal thickness, especially the inner retinal layer thickness, is a potentially early AD marker indicating neurodegeneration.
Keywords: Alzheimer's disease, Mild cognitive impairment, Retinal thickness, Brain atrophy, Biomarker
1. Introduction
Alzheimer's disease (AD) is a common neurodegenerative disorder characterized by an insidious and progressive worsening of cognitive function [1]. It is now well established that the disease entity presents as a continuous aggregation of pathological changes, and is thus defined in vivo by certain biomarkers and the results of a postmortem examination [2], [3], [4]. Jack et al. [5] proposed a research framework based on a biomarker-based definition of AD in living people; this framework is known as the A/T/N biomarker classification scheme. Since that time, biomarkers for AD have been generally classified into three main categories: (1) A: aggregated β amyloid peptide or an associated pathologic state, as shown by cerebrospinal fluid β amyloid peptide or amyloid positron emission tomography; (2) T: aggregated Tau (neurofibrillary tangles), including cerebrospinal fluid phosphorylated tau and Tau positron emission tomography; (3) N: neurodegeneration or neuronal injury markers consisting of structural magnetic resonance imaging (MRI), fluorodeoxyglucose, positron emission tomography, and cerebrospinal fluid total Tau. However, owing to various reasons, including high financial costs, procedure invasiveness, and inconsistency among laboratories, many of the aforementioned markers have not been widely used in clinical practice. The retina is embryonically derived from the cranial portion of the neural tube and can be used to noninvasively assess the central nervous system. During 1986-1987, Hinton and Sadun [6], [7] provided evidence that visual dysfunction and optic-nerve degeneration occurred during the early stage of AD. Spectral domain optical coherence tomography (SD-OCT) is a newly developed sophisticated imaging technique that can be used to rapidly obtain objective and reliable measurements of the retinal layers with an axial resolution of ≤5 μm. Recently, several studies that used OCT found that the thickness of the retinal nerve fiber layer (RNFL) [8], [9], [10], [11], and also the ganglion cell layer [12], [13], was reduced in patients with AD when compared with healthy subjects. Therefore, RNFL and macular measurements have been proposed as surrogate markers that can be used for identifying and monitoring AD. Because early detection of AD should allow physicians to identify patients who might benefit from therapy before experiencing the onset of apparent cognitive impairment [14], more attention had been paid to the prodromal or preclinical stage of AD.
Mild cognitive impairment (MCI) is defined as a status between normal aging and dementia [15]. MCI can evolve into AD, with an annual progression rate of 4∼23% [16], [17], [18], [19]. Among the various MCI subtypes, amnestic MCI (aMCI) is most likely to progress to AD dementia [20], [21]. However, unlike the OCT studies of AD, retinal structural changes in patients with MCI have remained a subject of controversy [13], [22], [23]. Recently, den Haan et al. [24] performed a meta-analysis to assess retinal layer thickness in patients with AD or MCI. Those data showed that RNFL thickness was moderately reduced in patients with MCI when compared with RNFL thickness in healthy control subjects. By contrast, other case-control studies and meta-analyses [25] have found no significant difference between the OCT measurements in aMCI and normal control (NC) subjects.
In this study, we used SD-OCT to investigate retinal thickness by measuring the following indexes in subjects with AD or MCI as well as NC: peripapillary retinal nerve fiber layer (p-RNFL) thickness, ganglion cell complex (GCC) thickness, and segmentation of macular thickness. By analyzing the cognitive and imaging data, we further explored the degree to which retinal measurements correlated with cognitive performance and imaging markers.
2. Materials and methods
2.1. Subjects
The patients were enrolled from the Memory Clinic in Huashan Hospital from June 2017 to March 2018. Cognitively NC subjects were recruited from a community-based aging cohort—Shanghai Aging Study.
Each participant completed an in-person evaluation that included three main components: (1) an interview by a research nurse who collected demographic information, a medical and neurologic history, and a risk factor profile. The interview also had the study candidate answer a short set of questions concerning their memory. (2) A neurologic evaluation performed by a physician. The evaluation included a medical history review and a complete neurologic examination. (3) Neuropsychological testing. All the participants underwent global cognitive screening and were administered the Mini-Mental State Examination (MMSE). Patients with mild AD or MCI completed a comprehensive battery of tests that covered 4 main domains: (1) memory (delayed recall from the Auditory Verbal Learning Test, Logic Memory Test); (2) executive function/attention (Trail Making Test, Stroop Color Word Test, Symbol Digit Modalities Test); (3) language (Boston Naming Test, Verbal Fluency Test); (4) visuospatial skills (Rey-Osterrieth Complex Figure Test, Clock Drawing Test). Normative data and detailed descriptions of these tests were reported elsewhere [26], [27]. AD and MCI were diagnosed based on NIA/AA 2011 criteria. Operationally, MCI was defined as (1) Scored 1.5 standard deviations below the norms for age and education-specific cutoffs in at least one neuropsychological domain; (2) without noticeable functional impairment in daily life. The NC subjects had no cognitive complaint or any evidence of a neurologic disorder. The control subjects were ascertained as cognitively normal according to a neuropsychological assessment.
Subjects who satisfied any of the following criteria were excluded from the study: (1) Personal or family history of psychiatric disorders, dementia associated with Lewy body dementia, frontotemporal dementia, vascular dementia, Parkinson's disease, or multiple sclerosis, among others; (2) History of glaucoma or increased intraocular pressure (IOP), retinal detachment, severe myopia, optic neuropathy, or other optic nerve disorders. Retinal vascular disease, early age-related macular degeneration, ocular trauma, cataract that prevented an ocular and OCT examination, and other ocular diseases (e.g., cornea disease or uveitis, etc); (3) History of alcohol abuse, carbon monoxide poisoning, hypothyroidism, or other serious chronic medical conditions; (4) Severe cognitive impairment that made the participant unable to cooperate during the eye examination.
2.2. Eye examinations
The time interval between the clinical assessment and eye examination was 1∼90 days. All subjects underwent the following eye examinations: visual acuity, IOP using noncontact tonometry, slit-lamp examination of the anterior segment, ophthalmoscope examination of the posterior segment, and fundus photography (Version 1.5.0.0, NIDEK Co., Ltd).
Because the retinal changes that occur in glaucoma can resemble those that occur in AD, we excluded patients with glaucoma to avoid a possible confounding bias. Subjects with any of the following conditions were excluded from the study: (1) a random IOP >22 mm Hg; (2) slit-lamp examination revealed a suspicious angle closure; (3) fundus photography combined with a fundus examination revealed the following changes in the optic papilla: (a) narrowing of the existential disk edge; (b) parapapillary hemorrhage; (c) rim widths that did not comply with inferior ≥ superior ≥ nasal ≥ temporal principles.
2.3. Optical coherence tomography imaging
OCT images were obtained with an Optovue AngioVue System (software RTVue version 2017.1.0.155, Optovue Inc., Fremont, CA, USA), and an ophthalmic evaluation was performed on the same day. The OCT images were obtained under conditions of normal room daylight, and no cycloplegic eye drop was administered. The following procedures were selected for use in our study: cross-line testing, optic nerve head and 3D disc examinations, GCC evaluation, and retinal mapping. The retina consists of ten layers; from the inside out, it comprises the internal limiting membrane (ILM), nerve fiber layer (NFL), ganglion cell layer, inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer, outer nuclear layer, outer limiting membrane, photoreceptor, and retinal pigment epithelium. The cross-line test was administered to exclude maculopathy. The optic nerve head and 3D disc assessments were performed to analyze the thickness of the p-RNFL. The retinal map divided the macular region of the retina into the inner retina and the outer retina, with the inner retina extending from the ILM to the IPL and the outer retina extending from the INL to the retinal pigment epithelium. The retinal map analysis circles had diameters of 1 mm, 3 mm, and 5 mm, respectively. The GCC included layers from the ILM to the IPL, and had a diameter of 7 mm. Therefore, the inner retina was equivalent to the core area of the GCC. All measurements were taken by the same ophthalmologist to ensure operative coherence among the subjects. Images were reviewed by two ophthalmologists independently, and only those with good quality were included (image clarity > 6). The values for all parameters were automatically calculated by the system's software. The ophthalmologist who collected OCT data performed any manual corrections needed to obtain accurate results in the optic nerve head and 3D disc scans for the p-RNFL.
Both eyes were examined for each subject; however, only data for the right eye of each subject was included in our statistical analysis, to avoid bias caused by an association between the eyes of each subject [28]. The left eye was selected if the right eye was ineligible.
2.4. Neuroimaging data acquisition and imaging analysis
The time interval between the OCT examination and MRI scan was ≤6 months. Neuroimaging was performed via standard procedures to ensure uniform data collection. The scan was conducted in a Siemens 3.0 T MAGNETOM Verio with a sequence of MPRAGE T1WI. The voxel size was 1 × 1 × 1 mm. The FreeSurfer (http://surfer.nmr.mgh.harvard.edu) Analysis software package was used to analyze and visualize structural neuroimaging data obtained from cross-sectional or longitudinal views. The procedures used for MRI structural analysis were as follows: (1) OsiriX (http://www.osirix-viewer.com) software was used to view the obtained brain MRI structure and exclude data produced by obvious lesions and imaging artifacts; (2) FreeSurfer software was used to analyze the brain MRI structure screened by recon-all instructions; (3) tKmedit and tKsurfer were used to check the division partition of white-gray matter after checking recon-all.log to exclude any errors; (4) Asegstats2table and aparcstats2table were used to extract data for the relevant structure.
2.5. Standard protocol approvals, registrations, and patient consent
The study protocol was approved by the Institutional Review Board of Huashan Hospital. Written informed consent was obtained from each study participant and/or the legal representative.
2.6. Data analysis and statistics
All data were analyzed using IBM SPSS Statistics for Windows, Version 21 (IBM Corp., Armonk, NY, USA). Values for continuous variables are expressed as the mean and standard deviation (SD), and values for categorical variables are expressed as a number and frequency (%). Histograms and Q-Q plots were used to test data for a normal distribution. Normally distributed data were analyzed by one way ANOVA, non-normally distributed data were analyzed with the Mann-Whitney U test, and binary variables were analyzed with Fisher's exact test. Between-group comparisons were performed using post hoc analysis. We performed a Bonferroni correction by dividing the critical P value (α) by the number of comparisons being made. We used a generalized linear model to assess the cross-sectional associations (beta estimates [β], standard error [SE]) of the AD group, aMCI group, and NC group based on retinal measurements. All models were adjusted for age, sex, hypertension, and diabetes. Pearson's coefficient was used to correlate parameters of the Angio-OCT with cognitive scores and MRI volume measurements. All P values and 95% CIs were estimated in a two-tailed manner. Differences with a P value < .05 were considered to be statistically significant.
3. Results
3.1. Clinical and demographic characteristics of the study participants
The clinical and demographic characteristics and MMSE scores of participants in the AD, MCI, and NC groups are shown in Table 1. After excluding diseases such as age-related macular degeneration, glaucoma, high myopia, diabetic retinopathy, vitreomacular traction syndrome, as well as subjects with an incomplete eye examination, a total of 191 eyes were evaluated; these included 5 left eyes (2 with a cataract and 3 with corneal macular) and 186 right eyes; among which 73 eyes were from 73 patients with AD, 51 eyes were from 51 patients with MCI, and 67 eyes were from 67 NC subjects.
Table 1.
Demographic characteristics of the study subjects
| Groups | AD | MCI | NC | P value |
|---|---|---|---|---|
| Subjects | 73 | 51 | 67 | |
| Eyes studied | 73 | 51 | 67 | |
| Sex M/F | 29/44 | 20/31 | 24/43 | .88 |
| Age, y, (SD) | 71.40 (7.82) | 71.67 (8.04) | 68.91 (5.88) | .063 |
| MMSE (SD) | 19.67 (4.58) | 28.33 (1.55) | 28.67 (1.00) | <.001∗ |
| VA (SD) | 0.68 (0.24) | 0.71 (0.23) | 0.71 (0.14) | .727 |
| IOP (SD) | 14.18 (2.57) | 14.49 (2.48) | 14.06 (2.14) | .735 |
| HBP | 26 | 22 | 25 | .686 |
| DM | 9 | 10 | 12 | .500 |
The bold value indicate the statistical significance.
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; M, male; F, female; y, years; SD, standard deviation; VA, visual acuity; MMSE, Mini-Mental State Examination; IOP, intraocular pressure; HBP, high blood pressure; DM, diabetes mellitus.
P < .01.
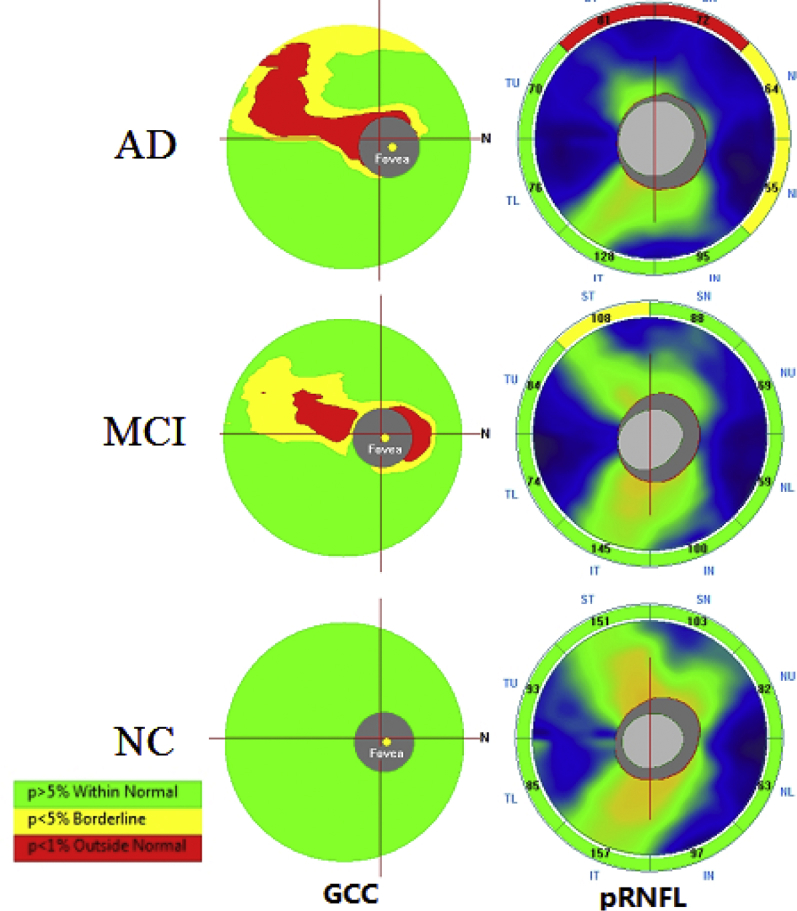
The mean age in the AD, MCI, and NC group was 71.40 ± 7.82 (55–87 years), 71.67 ± 8.04 (51–87 years), and 68.91 ± 5.88 (55–83 years), respectively. There was no significant difference in sex, visual acuity, IOP, or the incidence of high blood pressure or diabetes mellitus among the different groups. The mean age of the NC subjects was slightly less than that of the MCI and AD subjects (P = .043 and P = .044, respectively). Fig. 1 shows an SD-OCT image of a typical GCC and RNFL analysis of a 68-year-old patient with AD, a 65-year-old patient with MCI, and a 72-year-old NC subject.
Fig. 1.
SD-OCT image of the typical GCC and RNFL analysis figures of a 68-year-old patient with AD, a 65-year-old patient with MCI, and a 72-year-old NC subject. The software automatically compared the measured thickness to the age-matched database, generating the map and a significance color-coded to match thickness. The left line was GCC analysis, and the right line was p-RNFL analysis. The green zone means within the normal range (P = 5%–95%), the yellow zone means borderline values (1% < P < 5%), the red zone means outside the normal range (P < 1%). Abbreviations: AD, Alzheimer's disease; GCC, ganglion cell complex; MCI, mild cognitive impairment; RNFL, retinal nerve fiber layer; NC, normal control; SD-OCT, spectral domain optical coherence tomography.
3.2. Retinal thickness measurements and comparisons among groups
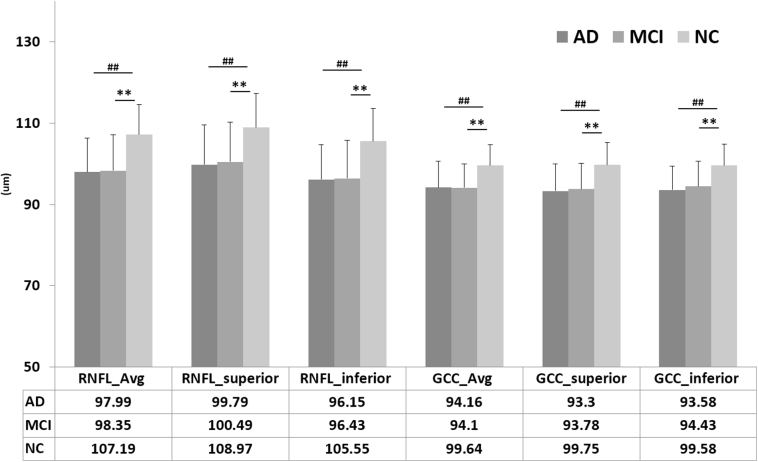
The p-RNFL and macular GCC thicknesses in the AD, MCI, and NC subjects are shown in Fig. 2. Both the p-RNFL and GCC were significantly thinner in the AD and MCI eyes than in the NC eyes. Although the mean thicknesses showed a trend of NC > MCI > AD, no significant difference was found between the AD and MCI groups.
Fig. 2.
Peripapillary retinal nerve fiber layer thickness and ganglion cell complex thickness. ∗∗ MCI versus NC, P < .01, ## AD versus NC, P < .01, AD versus MCI, P > .05. Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; SD, standard deviation; RNFL, retinal nerve fiber layer; GCC, ganglion cell complex; Avg, average; superior, superior hemisphere; inferior, inferior hemisphere. Data reported as mean and SD with P values from one-way ANOVA.
3.3. Retinal map analysis
The results of a macular retina map analysis are shown in Table 2. Forty-two eyes from 42 patients with AD, 48 eyes from 48 patients with aMCI, and 45 eyes from 45 NC subjects were included in the map analysis. Three circular areas from the center to the outside were calculated: fovea (Ø = 1 mm), parafovea (Ø = 3 mm), and perifovea (Ø = 5 mm). Our results showed that all the parameters were significantly thinner in the AD eyes than in the NC eyes. However, a comparison of the MCI group with the NC group showed that although the full retinal thickness and inner retinal thickness were both significantly reduced, there was no significant group difference in the outer retinal layer. Owing to the length constraint, we hereby presented the results of perifovea area only. Analyses of the parafovea and fovea regions showed a similar trend.
Table 2.
Retina map: segmentation analysis (perifovea area)
| Retinal thickness | AD (N = 42) | MCI (N = 48) | NC (N = 45) |
P value |
|
|---|---|---|---|---|---|
| AD versus NC | MCI versus NC | ||||
| Inner average perifovea | 107.79(6.19) | 108.23(7.39) | 113.36(6.00) | <.001∗ | <.001∗ |
| Inner superior perifovea | 107.60(5.54) | 107.63(6.59) | 113.22(5.77) | <.001∗ | <.001∗ |
| Inner inferior perifovea | 104.21(7.47) | 105.65(7.96) | 108.91(6.80) | .004∗ | .036† |
| Inner nasal perifovea | 118.12(9.92) | 118.10(10.68) | 125.04(8.50) | .001∗ | .001∗ |
| Inner tempo perifovea | 101(5.81) | 101.83(8.17) | 106.27(6.07) | <.001∗ | .002∗ |
| Outer average perifovea | 167.21(6.78) | 171.79(8.72) | 173.24(8.78) | .001∗ | .394 |
| Outer superior perifovea | 170.10(8.06) | 173.79(9.31) | 175.87(8.80) | .003∗ | .256 |
| Outer inferior perifovea | 161.24(6.82) | 165.54(8.27) | 167.53(9.14) | <.001∗ | .242 |
| Outer nasal perifovea | 173.05(8.05) | 177(11.69) | 178.78(11.95) | .015† | .429 |
| Outer tempo perifovea | 164.55(7.59) | 170.48(9.23) | 171.73(9.39) | <.001∗ | .494 |
The retina contains ten layers: internal limiting membrane (ILM), nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), outer limiting membrane (OLM), photoreceptor, retinal pigment epithelium (RPE). The retina map divide the macular region retina into inner retina and outer retina, inner retina is from ILM to IPL and outer retina is from INL to RPE.
Data reported as mean and SD.
The bold values indicate the statistical significance.
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; NC, normal control; SD, standard deviation; Avg, average; superior, superior quadrant; inferior, inferior quadrant; nasal, nasal quadrant; tempo, temporal quadrant.
P < .01.
P < .05.
A multivariate analysis was performed using the generalized linear model and with adjustments made for the confounding factors of sex, age, hypertension, and diabetes. Again, as shown in Table 3, similar results were obtained. Unlike the full and inner retinal thicknesses, no significant difference in outer retinal thickness was observed between the MCI and NC groups.
Table 3.
Multivariate analysis of retinal thickness: full and segmental measures
| Retinal thickness | AD versus NC |
MCI versus NC |
||||
|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | |
| Average p-RNFL | −8.826 | 1.377 | <.001∗ | −8.598 | 1.506 | <.001∗ |
| Average GCC | −6.316 | 1.825 | <.001∗ | −4.631 | 1.766 | <.001∗ |
| Inner average perifovea | −6.316 | 1.825 | <.001∗ | −4.631 | 1.766 | .001∗ |
| Outer average perifovea | −5.050 | 1.934 | .002∗ | −1.638 | 1.871 | .477 |
Generalized linear model was used to evaluate the relation between retinal measures and cognitive diagnosis, which was adjusted for confounders of sex, age, medical history of hypertension and diabetes.
The scale parameter was estimated by maximum likelihood.
The bold values indicate the statistical significance.
Abbreviations: RNFL, retinal nerve fiber layer; GCC, ganglion cell complex.
P < .01.
3.4. The correlation between retinal thickness and cognitive imaging measures
The correlation between OCT retinal measures and brain volume as measured on MR images is shown in Fig. 3. In general, we found a significant association between p-RNFL thickness, full perifovea thickness, inner perifovea thickness, and brain volume. An even stronger correlation was found between inner perifovea retinal thickness and the hippocampal and entorhinal cortex volume (r = 0.427–0.644, P < .01). We did not find a significant association between p-RNFL, GCC thickness, and cognitive performance in the AD, MCI, or NC group (P > .05).
Fig. 3.
Correlation analysis between SD-OCT measures and brain volume. The parameter was estimated using Pearson's correlation coefficients. Abbreviations: HIPPO, brain volume of hippocampus; ENTOR, brain volume of entorhinal cortex; L = left; R = right; SD-OCT, spectral domain optical coherence tomography. **P < .01.
4. Discussion
OCT is a simple, fast, and noninvasive optical biopsy and cellular-resolution imaging technology, which is based on the principle of low coherence interferometry [29]. The use of OCT has contributed to remarkable improvements in the diagnosis and monitoring of neurodegenerative diseases [30], [31]. In this study, we found GCC and p-RNFL thinning in patients with AD, and this finding was consistent with those in previous studies and a meta-analysis [24]. In addition, similar changes have been observed in patients with MCI. We found absolute decreases of 10 μm in p-RNFL thickness and 5 μm in GCC thickness in patients with MCI when compared with control subjects.
The clinical significance of the change in p-RNFL thickness in individuals with MCI remains controversial. Some researchers found that the p-RNFL was significantly thinner in subjects with MCI than in cognitively healthy control subjects [22], [23], whereas other researchers did not find such a difference after adjusting for confounding factors [13]. A recent meta-analysis found that RNFL thickness was significantly (inversely) related to cognitive scores [25]. The inconsistent results in these studies can be attributed to high degrees of variability in the study inclusion/exclusion criteria, cognitive testing scales, and the rigor of adjustment for confounding factors. Unlike previous studies [23], [32], our study showed that the p-RNFL in both MCI and AD patients was thinner when compared with the p-RNFL in cognitively normal elderly individuals, even after adjusting for the confounding factors of age, gender, diabetes, and hypertension. Moreover, there was no significant difference between the MCI and AD groups, suggesting that the reduction in p-RNFL thickness might be a relatively early pathological event in the continuous process of AD, and probably occurs before clinical dementia becomes evident.
Here, we utilized the GCC as an indicator of the retinal ganglion cell (RGC) layer, because the RGC layer is the main component of the GCC. The GCC refers to the complex of the macular fovea, 7 mm from the ILM to the IPL. Our study demonstrated an absolute 5 μm decrease in GCC thickness in the MCI group when compared with the control group. Snyder et al. [33] studied preclinical AD and found evidence of tissue loss in the IPL during a continued period of disease progression. The patients with MCI in our study showed a significant reduction in mean overall GCC thickness and reductions in the GCC thickness in all four quadrants. This indicated that the ganglion cells and their axons in the retina had changed not only during the AD stage but also during the MCI stage.
The human retina contains more than 1 million RGCs. Approximately, 50% of RGCs are concentrated within 4.5 mm of fovea [34], and unlike a population of RNFL cells, there is almost no variation in the RGC population within the parafoveal area [34]. Thus a quantitative evaluation of RGC structure in the parafoveal area may be more sensitive and reliable. The Optovue AngioVue System automatically segments the macular retina into regions of full, inner, and outer macula. The retina contains three types of neurons: RGCs, bipolar cells, and optic cells. The inner retina extends from the ILM to the IPL, and the outer retina extends from the INL to the retinal pigment epithelium. Thus the inner retina consists of small-sized ganglion cells, and the outer retina mainly includes two other types of neurons and the axons. Our results showed that the retinal layers in the macular area were differentially affected in the AD and MCI groups: in patients with AD, a general decrement in thickness was observed in both the inner and outer perifoveal layer. Meanwhile, in the MCI group, only the inner retina exhibited significant thinning, when compared with the inner retina of cognitively healthy, elderly control subjects. This finding is consistent with that in an earlier study, which showed that although the disease caused a general decrease in RNFL foveal thickness, only the inner retinal layers exhibited significant thinning when compared with those of healthy subjects [35]. The thinning of the outer layer indicates tissue loss extending from the INL to the outer nuclear layer and suggests retrograde synaptic degeneration.
The retina is an extension of nerve cells in the brain, and our study indicates that retinal measurements, and especially the thickness of the inner layer of the retina, is a more sensitive marker of early neurodegeneration than other segmental retinal indexes, such as outer retina thickness. We also analyzed how retinal changes correlated with cognitive performance and MRI volumetric measurements. No correlation was found between RNFL thinning and cognitive performance. However, retinal layer thickness, and especially the thickness of the inner retinal layer, was strongly correlated with volumetric measurements made by MRI imaging. Few studies have described the relation between retinal layers and MRI features. One such study found an association between decreased gray-matter volume and disrupted white-matter microstructure and retinal layer thickness, but only in NC subjects [36]. Another study in cognitively healthy subjects showed that the medial temporal lobe was related to both RNFL thickness and total macular thickness [37]. In patients with AD, retinal thickness was reported to be correlated with parietal cortical atrophy [38], most likely because of age-related changes [39].
Atrophy of the medial temporal lobe (including the hippocampus, entorhinal cortex, temporal lobe volume, etc.) predicts the conversion of MCI to AD [40], [41]. We analyzed correlations between the p-RNFL, GCC, macular thickness, and the brain volume. Significant associations were found between p-RNFL thickness, full perifovea (Ø = 5 mm) thickness, and MRI volume measurements. A further segmentation analysis revealed an even stronger association between inner perifovea retinal thickness and the hippocampal and entorhinal cortex volume, which once again suggested the inner layer of the retina to be an imaging-relevant biomarker.
This study included only patients with mild to moderate AD, and the mean MMSE score was 19.67 ± 4.58. Patients with severe AD were not able to follow instructions and successfully complete an OCT evaluation, which made OCT testing unreliable in that population. It is possible that changes in the RNFL are more apparent in patients with severe dementia; if so, this could explain why we found no differences in the mean p-RNFL and GCC measurements in the MCI and AD groups. It could also explain why we failed to find an association between cognitive scores and RNFL thickness. However, another possibility is that a degenerative change in the retina represents an earlier pathological change, and precedes a decline in cognitive function.
Under the latest ATN marker framework, we propose that measurements of retinal thickness be included in the “N” category, which reflects neurodegeneration or neuronal injury. Further studies are needed to determine whether retina thinning represents a specific Alzheimer's-related pathogenic process, or is just indicative of a general change that underlies various neurodegenerative diseases.
The present study has some limitations. Owing to the cross-sectional nature of the study, we could not substantiate the chronological of changes that occurred in the RNFL. The NC group might have included a few subjects with subtle cognitive impairment. Although not statistically significant, the mean age in the NC group was less than those in the two patient groups. The mean ages in the AD, MCI, and NC groups, were 71.40 years, 71.67 years, and 68.91 years, respectively. A previous study conducted with nonglaucomatous Asian subjects [42] showed that age-associated RNFL thinning became significant in participants older than 41 years, with the average reduction in RNFL thickness being 2.71 μm for every 10-year increase in age. In our study, we observed an absolute 10 μm decrease in p-RNFL thickness among subjects in the MCI and AD groups, and that decrease was much greater than the previously reported age-related reduction (2.71 μm). However, it is possible that age could have biased the results. Therefore, we performed a generalized linear model analysis to adjust for confounding factors such as age, hypertension, and diabetes, etc, but still obtained similar results. It should be mentioned that the overall sample size in our study was relatively small, which might partially explain the insignificant association between cognition and retinal measurements. In future study, we plan to expand our sample size to ensure comparability among groups. While there have been many similar studies, it is difficult to compare the results of previous studies to the results of our study, because different measurements and equipment were used to analyze changes in retinal thickness. Further research is also needed to develop a standardized method for detecting retinal changes. In addition, we did not enroll subjects who were in a preclinical phase of AD, such as subjects with subjective cognitive dysfunction. Finally, owing to the lack of the amyloid or Tau-related markers, we were unable to analyze the association between those relevant markers and the different retinal measurements.
Prospective studies are needed to validate whether retinal nerve fiber measurements can serve as a surrogate biomarker for patients with AD over time and to further examine whether they can serve as an early diagnostic marker or disease monitoring and prognostic marker.
Research in context.
-
1.
Systematic review: Alzheimer's disease (AD) is a continuous aggregation of pathological changes and is defined by in vivo biomarkers. The author reviewed recently publications using OCT in the patients with AD. The retinal nerve fiber layer (RNFL) thickness was found to be thinner in patients with AD than in healthy subjects, as well as the ganglion cell complex. Meanwhile, retinal structural changes in patients with MCI remained a subject of controversy. These relevant citations were appropriately cited.
-
2.
Interpretation: The retina thickness of the ganglion cell complex and p-RNFL decreased in both AD and MCI patients, as compared with NC subjects. The inner and outer retinal layers in macular area were differently affected by AD and MCI. The inner retinal layers in macular area in MCI exhibited significant thinning compared with NC. Remarkable association was found between the p-RNFL thickness, full perifovea thickness, and the brain volume measured on MRI (P < .05). Better correlation was seen between the inner perifovea retina thickness with the hippocampal and entorhinal cortex volume. We propose that the retina thickness measures may be a novel “N” marker under the Jack “A/T/N” framework, which reflects the neurodegeneration or neuronal injury.
-
3.
Future directions: The article suggests the retina thickness by OCT to be an early AD marker indicating neurodegeneration, whereas the inner layer of the perifovea retina might be a more sensitive index. Prospective studies are needed, to validate whether retina nerve fiber measurements can be a surrogate biomarker for the patients with AD over time, and to further examine whether it can function as an early diagnostic marker or a disease monitoring and prognostic marker.
Acknowledgments
The authors are grateful to the study subjects for their cooperation which made this research possible.
This work was supported by grants from National Chronic Disease Project (2016YFC1306402), Shanghai Science and Technology Municipality (17411950106, 19411961700), Scientific Research Project from STCSM (17411950701), Shanghai Brain-Intelligence Project from STCSM (16JC1420500), Natural Science Foundation and Major Basic Research Program of Shanghai (16JC1420100), and National Natural Science Foundation of China (81773513).
Footnotes
Conflict of interest: The authors have no actual or potential conflicts of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.04.003.
Contributor Information
Yiqin Xiao, Email: xiaoyiqin1028@163.com.
Qianhua Zhao, Email: applenasa@hotmail.com.
Supplementary Data
References
- 1.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Maccioni R.B., Munoz J.P., Barbeito L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch Med Res. 2001;32:367–381. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 3.Buerger K., Ewers M., Pirttila T., Zinkowski R., Alafuzoff I., Teipel S.J. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 4.Tapiola T., Alafuzoff I., Herukka S.K., Parkkinen L., Hartikainen P., Soininen H. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 5.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinton D.R., Sadun A.A., Blanks J.C., Miller C.A. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 7.Sadun A.A., Borchert M., DeVita E., Hinton D.R., Bassi C.J. Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol. 1987;104:113–120. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 8.Trebbastoni A., D'Antonio F., Bruscolini A., Marcelli M., Cecere M., Campanelli A. Retinal nerve fibre layer thickness changes in Alzheimer's disease: Results from a 12-month prospective case series. Neurosci Lett. 2016;629:165–170. doi: 10.1016/j.neulet.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Kesler A., Vakhapova V., Korczyn A.D., Naftaliev E., Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin Neurol Neurosurg. 2011;113:523–526. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Pillai J.A., Bermel R., Bonner-Jackson A., Rae-Grant A., Fernandez H., Bena J. Retinal nerve fiber layer thinning in Alzheimer's disease: a case-control study in comparison to normal aging, Parkinson's disease, and non-Alzheimer's dementia. Am J Alzheimers Dis Other Demen. 2016;31:430–436. doi: 10.1177/1533317515628053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marziani E., Pomati S., Ramolfo P., Cigada M., Giani A., Mariani C. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–5958. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 12.den Haan J., Balk L.J., Verbraak F.D. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol. 2018;96:e265–e266. doi: 10.1111/aos.13550. [DOI] [PubMed] [Google Scholar]
- 13.Cheung C.Y., Ong Y.T., Hilal S., Ikram M.K., Low S., Ong Y.L. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015;45:45–56. doi: 10.3233/JAD-141659. [DOI] [PubMed] [Google Scholar]
- 14.Becker R.E., Greig N.H., Giacobini E. Why do so many drugs for Alzheimer's disease fail in development? Time for new methods and new practices? J Alzheimers Dis. 2008;15:303–325. doi: 10.3233/jad-2008-15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Inzelberg R., Massarwa M., Schechtman E., Strugatsky R., Farrer L.A., Friedland R.P. Estimating the risk for conversion from mild cognitive impairment to Alzheimer's disease in an elderly Arab community. J Alzheimers Dis. 2015;45:865–871. doi: 10.3233/JAD-142871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma F., Wu T.F., Miao R.J., Xiao Y.Y., Zhang W.W., Huang G.W. Conversion of mild cognitive impairment to dementia among subjects with diabetes: a population-based study of incidence and risk factors with five years of follow-up. J Alzheimers Dis. 2015;43:1441–1449. doi: 10.3233/JAD-141566. [DOI] [PubMed] [Google Scholar]
- 18.Chan W.C., Lam L.C., Tam C.W., Lui V.W., Leung G.T., Lee A.T. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing. 2011;40:30–35. doi: 10.1093/ageing/afq151. [DOI] [PubMed] [Google Scholar]
- 19.Busse A., Hensel A., Guhne U., Angermeyer M.C., Riedel-Heller S.G. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 20.Larrieu S., Letenneur L., Orgogozo J.M., Fabrigoule C., Amieva H., Le Carret N. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 22.Ascaso F.J., Cruz N., Modrego P.J., Lopez-Anton R., Santabarbara J., Pascual L.F. Retinal alterations in mild cognitive impairment and Alzheimer's disease: an optical coherence tomography study. J Neurol. 2014;261:1522–1530. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 23.Gao L., Liu Y., Li X., Bai Q., Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer's disease. Arch Gerontol Geriatr. 2015;60:162–167. doi: 10.1016/j.archger.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 24.den Haan J., Verbraak F.D., Visser P.J., Bouwman F.H. Retinal thickness in Alzheimer's disease: a systematic review and meta-analysis. Alzheimers Dement (Amst) 2017;6:162–170. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoll B., Simonett J., Volpe N.J., Farsiu S., Ward M., Rademaker A. Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement (Amst) 2016;4:85–93. doi: 10.1016/j.dadm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding D., Zhao Q., Guo Q., Meng H., Wang B., Luo J. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement. 2015;11:300–309.e2. doi: 10.1016/j.jalz.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Q., Lv Y., Zhou Y., Hong Z., Guo Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One. 2012;7:e51157. doi: 10.1371/journal.pone.0051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdoch I.E., Morris S.S., Cousens S.N. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82:971–973. doi: 10.1136/bjo.82.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebolleda G., Munoz-Negrete F.J. [Optical coherence tomography, an inflection point in neuro-ophthalmology] Arch Soc Esp Oftalmol. 2007;82:731–732. doi: 10.4321/s0365-66912007001200001. [DOI] [PubMed] [Google Scholar]
- 31.Simao L.M. The contribution of optical coherence tomography in neurodegenerative diseases. Curr Opin Ophthalmol. 2013;24:521–527. doi: 10.1097/ICU.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 32.Paquet C., Boissonnot M., Roger F., Dighiero P., Gil R., Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2007;420:97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 33.Snyder P.J., Johnson L.N., Lim Y.Y., Santos C.Y., Alber J., Maruff P. Nonvascular retinal imaging markers of preclinical Alzheimer's disease. Alzheimers Dement (Amst) 2016;4:169–178. doi: 10.1016/j.dadm.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curcio C.A., Allen K.A. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Martin E., Bambo M.P., Marques M.L., Satue M., Otin S., Larrosa J.M. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol. 2016;94:e454–e459. doi: 10.1111/aos.12977. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Ong Y.T., Hilal S., Loke Y.M., Wong T.Y., Chen C.L. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2016;54:585–595. doi: 10.3233/JAD-160067. [DOI] [PubMed] [Google Scholar]
- 37.Casaletto K.B., Ward M.E., Baker N.S., Bettcher B.M., Gelfand J.M., Li Y. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiol Aging. 2017;51:141–147. doi: 10.1016/j.neurobiolaging.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Haan J., Janssen S.F., van de Kreeke J.A., Scheltens P., Verbraak F.D., Bouwman F.H. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer's disease and controls. Alzheimers Dement (Amst) 2018;10:49–55. doi: 10.1016/j.dadm.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckerstrom C., Klasson N., Olsson E., Selnes P., Rolstad S., Wallin A. Similar pattern of atrophy in early- and late-onset Alzheimer's disease. Alzheimers Dement (Amst) 2018;10:253–259. doi: 10.1016/j.dadm.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erten-Lyons D., Howieson D., Moore M.M., Quinn J., Sexton G., Silbert L. Brain volume loss in MCI predicts dementia. Neurology. 2006;66:233–235. doi: 10.1212/01.wnl.0000194213.50222.1a. [DOI] [PubMed] [Google Scholar]
- 41.Saka E., Dogan E.A., Topcuoglu M.A., Senol U., Balkan S. Linear measures of temporal lobe atrophy on brain magnetic resonance imaging (MRI) but not visual rating of white matter changes can help discrimination of mild cognitive impairment (MCI) and Alzheimer's disease (AD) Arch Gerontol Geriatr. 2007;44:141–151. doi: 10.1016/j.archger.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Peng P.H., Hsu S.Y., Wang W.S., Ko M.L. Age and axial length on peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography in nonglaucomatous Taiwanese participants. PLoS One. 2017;12:e0179320. doi: 10.1371/journal.pone.0179320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.