Abstract
Introduction
Researchers are searching for clinical instruments to predict amyloid positivity for disease classification. Informant-based reports could detect disease status. This study compares subjective memory complaints captured by informant-based reports between positron emission tomography (PET)–positive and PET-negative patients and hypothesizes that amyloid PET positivity associates with increased informant-based cognitive complaints.
Methods
Ninety-eight amnestic mild cognitive impairment or mild dementia subjects were studied. Subjective report was captured by the informant-driven Alzheimer's Questionnaire (AQ) administered before PET. Differences in demographics and AQ score by diagnostic status and amyloid status were measured, and a receiver-operating characteristic curve was calculated.
Results
Sixty-five mild cognitive impairment/Alzheimer's disease amyloid PET-positive and 33 amyloid PET-negative subjects were included. AQ was significantly higher (12.51 ± 4.95) for amyloid PET-positive subjects (9.06 ± 3.65; P = .001).
Conclusions
Amyloid PET-positive subjects with Alzheimer's disease or mild cognitive impairment have more informant-based reports of cognitive decline, indicating utility for a brief informant measure.
Keywords: Amyloid PET, Informant-based reporting, Alzheimer's disease, Dementia, Disease classification
1. Introduction
Amyloid β (Aβ) protein deposition in the brain is a hallmark feature of Alzheimer's disease (AD) [1]. With recent advances in neuroimaging, it has become possible to assess amyloid status in vivo as measured by Aβ positron emission tomography (PET) scan. The ability to determine brain amyloid pathology has substantial clinical benefits and allows for improved confidence in the diagnosis of cognitive decline due to AD, as many studies have shown a positive correlation between Aβ PET-positive individuals and their likelihood to progress to AD [2], [3]. Additionally, amyloid status can alter the treatment and care decisions for individuals with mild cognitive impairment (MCI) and dementia [4], [5]. The challenge with amyloid PET scanning is that it is not widely available [6] and is prohibitively expensive for many patients as it is not a covered service under Medicare [7], [8]. Thus, finding easily available, inexpensive tools capable of detecting amyloid status has significant potential for patient care.
Objective assessments of cognitive functioning have value in improving the accuracy of clinical diagnoses of early cognitive decline [9]. There is also increasing evidence suggesting that questionnaires eliciting subjective memory complaints (SMCs) are viable screening measures sensitive to objective cognitive impairment and, moreover, that qualitative analysis of SMCs is able to predict prodromal clinical manifestations of dementia [10]. Examples of published questionnaires for SMCs include the AD8 [11], the Informant Questionnaire on Cognitive Decline in the Elderly [12], and the Memory Functioning Questionnaire [13].
The Alzheimer's Questionnaire (AQ) [14] is an informant-based SMC questionnaire that evaluates five domains associated with AD including memory, orientation, functional ability, visuospatial, and language. Prior research with the AQ has demonstrated its ability to differentiate patients with clinically diagnosed mild cognitive impairment (MCI) and AD from normal aging with high sensitivity (89.0% for MCI and 99.0% for AD) and specificity (91.0% for MCI and 96.0% for AD) [15]. The AQ also demonstrated good internal consistency (Cronbach's α = 0.88). This pilot study was then followed by a larger validation study that included 300 individuals (100 with AD, 100 with amnestic mild cognitive impairment [aMCI], 100 normal) [16]. The results of the validation study were very similar to that of the pilot study with sensitivity and specificity for aMCI being 89% and 91%, respectively. Internal consistency remained high (Cronbach's α = 0.89) while the between-domain correlations were modest (r = 0.45–0.69), indicating that the individual domains of the AQ are measuring distinct constructs. In a subsequent study using a subset of aMCI and cognitively normal individuals from the validation study, analyses of the individual AQ items were carried out to determine which cognitive symptoms were most strongly associated with aMCI [15]. Four AQ items were strong indicators of aMCI which included repetition of statements and/or questions (odds ratio [OR] 13.20 [3.02, 57.66]); trouble knowing the day, date, month, year, and time (OR 17.97 [2.63, 122.77]); difficulty managing finances (OR 11.60 [2.10, 63.99]); and decreased sense of direction (OR 5.84 [1.09, 31.30]). These four items accounted for approximately 71% of the variance between aMCI and cognitively normal individuals after controlling for age and education. A more recent study carried out investigated how well the AQ correlates with traditional neuropsychological measures used to make clinical diagnoses of aMCI and AD. Using a large age-, education-, and sex-matched group, this study found that the AQ correlated strongly with the Mini-Mental State Examination (MMSE) (r = 0.71) and the Dementia Rating Scale-2 (r = 0.72). The AQ demonstrated strong correlations with the Rey Auditory Verbal Learning Test (r = -0.61) and the Brief Visuospatial Memory Test-Revised (r = −0.65). Moderate correlations between the AQ and measures of executive function were also found (Trails B, r = 0.53; Stroop Color/Word, r = −0.51) [17].
To date, very few studies have assessed the relationship between SMCs and pathological status. Some early studies with the Memory Functioning Questionnaire have shown that poorer subjective ratings of memory, executive functioning, and overall cognition were significantly correlated with greater PET tracer uptake indicating greater amyloid burden [18], [19]. Additionally, individuals with a greater number of SMCs were more likely to be amyloid positive even after controlling for neurodegeneration status (positive or negative), age, and education [18]. While the AQ has been shown to improve diagnostic accuracy, this informant measure has not yet been associated with Aβ protein positivity.
As such, the primary aim of the present study was to determine the ability of subjective reports captured by the informant-based AQ to differentiate amyloid status as determined by PET scan to determine if an increase in subjective memory complaints is associated with increased Aβ protein positivity in the brain. Given past research with the AQ showing that individuals with MCI and AD have higher scores than cognitively normal individuals, we hypothesized that individuals who are amyloid PET positive will have higher AQ scores, and AQ cutoffs might be used to predict amyloid PET positivity in the future. Establishing the sensitivity of the AQ to amyloid status will bolster its utility in the clinical setting and would offer an additional tool for clinicians to identify those patients with increased likelihood of amyloid pathology.
2. Methods
2.1. Subjects
Archival records from 98 patients seen at an outpatient neurology center in the Southwest United States who underwent an amyloid PET scan as part of the Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) study were reviewed. The minimal risk protocol was approved by the St Joseph's/Dignity Health/Barrow Neurological Institute Institutional Review Board prior to records being reviewed. All study procedures were reviewed and approved by the Institutional Review Board at Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona.
Prospectively evaluated patients with mild to moderate AD or MCI were assessed in the memory disorders clinic before being enrolled in the IDEAS study. All participants met inclusion and exclusion criteria for the IDEAS study. They were excluded from this sample if they did not meet the criteria for amyloid PET imaging through the IDEAS study. All participants over the age of 50 years with a diagnosis of mild to moderate AD or MCI, a completed an amyloid PET scan, data from the informant-based AQ, and had a MMSE [20] score of 15-30 were included. All participants with diagnoses of AD met the criteria established by the National Institute on Aging and the Alzheimer's Association [21], and the diagnosis of MCI was made based on the Petersen criteria [22]. We included MCI patients up to MMSE of 30 that had SMC and a clinical diagnosis of MCI with sufficient cognitive impairment to warrant a diagnosis and further evaluation including amyloid PET. Exclusion criteria included evidence of vascular, traumatic, or inflammatory causes of aMCI evident by noncontrast magnetic resonance imaging and history of major systemic diseases that could possibly affect cognitive function including other dementias (e.g., dementia with Lewy bodies, frontotemporal dementia, overt primary progressive aphasia (such as nonfluent progressive aphasia or semantic dementia), Parkinson's disease dementia, vascular dementia), cardiopulmonary failure, hepatic or renal failure, diabetes mellitus, head injury, stroke, or other neurodegenerative disease. Additional variables including age, sex, education level, clinical diagnosis (established by a behavioral neurologist), and additional significant medical history were gathered from the patient's medical record.
2.2. Procedure
2.2.1. Amyloid imaging
Amyloid PET status was acquired from patients seen as part of the IDEAS study between June 2016 and December 2017, which utilized the F-18 florbetapir compound. Imaging was read and reviewed by an expert radiologist who provided a qualitative status of Aβ positive (A+) or negative (A−) [23].
2.3. Alzheimer's questionnaire
The AQ was administered to an informant accompanying the patient during clinical consultation as the standard of care. Each question is answered either “yes” or “no” and the sum of the positively endorsed items add up to a total score from 0 to 27. Six AQ items are weighted (i.e., worth two points) given their status as highly predictive of a clinical diagnosis of AD (e.g., repeating questions, disorientation to time [14]).
2.3.1. Analyses
All data were analyzed using the IBM SPSS statistical package, version 23. Pearson bivariate correlations were conducted to measure the associations between demographic variables (age and education) and AQ total score. Chi-square analyses were conducted to measure potential differences in diagnostic status (aMCI vs. dementia) by amyloid status (positive vs. negative) and to measure differences in sex by diagnostic status and amyloid status. Independent samples t-tests were conducted to measure potential differences using age and education level as dependent variables and amyloid status, diagnostic status, and sex as independent variables. Of note, education data were unavailable for 18 patients who were thus excluded from the t-test analysis evaluating differences in education level. An analysis of covariance was conducted to measure differences in AQ total score with sex as the independent variable and education as a covariate. An independent samples t-test was conducted to measure differences in AQ total score using amyloid status (positive vs. negative) as the independent variable. Differences in AQ score by diagnostic status were excluded from analyses as the AQ was used, in part, to determine clinical diagnosis of MCI and AD. Finally, receiver-operating characteristic curves were plotted to assess overall classification accuracy of the AQ in predicting amyloid positivity.
3. Results
With regard to sample characteristics, men had significantly more years of education (M = 16.6, standard deviation = 3.0) than women (M = 14.2, standard deviation = 2.4), t (78) = 3.78, P < .001, but the sexes did not differ by age, t (96) = −0.23, P = .816. There were no significant sex differences in AQ score, accounting for education, F (1, 77) = 0.15, P = .699. There were also no significant correlations between the AQ total score and demographic variables (age and education). There were no differences between the MMSEs in amyloid PET+ and PET (−).
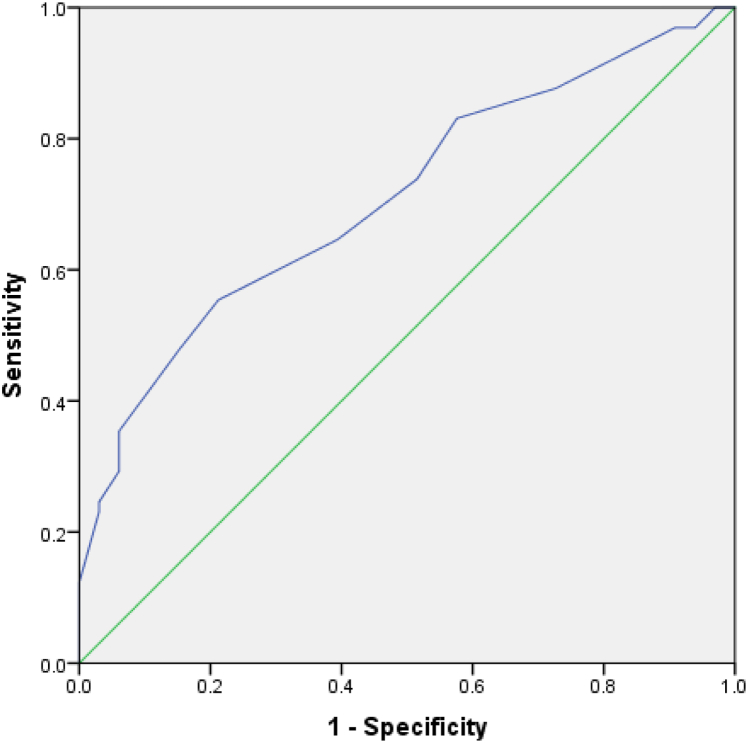
With regard to amyloid status, the sample included 65 aMCI/AD subjects that were amyloid PET positive and 33 subjects were reported as amyloid PET negative. Table 1 contains detailed demographic information by amyloid as well as diagnostic status. There was a greater proportion of patients diagnosed with dementia than those diagnosed with aMCI among the amyloid-positive individuals, χ = 5.10, P = .024. There were no significant differences in sex, age, or education level by amyloid status. Total AQ was significantly higher for patients who were amyloid PET positive versus those who were amyloid PET negative, t(96) = −3.54, P = .001, d = 0.79. See Table 2 for AQ total scores by amyloid status. Additionally, the AQ exhibited adequate overall classification in predicting amyloid status, with an area under the curve of 0.71 (95% confidence interval = 0.607–0.812), P = .001 (see Fig. 1). Additionally, sensitivity and specificity values corresponding to AQ cutoff scores are presented in Table 3.
Table 1.
Demographic characteristics by amyloid and diagnostic status
| Amyloid PET (+), n = 65 | Amyloid PET (−), n = 33 | P value | aMCI, n = 76 | Dementia, n = 22 | P value | |
|---|---|---|---|---|---|---|
| Age | 76.14 (7.12) | 75.67 (6.36) | .749 | 75.36 (6.22) | 78.14 (8.48) | .093 |
| Sex | Male = 64% | Male = 51% | .226 | Male = 55% | Male = 55% | .952 |
| Education | 15.5 (3.3) | 15.8 (2.3) | .589 | 15.8 (1.8) | 15.0 (3.5) | .362 |
| MMSE | 24.1 (5.8) | 24.4 (5.1) | .802 | 26.8 (2.1) | 21.3 (4.1) | .01 |
NOTE. Values are expressed as mean (standard deviation).
Abbreviations: aMCI, amnestic mild cognitive impairment; MMSE, Mini-Mental State Examination; PET, positron emission tomography.
Table 2.
Alzheimer's questionnaire total score by diagnosis and amyloid status
Abbreviations: AQ, Alzheimer's Questionnaire; PET, positron emission tomography.
Alzheimer's Questionnaire, scores range from 0 to 27.
P < .05.
Fig. 1.
ROC for AQ prediction of amyloid status. Area under the curve = 0.710, P = .001. Abbreviations: AQ, Alzheimer's Questionnaire; ROC, receiver-operating characteristic.
Table 3.
Sensitivity and specificity of AQ total score values
| AQ cutoffa | Sensitivity | Specificity |
|---|---|---|
| 6 | .923 | .182 |
| 7.5 | .877 | .273 |
| 8.5 | .831 | .424 |
| 9.5 | .738 | .485 |
| 10.5 | .646 | .606 |
| 11.5 | .554 | .788 |
Abbreviation: AQ, Alzheimer's Questionnaire.
Total AQ score.
4. Discussion
In this study, we find that amyloid PET positivity is associated with a greater number of informant-based subjective complaints of cognitive impairment, as measured by the AQ. This finding suggests that there is a higher likelihood of AD pathology in patients who are reported as having increasing levels of cognitive impairment by an informant. The AQ demonstrated moderate overall classification for predicting amyloid positivity suggesting that informant-based subjective reporting might be a potential screen for AD pathology with reasonable sensitivity and specificity.
Other groups have explored the relationship of subjective memory complaints and amyloid PET status. Several studies have found that subjective cognitive complaints were associated with amyloid status or amyloid burden in healthy older adults [18], [19], [24] although others have not found a significant relationship [25]. There is also some evidence that subjective memory complaints in patients with MCI are also associated with amyloid positivity [24].
However, these prior studies have utilized measures of patient-reported SMCs rather than informant-reported SMCs, and with increasing memory impairment, informant-based measures of subjective cognitive functioning become critical to clinical assessment. For example, Buckley et al. (2015) found that ratings of subjective cognitive complaints between healthy older adults and their informants tended to agree, but these ratings diverged as levels of patient cognitive impairment increased. Specifically, among patients with MCI and informants who expressed high concern for cognitive difficulties, their subjective ratings were negatively correlated. In other words, at greater levels of cognitive impairment (i.e., MCI) when patients reported few subjective complaints their informants endorsed observing a high degree of cognitive difficulty [24]. This highlights the importance of capturing informant ratings of cognitive difficulties as insight is more likely to be affected as memory impairment progresses.
While most research examining subjective cognitive complaints and amyloid status has focused on patient-reported complaints, there is at least one study that examined the relationship between an informant report and amyloid burden. Hollands et al. (2015) found that healthy older adults with high levels of Aβ did not significantly differ in the severity of cognitive complaints reported by informants than healthy older adults with low amyloid levels [25]. In contrast, our present findings indicate that amyloid status is associated with significantly greater subjective cognitive concerns endorsed by informants. Differences in diagnostic characteristics may account for this discrepancy as our study examined individuals with clinical diagnoses of aMCI and AD dementia rather than healthy older adults. Alternatively, differences in self report versus informant report may also account for the discrepancy. An informant report becomes important in these disease stages when insight can become impaired [26]. Additionally, Hollands et al. (2015) measured informant-based ratings using the Informant Questionnaire on Cognitive Decline in the Elderly [12], which, unlike the AQ, is not specific to the types of cognitive difficulties most common in AD.
The findings from this study indicate that the AQ shows promise as a tool to improve diagnostic accuracy in individuals suspected of AD (i.e., those with clinical diagnoses of amnestic MCI and Alzheimer's dementia). The patients included in this study all carried clinical diagnoses of aMCI or AD, and thus, it is notable that the AQ differentiates those that are amyloid positive from those who are amyloid negative despite a relatively homogenous clinical presentation. In other words, patients with clinical diagnoses of aMCI and AD are presumed to likely have Alzheimer's pathology, but the AQ was still able to classify these patients by amyloid status. However, those with atypical presentations of AD (e.g., posterior cortical atrophy or logopenic primary progressive aphasia) may not be accurately classified by the AQ as the types of cognitive complaints assessed by the measure as specific to typically presenting AD syndrome.
Overall, this study highlights the potential utility for informant-based subjective cognitive questionnaires in screening individuals for AD pathology. The AQ and other measures may provide cost-effective screening tools for inclusion in clinical trials and to improve diagnostic accuracy and appropriate referrals in settings where testing may be limited by resources and time demands such as primary care. Further research is needed to determine the accuracy of informant-based measures in predicting amyloid status, but our findings indicate that the AQ has promise as an important diagnostic screening tool in the identification of AD pathology.
Research in context.
-
1.
Systematic review: The authors did an extensive PubMed search to read and understand the literature that explores the connection between subjective cognitive reports of impairment and amyloid positron emission tomography (PET) status. In order to publish this paper, the content was submitted to the Imaging Dementia—Evidence for Amyloid Scanning study for review and approval as the scans were acquired as part of the Imaging Dementia—Evidence for Amyloid Scanning study.
-
2.
Interpretation: This study seeks to correlate amyloid PET scan status with a subjective report captured by the informant-based AQ to determine if an increase in subjective memory complaints is associated with increased Aβ protein positivity in the brain. The hypothesis is that the stronger the report of impairment, the more likely the Alzheimer's disease pathology. Our findings confirm our hypothesis.
-
3.
Future directions: If subjective reports of cognitive impairment could be a proxy of amyloid positivity, it would represent an inexpensive screening tool. This would need to be validated in a large study where the AQ is prospectively administered to a cohort undergoing amyloid PET scanning.
Acknowledgments
This study was supported by NIH COBRE 5P20GM109025 and the Barrow Neurological Foundation and the Keep Memory Alive Foundation.
References
- 1.Murphy M.P., LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatashita S., Wakabe D. Amyloid-β deposition and long-term progression in mild cognitive impairment due to Alzheimer's disease defined with amyloid PET imaging. J Alzheimers Dis. 2017;57:765–773. doi: 10.3233/JAD-161074. [DOI] [PubMed] [Google Scholar]
- 3.Laccarino H., Singer A., Martorell A., Rudenko A., Gao F., Gillingham T. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-Juan P., Ghosh P., Hagen J., Gesierich B., Henry M., Grinberg L. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology. 2014;82:230–238. doi: 10.1212/WNL.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidman D.A., Zamrini E., Sabbagh M.N., Jacobson S., Burke A., Belden C. Added value and limitations of amyloid-PET imaging: review and analysis of selected cases of mild cognitive impairment and dementia. Neurocase. 2017;23:41–51. doi: 10.1080/13554794.2017.1290806. [DOI] [PubMed] [Google Scholar]
- 6.Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med. 2013;54:1011–1013. doi: 10.2967/jnumed.113.127068. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicaid and Medicare Services Amyloid PET. https://www.cms.gov/medicare/coverage/coverage-with-evidence-development/amyloid-pet.html
- 8.Mitka M. PET imaging for Alzheimer disease: are its benefits worth the cost? JAMA. 2013;309:1099–1100. doi: 10.1001/jama.2013.2101. [DOI] [PubMed] [Google Scholar]
- 9.Bondi M., Glenn E. Mild cognitive impairment: a concept and diagnostic entity in need of input from neuropsychology. J Int Neuropsychol Soc. 2014;20:129–134. doi: 10.1017/S1355617714000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsumimoto K., Doi T., Makizako H., Hotta R., Nakakubo S., Makino K. Association of Social Frailty With Both Cognitive and Physical Deficits Among Older People. J Am Med Dir Assoc. 2017;18:603–607. doi: 10.1016/j.jamda.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Galvin J.E., Roe C.M., Powlishta K.K., Coats M.A., Muich S.J., Grant E. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 12.Jorm A.F. Centre for Mental Health Research The Australian National University Canberra; Australia: 1996. Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly (Short IQCODE) PDF. [Google Scholar]
- 13.Gilewski M.J., Zelinski E.M., Schaie K.W. The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 14.Sabbagh M., Malek-Ahmadi M., Kataria R., Belden C., Connor D., Pearson C. The Alzheimer's questionnaire: a proof of concept study for a new informant-based dementia assessment. J Alzheimers Dis. 2010;22:1015–1021. doi: 10.3233/JAD-2010-101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek-Ahmadi M., Davis K., Christine M., Belden, Jacobson S., Sabbagh M. Informant-reported cognitive symptoms that predict amnestic mild cognitive impairment. BMC Geriatr. 2012;12:3. doi: 10.1186/1471-2318-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek-Ahmadi M., Davis K., Belden C.M., Laizure B., Jacobson S.A., Yaari R. Validation and diagnostic accuracy of the Alzheimer's Questionnaire (AQ) Age and Ageing. 2012;41:396–399. doi: 10.1093/ageing/afs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budolfson K., Malek-Ahmadi M., Belden C., Powell J., Davis K., Jacobson S.A. Neuropsychological Correlates of the Alzheimer's Questionnaire (AQ) J Alzheimer's Dis. 2015;46:389–397. doi: 10.3233/JAD-142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amariglio R., Becker J.A., Carmasin J., Wadsworth L., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen R., Caracciolo B., Brayne C., Gauthier S., Jelic V., Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barthel H., Sabri O. Clinical Use and Utility of Amyloid Imaging. J Nucl Med. 2017;58:1711–1717. doi: 10.2967/jnumed.116.185017. [DOI] [PubMed] [Google Scholar]
- 24.Buckley R.F., Villemagne V.L., Masters C.L., Ellis K.A., Rowe C.C., Johnson K. A conceptualization of the utility of subjective cognitive decline in clinical trials of preclinical Alzheimer's disease. J Mol Neurosci. 2016;60:354–361. doi: 10.1007/s12031-016-0810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollands S., Lim Y.Y., Buckley R., Pietrzak R.H., Snyder P.J., Ames D. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis. 2015;43:677–686. doi: 10.3233/JAD-140678. [DOI] [PubMed] [Google Scholar]
- 26.Desikan R.S., Thompson W.K., Holland D., Hess C.P., Brewer J.B., Zetterberg H., Alzheimer's Disease Neuroimaging Initiative Group The role of clusterin in amyloid-β-associated neurodegeneration. JAMA Neurol. 2014;71:180–187. doi: 10.1001/jamaneurol.2013.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]