Abstract
Background
Proton radiotherapy (PRT) reduces the volume of normal tissue receiving radiation dose, which may lead to better neurocognitive outcomes. We examined change in neurocognitive scores over time in pediatric brain tumor patients treated with proton craniospinal irradiation (CSI), proton focal RT, or surgery only.
Methods
Patients received annual neurocognitive evaluations for up to 6 years. We examined Full Scale IQ (FSIQ), Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI) scores. General linear mixed models examined change in scores over time by treatment group, adjusting for significant covariates.
Results
Scores from 93 patients treated between 2012 and 2017 (22 proton CSI, 31 proton focal, and 40 surgery only) were examined. Treatment groups were similar on gender (51.6% male), age at treatment (median = 9.7 y), and length of follow-up (median = 2.9 y). The surgery only group had proportionately more gliomas (P < 0.001), and the proton CSI group had more infratentorial tumors (P = 0.001) and higher total RT dose (P = 0.004). The proton focal and surgery only groups exhibited stable neurocognitive scores over time across all indexes (all P > 0.05). In the proton CSI group, WMI, PSI, and FSIQ scores declined significantly (P = 0.036, 0.004, and 0.017, respectively), while VCI and PRI scores were stable (all P > 0.05).
Conclusions
Focal PRT was associated with stable neurocognitive functioning into survivorship. Outcomes were similar whether patients received focal PRT or no radiotherapy, even in neurocognitive domains known to be particularly radiosensitive. Proton CSI emerged as a neurocognitive risk factor, consistent with photon outcomes research.
Keywords: cognitive, IQ, processing speed, pediatric brain tumor, proton radiotherapy
Key Point.
1. There is no difference in neurocognitive outcomes between proton focal radiotherapy and no radiotherapy.
Importance of the Study.
To our knowledge, this is the first prospective, longitudinal study comparing neurocognitive change over time in pediatric brain tumor patients treated with focal proton radiotherapy, craniospinal proton radiotherapy, or no radiotherapy. Our findings are suggestive of neurocognitive sparing benefits for patients treated with focal proton radiotherapy, with outcomes comparable to patients treated without radiotherapy. In contrast, craniospinal proton radiotherapy was associated with some neurocognitive risk in certain domains, although profound neurocognitive impairment was not evident. Our findings fill a critical gap in our knowledge regarding the range of neurocognitive outcomes to expect following treatment with proton radiotherapy. The stable outcomes observed following focal proton radiotherapy, in domains known to be particularly sensitive to decline in photon outcomes research, are notable. While these findings are suggestive of neurocognitive sparing, additional research with larger samples and late survivorship outcomes is needed and ongoing.
While cranial radiotherapy (RT) is an essential treatment for many pediatric brain tumors, it is associated with significant neurocognitive morbidity.1–3 Greater neurocognitive risk is associated with younger age at RT, higher RT dose, and larger RT field size.4–6 Advanced RT techniques, including 3-D conformal and intensity-modulated (IM)RT, have improved the ability to reduce photon radiation (XRT) dose to nontarget tissues.7 Even so, dose is inevitably deposited in surrounding normal brain tissue, which may impair brain functioning, growth, and development.8
Particle therapy, including proton RT (PRT), offers physical properties superior to photons that may further improve techniques for medical radiation.9 Protons deposit maximum dose at the desired depth of tissue, which can be precisely conformed to the clinical target. Compared with XRT, PRT deposits less entrance dose and no exit dose, thereby minimizing irradiation of healthy surrounding tissue. In this way, it is anticipated that PRT will result in better neurocognitive sparing in children treated for brain tumors; however, there is limited available evidence, as of yet, to support this prevailing clinical assumption.
Currently, empirical support for neurocognitive sparing with PRT is encouraging but not definitive. Thus far, only one study has compared cognitive change between pediatric brain tumor patients treated with PRT versus XRT.10 No significant IQ decline or impairment was found among early survivors treated with PRT, while survivors treated with XRT exhibited significant IQ decline. Even so, IQ trajectories were not statistically different between PRT and XRT groups. Across the few PRT outcome studies available to date, PRT-treated patients in early survivorship, as a group, do not exhibit profound neurocognitive change or impairment.10–13 Still, processing speed has emerged as a domain of neurocognitive vulnerability,11–13 while younger age11,13 and craniospinal irradiation (CSI)10–13 have emerged as clinical risk factors for neurocognitive change post-PRT.
With few outcome studies available, uncertainty remains regarding the neuroprotective benefits of PRT and the range of neurocognitive outcomes to expect following treatment with PRT. To our knowledge, this is the first prospective, longitudinal study comparing neurocognitive change over time in early survivorship between pediatric brain tumor patients treated with PRT versus patients treated without radiotherapy (ie, surgery only). Inclusion of this “surgery only” treatment comparison group provides a natural control for the mass effect of a brain tumor and related disruptions that could impact development (eg, school absences). While neurosurgery is associated with neurocognitive risk, this group is unlikely to show the progressive neurocognitive declines traditionally associated with RT.14–17 We hypothesized that survivors treated with PRT would show greater declines in IQ, working memory, and processing speed compared with survivors treated with surgery only. Further, we expected to find the largest neurocognitive declines among survivors treated with CSI PRT versus focal PRT or surgery only.
Materials and Methods
Patients
This study reports on outcomes in early survivorship from an ongoing prospective, longitudinal study of neurocognitive outcomes in pediatric brain tumor patients treated with PRT or surgery only between 2012 and 2017. Following approval from the institutional review board, potentially eligible patients were identified following a new brain tumor diagnosis or when scheduled for neurosurgery or radiation therapy. All eligible patients were approached for the study. Informed written consent was obtained prior to participation. Participants received annual neurocognitive testing from the start of treatment for up to 6 years posttreatment. The baseline neurocognitive evaluation was completed within 6 months of PRT or surgery.
Eligibility criteria for the PRT group included: (1) diagnosis of a primary brain tumor, (2) age 3–18 years (inclusive) at enrollment, and (3) cranial PRT (focal or CSI) within the last 6 months with no history of prior courses of RT. Eligibility criteria for the surgery only group included: (1) diagnosis of a primary brain tumor, (2) ages 3–18 years (inclusive) at enrollment, and (3) surgical resection or biopsy within the last 6 months with no history of RT or plan for future RT at the time of enrollment. Four patients in the sample originally received surgery only, but later required PRT due to disease progression or relapse. Those patients are included in the PRT group, and only their PRT baseline and post-PRT follow-up evaluations were included in analyses. Patients diagnosed with brainstem glioma, high-grade glioma, or atypical teratoid/rhabdoid tumor were excluded from participation due to our interest in long-term outcomes.
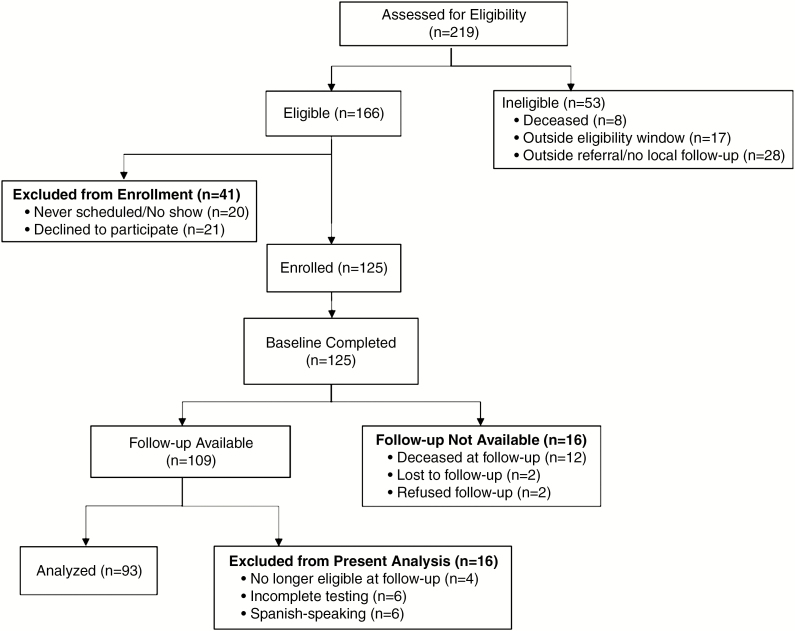
Eligibility and enrollment numbers are reported in the consort flow diagram (Fig. 1). A total of 125 patients were enrolled on study. Out of 166 eligible patients, 21 declined participation (12.7%) and 20 did not schedule and/or show for study testing within the baseline window (12.0%). Patients who declined to participate did not differ from enrolled participants on treatment type, age at treatment, sex, race, or histology (data not shown, all P > 0.05). Overall, of 125 enrolled patients who completed baseline testing, 109 also completed follow-up testing (87.2% retention).
Fig. 1.
CONSORT flow diagram.
The present study examines neurocognitive scores from 93 patients with data at baseline and follow-up. Data were analyzed for English-speaking patients only, given the limited number of Spanish-speaking patients with available data in this sample (n = 6). Surveillance of our Spanish-speaking population is ongoing. Patients with incomplete testing (n = 6) or who were no longer eligible at follow-up (ie, received reirradiation for relapse; n = 4) were also excluded from analysis.
Measures
Participants were administered the age-appropriate version of the Wechsler Scales of Intelligence, the most widely used instruments for measuring cognitive ability.18–22 The Full Scale IQ (FSIQ) score provides a measure of global intellectual functioning. Index scores include: Verbal Comprehension (VCI), Perceptual Reasoning (PRI), Working Memory (WMI), and Processing Speed (PSI). The VCI measures verbal reasoning ability. The PRI measures nonverbal and fluid reasoning ability. The WMI measures the ability to store and manipulate information in short-term memory. The PSI measures the speed, efficiency, and accuracy of information processing. FSIQ and index scores are reported as standard scores (mean = 100, standard deviation = 15), which are computed from age norms. Lower scores indicate worse functioning. FSIQ reliabilities range 0.83–0.91 across versions of the Wechsler Scales of Intelligence used in this study.18,23–26 The Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) does not generate a PRI score. At our request, NCS Pearson (publisher of the WISC-V) generated the norms for calculating PRI scores from WISC-V subtests to enable comparison of scores across versions. WISC-V PRI reliabilities ranged 0.93–0.95 across ages 6–16.27
Statistical Analyses
Analyses were conducted using SAS for Windows version 9.4. Summary statistics were stratified by treatment group (proton CSI, proton focal, surgery only) and compared using Fisher’s exact tests, Kruskal–Wallis tests, or Wilcoxon rank sum tests. General linear mixed models compared change in neurocognitive scores over time between treatment groups. The models included fixed effects for treatment group, time, and a group-by-time interaction term as well as a random intercept and slope. Baseline characteristics significant at P < 0.10 in univariable analysis were included in the multiple regression models. Covariates remained in the models even if they were not significant in the multiple regressions. Regression coefficients for the multiple regression models were assessed at P < 0.05. Residuals plots (residuals vs predicted values) and respective quantile plots assessed model fit. No substantial departures from model assumptions were evident for any of the outcome measures.
Results
Demographic, clinical, and baseline characteristics are summarized and compared across treatment groups in Table 1. As expected, the distribution of tumor pathology differed across treatment groups, with greater proportions of medulloblastoma/primitive neuroectodermal tumors in the proton CSI group and gliomas in the surgery only group (P < 0.001). The tumor types represented in the surgery only group included: pilocytic astrocytoma (n = 18), dysembryoplastic neuroepithelial tumor (n = 7), craniopharyngioma (n = 4), meningioma (n = 3), ganglioglioma (n = 2), glioneuroma (n = 2), giant cell astrocytoma (n = 1), optic pathway glioma (n = 1), pleomorphic xanthoastrocytoma (n = 1), and schwannoma (n = 1). More patients in the proton CSI group had infratentorial tumors (P = 0.001) and experienced posterior fossa syndrome (P = 0.0004). More patients in the proton focal group received a ventriculoperitoneal (VP) shunt (P = 0.007). Total prescribed RT dose to tumor was higher in the proton CSI group (P = 0.004). All patients in the proton CSI group received a tumor bed plus margin boost. Most patients across groups received craniotomy (80.6%) or other tumor-directed neurosurgical procedures (16.2%). Three PRT patients never received neurosurgery for resection or biopsy. Treatment groups did not differ significantly by sex, race, insurance status, annual household income, tumor size, or Lansky/Karnofsky performance score at first clinic visit post diagnosis. Also, there were no significant differences in median age at diagnosis, age at treatment, follow-up interval, and follow-up frequency. Treatment groups did not differ on neurocognitive index scores at baseline.
Table 1.
Demographic, clinical, and baseline characteristics by treatment group (N = 93)
| Proton CSI (n = 22) | Proton Focal (n = 31) | Surgery Only (n = 40) | |||||
|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | P | |
| Sex | 0.622 | ||||||
| Male | 13 | 59.1 | 14 | 45.2 | 21 | 52.5 | |
| Female | 9 | 40.9 | 17 | 54.8 | 19 | 47.5 | |
| Race/ethnicity | 0.193 | ||||||
| White | 16 | 72.7 | 15 | 48.4 | 24 | 60.0 | |
| Black | 1 | 4.5 | 6 | 19.4 | 5 | 12.5 | |
| Hispanic/Latino | 3 | 13.6 | 9 | 29.0 | 11 | 27.5 | |
| Asian | 2 | 9.1 | 1 | 3.2 | 0 | 0.0 | |
| Insurance status†‖ | 0.281 | ||||||
| Private/commercial insurance | 17 | 77.3 | 19 | 61.3 | 22 | 55.0 | |
| Medicaid | 4 | 18.2 | 8 | 25.8 | 14 | 35.0 | |
| CHIP | 0 | 0.0 | 3 | 9.7 | 1 | 2.5 | |
| TRICARE | 0 | 0.0 | 0 | 0.0 | 2 | 5.0 | |
| Histology | <0.001 | ||||||
| Glioma | 1 | 4.5 | 16 | 51.6 | 32 | 80.0 | |
| Medulloblastoma/PNET | 17 | 77.3 | 1 | 3.2 | 0 | 0.0 | |
| Ependymoma | 0 | 0.0 | 6 | 19.4 | 0 | 0.0 | |
| Germ cell tumor | 3 | 13.6 | 3 | 9.7 | 0 | 0.0 | |
| Craniopharyngioma | 0 | 0.0 | 4 | 12.9 | 4 | 10.0 | |
| Other‡ | 1 | 4.5 | 1 | 3.2 | 4 | 10.0 | |
| Tumor location | 0.001 | ||||||
| Supratentorial | 6 | 27.3 | 21 | 67.7 | 30 | 75.0 | |
| Infratentorial | 16 | 72.7 | 10 | 32.3 | 10 | 25.0 | |
| VP shunt | 0.007 | ||||||
| Yes | 0 | 0.0 | 9 | 29.0 | 4 | 10.0 | |
| No | 22 | 100.0 | 22 | 71.0 | 36 | 90.0 | |
| Posterior fossa syndrome | 0.0004 | ||||||
| Yes | 9 | 40.9 | 1 | 3.2 | 3 | 7.5 | |
| No | 13 | 59.1 | 30 | 96.8 | 37 | 92.5 | |
| Median | Min-Max | Median | Min-Max | Median | Min-Max | P | |
| Maximum tumor diameter (cm)† | 4.3 | 2.5–7.0 | 4.0 | 1.5–7.4 | 4.6 | 0.9–7.8 | 0.670 |
| Total RT dose to tumor (Gy) | 54.0 | 45.0–54.0 | 50.4 | 30.0–59.4 | — | — | 0.002 |
| CSI dose | 23.4 | 15.0–36.0 | — | — | — | — | — |
| Age at diagnosis, y | 10.0 | 2.2–17.8 | 8.4 | 1.0–16.5 | 9.3 | 2.2–18.6 | 0.831 |
| Age at treatment, y | 10.1 | 4.0–18.2 | 9.2 | 3.4–17.1 | 9.7 | 2.9–18.6 | 0.763 |
| Baseline to last evaluation, y | 2.8 | 0.8–5.4 | 2.9 | 0.5–6.1 | 3.1 | 1.0–6.0 | 0.677 |
| Number of Evaluations | 3.5 | 2–5 | 3.0 | 2–6 | 3.0 | 2–6 | 0.438 |
| Performance score†⍑ | 90.0 | 40.0–100.0 | 100.0 | 70.0–100.0 | 90.0 | 50.0–100.0 | 0.133 |
| Annual household income† | $60,000-$79,999 | $10,000-≥$200,000 | $40,000- $59,999 | <$10,000-≥$200,000 | $60,000- $79,999 | <$10,000-≥$200,000 | 0.653 |
| Baseline neurocognitive scores† | Mean (sd) | Min-Max | Mean (sd) | Min-Max | Mean (sd) | Min-Max | P |
| VCI | 96.3 (11.8) | 65.0–116.0 | 98.0 (18.3) | 64.0–138.0 | 93.6 (16.7) | 45.0–127.0 | 0.516 |
| PRI | 98.3 (15.9) | 61.0–129.0 | 96.7 (17.2) | 65.0–129.0 | 96.3 (20.3) | 45.0–137.0 | 0.921 |
| WMI | 95.8 (14.8) | 59.0–116.0 | 100.9 (20.0) | 68.0–138.0 | 92.5 (19.6) | 45.0–124.0 | 0.217 |
| PSI | 88.2 (19.6) | 45.0–128.0 | 91.7 (18.5) | 53.0–132.0 | 88.1 (18.4) | 50.0–120.0 | 0.725 |
| FSIQ | 94.2 (16.3) | 54.0–129.0 | 95.9 (19.5) | 61.0–128.0 | 93.1 (17.3) | 53.0–128.0 | 0.807 |
Note. CHIP = Children’s Health Insurance Program; PNET = primitive neuroectodermal tumor; sd = standard deviation.
‡The other histology category includes meningioma (n = 4), choroid plexus carcinoma (n = 1), and schwannoma (n = 1).
†Data were missing for insurance status (n = 3), tumor diameter (n = 1), performance score (n = 12), annual household income (n = 3), VCI (n = 1), PRI (n = 5), WMI (n = 3), PSI (n = 4), and FSIQ (n = 2).
⍑Performance score was obtained from Lansky/Karnofsky rating at the first clinic visit after diagnosis.
‖Comparison of insurance status across groups compared government-funded coverage for low-income families (Medicaid and CHIP) vs other (private/commercial insurance and TRICARE).
For each neurocognitive index score (VCI, PRI, WMI, PSI, and FSIQ), we created separate linear mixed models to examine and compare score change over time by treatment group. All clinical variables that differed across groups at baseline (including histology, tumor location, total prescribed RT dose to tumor, history of posterior fossa syndrome, and shunt, as indicated in Table 1) were included as covariates in the models. Histology was dichotomized as glioma versus all other histologies for regression analyses.
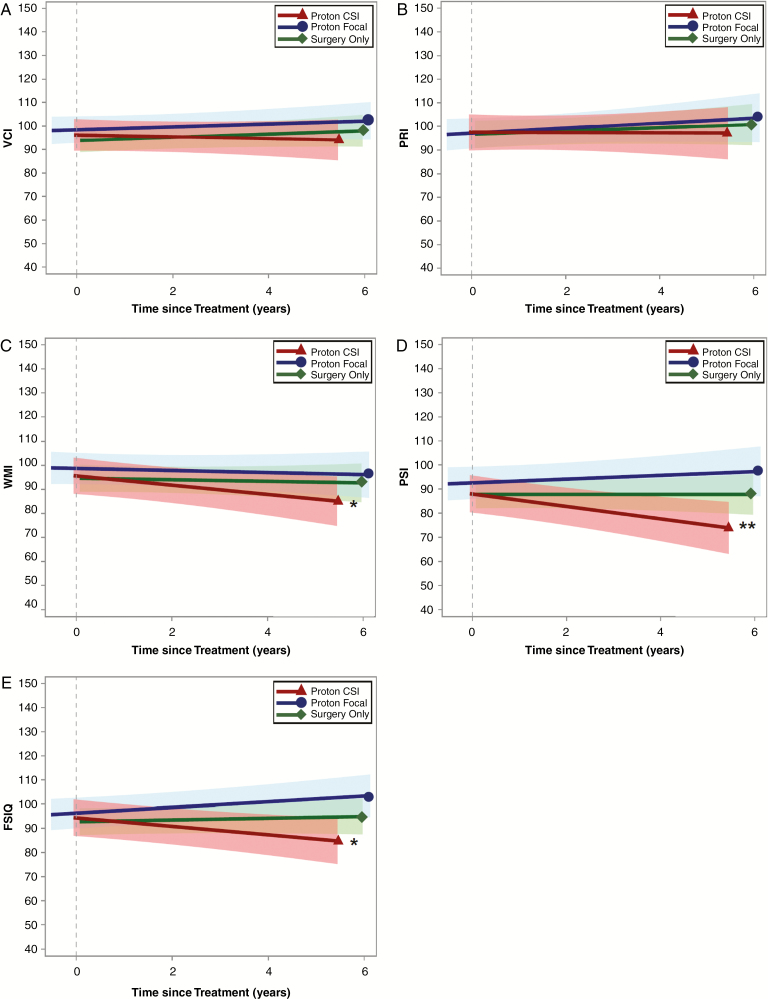
Model results are presented in Table 2 by neurocognitive index. Fig. 2A–E illustrates change in neurocognitive scores over time since PRT or surgery for all 3 groups. In the proton focal and surgery only groups, scores remained stable over time across all neurocognitive indexes (all P > 0.05). In the proton CSI group, VCI and PRI scores remained stable over time (both P > 0.05). WMI scores tended to decline by 1.8 points per year on average (P = 0.036) in the proton CSI group. Even so, WMI change over time (ie, slope) did not differ significantly across treatment groups (all P > 0.05). Patients in the proton CSI group lost a statistically significant 2.6 PSI points per year (P = 0.004) and 1.7 FSIQ points per year (P = 0.017) on average. PSI and FSIQ score declines (ie, slopes) were significantly greater for the proton CSI group compared with the proton focal group (P = 0.005 and P = 0.003, respectively) and the surgery only group (P = 0.019 and P = 0.020, respectively).
Table 2.
Linear mixed models of neurocognitive change over time by treatment group and index
| Index Score Change | Beta | 95% CI | P |
|---|---|---|---|
| VCI Change | |||
| Slope: proton CSI | −0.3 | −1.8, 1.1 | 0.665 |
| Slope: proton focal | 0.6 | −0.6, 1.9 | 0.298 |
| Slope: surgery only | 0.7 | −0.4, 1.8 | 0.211 |
| Slope difference: proton CSI vs proton focal | −1.0 | −2.9, 0.9 | 0.316 |
| Slope difference: proton CSI vs surgery only | −1.0 | −2.8, 0.8 | 0.274 |
| Slope difference: proton focal vs surgery only | 0.0 | −1.7, 1.6 | 0.963 |
| PRI Change | |||
| Slope: proton CSI | 0.0 | −2.0, 2.0 | 0.998 |
| Slope: proton focal | 1.1 | −0.6, 2.7 | 0.192 |
| Slope: surgery only | 0.7 | −0.8, 2.1 | 0.366 |
| Slope difference: proton CSI vs proton focal | −1.1 | −3.6, 1.5 | 0.399 |
| Slope difference: proton CSI vs surgery only | −0.7 | −3.1, 1.8 | 0.591 |
| Slope difference: proton focal vs surgery only | 0.4 | −1.8, 2.6 | 0.699 |
| WMI Change | |||
| Slope: proton CSI | −1.8 | −3.6, −0.1 | 0.036* |
| Slope: proton focal | −0.4 | −1.9, 1.0 | 0.574 |
| Slope: surgery only | −0.4 | −1.7, 0.9 | 0.567 |
| Slope difference: proton CSI vs proton focal | −1.4 | −3.7, 0.8 | 0.205 |
| Slope difference: proton CSI vs surgery only | −1.5 | −3.6, 0.7 | 0.177 |
| Slope difference: proton focal vs surgery only | 0.0 | −2.0, 1.9 | 0.973 |
| PSI Change | |||
| Slope: proton CSI | −2.6 | −4.3,−0.9 | 0.004** |
| Slope: proton focal | 0.7 | −0.7, 2.2 | 0.315 |
| Slope: surgery only | 0.0 | −1.3, 1.3 | 0.993 |
| Slope difference: proton CSI vs proton focal | −3.3 | −5.6, −1.1 | 0.005** |
| Slope difference: proton CSI vs surgery only | −2.6 | −4.7, −0.5 | 0.019* |
| Slope difference: proton focal vs surgery only | 0.7 | −1.2, 2.7 | 0.450 |
| FSIQ Change | |||
| Slope: proton CSI | −1.7 | −3.1, −0.3 | 0.017* |
| Slope: proton focal | 1.2 | 0.0, 2.4 | 0.054 |
| Slope: surgery only | 0.4 | −0.7, 1.4 | 0.494 |
| Slope difference: proton CSI vs proton focal | −2.9 | −4.7, −1.1 | 0.003** |
| Slope difference: proton CSI vs surgery only | −2.1 | −3.8, −0.3 | 0.020* |
| Slope difference: proton focal vs surgery only | 0.8 | −0.8, 2.4 | 0.302 |
Note. Models were adjusted for clinical covariates significant on univariate comparisons: histology (glioma vs other), tumor location, total RT dose to tumor, posterior fossa syndrome, and shunt. Beta reported for each slope represents the increase/decrease in points per year on each index by treatment group. Slope difference compares slopes (ie, change in scores over time) between treatment groups on each index. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 2.
Bold lines are unadjusted mean regressions showing change in neurocognitive scores over time since treatment by group. Shaded bands represent the 95% confidence interval for each regression line. *WMI and FSIQ decline, P < 0.05. **PSI decline, P < 0.01.
Few risk factors, other than CSI, were identified in the overall sample (data presented in Supplementary Table 1). A history of posterior fossa syndrome was consistently associated with lower scores on all 5 indexes (VCI: P = 0.027, PRI: P = 0.008, WMI: P = 0.014, PSI: P = 0.014, FSIQ: P = 0.005), even after accounting for CSI and all covariates. History of VP shunt remained associated with lower PRI and PSI scores in the final models (P = 0.029 and 0.024, respectively). A group-by-time since treatment interaction was identified, with longer time from treatment being associated with lower PSI and FSIQ scores in the proton CSI group only (P = 0.019 and 0.020, respectively). No other statistically significant associations were identified in the models. Within the proton CSI group, change in index scores over time did not depend on CSI dose, all P > 0.05 (data not shown).
Discussion
In early survivorship (ie, within the first 3 years posttreatment on average), focal PRT was not associated with neurocognitive decline, even in domains known to be particularly radiosensitive (eg, processing speed, working memory). Neurocognitive trajectories were similarly stable whether patients received focal PRT or no RT. Group performance was within normal limits for age across indexes, remaining stable for up to 6 years posttreatment, in both focal PRT and surgery only groups. These findings offer strong support for the neurocognitive sparing benefit of focal PRT.
In contrast, as expected, PRT CSI was associated with some evidence of neurocognitive risk. In particular, processing speed showed the greatest vulnerability post-PRT CSI, consistent with XRT outcomes research associating higher RT doses to larger volumes of brain with less favorable neurocognitive outcomes.6,28–31 Working memory vulnerability was also identified. The solidly stable performance in verbal and perceptual reasoning domains in this group suggests that the decline observed in FSIQ is likely driven by declines in processing speed and working memory. This pattern of vulnerability in cognitive efficiency domains, rather than a pattern suggestive of global intellectual decline, has been observed previously among patients treated with XRT.31–33
Baseline scores in the PRT CSI group were broadly within normal limits for age across indexes. Average decline of 1.7 IQ points per year falls at the low end of rates of IQ decline reported in other longitudinal studies of patients treated with XRT CSI,1,2,34,35 although our findings do not provide definitive evidence of a relative neurocognitive sparing benefit of PRT for patients treated with CSI. Still, the conformality of the tumor bed plus margin boost achieved with PRT results in more normal tissue sparing and, potentially, protection of total brain from achieving a threshold dose that could result in global impairment. In a prior study,10 our data suggested the greatest IQ insult from CSI occurred early and persisted. Following these patients into late survivorship will determine whether the decline detected here reflects the greatest degree of change that will be experienced by these survivors or whether continued decline will be evidenced in late survivorship.
The pattern of findings in our PRT CSI subsample differs in some ways from neurocognitive change observed in a medulloblastoma sample treated with PRT CSI reported by Yock and colleagues.11 Yock et al identified significantly lower FSIQ, VCI, and PSI scores at follow-up (median follow-up = 5.2 y), while WMI and PRI scores remained stable. The difference in VCI vulnerability between studies is notable. Possible differences in clinical characteristics between PRT CSI groups may explain differences in outcomes. For example, the Yock et al sample was younger at RT (median age = 6.6 y), and many patients received full posterior fossa boost (39%), both factors associated with neurocognitive risk.4–6 This contrasts with our CSI subsample, which included patients who were older at RT (median age = 10.0 y) and who received boost dose to the tumor bed plus margin only.
Our present study replicates the finding of stable IQ among pediatric brain tumor patients treated with focal PRT reported in a prior retrospective study.10 This replication of stability is notable and stands in contrast to the significant IQ decline observed among patients treated with focal XRT as reported by Kahalley et al in 2016.10 Together, these reports are suggestive of neurocognitive sparing with focal PRT. Kahalley et al10 did not detect significant IQ decline in patients treated with either PRT or XRT CSI, although the XRT CSI group exhibited persistently lower IQ scores, suggestive of an initial neurocognitive insult from XRT CSI that resulted in lasting but stable impairment. Since the present study identified significant FSIQ decline in the PRT CSI group, it is important to consider methodological differences that could explain the discrepant PRT CSI findings between the present prospective study and our prior retrospective study. Differences that could contribute to the discrepancy include: the use of standard, consistent neurocognitive instruments, systematic baseline and follow-up timing, and up to 6 years of follow-up surveillance in the present prospective study compared with the use of retrospective clinical data, inclusion of scores from different neurocognitive instruments, and variable baseline data acquisition in the prior retrospective study. It should also be noted that a relatively high proportion of patients in the PRT CSI group in the present study (40.9%) had a history of posterior fossa syndrome, which is associated with lasting neurocognitive risk.36 This proportion is at the high end of incidence rates reported historically in samples of pediatric posterior fossa tumors (24–39%).37,38 (Incidence of posterior fossa syndrome was not reported in the Kahalley et al retrospective study.10) Differences in PRT findings between studies underscore the importance of long-term outcomes research in this population. Despite growing availability of and interest in PRT, the number of pediatric brain tumor patients treated with PRT who have achieved late survival status to date remains relatively small, and our understanding of PRT-related outcomes is limited and preliminary.
Factors other than RT modality continue to influence neurocognitive trajectories following treatment for pediatric brain tumors. Both history of a VP shunt and history of posterior fossa syndrome were associated with neurocognitive risk in the overall sample. Even if PRT is found to be associated with neurocognitive sparing (relative to conventional XRT), hydrocephalus and postoperative complications (including posterior fossa syndrome and/or cerebellar mutism) will likely continue to exert an independent influence on outcomes, consistent with findings of XRT outcomes research.36,39 Although CSI dose was not associated with neurocognitive risk after accounting for all other variables in our models, it is noteworthy that more of the patients diagnosed with posterior fossa syndrome were treated with low CSI dose (≤23.4 Gy; n = 7) than high CSI dose (>23.4 Gy; n = 2). A higher number of posterior fossa syndrome cases in the low dose CSI subgroup is expected, since fewer patients presented with high risk disease in our sample, but our findings may indicate that posterior fossa syndrome carries greater neurocognitive risk than RT dose for patients receiving CSI.
Study limitations must be considered. Randomization to treatment groups was not practical or ethical. Without an XRT comparison group, we are limited in our ability to draw conclusions about PRT outcomes relative to outcomes expected in patients treated with contemporary XRT. Nonetheless, the dearth of outcomes data on pediatric brain tumor patients treated with PRT is notably disproportionate to the broad awareness of this treatment within the medical community and among parents of children receiving a brain tumor diagnosis, and our findings are among the first to characterize this population. Additionally, future studies should consider a more robust assessment of processing speed given its consistent vulnerability to RT effects in both PRT and XRT samples.11–13,30,31,40 Processing speed is a complex construct, and our measurement approach did not allow for parsing out involved components (eg, fine motor skills, visual-motor integration) that could differ across groups. Still, the longitudinal design, use of standardized assessment, and excellent follow-up rates achieved in this study are strengths that outweigh many of these unavoidable limitations.
These findings provide preliminary evidence of favorable neurocognitive outcomes associated with PRT in early survivorship, particularly in patients treated with focal PRT. Neurocognitive stability was observed whether patients received focal PRT or no RT. CSI remains associated with neurocognitive risk in domains traditionally associated with RT vulnerability (ie, processing speed and working memory); however, PRT CSI was not associated with profound impairment in our sample followed for up to 6 years post-RT. Despite these encouraging early findings, neurocognitive monitoring remains essential for all patients who receive RT (regardless of modality), consistent with current survivorship guidelines from the Children’s Oncology Group.41 Additional research with larger samples and longer follow-up is needed and ongoing.
Funding
This work was supported by the National Institutes of Health/National Cancer Institute (R01CA187202 to LSK); National Institutes of Health/National Cancer Institute (K07CA157923 to LSK).
Conflict of interest statement. No conflicts of interest.
Authorship statement. Study design: LSK, MDR, AM, MFO, MC, ACP, WEW, DRG. Data collection: LSK, MDR, MFO, MC, DRG, HHS, JO, JJX, and EAW. Data analysis: LSK and CGM. Data interpretation: LSK, CGM, MDR, MFO, MC, and DRG. All authors contributed to the writing and editing of the manuscript and agreed with the final version of the report.
Supplementary Material
Previous presentations. We presented these findings at the International Society of Pediatric Neuro-Oncology meeting in July 2018 (Denver, CO).
References
- 1. Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. [DOI] [PubMed] [Google Scholar]
- 2. Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol. 2001;19(15):3470–3476. [DOI] [PubMed] [Google Scholar]
- 3. Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. [DOI] [PubMed] [Google Scholar]
- 4. Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 6. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 7. Kun LE, Beltran C. Radiation therapy for children: evolving technologies in the era of ALARA. Pediatr Radiol. 2009;39(Suppl 1):S65–S70. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24(11):1387–1396. [DOI] [PubMed] [Google Scholar]
- 9. Yock TI, Tarbell NJ. Technology insight: proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1(2):97–103; quiz 1 p following 111. [DOI] [PubMed] [Google Scholar]
- 10. Kahalley LS, Ris MD, Grosshans DR, et al. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol. 2016;34(10):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. [DOI] [PubMed] [Google Scholar]
- 12. Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93(2):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ris MD, Beebe DW, Armstrong FD, et al. ; Children’s Oncology Group Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(29):4765–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Rocco C, Chieffo D, Pettorini BL, Massimi L, Caldarelli M, Tamburrini G. Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: the role of the tumor and the impact of the surgical treatment. Childs Nerv Syst. 2010;26(9):1173–1188. [DOI] [PubMed] [Google Scholar]
- 16. Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 17. Turner CD, Chordas CA, Liptak CC, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. [DOI] [PubMed] [Google Scholar]
- 18. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence‒Third Edition Technical Manual. San Antonio: Pearson; 2002. [Google Scholar]
- 19. Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 20. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: NCS Pearson, Inc; 2008. [Google Scholar]
- 21. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th ed. San Antonio, TX: NCS Pearson, Inc.; 2012. [Google Scholar]
- 22. Wechsler D. Wechsler Intelligence Scale for Children. 5th ed. Bloomington, MD: NCS Pearson, Inc; 2014. [Google Scholar]
- 23. Wechsler D. Wechsler Intelligence Scale for Children‒Fourth Edition Technical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 24. Wechsler D. Wechsler Adult Intelligence Scale‒Fourth Edition Technical and Interpretive Manual. San Antonio, TX: NCS Pearson, Inc; 2008. [Google Scholar]
- 25. Wechsler D. Wechsler Intelligence Scale for Children‒Fourth Edition Administration and Scoring Manual. Bloomington, MN: NCS Pearson, Inc; 2003. [Google Scholar]
- 26. Wechsler D. Wechsler Intelligence Scale for Children‒Fifth Edition Technical and Interpretive Manual. Bloomington, MN: NCS Pearson, Inc; 2014. [Google Scholar]
- 27. Standardization data from the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) Copyright © 2014 NCS Pearson, Inc. Used with permission. All rights reserved. [Google Scholar]
- 28. Raghubar KP, Lamba M, Cecil KM, et al. Dose-volume metrics and their relation to memory performance in pediatric brain tumor patients: a preliminary study. Pediatr Blood Cancer. 2018;65(9):e27245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ris MD, Ryan PM, Lamba M, et al. An improved methodology for modeling neurobehavioral late-effects of radiotherapy in pediatric brain tumors. Pediatr Blood Cancer. 2005;44(5):487–493. [DOI] [PubMed] [Google Scholar]
- 30. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahalley LS, Conklin HM, Tyc VL, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22(9):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kahalley LS, Winter-Greenberg A, Stancel H, Ris MD, Gragert M. Utility of the General Ability Index (GAI) and Cognitive Proficiency Index (CPI) with survivors of pediatric brain tumors: comparison to full scale IQ and premorbid IQ estimates. J Clin Exp Neuropsychol. 2016;38(10):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mabbott DJ, Monsalves E, Spiegler BJ, et al. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011;117(23):5402–5411. [DOI] [PubMed] [Google Scholar]
- 34. Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. [DOI] [PubMed] [Google Scholar]
- 35. Ris MD, Walsh K, Wallace D, et al. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer. 2013;60(8):1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robertson PL, Muraszko KM, Holmes EJ, et al. ; Children’s Oncology Group Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(Suppl 6):444–451. [DOI] [PubMed] [Google Scholar]
- 38. Wells EM, Khademian ZP, Walsh KS, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. [DOI] [PubMed] [Google Scholar]
- 39. Hardy KK, Bonner MJ, Willard VW, Watral MA, Gururangan S. Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology. 2008;17(11):1157–1161. [DOI] [PubMed] [Google Scholar]
- 40. Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. [DOI] [PubMed] [Google Scholar]
- 41. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 4.0. Monrovia, CA: Children’s Oncology Group; 2013. www.survivorshipguidelines.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.