Abstract
A major obstacle for treatment of HCC is the inadequate efficacy and limitation of the available therapeutic options. Despite the recent advances in developing novel treatment options, HCC still remains one of the major causes of cancer morbidity and mortality around the world. Achieving effective treatment and eradication of HCC is a challenging task, however recent studies have shown that targeting Natural Killer cells, as major regulators of immune system, can help with the complete treatment of HCC, restoration of normal liver function and subsequently higher survival rate of HCC patients. Studies have shown that decrease in the frequency of NK cells, their dysfunction due to several factors such as dysregulation of receptors and their ligands, and imbalance of different types of inhibitory and stimulating microRNA expression is associated with higher rate of HCC progression and development, and poor survival outcome. Here in our review, we mainly focused on the importance of NK cells in HCC development and treatment.
Introduction
Primary hepatic cancer is ranked as fifth common cancer which has a high incidence and prevalence worldwide. Epidemiological data shows that mortality rate due to hepatic cancer is considered to be the second among different types of cancers, which is approximately 9.1% of total cancer deaths [1]. The most common type of hepatic cancer is hepatocellular carcinoma consisting 70%–90% of primary hepatic cancer cases with 75% of cases occurring in Asia [2]. HCC is strongly associated with chronic infection with HBV, however the specific mechanism is still unknown. Some other risk factors that have a major role in development and progression of HCC are HCV infection, diabetes, alcoholism, chronic exposure to aflatoxin, nonalcoholic steatohepatitis and inherited disorders such as alpha-1 antitrypsin deficiency [3]. In human body, intra and extra-hepatic NK cells, as major cells of our innate immune system, have a critical role in body's immune responses against cells infected with HBV or HCV and also tumors like HCC [4]. These NK cells have various functions such as granzyme/perforin-mediated apoptosis, Fas/Fasl-mediated cell death, production and secretion of different types cytokines, and activation of NK and cytotoxic T lymphocytes by cytokines [5].
NK Cells in Initiation, Progression and Death of HCC
Both HBV and HCV infections cause liver cell injury and ultimately result in the liver cirrhosis, fibrosis and even HCC [6]. NK cells kill the virus-infected cells by NK cell-mediated cytolysis, which requires direct contact of the NK cell with the target cell, and immunological synapse formation. Several mechanisms regulate NK cell-mediated cytolysis such as the activation of apoptosis via the extrinsic pathway mediated by Fas-L and Fas [7], NK cell release of granzymes and perforins at the immunological synapses [8], which leads to elimination of HBV-infected cells, thus our body can protect itself against HBV infection [9]. Continuous destruction of target cells by NK cells leads to a nearly complete lytic granule and cytotoxic effector molecules depletion, which can lead to an exhausted state until they detach and get exposed to the activating factors such as interleukin-2, which can lead to restoration of their cytotoxic function [10]. Intra-hepatic NK cells have an important role in fighting against HBV infection and prevention of further complications caused by hepatitis B such as liver fibrosis. They exert their effect by inducing hepatic stellate cells apoptosis [11], [12], production and release of various pro- and anti-inflammatory cytokines such as TNF-α, granulocyte monocyte-colony stimulatory factor, interleukin-2, interleukin-10, interleukin-13, and interleukin-22 [5], [9], [13]and an intricate balance among these factors is necessary for their normal function. During chronic hepatitis B infections, there is an abnormal serum level of cytokines along with a rise of anti-inflammatory cytokine levels and a decrease of pro-inflammatory cytokines. This change in cytokine release is proposed to suppress normal immune responses against HBV, thus disrupting normal NK cell function [5], [14]. Both TNF-α and IL-6 are produced and secreted by macrophages, play an important role in liver pathological and physiological reactions such as regeneration and HCC. Liver progenitor cells, also known as oval cells, can differentiate into both cholangiocytes and hepatocytes, which are important for restoring liver mass under pathological conditions. Ji et al. [43] demonstrated that IL-6 promotes oval cell regeneration and proliferation, while TNF-α does not do so. Hence, deletion of IL-6 leads to an increased HCC development and tumor burden along with a significant reduction of NK cell levels. This suggests that NK cell-mediated HCC suppression is mediated by IL-6, however the underlying mechanism is yet to be elucidated [43]. IL-1α has multiple forms in vivo with each proposed to have varying functions. Membrane IL-1α inhibits HCC growth by promoting in vivo activation of NK and T cells, while increasing cytotoxic T and NK cells cytotoxicity [44] Overall, increased release of IL-6 and membrane IL-1α are considered protective against HCC via NK cell related mechanisms. Furthermore, these cytokines might have an important role in complications associated with HBV infection such as HCC and liver cirrhosis, demonstrating NK cells` importance in the development of HCC [11], [12] .The study done by Yoon and colleagues [15] demonstrated that NK cells play a critical role not only during the acute phase, but also during every stage of HCV infection Apart from inducing T cell activation, NK cells protect the liver from chronic hepatitis C-induced fibrosis. They act by inducing hepatic stellate cell apoptosis [16] (Fig. 1).
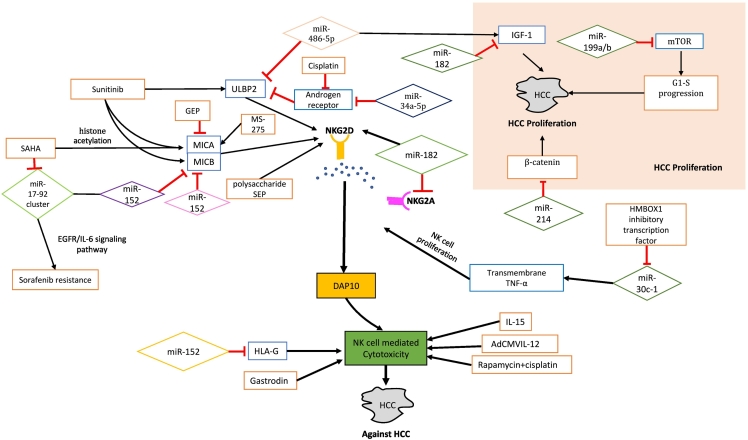
Fig. 1.
Summary of important cells, ligands, cytokines and their receptors involved in the growth, development and death of HCC cells.
NK cells, as important components of our immune system, play a major role in different stages of HCC development. Their function is mainly regulated through interaction with other immune cells such as macrophages, dendritic cells and B-lymphocytes, which is mediated by different types of cytokines, ligands and their receptors. Macrophages and dendritic cells stimulate the NK cells by secretion pro-inflammatory cytokines such as IL-12 and IL-18. On the other hand, NK cells are also stimulated by the dendritic cells via their CD30 ligand. Interactions between B cells and NK cells occur via CD40 and CD40L leading to B cell maturation, isotype switching, antibody secretion, and NK cell-mediated IFN-ɤ production. Along with the abovementioned cytokines and ligands, IL-2, type I IFNs and TLR ligand cause further stimulation and increase in cytotoxicity of NK cells. Upon activation, NK cells secrete pro-inflammatory cytokines and use Fas/FasL and Granzyme/perforin- mediated mechanism to induce apoptosis of hepatic stellate cells and lysis of HBV/HCV-infected cells. NKG2A is an inhibitory receptor which suppresses NK cells through CD16 and CD56dim, thus leading to increased growth and development of HCC. NKG2D is another important receptor, which is activated upon binding to its ligand MICA/B on HCC cells, thus leading to increased cytotoxicity of NK cells and blockade of STAT3. All of these lead to the apoptosis of HCC cells and prevention of HCC progression and metastasis.
Distribution of NK Cell Subtypes in Liver Diseases
Some of the NK cell subtypes that make the NK cell population are not as homogeneous as previously believed. These subtypes are classified according to their relative expression of CD16 and CD15 including CD56dimCD16+, CD56brightCD16+, thymic, NK22, and memory NK cells [17].
The main active subtype cytoxic in viral infections and tumors is CD56dimCD16+, characterized by high expression of CD16 [17]. Studies show that HBV-infected patients have significantly lower NK cell expression of CD56, and that this is associated with prolonged HBV infection. This subpopulation is developmentally mature and is the majority of Peripheral blood NK cells [5], [18].
NK cells which can be found in secondary lymphoid tissues and also other tissues are predominantly CD56brightCD16+, which are the predominant cytokine producing subtypes [19]. They are the major source of IFN-ɤ out of all the NK cell subsets, as mentioned before important for stimulation of APCs, pro-inflammatory cytokine production and viral antigen presentation. Although they are classically believed to be the immature precursors of CD56dimCD16+, recent evidence has cast doubt upon this hypothesis as described by Michel et al. [19]. Thus, activated of NKG2A can further suppress NK cell's response against HCC's immune escape through stimulation of CD56 dim and CD16. However, expression of CD56dim along with a rise in NKG2A expression level is highly correlated with poor prognosis of HCC patients [20].
Thymic NK cells produce IFN-γ and TNF-α, which are similar to CD56bright NK cells [21] and characteristically express CD127 (IL-7 receptor alpha) and require both IL-15 and GATA-3, a transcription factor, for development. The decreased cytotoxicity makes thymic NK cells less efficient at lysing HBV-infected cells [22], [23].
NK22 cells are distinguishable from the other NK subpopulations by being the main IL-22 secreting subtype. They showed to play a role in hepatocyte proliferation and abnormalities are usually associated with the HCC development. However, little is known about NK22 role in HBV infections. These NK22 cells also express granzyme-B and different types of cytokines, but at relatively lower amounts comparing with other NK cell subtypes [23], [24] .
A subset of NK cells, paradoxically as a member of innate immune system, can also mediate memory responses which are called memory NK cells. It has been reported following a number of viral infections such as influenza, HSV-2, and HIV type 1, these memory NK cells are present in the liver. This is suggestive that the liver may be hub for these memory NK cells; however, it is still unclear whether these are resident liver cells that exist in a stable state in liver sinusoids or just passer-by cells that enter the liver via the bloodstream [25]. Choreño et al. [26] favor the hypothesis that these cells circulate within lymph nodes and lymphatic vessels, which are the major sites of antigen priming. Following activation in these secondary lymphoid organs, the NK cells enter the liver in which they then reside until the next re-exposure to the antigen. Re-exposure at secondary sites makes them migrate to the sites of the antigen challenge and exert their memory functions [26]. Jiang et al. hypothesized that because memory NK cells are unable to be measured outside of the liver, the second antigenic challenge at distal locations causes memory NK cell differentiation into effector NK cells triggering their exit from the liver [25]. Liver specific tissue resident DX5−CD49a+ NK cells with memory potential exist in the liver whilst being developmentally distinct from NK cells derived from bone marrow. These are a subset of liver NK cells that can exert secondary immune responses to haptens, which is dependent upon the activity of the NLRP3 inflammasome [25], [27]. However, their relationship with hepatitis B has not been uncovered yet, but provides potential as a novel new avenue for treatment.
Pathophysiological Changes of NK Cells in HCC
Antigen-Presenting Cell Activation
NK cells interact with antigen presenting cells such as macrophages, B-lymphocytes, and Dendritic cells, which can result in immune reactions such as the triggering of cytokine release. Interactions between B cells and NK cells occur via CD40 on the B cell and CD154 or CD40L on the NK cell leading to B lymphocyte maturation, isotype switching, antibody secretion, and NK cell-mediated IFN-ɤ production. CD40 expressing B cells express MHC-I in order to inhibit NK cell-mediated cytolysis of cells expressing CD40 [5]. Furthermore, the spleen, where NK cells interact with B cells to produce antibodies, is heavily implicated in normal NK cell function [28]. Macrophages and DCs interaction with NK cells lead to IL-12 and IL-18 production. These interleukins have a compounding effect with normal cytotoxic NK cell function [29]. Following viral infection, macrophages produce an additional amount of IL-15 and IL-27, which are pro-inflammatory types of cytokines, leading to NK cell activation and differentiation [5]. In summary, NK cells can directly kill cells infected with HBV and inhibit replication of HBV, via interaction with macrophages and DCs which leads to production and release of pro-inflammatory cytokines such as IL-12, which is important in immune responses targeting HBV infection [10].
A number of immune cells such as dendritic cells, B and T lymphocytes regulate human NK cells by their precise interactions with these cells. Furthermore, they are regulated via interactions with cancer cells and cytokines in the local tumor microenvironment.
T regulatory cells are intricately linked with NK cell homeostasis and attenuating target cell sensitivity via minimizing IL-2 to NK cells. The converse is also true as NK cells regulate antigen-specific T lymphocytes during viral infections. NK cell depletion results in an increase in antigen-specific T lymphocytes, thus these NK cells are proposed to lower the immune reactions against tumors through reduction of T cell levels.
B cell secretion of antibodies can result in ADCC, which utilizes NK cells through CD16 and Fc receptors. Reciprocally, NK cells can activate B cells and induce class switching via IFN-ɤ cytokine release.
There is also two-way regulation present between Natural Killer and Dendritic cells through multitude of multifaceted indirect and direct mechanisms. Dendritic cells can directly activate Natural Killer cells via IL-15 trans-representation, cytokine secretion, which includes type I IFNs, and direct binding of CD30L on DCs and CD30 on NK cells. NK cells can also indirectly modulate DC function, and thus the body's immune response. One example is that NK cells can lyse antigen presenting DCs which thus reduces the number of APCs and subsequent T cell activation. This further decreases DC cytokine secretion leading to a decreased the immune response [30].
NK cells play an integral anti-tumor role in preventing liver fibrosis by inducing hepatic stellate cells' apoptosis [11], [12]. These Natural Killer cells, which are widely distributed in lymphoid and non-lymphoid tissues, perform immunosurveillance via their inhibitory receptors killer-immunoglobulin-like receptor and NKG2A to check if the right MHC is expressed.
Important NK cell receptors
Natural Killer cells express different types of receptors for regulation of inhibition and stimulation [31]. There is a group of receptors that cause inhibition of NK cells` function, via immunoreceptor tyrosine-based inhibition motifs (ITIMs) leading to phosphatase recruitment. These inhibitory receptors include NKG2A, KLRG1, and LIR-1/ILT2 [32]. The other main groups of receptors that are associated with stimulation of NK cells are as follow: 1) receptors that are associated with immunoreceptor tyrosine-based activation motif (ITAM)-coupled adaptor proteins including CD16, NKP46, KIR2DS1); 2) receptors not associated with ITAM motifs including NKG2D, CD2, CD7, DNAM-1); and 3) integrin adhesion molecules including VLA-4, VLA-5, CR3 [32]. Apart from the previously mentioned receptors, NK cell signaling and regulation is also controlled by chemokines and cytokines. They aid in recruiting NK cells to the areas of inflammation, which aids in combating infections. Some examples of the chemokine receptors are as follow, CCR1, CCR2, CCR3, CXCR1, CXR2, and XCR1 and similarly for receptors for cytokines such as IL-2R, IL-2R, IL-12R, IL-23R, and TNF-R [31]. A balance between inhibitory and stimulating signals at their respective receptors is important in NK cell activation and subsequent functioning.
IL-1R8 is another important receptor which serves as NK cell checkpoint. Molgora and colleagues showed that IL-1R8 inhibition results in higher NK cell susceptibility of hepatic cancer cells, thus plays a critical role in body's immune defense against tumors, whilst implicated in metastases [33].
NKG2D is an important activating receptor, which is expressed on Natural Killer cells, yδ, and some CD4+ and CD8+ T lymphocytes [34]. Its predominant ligands include UL-16 binding proteins such as ULBP2, and the MHC-I-related chain molecules A and B (MICA/B) which all are important for mediating cytotoxicity of NK cells [35]. NKG2D ligands expression is usually minimal in healthy cells, however stressful events such as transformation into malignancy or infections can cause an upregulation [34] . For example, MICA/B expression in normal tissues is limited predominantly to the gastrointestinal epithelium and thymus, however MICA/B have been found in several types of cancer cells such as colon, liver, and prostate cancer [36]. Following ligand-receptor engagement, association with the adaptor molecule DAP10 is integral for signal transduction. This occurs mechanistically through tyrosine phosphorylation of the YxxM motif leading to receptor complex coupling to the PI3K/Grb-2-Vav pathway [37]. Activation ultimately triggers cell-mediated cytotoxicity, thus promoting killing of tumor and infected cells [34], [38].
Tumor microenvironment
The tumor microenvironment (TME) of HCC has been found to have raised resident NK cells, tissue-resident memory CD8+ T cells, regulatory T cells, and tumor-associated macrophages levels. Analysis of both TME and non-tumor microenvironment (NTME) elucidated a chemotactic gradient for tumor-associated macrophages and resident NK cells via CXCR3/CXCl10 and CCR6/CCL20 pathways respectively [39]. For most solid tumors, cancer-associated fibroblasts (CAFs) play a general role in tumorigenesis by taking part in stimulation of proliferation of cancer cell, angiogenesis, and invasion. In a research done by Jia and colleagues [40], CAFs isolated from MHCC97L and HEP3B HCC cell lines caused a suppression of NK cell activation, thus providing a better condition for HCC progression [40]. CD4+ T lymphocytes have been demonstrated to play a key role in NK cells` activation through modulation of Th1 and Th2 cytokines. Even splenic NK cell cytotoxicity is reduced following CD4+ depletion [41]. Wu et al. [42] discovered that a number of NK cells in advanced-stage HCC patients were decreased along with a decreased TNF-α and IFN-ɤ expression level. They also found that monocytes/macrophages infiltration of peritumoral stroma was associated with reduced intratumoral NK cell activity [42].
Dysfunction of NK cells
Cytolytic Natural Killer cells play an integral role in body's immune defense mechanism against malignant tumors such as HCC. However, earlier studies have demonstrated that the frequency of NK cells, lymphokine-activated killer cells and IFN-ɤ production is significantly decreased in malignancies [43]. Further studies on HCC patients suggest that activity of NK cell is negatively correlated with the disease progression [44].
Different studies have shown that modulation in the secretion of several types of cytokines, i.e. IL-12, IL-2, TGF-β1 and their related receptors, may affect NK cell activity, thus contributing to the progression of the disease.
Tomoyuki et al. demonstrated that the pulsatile procedure of IL-12 combined with IL-2 leads to higher production of IFN-ɤ and TNF-α, increased activation, proliferation, and NK cell cytolytic activity [45]. From HCC associated fibroblasts, PGE2 and IDO have been shown to be immunosuppressive, leading to decreased activation and increased NK cell dysfunction [46]. Natural Killer cells and plasmacytoid Dendritic cells also increase IDO activity via IFN-α and also IFN-ɤ- dependent mechanisms [47].
Interestingly, HCC cells are can also produce different types of cytokines, such as TGF-β1. To determine its role, Mouri and colleagues used TGF-β1 in the culture supernatants conditioned by PRF / PLC /5, HepG2, and Hep3B HCC cell lines to assess its effect on IFN-ɤ production. The results showed that TGF-β1 may reduce the activation and NK cell and Cytotoxic T lymphocyte cytolytic activity through reduction of IFN-ɤ production [48], this can thus attenuate the immune response.
Experiments on co-cultured NK K562 cells and Huh7 cells with AFP-treated dendritic cells showed NK cells cytolytic activity compared with albumin-treated DCs was decreased. Subsequently, interaction of AFP with DCs can cause an impairment of IL-12 production, leading to inhibition of DC-dependent Natural Killer cell activation, suggesting the immunosuppressive role of AFP in HCC development [49]. Of interest, although tAFP indirectly impairs DC-dependent NK cell activation by impairing IL-12 production, recent studies suggest that the short-term exposure of Natural Killer cells to tAFP and nAFP (cord blood-derived AFP) leads to a pro-inflammatory IL-2-hyperresponsive phenotype in Natural Killer cells, which is characterized by CD-69 upregulation and enhanced tumor surveillance function. However, long-term co-culture of NK cells with t-AFP is negatively correlated with long-term viability of NK cells. It's noteworthy to mention that AFP itself can directly activate NK cells [50].
Further studies by Tong et al. has shown that HCC suppression is impaired by the absence of IL-6, leading to a decreased number of functioning NK cells [51]. Moreover, Zhang et al. discovered that subsets of liver-infiltrating CD11b−CD27− NK cells with decreased cytolytic activity and deficient potential of IFN-ɤ production may result in the tumor-infiltrating Natural Killer cell dysfunction in HCC patients [52]. Further assessment of peripheral blood Ntural Killer cells of patients with HCC receiving hepatectomy showed that the number of NKG2DCCD56dimNK cells is significantly decreased, which is accompanied with raised serum TGF-β and soluble MICA (sMICA) levels. These findings are correlated with a poor prognosis of HBV-related patients with HCC at one month post-hepatectomy [53].
Several attempts to establish a link between dysregulation of different receptors, their ligands, and dysfunction of NK cells have been made. Assessment of patients of with primary HCC showed that increased dysfunction of NK cells is associated with the lower expression of NKG2D, which is mainly affected by the liver function and NK cells activity [54]. Another study by Sun et al. demonstrated that factors from cancer nests in HCC can cause an increase in NKG2A expression contributing to NK cells exhaustion. Therefore blockade of NKG2A can be used as the potential therapeutic pathway to restore the normal Natural Killer cell cytolytic activity to fight the tumor cells [20].
Interestingly, assessing a subset of monocytes from advanced-staged HCC tissues has shown that increased expression of CD48 proteins on these monocytes is negatively correlated with NK cell TNF-α and IFN-ɤ production. Further experiments, showed that blocking 2B4 NK cell CD48 receptor, but not NKG2D and NKp30, is associated with the attenuation of monocyte/macrophage-elicited NK cell dysfunction [42]. This CD40 blockade also provides a potential therapeutic pathway for treatment. Another study done by Zhang and colleagues suggests that CD24 expression on HCC tumor tissue and its subsequent interaction with siglec-10 receptor on NK cells may lead to decreased survival and increased dysfunction of NK cells [55]. CD133 is another important ligand which is expressed in low frequency on HCC tissue cells. Studies have shown that CD133-expressing cells are insensitive to NK cell cytolytic activity. Normally, ADAM9 protease is engaged in the shedding of MICA. However, experiments showed that decreased ADAM9 protease expression on CD133si-PLC/PRF/5 and CD133-Huh7 cells led to increased membrane-bound MICA and decreased sMICA production [56]. These findings suggest that CD133 expression of tumor cells is predictive of a poor prognosis in patients with HCC [56]. Therefore CD48, CD24, and CD133 can all be considered immunosuppressive via mechanisms involving NK cells which can lead to the increased progression and ultimately poor prognosis of HCC.
Endoplasmic reticulum (ER) adaptor protein (ERAdP) is a novel ER membrane protein similarly implicated in NK cell dysfunction. It is constitutively expressed in both mouse and human Natural Killer cells, and its expression is attenuated in peripheral Natural Killer cells of HBV- associated HCC patients. ERAdP enhances Ubc13-mediated NF-kB ubiquitination to stimulate the Ubc13-mediated NF-kB pathway, thus resulting in activation of Natural Killer cells [57].
Discovering new ligands, receptors and pathways can unravel new therapeutic methods to fight against HCC and increase the survival rate of these patients. Apart from the other factors, it's important to note that any change in the tumor microenvironment, such as liver fibrosis, can disrupt the normal interaction between NK cells and tumor cells. This disruption can cause an inhibition of matrix metalloproteinase expressed on NK cells by matrix metalloproteinase inhibitors, leading to the slower migration of these cells toward their target (tumor and stellate cells). That could be a reason for decreased NK cell activity in conditions like extensive liver fibrosis leading to higher risk of HCC development [58].
The Interaction of Oncogenes With NK and HCC Cells
HCC Oncogenic Pathway
HCC development requires a complex interaction between numerous cellular events. Tumorigenesis is dependent on the tumor microenvironment and its interaction with different types of immune cells such as macrophages, neutrophils, myeloid-derived suppressor cells, NK cells, and tumor-infiltrating cells lymphocytes such as CD8+, CD4+ and regulatory T lymphocytes, altogether exhibit pro-tumor and/or anti-tumor effects. In addition, tumor cells activation and proliferation require oncogenic cellular pathways such as Wnt/beta-catenin, nuclear factor-kappaB, JAK/STAT, cMET, IGF, phosphatidyl-3 kinase/AKT/mammalian target of rapamycin and Raf/MAPK along with transcription activators and signal transducers and all of which are considered to be fundamental aspects of tumorigenesis [59].Immunoevasion is a hallmark of tumorigenesis, which can be achieved by amelioration or impairment of NK cell cytotoxicity as a method to evade host immunity [60].
STAT3 is another important transcriptional factor that plays an integral role in different cell functions, which is associated with tumor cell inflammation, cell proliferation and HCC development [15]. High levels of STAT3 is correlated with enhanced HCC progression, recurrence, and metastasis. Yang et al. showed that inhibit HCC cell growth and decrease HBV infection-related HCC by blocking STAT3 signaling using shRNAs [61]. Further studies by Sun et al. also showed that blockade of HCC tumor STAT3 resulted in TGF-β downregulation and type I IFN upregulation of HCC cells and restoration of the normal function of NK cells, suggesting the importance of STAT3 blockade as a novel strategy for HCC treatment. Thus, blocking of STAT3 in HCC cells caused an enhancement Natural Killer cell cytolytic activity through NKG2D ligand upregulation and facilitating recognition of HCC cells by NK cells. These results show the importance of STAT3 blockade for the improvement of NK cell cytotoxicity and its direct impact on tumor cells [62].
The expression of CD44 is upregulated in HCC patients, however higher expression levels of CD44 is associated with worse prognosis compared to that of patients with lower level of CD44. Kim et al. found that inhibition of mTOR resulted in downregulation of CD44 expression, thus inhibition of PI3K-AKT–mTOR pathway is associated with a better prognosis of HCC. [63], [64]. On the other hand, a series of experiments by Liu and colleagues showed that Ras activation is associated with NKG2D ligand upregulation which is dependent on Raf, MAPK/MEK and PI3K pathways. This NKG2D ligand upregulation leads to increased sensitivity of NK cells to tumor cells, thus leading to increased cytotoxic activity of NK cells against HCC cells [65].
Astrocyte elevated gene-1 (AEG-1) is implicated in HCC as a key driver of HCC progression and development. AEG-1 shRNA augments the anti-proliferative effect of Huaier polysaccharide (HP) in the human HCC MHCC97-H cell lines. This could be in part via the inhibition of the PI3K/Akt pathway and enhancement of the Natural Killer cell-mediated response, which causes a decrease in tumorigenesis and progression of HCC [66].
Progression of HCC. MHC-I Polypeptide-Related Sequence A /B (MICA/B)
The MHC-I polypeptide-related sequence A /B (MICA/B) play an integral role in body's immune mechanism against different types tumors. One of their major roles is to tag cancer cells for further recognition by cytotoxic NK and T lymphocytes [67]. MICB is a protein imperative for modulation of NK and activation of T lymphocyte via NKG2D receptor. Tong and colleagues found that Soluble MICB (sMICB) level was strongly associated with platelet counts and were both elevated in HCC subjects compared to that of healthy controls [68].
Fang et al. [69] found in a cohort of 96 patients with HCC, those with low expression of MICA/B in hepatoma cells tended to have a shorter survival time [69]. miR-889 overexpression inhibits mRNA and protein expression of MICB in SMMC7221 and HepG2 HCC cells. Therefore miR-889 upregulation attenuates the susceptibility of HCC cells to Natural Killer cell-based cytolysis [70].
Experiments on multiple immunocompetent mouse models showed that treatment with antibodies targeting MICA α3 domain induces NK cell mediated immunity [67].
Important NK Cell-Related microRNAs in HCC
MicroRNAs (miRNAs) are a category of small non-coding RNAs, which have important roles as key modulators of post-transcriptional gene expression. MicroRNAs are known to affect many physiological and pathological conditions via mechanisms such as translational repression, activation, and mRNA degradation, usually by imperfect complementary base pairing to 3′-untranslated regions [58], [59]. Impaired regulation of miRNAs frequently occurs in cancers, and can modulate different aspects of tumor biology such as immune avoidance, metastasis, proliferation, and survival [60]. Historically, miRNAs relating to cancer have been classified as either oncogenic (oncomiRs), which are overexpressed in tumors, or tumor suppressive, which are underexpressed. However, certain miRNAs such as miR-125b and miR-155 can act as either a tumor suppressor or oncomiR depending on the context, such as different types of malignancies. This can be understood by the fact that a single miRNA can target many different mRNAs, which can have opposing functions. Therefore, Svoronos et al. suggested a more holistic approach in examining the effects of miRNAs in cancer by considering miRNAs multiple targets, different cell types, and different therapies [60]. In this review, however, we specifically examine the effects of miRNAs on NK cell regulation in hepatocellular carcinoma (HCC) (Table.1).
Table 1.
Summary of Important microRNAs Involved in HCC Pathogenesis.
| MiRNA | Target Gene(s) | Mechanism | References |
|---|---|---|---|
| miR-20a↑ | EGFR/IL-6,MICB | Chemoresistance, Cytolysis | [38], [70], [75], [76] |
| miR-889↑ | MICB | Cytolysis | [70] |
| miR-106b, miR-93↑ | MCM7 | Cell cycle | [38], [77], [78] |
| miR-615-5p↑ | IGF-1, SHMT2 | NK cytolytic activity, proliferation, migration | [96], [145] |
| miR-30c-1↓ | TNF-α | Proliferation | [145] |
| miR-122↓ | CCL2 | Apoptosis, angiogenesis, metastasis | [87], [146] |
| miR-146a↓ | EGFR, ERK1/2, Stat5 |
Cell growth, apoptosis | [92], [93], [146] |
| miR-199a/b↓ | mTor,c-Met, PAK4 | Proliferation, metastasis, cell growth | [92], [93] |
| miR-214↓ | Ctnnb1 | Cell growth, invasion | [91, 92,95] |
| miR-486-5p↓ | IGF-1, ULBP2 | NK cytolytic activity | [95] |
Upregulation:↑ Downregulation: ↓.
A number of miRNAs have been studied regarding their effects on NK cell regulation. MicroRNAs such as miR-30c-1, miR-17-92, miR-889, miR-182, miR-34a-5p, miR-122, miR-146a possess the ability to enhance NK cells activation in hepatocellular carcinoma [71]. For instance, upregulation of miR-30c-1 would activate Transmembrane TNF-α, which then leads to enhanced NK cell function in the hepatoma cell lines mmc-7721 and HepG2 cell lines. HMBOX1 inhibitory transcription factor have been known to have an impact on miR-30c-1 expression in NK cells, ultimately lowering its activity, therefore upregulation of miR-30c-1 results in raised mTNF-α expression, increased proliferation of NK cells; thus by targeting HMBOX1 inhibitory transcription factor, NK cell function on hepatoma cells within the peripheral blood is increased [13].
Six cellular miRNAs (miR-20a, miR-93, miR-106b, miR-372, miR-373 and miR-520d) that target MHC-I related chain molecules A and B (MICA/B) mRNA were identified by Stern-Ginossar and colleagues [72]. Proteins from the MICA/B loci stimulate NKG2D receptor which is constitutively expressed on almost all Natural Killer and CD8+ cells. NKG2D receptor is generally believed to recognize “induced self” such as hyper-proliferative cells, infected cells, and transformed cells, then stimulate cell-mediated cytotoxicity, however this mechanism is complex and not clearly understood [73]. miR-20a is known to be a part of miR-17-92 cluster, designated as oncomiR-1, has been characterized to accumulate in a number of malignancies such as HCC [74]. Increased miR-17-92 cluster expression in HCC has shown to be involved in sorafenib resistance. The exact mechanism has not fully elucidated, however it is proposed to be related to the activation and inhibition of specific parts of the downstream EGFR/IL-6 signaling pathway [75]. Additionally, miR-20a has been shown to decrease MICB mRNA in HepG2 and H7402 cells, while also being implicated in tumorigenesis [76]. Similarly, miR-889 overexpression in-vitro inhibits MICB mRNA and protein expression in SMMC7221 and HepG2 HCC cells and a negative correlation was also found between the two in-vivo experiments. Thus, upregulation of both miR-20a and miR-889 decreases MICB expression which then, via decreased activation of NKG2D, attenuates HCC cell susceptibility to Natural Killer cell-based cytolysis [38], [70], [76]. Moreover, both miR-93 and miR-106b are members of miR-106b-93-25 cluster, with the high expression levels of its host gene minichromosome maintenance complex component 7 (MCM7) which is regarded as poor prognosis of HCC indicator [38], [77], [78] . Mechanistically, inhibition of MCM7 upregulates cyclin D1 via MAPK pathway modulation, thus restraining cell cycle progression by inducing G1 arrest in HCC cell lines [38], [78]. miR-182, as a part of miR-96/182/183 family, is known to be an oncogenic miRNA in HCC. It increases invasion, metastasis, proliferation, and resistance to chemotherapy in hepatoma cells [79], [80]. Abdelrahman and colleagues discovered that miR-182 is upregulated in NK cells of non-metastatic, early-stage HCC patients which resulted in an upregulation and downregulation of NKG2D and NKG2A respectively [1]. In contrast, during late-stage HCC, the relative expression levels of NKG2A and NKG2D was found to be reversed, which decreases the cytotoxicity of Natural Killer cells [81], [82]. It was posited that this was due to a compensatory mechanism during the early- stage HCC where NK cells act against hepatoma cells which is also in concordance with their expression in early and late stages of non-small cell lung carcinoma [1], [83].
miR-34a-5p has shown to lower androgen receptor (AR) levels in prostate cancer [84]. Shi et al. found that an increase in miR-34a-5p led to suppression of AR expression in SNU423 and HCC SK-Hep1 cell lines. This suppressed AR levels is predicted to cause an upregulation of ULBP2, a NKG2D ligand, thus enhancing NK cell cytotoxicity [85]. A previous report demonstrated the possibility that miR-34a serves as an ULBP2 repressor in melanoma cells, however AR levels were not measured, therefore it might be due to another mechanism [86].
The chemokine C-C motif chemokine ligand 2 (CCL2) is a chemokine that is upregulated in primary human HCC. It recruits CCR2-positive immune cells to promote inflammation via pro-inflammatory cytokines such as TNF-α and IL-6 production [87]. Decreased level of miR-122 is correlated with poor prognosis and metastasis of HCC [88]. Furthermore, miR-122 depletion in mouse liver results in an upregulation of CCL2, recruitment of CCR2 + CD11bhighGr1+, and hepatitis. Treatment of KO mice with CCL2 neutralizing antibody reduced liver damage, HCC incidence, along with decreased expression of IL-6 and TNF-α downstream targets phospho-STAT3 and c-MYC [87].
miR-146a is a key immune response regulator, with varying roles in different tumor cells. In HCC cells, it is downregulated through the blockage of activated STAT3. Constitutively, activated STAT3 led to an upregulation of inflammatory and immunosuppressive cytokines along with an increased miR-146a expression by binding to the miR-146a promoter. The STAT3-regulated miRNA-146as are posited to be the important modulators of immunosuppression and progression of liver cancer [89]. Lower miR-199a/b and miR-214 expression in NK cells is also associated with the development and progression of HCC [90], [91], [92].
miR-199a/b can directly degrade mRNA of proto-oncogenes MET and mammalian target of rapamicin (mTOR), in addition to reducing their encoded protein products. The mTOR pathway is activated by extracellular signaling, causing phosphorylation of its translational regulator phospho-p70S6 kinase, leading to proliferation and progression of cell from G1 to S phase. Thus, miR-199a/b up-regulation inhibits proliferation and invasiveness of HCC cell lines [90], [92]. MET, a transmembrane tyrosine kinase receptor of hepatocyte growth factor (HGF) ligand, is involved in the control of the invasive growth during embryogenesis, tumorigenesis, organogenesis, wound healing, and inflammatory responses. Decreased expression of MET results in inhibition of cell proliferation, invasion, and ultimately apoptosis of HCC cells [92], [93].
miR-214 reduces β-catenin protein levels, not by mRNA degradation, rather through inhibition of mRNA translation (Ctnnb1). Downregulation of miR-214 causes higher β-catenin expression, which is associated with HCC cell growth, progression, invasion, and early recurrence [91], [92], [94] .
HLA-G is an immune inhibitory molecule which is a part of the non-classic MHC-I family. In HCC, it regulates anti-cancer immune response by affecting dendritic cells, CD8+ T lymphocytes, and Natural Killer cells. miR-152 targets and downregulates HLA-G, thus leading to the attenuation of NK-mediated cytotoxicity toward tumor cells [94], [95].
Insulin-like growth factor 1 (IGF-1) is the main ligand of IGF-signaling pathway characterized by tumorigenic and proliferative activity in carcinomas such as HCC. It acts by binding to the tyrosine kinase IGF Receptor, undergoing a conformational change, autophosphorylation, and activation of various signaling pathways including PI3K/AKT/mTOR, RAS/RAF/ERK and the JAK/STATs [96]. Its expression showed to be lower in HCC patients together with a reduction of Natural Killer-mediated cytolytic activity; However, in NK cells, it is essential for NK-mediated cytotoxicity. Youness et al. [97]found that miR-486-5p ectopic expression in Natural killer cells of HCC patients led to higher IGF-1 mRNA levels. Although usually inhibitory, there have been reports in the literature of miRNA that miR-486-5p act by activating genes rather than repressing [43], [98], [99]. Thus, miR-486-5p is essential for increasing NK-mediated cytolytic activity which contrasts its oncogenic role in hepatocytes overwhelming its ULBP2 mRNA repression in hepatocytes [97]. miR-615-5p downregulates IGF-1, thus repressing IGF-1R; however, it was found to have an anti-cytotoxic effect in NK cell lines along with a tumor suppressive effect in HCC cell lines.
Importance of NK Cells in HCC Metastasis Prevention
As previously mentioned, NK cell loss of function is highly associated with poor outcome and lower survival rate of HCC patients, which could be due to the absence of adequate amount of Natural Killer cell-based immune response to cancerous cells. Some studies suggest that Natural Killer cells might have a role in the removal of micro-metastatic cells from the circulation and also cytolysis of HCC metastatic cells in the other organs. In an experiment done by Barajas et al. they showed that gene therapy using an adenovirus that carries the IL-12 gene (AdCMVIL-12) prevents spontaneous lung metastasis by stimulating the activation of NK cells in HCC rat models [100].
Further experiments on IL-1R8-deficient mice showed that IL-1R8 serves as NK cell checkpoints, removal of which results in increased TLR and IL-1 mediated inflammation, thus resulting in prevention of liver and lung metastasis [33]. Granulin-epithelin precursor (GEP), which is a liver oncofetal protein found in HCC, is responsible for its growth, chemoresistance, and metastasis. In HCC cell lines, GEP overexpression causes a reduction in sensitivity of HCC cell lines to NK cell cytoxicity and vice versa. This is due to the downregulation of MICA, upregulation of human leukocytes antigen-E, and decreased production of soluble MICA, thus repressing NK cell activation. Additionally, in HCC patients, high levels of serum GEP and/or MICA showed to be associated with poor survival rate [60].Thus, Natural Killer cells play a major role in the inhibition of tumor progression, migration and invasion [101].
HCC Treatment Strategies
NK cells as major immunoregulatory components of our immune system can directly kill tumor cells without antigen sensitization. Previous data showed the potential of cancer immunotherapy for manipulation of NK cell activation [12], [102]. Thus, targeting NK cells can be used as a promising treatment strategy which can significantly improve the process of treatment and that includes the use of adoptive transfer of allogeneic Natural killer cells, genetic engineered NK cells, NK cell-targeted chemotherapy and so on [103]. Previous studies suggest that targeting Natural Killer cells alone or with other immune cells such DCs can provide a powerful treatment method to help the body fight against HCC cells and is it associated with better outcome and higher survival rate of HCC patients [104](Fig. 2).
Fig. 2.
Summary of important microRNAs, cytokines and drugs used to treat HCC through stimulation of NK cells.
MicroRNAs, involved in HCC death and development, are classified as oncogenic such as miR-20a and miR-889 or tumor suppressive such as miR-34a-5p and miR-122. There is another group of microRNAs which can act both as either tumor suppressor or oncogenic microRNA such as miR-125b and miR-155. These microRNAs have both direct and indirect impact on the cytotoxicity of NK cells, thus playing an important role in determination of HCC prognosis.
Immunotherapy
There is a characterized reduction in TRAIL expressing NK cells occurring via decreased expression of CXCR3 ligand CXCL9 following hepatectomy. TRAIL is immunoprotective by increasing Natural Killer cells infiltration in the tumor environment. Hepatectomy and partial hepatectomy both lead to downregulation of the CXCL9-CXCR3 axis, which then decreases TRAIL expressing NK cells, and this is correlated with poor prognosis and early HCC recurrence [105]. However, recent studies have also shown that combined oncolytic adenoviruses that encode IL-12 and TRAIL genes can suppress hepatocellular carcinoma by increasing infiltration of NK cells in tumor microenvironment [106]. Therefore there is therapeutic potential for stimulation of TRAIL post hepatectomy in order to improve outcomes.
Peripheral blood Natural Killer cells can be expanded and activated by the use of different types of pro-inflammatory cytokines such as IL-21, IL-15, IL-12, IL-2 and type I IFNs [11]. Effects of Natural killer cell infusions were examined by Kamiya and colleagues, who discovered that expanded Natural Killer cells generally had a higher level of cytotoxicity against HCC cell lines than unstimulated or IL-2-activated NK cells. Furthermore, experiments demonstrated that using the chimeric NKG2D-CD3ζ-DAP10 receptor enhanced the signaling capacity of the NKG2D receptor leading to increased cytotoxic activity of expanded Natural Killer cells in vitro and also in immunodeficient mouse models [110]. TLR7 and TLR8 agonists can also increase the efficacy of NK cell-mediated immune surveillance by promoting NK-DC cross-talk. DCs also cause IL-12 and type I IFN dependent enhancement of NK cells function, thus leading to an increased anti-tumor immune response to HCC [107]. Yu et al. have developed GPC3-Specific chimeric AR-engineered NK cells which exhibit highly cytotoxic activity and could be used as an effective immunotherapy option for GPC3+ HCC patients [109].
Chemotherapeutic Agents
A number of chemotherapeutic agents have been described to affect NK cells. Multitargeted tyrosine kinase inhibitors (MTKIs) mediate the NKG2D ligands upregulation to sensitize cancer cells to Natural Killer cell therapy. Yet, the exact underlying mechanism is still unkown. Sunitinib, an MTKI, showed to cause increase in activation and cytotoxic capacity of Natural killer cells in HepG2 cell lines. It causes an upregulation of NKG2DLs, NF-kb family genes via NF-kb signaling noncanonical pathways, apoptotic genes, and DNA damage repair genes [111]. Muromonab-CD3 is a discontinued anti-CD3 antibody due to its side effect profile, and it along with its alternative GMP CD3 which has similar impacts on hepatic mononuclear cells, cause enhancement of Natural Killer cell activation and T lymphocyte depletion [112].
The polysaccharide SEP, acting via TLR2/4, has shown to activate Natural Killer cells and T lymphocytes. It also enhances the antitumor activity of the pyrimidine antimetabolite gemcitabine (GEM) against HCC by stimulation of NKG2D and DAP10/Akt pathways resulting in the activation of NK cells. It is also posited that NKG2D upregulation improves NK-92 cells sensitivity targeting to its ligand MICA expressed on HepG2 cells. GEM upregulates expression of MICA and decreases secretion of sMICA via inhibition of ADAM10, thus enhancing NK-92 cells cytolytic activity against HepG2 cells [113].
The proteasome inhibitor bortezomib has dual mechanisms for its antitumor effects in HCC. It inhibits proliferation of cancer cells and primes hepatoma cells for reactivity of Natural Killer cell. Therefore there is therapeutic potential if it is combined with NK immunotherapy which would cause synergistic effects [114]. Cisplatin has also showed it increases the efficacy of immunotherapy by increasing NK cell cytotoxicity through modulation of AR-ULBP2 signals [85].
Sorafenib administration triggers proinflammatory activities of tumor-associated macrophages by sensitizing it toward exogenous immune stimuli. This leads to induction of anti-tumor Natural Killer cell responses in a NF-kB and cytokine-dependent manner. Natural Killer cell activation, triggered by Sorafenib, is dependent on LPS, which is an exogenous factor and can be found in abundant number in the liver portal system [115]. Shi et al. found that sorafenib possibly enhances the cytotoxicity via inhibition of AR, thus increasing a subtype of IL-12, IL-12A [116]. Regorafenib, a newly recognized multikinase inhibitor anticancer drug for the treatment of advanced-stage HCC, acts by inhibiting RET, c-KIT, c-RAF, TIE-2, PDGFR, VEGFR1–3, FGFR-1, p38 MAP kinase and BRAF. A series of experiments performed by Tai and colleagues showed that Regorafenib causes inhibition of STAT3 signaling pathway, thus leading to the enhancement of Natural Killer cell cytolytic activity through NKG2D ligand upregulation and facilitating recognition of HCC cells by NK cells and ultimately apoptosis of HCC cells [64]. Regorafenib has a higher efficacy in induction of HCC cell apoptosis compared to that of Sorafenib, but their side effects and toxicity are almost the same. [118], [119], [120]. A pro-inflammatory TME with expression of TLR3 is associated with higher survival rate of HCC patient, occurring via activation of tumor infiltrating Natural Killer cells and T lymphocytes. TLR3 expression in HCC patients is positively correlated with NK cell activation, tumor infiltration, and is negatively correlated with viability of tumor parenchymal cells [108]. Ho and colleagues showed that the combination treatment of sorafenib and the TLR3 agonist, lysine-stabilized polyinosinic-polycytidylic acid, enhances in vivo local NK cell, T lymphocyte, macrophage and DC activation. Ligation of TLR3 alone might be the reason for augmented NK and T cell activation, however there is also a synergistic Natural Killer cell activation enhancement caused by combined therapy. It is posited that enhancement of tumor cell death by usage of two drugs results in increased free RNAs, triggering TLR3, leading to an increased immune activation [117].
HDACIs
Histone deacetylase inhibitors (HDACIs) also affect NK cell function in HCC. Suberoylanilide hydroxamic acid (SAHA) treatment was found to significantly increase the susceptibility of HepG2 and H7402 HCC cell lines to NK cytolysis. Yang et al. demonstrated that MCM7and miR-17-92 cluster expression were decreased in a dose-dependent manner. The mechanism of this effect was posited to be two-fold as MICA mRNA transcription induced by SAHA via an increase in MICA-associated histone acetylation. SAHA causes suppression of the miRNAs targeting MICA/B in order to lower the threshold of MICA/B expression [38]. Given that the miR-17-92 cluster is characterized to play a key in sorafenib resistance, there is potential for dual therapy for increased efficacy.
HDACIs also induces MICA expression, which as previously mentioned enhances NK cell mediated HCC cells eradication [35], [121]. Sodium valproate, can increase the cytotoxic activity of NK cells [59]. The HDACI MS-275 was found to upregulate expression of MICA, MICB and HSP70 which thus enhances NK cytotoxicity in HepG2 lines [122]. Further studies by Goto and colleagues have shown that disulfiram enhances antitumor Natural Killer cell activity by suppressing sMICA production with enzymatic inhibition of ADAM10 [123]. Therefore HDACIs have potentials as HCC vaccines.
Antimicrobials
Recent research has uncovered possibilities for using existing antimicrobials in the treatment of HCC. The antifungal HDACI trichostatin A indirectly kills HCC cells by increasing NK cell cytotoxicity of HCC cells via transcriptional modulation of important genes such as ULBP1 and RAET1G. Directly, it can increase apoptosis of HCC cells [124]. Lomofungin is an FDA approved antifungal,that decreases ADAM17 activity which then decreases sMICA in a dose dependent manner [125]. Anisomycin is an antibiotic that is a eukaryotic protein synthesis inhibitor produced by Streptomyces griseolus. A study done by Kim and colleagues has shown its potential as a novel therapeutic HCC drug as it modulates a broad range of genes such as lymphocyte-associated antigen-3, MHC-1, CD58, and ICAM4 in order to improve formation of immunological synapses between Natural killer and Hepatocellular carcinoma cells [126].
Novel Treatments
Melittin, isolated from honeybee venom, has potential anti-arthritic, antimicrobial, anti-inflammatory and also anti-tumor properties. A synthetic novel peptide analog of this, known as TT-1, was developed to have more anti-tumor effects. The combination of TT-1 with IFN-α enhances NK cells activity against HCC cells by promoting NKG2D and MICA interaction [127]. Another melittin analog Mel-P15 was also found to have potential in the treatment of HCC. It directly stimulates NK cytotoxicity, and also increased secretion of IL-2, TNF-α and IFN-γ [128]. Gastrodin is a compound extracted from the well-known East Asian restorative food Gastrodia elata Blume. It can reduce growth of transplanted H22 hepatic tumor cells with low toxicity in mice. Gastrodin causes enhancement of cytotoxicity of Natural killer cells and CD8+ T lymphocytes via a mechanism that has not yet been understood [129]. Interestingly, Chen et al. showed that polypeptides extracted from scorpion venom (PESV) causes inhibition of HCC cell proliferation in mice. They also found that survival time of these cancer mouse models was increased following treatment with PESV. The inhibitory effects of PESV in HCC is likely due to MICA-NKG2D pathway activation resulting in the upregulation of NK cell activity [130]. These more novel treatments demonstrate the therapeutic potential and importance of research into less established medications.
Other potential treatment options apart from the listed types of immunotherapy, anticancer drugs, HDACIs, antimicrobials, and novel treatments, such as recombinant plasmid DNA, chimeric virus-like particles, viral vectors such as different types of Adeno-associated virus (AAV), adoptive transfer of tumor-specific T lymphocytes and cancer cell vaccines which can also enhance the NK cell-mediated immunosurveillance against HCC [131]. Even nano-pulse stimulation, which induces upregulation of CD8 expressing NK cells, demonstrated to be an effective treatment modality for Hepatocellular carcinoma [132]. Despite the advances in NK cell-based research and treatment, much is left to learn about the correct use of NK cells for HCC treatment. One of the most important steps to develop novel treatments against HCC is to better understand the properties and differences of intra-hepatic Natural Killer cells from that of peripheral blood in order to increase specificity of treatments [133]. (Table 2)
Table 2.
A Brief Summary of Novel and Currently Used HCC Treatment Strategies.
| Treatment Strategy | Mechanism | Stage | Country | Clinical Trials.gov Identifier | Refrence | |
|---|---|---|---|---|---|---|
| Immunotherapy | Combined oncolytic adenovirus encoding IL-12 and TRAIL genes | Increasing infiltration of TRAIL expressing NK cells in tumor microenvironment Upregulation of CXCL9-CXCR3 axis |
[1], [105], [106] | |||
| type I IFNs | Expansion and activation of peripheral blood NK cells Increasing cytotoxicity of NK cells |
[11] | ||||
| IL-2 | Expansion and activation of peripheral blood NK cells Increasing cytotoxicity of NK cells |
Phase1 | China | NCT03175679 | [11] | |
| IL-21, IL-15, IL-12 | Expansion and activation of peripheral blood NK cells Increasing cytotoxicity of NK cells |
Phase3 | China | NCT01681446 | [11] | |
| TLR7 and TLR8 agonists | Promotion of NK-DC cross-talk IL-12 and type I IFN dependent enhancement of NK cells function caused by dendritic cells |
Phase 1 | Sweden China |
NCT01974661 NCT02882659 |
[107] | |
| TLR3 agonists (such as lysine-stabilized polyinosinic-polycytidylic acid) | Activation of tumor infiltrating Natural Killer cells and T lymphocytes | Phase 1 Phase 2 |
China | NCT02562963 | [108] | |
| GPC3-Specific chimeric AR-engineered NK cells | Highly cytotoxic NK cells fighting against GPC3+ HCC cells | [109] | ||||
| chimeric NKG2D-CD3ζ-DAP10 receptor | Enhancement of the signaling capacity of the NKG2D receptor Increasing the cytotoxic activity of expanded Natural Killer cells |
[110] | ||||
| `Chemotherapy | Multitargeted tyrosine kinase inhibitors (MTKIs) | Upregulation of NKG2DLs, NF-kb family genes via NF-kb signaling noncanonical pathways, apoptotic genes, and DNA damage repair genes Sensitization of cancer cells to Natural Killer cell therapy |
Phase 3 | Netherlands | NCT01265810 | [111] |
| Muromonab-CD3 (a discontinued anti-CD3 antibody) and its alternative GMP CD3 | Enhancement of Natural Killer cell activation T lymphocyte depletion |
Phase 3 | Egypt | NCT02568748 | [112] | |
| The polysaccharide SEP | Activation of Natural Killer cells and T lymphocytes via TLR2/4 Enhancement of the antitumor activity of the pyrimidine antimetabolite gemcitabine (GEM) against HCC by stimulation of NKG2D and DAP10/Akt pathways resulting in the activation of NK cells |
[113] | ||||
| Gemcitabine (GEM) (a pyrimidine antimetabolite) | Upregulation of MICA expression Decreasing secretion of sMICA via inhibition of ADAM10 Enhancement of NK-92 cells cytolytic activity against HepG2 cells |
Phase 2 | China | NCT02527772 | [113] | |
| Bortezomib (a proteasome inhibitor) | Inhibition of cancer cell proliferation Priming hepatoma cells for reactivity of NK cells |
Phase 2 | USA | NCT03607643 | [114] | |
| Cisplatin | Increasing the efficacy of immunotherapy by increasing NK cell cytotoxicity through modulation of AR-ULBP2 signals | [85] | ||||
| Sorafenib | Triggering pro-inflammatory activities of tumor-associated macrophages by sensitizing it toward exogenous immune stimuli leading to induction of anti-tumor Natural Killer cell responses in a NF-kB and cytokine-dependent manner Enhancement of the NK cell cytotoxicity via inhibition of AR, thus increasing a subtype of IL-12, IL-12A Combination treatment of sorafenib and the TLR3 agonist, lysine-stabilized polyinosinic-polycytidylic acid, enhances in vivo local NK cell, T lymphocyte, macrophage and DC activation |
[108], [115], [116], [117] | ||||
| Regorafenib | 1. Inhibition of RET, c-KIT, c-RAF, TIE-2, PDGFR, VEGFR1–3, FGFR-1, p38 MAP kinase and BRAF 2. Inhibition of STAT3 signaling pathway leading to the enhancement of NK cell cytolytic activity and apoptosis of HCC cells |
Phase 2 |
Germany |
NCT03644511 | [118], [119], [120] | |
| HDACIs | Suberoylanilide hydroxamic acid (SAHA) | Suppression of the miRNAs targeting MICA/B in order to lower the threshold of MICA/B expression Induction of MICA expression causing enhancement of NK cell-mediated HCC cell eradication |
Phase 1 | USA | NCT01075113 | [35], [38], [121] |
| Sodium valproate | Increasing cytotoxic activity of NK cells | [59] | ||||
| HDACI MS-275 | Upregulate expression of MICA, MICB and HSP70 leading to enhanced NK cytotoxicity in HepG2 lines | [122] | ||||
| Disulfiram | Enhancement of antitumor activity of Natural Killer cells by suppressing sMICA production with enzymatic inhibition of ADAM10 | [123] | ||||
| Antimicrobials | Trichostatin A (an antifungal HDACI) | Indirectly kills HCC cells by increasing NK cell cytotoxicity of HCC cells via transcriptional modulation of important genes such as ULBP1 and RAET1G Directly kills HCC cells by increasing apoptosis of HCC cells |
[124] | |||
| Lomofungin (antifungal) | Decreasing ADAM17 activity which then decreases sMICA in a dose dependent manner | [125] | ||||
| Anisomycin (antibiotic) | Modulation of a broad range of genes such as lymphocyte-associated antigen-3, MHC-1, CD58, and ICAM4 in order to improve formation of immunological synapses between Natural killer and Hepatocellular carcinoma cells | [126] | ||||
| Novel Treatment options | TT-1 (synthetic novel peptide analog of Melittin, an anti-arthritic, anti-microbial, anti-inflammatory and anti-tumor drug) | Combination of TT-1 with IFN-α enhances NK cells activity against HCC cells by promoting NKG2D and MICA interaction | [127] | |||
| Mel-P15 (Melittin analog) | Direct stimulation of NK cytotoxicity Increasing secretion of IL-2, TNF-α and IFN-γ |
[128] | ||||
| Gastrodin | Enhancement of cytotoxicity of Natural killer cells and CD8+ T lymphocytes | [129] | ||||
| polypeptides extracted from scorpion venom (PESV) | MICA-NKG2D pathway activation resulting in the upregulation of NK cell activity | [130] | ||||
| Recombinant plasmid DNA, chimeric virus-like particles, viral vectors such as different types of Adeno-associated virus (AAV), adoptive transfer of tumor-specific T lymphocytes and cancer cell vaccines | Enhancement of NK cell-mediated immunosurveillance against HCC | [131] | ||||
| Nano-pulse stimulation | Inducing upregulation of CD8 expressing NK cells | [132] | ||||
NK Cells in Other Tumors
As previously mentioned, NK cells play a critical immunodefensive role in different types of malignancies such as lymphoma, HCC, gastric, renal, lung and endometrial cancer. As we discover more about their important role in body's immunodefense mechanism against malignancies, we can develop more treatment options with better and higher efficacy.
Assessment of a group of activating surface receptors such as NKp30, NKp46, NKG2D, and DNAM-1 on peripheral blood NK cells of gastric cancer patients showed that decreased expression of these surface receptors is highly associated with decreased NK-cell mediated immunity in these patients, which is negatively correlated with survival rate [134].
Wang and colleagues found that utilization of IL-15 leads to increased cytotoxicity of NK cells, which can help to prevent or reduce hepatic metastasis in gastric cancer patients [135].
On the other hand, Misumi T et al. also found that using rhCD137 ligand, which serves as a costimulatory molecule, induces further activation of NK cells, thus increases the efficacy of treatment with tumor-targeting mAbs in gastric cancer patients [136] . Experiments on Ishikawa-xenografted nude mouse model of uterine endometrial cancer showed that combined use of rapamycin with cisplatin restricted tumor growth through increasing cytotoxic activity of NK cells. [137]. Furthermore, Langers et al. demonstrated that cross-talk between NK and Dendritic cells, which is mediated by CD40 interaction and IL-12p70 secretion, leads to an increased immunity against HPV vaccine viral particles, thus lowers the chance of developing uterine cervical cancer in the future. [138]. It was reported that mutations in β-catenin exon 3 and the subsequent activation of Wnt pathway is associated with lower survival rate and poor prognosis of NK/T-cell lymphoma patients [139]. Use of genetically engineered NK cells as an effective antitumor treatment option for treating Renal Cell Carcinoma has attracted many scientists` attention. Zhang and colleagues examined the efficacy of combined therapy with Cabozantinib and epidermal growth factor- (EGFR-) specific third-generation CAR-NK-92 cells for treatment of renal cell carcinoma. They found that Cabozantinib could promote CAR-NK-92 cell cytolytic ability through increasing EGFR and decreasing PD-L1 surface receptors of RCC cells. [140]. On the other hand, Kremer V et al. reported that treatment of RCC with genetically engineered CXCR2- expressing NK cells has higher efficacy due to increased migration these NK cells toward RCC cells. [141]. NK cells also play an important role in the control of lung cancer, however the underlying mechanism is not well understood. Shi L et al. reported that exposure of NK cells to the environmental major histocompatibility class I (MHCI) causes an upregulation of activating surface receptors such as NKG2D, NKp46, which leads to a better control of lung cancer by NK cells. [142]
As we mentioned before, enhancing the cytotoxic ability of NK cells is the hallmark of most cancer and infection treatments, however in some conditions such as organ transplantation, NK cells need to be suppressed in order to prevent organ rejection. NK cells are one of the major causes of allograft rejection and this is due to their cytotoxic effect on allograft tissue through ligation of immunoglobulain-like receptor (KIR) or NKG2D. [143]
It's noteworthy to know that NK cells recent studies have shown that NK cells are also involved in varying pathological and physiological processes in preganancy such as pregnancy induced- hypertension and some cancers. There is also a distinct group of small lymphocytes known as the uterine-specific uNK cells, which are different from the peripheral blood NK cells and are involved in mediating trophoblast invasion. [144]
Conclusion
The literature includes overwhelming evidence regarding the importance of Natural Killer cells in HCC. Dysfunction of Natural Killer cells is important and variant between control, early, and late stage HCC. It generally involves decreased cytolytic activity of NK cells, whilst there are a number of different mechanisms causing this including the complex interplay between receptors, ligands, and the NK cells themselves. The exact pathophysiological mechanism underlying this change is still not completely understood however this is an important area for research. The research into miRNA adds another layer of complexity, however this also holds promise as a way of tailoring novel HCC treatment to the individual – realizing the ideal of precision medicine.
Declaration of Competing Interest
There is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Acknowledgments
Acknowledgments
The authors thank the authors of the primary studies. This work is funded by Zhejiang Provincial Natural Science Foundation of China (2015C03026).
Contributor Information
Sarun Juengpanich, Email: 21718716@zju.edu.cn.
Liang Shi, Email: liang_shi@zju.edu.cn.
Yasaman Iranmanesh, Email: 3140300496@zju.edu.cn.
Jiang Chen, Email: JCHEN106@mgh.Harvard.edu.
Zhenzhe Cheng, Email: 3140102050@zju.edu.cn.
Aaron Kah-Jin Khoo, Email: Aaron.khoo@uqconnect.edu.au.
Long Pan, Email: panlong1994@zju.edu.cn.
Yifan Wang, Email: anwyf@zju.edu.cn.
Xiujun Cai, Email: srrsh_cxj@zju.edu.cn.
References
- 1.Abdelrahman MM, Fawzy IO, Bassiouni AA, Gomaa AI, Esmat G, Waked I, Abdelaziz AIJHi. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. 2016;77:667–673. doi: 10.1016/j.humimm.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Petrick JL. W.T.J.C.i.l.d. London. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. 2015;19:223–238. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KLJG. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Nomura M, Tsuge M, Uchida T, Hiraga N, Kurihara M, Tsushima K, Fujino H, Nakahara T, Nurakami E, Abe-Chayama HJJovh. CTL-associated and NK cell-associated immune responses induce different HBV DNA reduction patterns in chronic hepatitis B patients. 2018;25:1555–1564. doi: 10.1111/jvh.12970. [DOI] [PubMed] [Google Scholar]
- 5.Shabani Z, Bagheri M, ZareBidaki M, Hassanshahi G, Arababadi MK, Nejad MM. D.J.A.o.v. Kennedy. NK cells in hepatitis B virus infection: a potent target for immunotherapy. 2014;159:1555–1565. doi: 10.1007/s00705-013-1965-3. [DOI] [PubMed] [Google Scholar]
- 6.Ortega-Prieto A, Dorner MJV. Immune evasion strategies during chronic hepatitis B and C virus infection. 2017;5:24. doi: 10.3390/vaccines5030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S-f, Wang W-j. Y.-q.J.B.J.o.I.D. Gao. Natural killer cells in hepatitis B virus infection. 2015;19:417–425. doi: 10.1016/j.bjid.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mace EM, Dongre P, Hsu H-T, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JSJI. c. biology, Cell biological steps and checkpoints in accessing NK cell cytotoxicity. 2014;92:245. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun C, Sun H-y, Xiao W-h, Zhang C, Tian Z-gJAPS. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. 2015;36:1191. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arababadi MK, Pourfathollah AA, Jafarzadeh A, Hassanshahi G, Daneshmandi S, Shamsizadeh A, Kennedy DJSjogojotSGA. Non-association of IL-12+ 1188 and IFN-γ+ 874 polymorphisms with cytokines serum level in occult HBV infected patients. 2011;17:30. doi: 10.4103/1319-3767.74461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsumi T, Takehara TJHR. Impact of natural killer cells on chronic hepatitis C and hepatocellular carcinoma. 2016;46:416–422. doi: 10.1111/hepr.12619. [DOI] [PubMed] [Google Scholar]
- 12.Tosello-Trampont A, Surette FA, Ewald SE, Hahn YSJFii. Immunoregulatory role of NK cells in tissue inflammation and regeneration. 2017;8:301. doi: 10.3389/fimmu.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Tian Z, Wei HJFii. Developmental and functional control of natural killer cells by. Cytokines. 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arababadi MK, Pourfathollah AA, Jafarzadeh A, Hassanshahi GJHM. Serum levels of IL-10 and IL-17A in occult HBV-infected South-East Iranian patients. 2010;10:31. [PMC free article] [PubMed] [Google Scholar]
- 15.J.C.YoonC.M.Yang,Y.Song,J.M.J.W.j.o.g. Lee,Natural killer cells in hepatitis C: Current progress, 22 (2016) 1449. [DOI] [PMC free article] [PubMed]
- 16.Glässner A, Eisenhardt M, Krämer B, Körner C, Coenen M, Sauerbruch T, Spengler U, Nattermann JJLi. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL-and NKG2D-dependent manner. 2012;92:967. doi: 10.1038/labinvest.2012.54. [DOI] [PubMed] [Google Scholar]
- 17.Mondelli MU, Oliviero B, Mele D, Mantovani S, Gazzabin C, Varchetta SJFii. Natural killer cell functional dichotomy: a feature of chronic viral hepatitis? 2012;3:351. doi: 10.3389/fimmu.2012.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy M, MacNicholas R, Grealy R, Taylor M, Otegbayo J, O'Dea S, Mulcahy F, Ryan T, Norris S, Doherty D. Circulating CD56 dim natural killer cells and CD56 T cells that produce interferon-c or interleukin-10 are expanded in asymptomatic. 2014. E antigen-negative patients with persistent hepatitis B virus infection. [DOI] [PubMed] [Google Scholar]
- 19.Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M, Zimmer JJTJoI. Human CD56bright NK cells: an update. 2016;196:2923–2931. doi: 10.4049/jimmunol.1502570. [DOI] [PubMed] [Google Scholar]
- 20.Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, Wang J, Song J, Zheng M, Sun HJO. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. 2017;6 doi: 10.1080/2162402X.2016.1264562. e1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrielli S, Sun M, Bell A, Zook EC, de Pooter RF, Zamai L, Kee BLJEjoi. Murine thymic NK cells are distinct from ILC1s and have unique transcription factor requirements. 2017;47:800–805. doi: 10.1002/eji.201646871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu JJE. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. 2014;3 doi: 10.7554/eLife.01659. e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Q.TangY.-O.AhnP.SouthernB.R.BlazarJ.S.MillerM.R.J.B.Verneris,Development of IL-22 producing NK lineage cells from umbilical cord blood hematopoietic stem cells in the absence of secondary lymphoid tissue, (2011) blood-2010-2009-303081. [DOI] [PMC free article] [PubMed]
- 24.Fukui A, Kamoi M, Funamizu A, Fuchinoue K, Chiba H, Yokota M, Fukuhara R, Mizunuma HJRm. biology, NK cell abnormality and its treatment in women with reproductive failures such as recurrent pregnancy loss, implantation failures, preeclampsia, and pelvic endometriosis. 2015;14:151–157. doi: 10.1007/s12522-015-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Chen Y, Peng H, Tian ZJC. m. immunology, Memory NK cells: why do they reside in the liver? 2013;10:196. doi: 10.1038/cmi.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choreño Parra JA, Martínez Zúñiga N, JiménezZamudio LA, Jiménez Álvarez LA, Salinas Lara C, Zúñiga JJFii. Memory of natural killer cells: a new chance against. Mycobacterium tuberculosis. 2017;8:967. doi: 10.3389/fimmu.2017.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Boorn JG, Jakobs C, Hagen C, Renn M, Luiten RM, Melief CJ, Tüting T, Garbi N, Hartmann G, Hornung VJI. Inflammasome-dependent induction of adaptive NK cell memory. 2016;44:1406–1421. doi: 10.1016/j.immuni.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Hulikova K, Svoboda J, Benson V, Grobarova V, Fiserova AJIi. N-acetyl-D-glucosamine-coated polyamidoamine dendrimer promotes tumor-specific B cell responses via natural killer cell activation. 2011;11:955–961. doi: 10.1016/j.intimp.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Andrews DM, Andoniou CE, Scalzo AA, van Dommelen SL, Wallace ME, Smyth MJ, Degli-Esposti MAJMi. Cross-talk between dendritic cells and natural killer cells in viral infection. 2005;42:547–555. doi: 10.1016/j.molimm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Sungur CM, Murphy WJJCRiO. Positive and negative regulation by NK cells in. Cancer. 2014;19 doi: 10.1615/critrevoncog.2014010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biassoni RJCPiI. Human natural killer receptors, co-receptors, and their ligands. 2009;84 doi: 10.1002/0471142735.im1410s84. 14.10. 11–14.10. 40. [DOI] [PubMed] [Google Scholar]
- 32.Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J, Wood SMJJoii. Molecular mechanisms of natural killer cell activation. 2011;3:216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- 33.Molgora M, Bonavita E, Ponzetta A, Riva F, Barbagallo M, Jaillon S, Popović B, Bernardini G, Magrini E, Gianni FJN. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. 2017;551:110. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espinoza JL, Nguyen VH, Ichimura H, Pham TT, Nguyen CH, Pham TV, Elbadry MI, Yoshioka K, Tanaka J, Trung LQJSr. A functional polymorphism in the NKG2D gene modulates NK-cell cytotoxicity and is associated with susceptibility to human papilloma virus-related cancers. 2016;6 doi: 10.1038/srep39231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies TJS. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 36.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki YJIjoc. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 37.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JEJIr. The NKG2D receptor: immunobiology and clinical implications. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, Tian Z, Zhang CJBjoc. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. 2015;112:112. doi: 10.1038/bjc.2014.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, Chua C, Leong JY, Lim KH, Toh HCJPotNAoS. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. 2017;114:E5900–E5909. doi: 10.1073/pnas.1706559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia C-C, Wang T-T, Liu W, Fu B-S, Hua X, Wang G-Y, Li T-J, Li X, Wu X-Y, Tai YJPO. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. 2013;8 doi: 10.1371/journal.pone.0063243. e63243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Q, Lan P, Yu X, Han Q, Zhang J, Tian Z, Zhang CJMct. Immunotherapy for hepatoma using a dual-function vector with both immunostimulatory and pim-3-silencing effects. Mol Cancer Ther. 2014;13(6):1503–1513. doi: 10.1158/1535-7163.MCT-13-0722. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng LJH. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. 2013;57:1107–1116. doi: 10.1002/hep.26192. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima JJCr. Thioredoxin 1 negatively regulates angiotensin II–induced cardiac hypertrophy through upregulation of miR-98/let-7. 2011;108:305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saibara T, Maeda T, Miyazaki M, Onishi S, Ymamot YJH. Depressed immune function in patients with cirrhosis before emergence of hepatocellular carcinoma. 1993;18:315–319. [PubMed] [Google Scholar]
- 45.Sawayama T, Sakaguchi K, Senoh T, Ohta T, Nishimura M, Takaki A, Tsuji T, Shiratori YJAMO. Effects of pulsing procedure of interleukin-12 in combination with interleukin-2 on the activation of peripheral blood lymphocytes derived from patients with hepatocellular carcinoma. 2003;57:285–292. doi: 10.18926/AMO/32813. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen GJCl. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. 2012;318:154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Asghar K, Farooq A, Zulfiqar B, Rashid MUJWjog. Indoleamine 2, 3-dioxygenase: As a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma. 2017;23:2286. doi: 10.3748/wjg.v23.i13.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mouri H, Sakaguchi K, Sawayama T, Senoh T, Ohta T, Nishimura M, Fujiwara A, Terao M, Shiratori Y, Tsuji TJAMO. SuppressiveEffects ofTransforming Growth Factor-Produced by Hepatocellular Carcinoma Cell Lines on Interferon-Production by Peripheral Blood Mononuclear Cells. 2002;56:309–315. doi: 10.18926/AMO/31688. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Tatsumi T, Miyagi T, Tsunematsu H, Aketa H, Hosui A, Kanto T, Hiramatsu N, Hayashi N, Takehara TJC. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. 2011;165:211–219. doi: 10.1111/j.1365-2249.2011.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vujanovic L, Stahl EC, Pardee AD, Geller DA, Tsung A, Watkins SC, Gibson GA, Storkus WJ, Butterfield LHJCir. Tumor-derived α-fetoprotein directly drives human natural killer-cell activation and subsequent cell death. Cancer Immunol Res. 2017;5(6):493–502. doi: 10.1158/2326-6066.CIR-16-0216. [DOI] [PubMed] [Google Scholar]
- 51.Ji T, Li G, Chen J, Zhao J, Li X, Lin H, Cai X, Cang YJO. Distinct role of interleukin-6 and tumor necrosis factor receptor-1 in oval cell-mediated liver regeneration and inflammation-associated hepatocarcinogenesis. 2016;7:66635. doi: 10.18632/oncotarget.11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q-F, Yin W-W, Xia Y, Yi Y-Y, He Q-F, Wang X, Ren H, Zhang D-ZJC. m. immunology, Liver-infiltrating CD11b− CD27− NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. 2017;14:819. doi: 10.1038/cmi.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J, Duan Z, Zhang L, Huang X, Long L, Tu J, Liang H, Zhang Y, Shen T, Lu FJO. Failure recovery of circulating NKG2D+ CD56dimNK cells in HBV-associated hepatocellular carcinoma after hepatectomy predicts early recurrence. 2016;5 doi: 10.1080/2162402X.2015.1048061. e1048061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sha W-H, Zeng X-H, Min LJG. The correlation between NK cell and liver function in patients with primary hepatocellular carcinoma. 2014;8:298. doi: 10.5009/gnl.2014.8.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang P, Lu X, Tao K, Shi L, Li W, Wang G, Wu KJjosr. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. 2015;194:107–113. doi: 10.1016/j.jss.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Kohga K, Tatsumi T, Takehara T, Tsunematsu H, Shimizu S, Yamamoto M, Sasakawa A, Miyagi T, Hayashi NJJoh. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. 2010;52:872–879. doi: 10.1016/j.jhep.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Hao L, Li C, Ye B, Du Y, Zhang H, Long B, Zhu P, Liu B, Yang LJTJoI. The endoplasmic reticulum adaptor protein ERAdP initiates NK cell activation via the Ubc13-mediated NF-κB pathway. 2015;194:1292–1303. doi: 10.4049/jimmunol.1402593. [DOI] [PubMed] [Google Scholar]
- 58.Zhang DY. S.L.J.H. Friedman. Fibrosis-dependent mechanisms of hepatocarcinogenesis. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel P, Schutzer SE, Pyrsopoulos NJWjogp. Immunobiology of hepatocarcinogenesis: Ways to go or almost there? 2016;7:242. doi: 10.4291/wjgp.v7.i3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung PF, Yip CW, Wong NC, Fong DY, Ng LW, Wan AM, Wong CK, Cheung TT, Ng IO, Poon RTJCir. 2014. Granulin–epithelin precursor renders hepatocellular carcinoma cells resistant to natural killer cytotoxicity. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Zheng B, Han Q, Zhang C, Tian Z, Zhang JJCb. therapy, Targeting blockage of STAT3 inhibits hepatitis B virus-related hepatocellular carcinoma. 2016;17:449–456. doi: 10.1080/15384047.2016.1156257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X, Sui Q, Zhang C, Tian Z, Zhang JJMct. Targeting Blockage of STAT3 in Hepatocellular Carcinoma Cells Augments NK Cell Functions via Reverse Hepatocellular Carcinoma–Induced Immune Suppression. 2013;12:2885–2896. doi: 10.1158/1535-7163.MCT-12-1087. [DOI] [PubMed] [Google Scholar]
- 63.Swamy SG, Kameshwar VH, Shubha PB, Looi CY, Shanmugam MK, Arfuso F, Dharmarajan A, Sethi G, Shivananju NS, Bishayee A. Targeting multiple oncogenic pathways for the treatment of hepatocellular carcinoma. Target Oncol. 2017;12:1–10. doi: 10.1007/s11523-016-0452-7. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Jiang J, Badawi M, Schmittgen TD. miR-221 regulates CD44 in hepatocellular carcinoma through the PI3K-AKT-mTOR pathway, Biochem. Biophys Res Commun. 2017;487:709–715. doi: 10.1016/j.bbrc.2017.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu XV, Ho SS, Tan JJ, Kamran N, Gasser S. Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol. 2012;189:1826–1834. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 66.Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y, Hao M, Sheng S, Sun Y, Zhang HJTB. Astrocyte elevated gene-1 (AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced anti-metastatic potency via inactivating downstream P13K/Akt pathway as well as augmenting cell-mediated immune response. 2014;35:4219–4224. doi: 10.1007/s13277-013-1552-y. [DOI] [PubMed] [Google Scholar]
- 67.de Andrade LF, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, Tsoucas D, Franz B, May KF, Harvey CJJS. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. 2018;359:1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Tong H, Hoan NX, Cuong BK, Sy BT, Son HA, Quyet D, Binh VQ, Kremsner PG, Bock CT, Velavan TPJBid. Soluble MICB protein levels and platelet counts during hepatitis B virus infection and response to hepatocellular carcinoma treatment. 2015;15:25. doi: 10.1186/s12879-015-0754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang L, Gong J, Wang Y, Liu R, Li Z, Wang Z, Zhang Y, Zhang C, Song C, Yang AJJoE. MICA/B expression is inhibited by unfolded protein response and associated with poor prognosis in human hepatocellular carcinoma. 2014;33:76. doi: 10.1186/s13046-014-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie H, Zhang Q, Zhou H, Zhou J, Zhang J, Jiang Y, Wang J, Meng X, Zeng L, Jiang XJC. microRNA-889 is downregulated by histone deacetylase inhibitors and confers resistance to natural killer cytotoxicity in hepatocellular carcinoma cells. 2018;70:513–521. doi: 10.1007/s10616-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paladini L, Fabris L, Bottai G, Raschioni C, Calin GA, Santarpia LJJoE, Research CC. Targeting microRNAs as key modulators of tumor immune response. 2016;35:103. doi: 10.1186/s13046-016-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim OJNi. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. 2008;9:1065. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 73.Lanier LLJCir. NKG2D receptor and its ligands in host defense. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJJn. A microRNA polycistron as a potential human oncogene. 2005;435:828. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Awan FM, Naz A, Obaid A, Ikram A, Ali A, Ahmad J, Naveed AK, Janjua HAJSR. MicroRNA pharmacogenomics based integrated model of miR-17-92 cluster in sorafenib resistant HCC cells reveals a strategy to forestall drug resistance. 2017;7 doi: 10.1038/s41598-017-11943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie J, Liu M, Li Y, Nie Y, Mi Q, Zhao SJC. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. m immunology. 2014;11:495. doi: 10.1038/cmi.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele GJSs. Identification of the miR-106b~ 25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. 2010;3 doi: 10.1126/scisignal.2000594. ra29-ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu K, Wang Z, Fan H, Li J, Liu J, Li P, Liang Z, An H, Jiang Y, Lin QJCd. disease, MCM7 promotes cancer progression through cyclin D1-dependent signaling and serves as a prognostic marker for patients with hepatocellular carcinoma. 2017;8 doi: 10.1038/cddis.2016.352. e2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C, Ren R, Hu H, Tan C, Han M, Wang X, Zheng YJCjocr. MiR-182 is up-regulated and targeting Cebpa in hepatocellular carcinoma. 2014;26:17. doi: 10.3978/j.issn.1000-9604.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Li J, Shen J, Wang C, Yang L, Zhang XJBc. MicroRNA-182 downregulates metastasis suppressor 1 and contributes to metastasis of hepatocellular carcinoma. 2012;12:227. doi: 10.1186/1471-2407-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi NJJoh. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. 2005;43:1013–1020. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 82.Jiang J, Zhou Z, Li J, Ye Y, Chen QJXbyfzmyxzzCjoc. Expression and significance of the NK cell receptors in primary hepatocellular carcinoma and paracancerous tissues. m immunology. 2012;28:529–532. [PubMed] [Google Scholar]
- 83.Yu DP, Han Y, Zhao QY, Liu ZD. CD3+ CD4+ and CD3+ CD8+ lymphocyte subgroups and their surface receptors NKG2D and NKG2A in patients with non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(6):2685–2688. doi: 10.7314/apjcp.2014.15.6.2685. [DOI] [PubMed] [Google Scholar]
- 84.P. Östling, S.-K. Leivonen, A. Aakula, P. Kohonen, R. Mäkelä, Z. Hagman, A. Edsjö, S. Kangaspeska, H. Edgren, D.J.C.r. Nicorici, Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells, (2011). [DOI] [PubMed]
- 85.Shi L, Lin H, Li G, Sun Y, Shen J, Xu J, Lin C, Yeh S, Cai X, Chang CJCl. Cisplatin enhances NK cells immunotherapy efficacy to suppress HCC progression via altering the androgen receptor (AR)-ULBP2 signals. 2016;373:45–56. doi: 10.1016/j.canlet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen AJCr. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress induced ligand of the natural killer cell receptor NKG2D. canres. 2011:1977–2011. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 87.Teng K-Y, Han J, Zhang X, Hsu S-H, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MAJMct. Blocking the CCL2–CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. 2017;16:312–322. doi: 10.1158/1535-7163.MCT-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thakral S, Ghoshal KJCgt. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. 2015;15:142–150. doi: 10.2174/1566523214666141224095610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun X, Zhang J, Hou Z, Han Q, Zhang C, Tian ZJCc. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. 2015;14:243–252. doi: 10.4161/15384101.2014.977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.F. Fornari, M. Milazzo, P. Chieco, M. Negrini, G.A. Calin, G.L. Grazi, D. Pollutri, C.M. Croce, L. Bolondi, L.J.C.r. Gramantieri, MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells, (2010) 0008–5472. CAN-0010-0145. [DOI] [PubMed]
- 91.Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z, Jiang JJB. b.r. communications, MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of β-catenin. 2012;428:525–531. doi: 10.1016/j.bbrc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 92.Ip BC, Liu C, Ausman LM, von Lintig J, Wang XD. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014;7(12):1219–1227. doi: 10.1158/1940-6207.CAPR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim S, Lee UJ, Kim MN, Lee E-J, Kim JY, Lee MY, Choung S, Kim YJ, Choi Y-CJJoBC. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) 2008;283:18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 94.Bian X, Si Y, Zhang M, Wei R, Yang X, Ren H, Zheng G, Wang C, Zhang YJTB. Down-expression of miR-152 lead to impaired anti-tumor effect of NK via upregulation of HLA-G. 2016;37:3749–3756. doi: 10.1007/s13277-015-3669-7. [DOI] [PubMed] [Google Scholar]
- 95.Amiot L, Vu N, Samson MJJoh. Biology of the immunomodulatory molecule HLA-G in human liver diseases. 2015;62:1430–1437. doi: 10.1016/j.jhep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Rahmoon MA, Youness RA, Gomaa AI, Hamza MT, Waked I, El Tayebi HM, Abdelaziz AIJGF. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 97.Youness RA, Rahmoon MA, Assal RA, Gomaa AI, Hamza MT, Waked I, El Tayebi HM, Abdelaziz AIJGF. Contradicting interplay between insulin-like growth factor-1 and miR-486-5p in primary NK cells and hepatoma cell lines with a contemporary inhibitory impact on HCC tumor progression. 2016;34:128–140. doi: 10.1080/08977194.2016.1200571. [DOI] [PubMed] [Google Scholar]
- 98.Stadthagen G, Tehler D, Høyland-Kroghsbo NM, Wen J, Krogh A, Jensen KT, Santoni-Rugiu E, Engelholm LH, Lund AHJPg. Loss of miR-10a activates lpo and collaborates with activated Wnt signaling in inducing intestinal neoplasia in female mice. 2013;9 doi: 10.1371/journal.pgen.1003913. e1003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Place RF, Li L-C, Pookot D, Noonan EJ, Dahiya RJPotNAoS. MicroRNA-373 induces expression of genes with complementary promoter sequences. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto JJH. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. 2001;33:52–61. doi: 10.1053/jhep.2001.20796. [DOI] [PubMed] [Google Scholar]
- 101.Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP, Chi XQ, Zhang S, Wang XMJOl. Natural killer cells inhibit pulmonary metastasis of hepatocellular carcinoma in nude mice. 2016;11:2019–2026. doi: 10.3892/ol.2016.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qin Z, Chen J, Zeng J, Niu L, Xie S, Wang X, Liang Y, Wu Z, Zhang MJCb. therapy, Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: A preliminary clinical study. 2017;18:323–330. doi: 10.1080/15384047.2017.1310346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cariani E, Missale GJO. KIR/HLA immunogenetic background influences the evolution of hepatocellular carcinoma. 2013;2 doi: 10.4161/onci.26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukaida N, Nakamoto YJWjog. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. 1839;24(2018) doi: 10.3748/wjg.v24.i17.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yano T, Ohira M, Nakano R, Tanaka Y, Ohdan HJPo. Hepatectomy leads to loss of TRAIL-expressing liver NK cells via downregulation of the CXCL9-CXCR3 axis in mice. 2017;12 doi: 10.1371/journal.pone.0186997. e0186997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Shemi AG, Ashshi AM, Na Y, Li Y, Basalamah M, Al-Allaf FA, Oh E, Jung B-K, Research YJJoE Chae-Ok CC. Combined therapy with oncolytic adenoviruses encoding TRAIL and IL-12 genes markedly suppressed human hepatocellular carcinoma both in vitro and in an orthotopic transplanted mouse model. 2016;35:74. doi: 10.1186/s13046-016-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Z, Yu X, Zhang J, Tian Z, Zhang CJCl. TLR7/8 agonists promote NK–DC cross-talk to enhance NK cell anti-tumor effects in hepatocellular carcinoma. 2015;369:298–306. doi: 10.1016/j.canlet.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 108.Chew V, Tow C, Huang C, Bard-Chapeau E, Copeland NG, Jenkins NA, Weber A, Lim KH, Toh HC, Heikenwalder MJJJotNCI. Toll-like receptor 3 expressing tumor parenchyma and infiltrating natural killer cells in hepatocellular carcinoma patients. 2012;104:1796–1807. doi: 10.1093/jnci/djs436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li ZJMT. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. 2018;26:366–378. doi: 10.1016/j.ymthe.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamiya T, Chang YH, Campana D. Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res. 2016;4(7):574–581. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 111.Huang Y-x, Chen X-t, Guo K-y, Li Y-h, Wu B-y, Song C-y, He Y-jJJoI. Sunitinib Induces NK-κB-dependent NKG2D Ligand Expression in Nasopharyngeal Carcinoma and Hepatoma Cells. 2017;40:164–174. doi: 10.1097/CJI.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 112.M.OhiraS.NishidaT.MatsuuraI.MuraokaP.TryphonopoulosJ.FanA.TekinG.SelvaggiD.LeviP.Ruiz Comparative Analysis of T-Cell Depletion Method for Clinical Immunotherapy—Anti–Hepatitis C Effects of Natural Killer Cells Via Interferon-γ Production, Transplant Proc, Elsevier, 2013, pp. 2045–2050. [DOI] [PubMed]
- 113.Xie X, Zhou Y, Wang X, Guo J, Li J, Fan H, Dou J, Shen B, Zhou CJCp. Enhanced antitumor activity of gemcitabine by polysaccharide-induced NK cell activation and immune cytotoxicity reduction in vitro/vivo. 2017;173:360–371. doi: 10.1016/j.carbpol.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 114.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, Lauer UM, Bitzer M. H.R.J.C.C.R. Salih. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. 2008;14:3520–3528. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 115.Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR. M.J.H. Schuchmann. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. 2013;57:2358–2368. doi: 10.1002/hep.26328. [DOI] [PubMed] [Google Scholar]
- 116.Shi L, Lin H, Li G, Jin R-A, Xu J, Sun Y, Ma W-L, Yeh S, Cai X, Chang CJMct. Targeting Androgen Receptor (AR)→IL12A Signal Enhances Efficacy of Sorafenib plus NK Cells Immunotherapy to Better Suppress HCC Progression. Mol Cancer Ther. 2016;15(4):731–742. doi: 10.1158/1535-7163.MCT-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ho V, Lim TS, Lee J, Steinberg J, Szmyd R, Tham M, Yaligar J, Kaldis P, Abastado J-P, Chew VJO. TLR3 agonist and Sorafenib combinatorial therapy promotes immune activation and controls hepatocellular carcinoma progression. 2015;6 doi: 10.18632/oncotarget.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trojan J, Waidmann O. Role of regorafenib as second-line therapy and landscape of investigational treatment options in advanced hepatocellular carcinoma. Journal of hepatocellular carcinoma. 2016;3:31. doi: 10.2147/JHC.S112537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tai W-T, Chu P-Y, Shiau C-W, Chen Y-L, Li Y-S, Hung M-H, Chen L-J, Chen P-L, Su J-C, Lin P-Y. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20:5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Q, Zhang H, Ding J, Liu H, Li H, Li H, Lu M, Miao Y, Li L, Zheng J. 2018. Combination Therapy with EpCAM-CAR-NK-92 Cells and Regorafenib against Human Colorectal Cancer Models, Journal of immunology research, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goto K, Annan DA, Morita T, Li W, Muroyama R, Matsubara Y, Ito S, Nakagawa R, Tanoue Y, Jinushi MJSR. Novel chemoimmunotherapeutic strategy for hepatocellular carcinoma based on a genome-wide association study. 2016;6:38407. doi: 10.1038/srep38407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiao W, Dong W, Zhang C, Saren G, Geng P, Zhao H, Li Q, Zhu J, Li G, Zhang SJEjomr. Effects of the epigenetic drug MS-275 on the release and function of exosome-related immune molecules in hepatocellular carcinoma cells. 2013;18:61. doi: 10.1186/2047-783X-18-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goto K, Arai J, Stephanou A, Kato NJO. Novel therapeutic features of disulfiram against hepatocellular carcinoma cells with inhibitory effects on a disintegrin and metalloproteinase 10. 2018;9:18821. doi: 10.18632/oncotarget.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin S, Kim M, Lee SJ, Park KS, Lee CH. Trichostatin A Sensitizes Hepatocellular Carcinoma Cells to Enhanced NK Cell-mediated Killing by Regulating Immune-related Genes. Cancer genomics & proteomics. 2017;14(5):349–362. doi: 10.21873/cgp.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arai J, Goto K, Tanoue Y, Ito S, Muroyama R, Matsubara Y, Nakagawa R, Kaise Y, Lim LA, Yoshida HJIjoc. 2018. Enzymatic inhibition of MICA sheddase ADAM17 by lomofungin in hepatocellular carcinoma cells. [DOI] [PubMed] [Google Scholar]
- 126.Kim M, Lee SJ, Shin S, Park KS, Park SY, Lee CHJSr. Novel natural killer cell-mediated cancer immunotherapeutic activity of anisomycin against hepatocellular carcinoma cells. 2018;8:10668. doi: 10.1038/s41598-018-29048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wan L-l, Zhang D-q, Zhang J-n, Ren L-qJJoZU-SB. Vol. 18. 2017. Anti-hepatocarcinoma activity of TT-1, an analog of melittin, combined with interferon-α via promoting the interaction of NKG2D and MICA Journal of Zhejiang University-SCIENCE B. 2017; pp. 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu T, Cui T, Peng L, Kong S, Zou J, Tian XJOl. The anti-hepatocellular carcinoma activity of Mel-P15 is mediated by natural killer cells. 2017;14:6901–6906. doi: 10.3892/ol.2017.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shu G, Yang T, Wang C, Su H, Xiang MJT. Gastrodin stimulates anticancer immune response and represses transplanted H22 hepatic ascitic tumor cell growth: Involvement of NF-κB signaling activation in CD4+ T cells. a pharmacology. 2013;269:270–279. doi: 10.1016/j.taap.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, Zhidan W, Xia R, Zhaoxia W, Qing J, Qiang G, Haipeng Y, Hengxiao WJIi. Scorpion venom activates natural killer cells in hepatocellular carcinoma via the NKG2D-MICA pathway. 2016;35:307–314. doi: 10.1016/j.intimp.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 131.Wang X, Wang QJCJoG. 2018. Hepatology, Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity; p. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lassiter BP, Guo S, Beebe SJJC. Nano-Pulse Stimulation Ablates Orthotopic Rat Hepatocellular Carcinoma and Induces Innate and Adaptive Memory Immune Mechanisms that Prevent Recurrence. 2018;10:69. doi: 10.3390/cancers10030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar N, Khakoo SIJH. 2018. Hepatocellular carcinoma: Prospects for natural killer cell immunotherapy. [DOI] [PubMed] [Google Scholar]
- 134.Han B, Mao F-y, Zhao Y-l, Lv Y-p, Teng Y-s, Duan M, Chen W, Cheng P, Wang T-t, Liang Z-y. 2018. Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer, Journal of immunology research, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang W, Jin J, Dai F, Long Z, Liu X, Cai H, Zhou Y, Chen Z, Huang H. Interleukin-15 suppresses gastric cancer liver metastases by enhancing natural killer cell activity in a murine model. Oncol Lett. 2018;16:4839–4846. doi: 10.3892/ol.2018.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Misumi T, Tanabe K, Fujikuni N, Ohdan H. Stimulation of natural killer cells with rhCD137 ligand enhances tumor-targeting antibody efficacy in gastric cancer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou W-J, Chang K-K, Wu K, Yang H-L, Mei J, Xie F, Li D-J, Li M-Q. Rapamycin Synergizes with Cisplatin in Antiendometrial Cancer Activation by Improving IL-27–Stimulated Cytotoxicity of NK Cells. Neoplasia. 2018;20:69–79. doi: 10.1016/j.neo.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Langers I, Renoux V, Reschner A, Touzé A, Coursaget P, Boniver J, Koch J, Delvenne P, Jacobs N. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur J Immunol. 2014;44:3585–3595. doi: 10.1002/eji.201444594. [DOI] [PubMed] [Google Scholar]
- 139.Qin B, Tang D, Ni M, Gao W, Zhang M. The aberrant activation of Wnt pathway caused by β-catenin mutation and its prognostic significance in NK/T-cell lymphoma. Neoplasma. 2019;66:20–27. doi: 10.4149/neo_2018_170929N619. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Q, Tian K, Xu J, Zhang H, Li L, Fu Q, Chai D, Li H, Zheng J. 2017. Synergistic effects of cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma, Journal of immunology research, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, Colón E, Scherman-Plogell A-H, Lundqvist A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. Journal for immunotherapy of cancer. 2017;5:73. doi: 10.1186/s40425-017-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi L, Li K, Guo Y, Banerjee A, Wang Q, Lorenz UM, Parlak M, Sullivan LC, Onyema OO, Arefanian S. Modulation of NKG2D, NKp46, and Ly49C/I facilitates natural killer cell-mediated control of lung cancer. Proc Natl Acad Sci. 2018;115:11808–11813. doi: 10.1073/pnas.1804931115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Calabrese DR, Lanier LL, Greenland JR. Natural killer cells in lung transplantation. Thorax. 2019;74:397–404. doi: 10.1136/thoraxjnl-2018-212345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Small HY, Cornelius DC, Guzik TJ, Delles C. Natural killer cells in placentation and cancer: Implications for hypertension during pregnancy. Placenta. 2017;56:59–64. doi: 10.1016/j.placenta.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 145.Wu X, Deng L, Tang D, Ying G, Yao X, Liu F, Liang GJTB. miR-615-5p prevents proliferation and migration through negatively regulating serine hydromethyltransferase 2 (SHMT2) in hepatocellular carcinoma. 2016;37:6813–6821. doi: 10.1007/s13277-015-4506-8. [DOI] [PubMed] [Google Scholar]
- 146.S.HuangR.HeM.RongY.DangG.J.B.r.i.ChenSynergistic effect of MiR-146a mimic and cetuximab on hepatocellular carcinoma cells, 2014 (2014). [DOI] [PMC free article] [PubMed]