Abstract
Mesenchymal stem cells (MSCs) are one of the most useful cell resources for clinical application in regenerative medicine. However, standardization and quality assurance of MSCs are still essential problems because the stemness of MSCs depends on such factors as the collection method, individual differences associated with the source, and cell culture history. As such, the establishment of culture techniques which assure the stemness of MSCs is of vital importance. One important factor affecting MSCs during culture is the effect of the mechanobiological memory of cultured MSCs built up by their encounter with particular mechanical properties of the extracellular mechanical milieu. How can we guarantee that MSCs will remain in an undifferentiated state? Procedures capable of eliminating effects related to the history of the mechanical dose for cultured MSCs are required. For this problem, we have tried to establish the design of microelastically patterned cell-culture matrix which can effectively induce mechanical oscillations during the period of nomadic migration of cells among different regions of the matrix. We have previously observed before that the MSCs exposed to such a growth regimen during nomadic culture keep their undifferentiated state—with this maintenance of stemness believed due to lack of a particular regular mechanical dosage that is likely to determine a specific lineage. We have termed this situation as “frustrated differentiation”. In this minireview, I introduce the concept of frustrated differentiation of MSCs and show possibility of purposeful regulation of this phenomenon.
Keywords: Frustrated differentiation, Mesenchymal stem cells, Micro elasticity patterning, Matrix stiffness, Stemness of MSCs
Introduction
Matrix rigidity-influenced control of stem cell differentiation has attracted considerable interest in the research fields of mechanobiology and biomedical engineering, triggered by a report from Engler et al. on mechanobiological lineage specification of mesenchymal stem cells (MSCs) (Engler et al. 2006). Since then, stem cell mechanobiology has been extensively studied, with particular focus on the substrate/matrix mechanics-regulated differentiation of MSCs (Smith et al. 2017), and some essential factors for mechanotransduction have been revealed (Dupont et al. 2011; Piccolo et al. 2014; Dupont 2016; Nardone et al. 2017). The effects of typical mechanobiological parameters of the matrix, such as elasticity (Engler et al. 2006) and viscosity (Chaudhuri et al. 2015a, b; Bennett et al. 2018), on the lineage of MSCs have been scrutinized and applied in biomaterials engineering (Li et al. 2017).
On the other hand, MSC-based cell therapy has become more and more important in actual clinical applications (Galipeau and Sensébé 2018; Martin et al. 2019). The therapeutic effect of MSCs is derived not only from their differentiation potential or multipotency but also from their ability to secrete various useful cytokines and proteins with tissue protective effect such as BDNF, NGF, neurogulin-1, BNP, IL-6, FGF-2, GDNF, FGF-20, HGF, G-CSF, VEGF, and angiopoietin-1,2 (Crigler et al. 2006; Yoshihara et al. 2007; Phinney 2007; Fan et al. 2011; Kuroda et al. 2011), and with immune-modulating effects such as sTNFR and TSG-6 (Prockop et al. 2010). Indeed, most of current cell therapy with MSCs relies on this latter cytokine effect, in which MSCs are typically used in an undifferentiated state prior to specifying a certain lineage. Therefore, maintenance of the stemness of MSCs is an essential requirement for actual clinical application.
Although mechanobiological investigations and actual clinical applications of MSCs are expanding as mentioned above, the same fundamental problems with MSCs remain (Shen 2013; Sipp et al. 2018): i.e., problems regarding the assurance of MSC quality and their corresponding therapeutic effectiveness. Regarding the first point, MSCs are still not perfectly defined as a cell population and not enough reliable markers have been established (Mendicino et al. 2014; Lv et al. 2014). In fact, the situation is so confusing that the term “mesenchymal stem cells” itself has been reconsidered as “mesenchymal signaling cells” (Calpan 2017). Secondly, the population of MSCs in a sample is heterogeneous in principle due to differences between the source tissues and individuals. Thirdly, due to the nature of the asymmetric division of a MSC, i.e., a genuine MSC proliferates very slowly, while transiently amplifying cells grow fast, most MSC samples contain very few genuine MSCs (ca. 0.2%) with three-way differentiation potential (adipo-, osteo-, and chondrogenic differentiation) and the remainder are MSCs with some bias for specific lineages (Okamoto et al. 2002). Fourthly, the stemness of MSCs varies depending on the culture method and the environment during maintenance culture (Yang et al. 2014). Although these problems hinder the precise definition of MSCs, there have been a growing number of mechanobiological investigations. However, are the results really sufficiently reproducible and realistically fundamental for understanding the mechanobiology of cells with such an unidentified character, so-called MSCs?
As most researchers who actually handle MSCs know well, it can sometimes be difficult to reproduce the mechanobiological behaviors of MSCs. One of the essential reasons for this is the changes in the MSC population during passage culture on conventional plastic dishes. For example, Young and Anseth reported that the memory of the mechanical dose provided by the culture substrate critically modulates the differentiation propensity of an MSC sample (Yang et al. 2014). As they clearly demonstrated, long-term culture of MSCs on a stiff substrate induces a quasi-irreversible bias of lineages of MSCs to osteogenic differentiation, which means a deterioration of the stemness of MSCs and may also cause loss or reduction of undifferentiated MSC-based therapeutic effects. Such an unsteady property of differentiation propensity should inevitably affect the mechanobiological behaviors of MSCs. MSC samples used in basic and clinical studies must be well-defined in terms of their culture history and mechanical dose received from extracellular milieu. How should we assure the stemness and quality of MSC samples so that we can perform reliable experiments on the mechanobiology of MSCs?
Here, we should divide the problem into two parts: the heterogeneous population of MSCs and the lack of a methodology for maintaining the stemness of MSCs. To address the former issue, the marker expression profiles in MSC samples must be characterized. Recently, single cell-based RNA-seq analysis has been applied to characterize the heterogeneous populations of MSCs and to clarify the actual features of the cell ensemble of so-called MSCs (Freeman et al. 2015; McLeod and Mauck 2017; Liu et al. 2019). However, even if the general definition of MSCs is established in the future based on such an analysis, the latter issue of time- and environment-dependent deterioration of stemness should prevent the reliable experimental use of MSCs. In this sense, the development of a culture substrate or matrix to maintain the stemness of MSCs is essential for fundamentally supporting sound mechanobiological science and technology for MSCs.
Below, I discuss a design concept for such a culture substrate and introduce a proof of concept which we recently developed.
Methodology to avoid accumulating a culture history for MSCs
How can we guarantee that MSCs will remain in an undifferentiated state? In principle, elimination of the history of the mechanical dose for cultured MSCs should be effective. Considering that MSCs can sense and memorize mechanical signals and their dose from the culture substrate, avoidance of the accumulation of mechanical signals or initialization of the mechanical history may help to avoid biasing a stem cell toward a specific lineage. To establish a technology that can maintain MSCs in an undifferentiated state, it is essential to modulate the dynamics of mechanical signal input to cells from the culture substrate. How should we design an extracellular mechanical environment to avoid accumulating the culture history for MSCs?
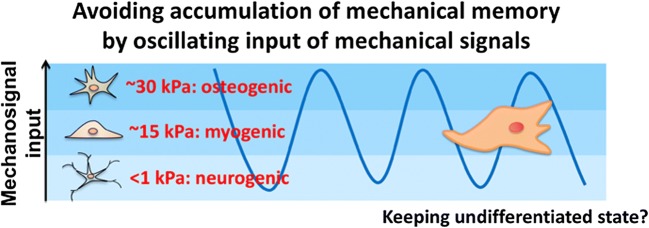
An effective approach is to make the mechanical signals oscillate (Fig. 1). If the mechanical signals are continuously changed within a short period, in succession, the cells should not accumulate enough of a mechanical dose to determine specific lineages. Indeed, they could not differentiate even if they wanted to. We referred to this state as “frustrated differentiation” (Kidoaki and Jinnouchi 2012; Kidoaki et al. 2017). How can we drive such oscillation of mechanosignal input from the culture substrate? The use of a mechanically oscillating substrate should be effective, but requires equipment for stretching the substrate and special incubators. Thus, from a practical viewpoint, we used a method that takes advantage of spontaneous cell migration. Since MSCs typically exhibit random crawling on the substrate during usual culture, if we could prepare a patterned substrate with a heterogeneous distribution of elasticity, as in stiff and soft regions, MSCs should encounter regions with different levels of elasticity when they move and thus receive different mechanosignals. Such migration on heterogeneous matrix elasticity is expected to realize the quasi-oscillatory input of mechanosignals to MSCs in stand-alone static culture systems.
Fig. 1.
Schematic representation of strategy for MSCs to avoid accumulating mechanical memory from the culture substrate through oscillating input of mechanical signals
Such nomadic culture of MSCs on a heterogeneous matrix elasticity could provide another principle for the inhibition of undesired differentiation. Mechanoresponsive lineage specification is regulated by mechanotransducing co-transcriptional factors such as YAP/TAZ interacting with LATS1/2 and AMOT (Piccolo et al. 2014). YAP/TAZ and its binding proteins such as RUNX2 shows characteristic cytoplasmic localization in MSCs cultured on softer substrates, but nuclear translocation on a stiffer substrate (Dupont et al. 2011). These behaviors are associated with lineage specification toward adipogenic and osteogenic differentiation, respectively (Dupont et al. 2011). If the localization of these factors could be prevented for both the cytosol and nucleus in an oscillatory shuttling manner, signal pathways for adipogenic and osteogenic lineages could show short-term alternation during nomadic culture and induce frustrated differentiation. To induce such nucleocytoplasmic shuttling of mechanotransducing factors in MSCs, the elastic moduli of soft and stiff regions should be appropriately designed across a threshold modulus that divides localization between the nucleus and cytoplasm. The threshold value is typically around 10 to 20 kPa, but depends on the lot of MSCs. In addition, the scale of the heterogeneous distribution of elasticity should also be carefully designed to be comparable to the size of a single cell. If the soft/stiff domains are as small as a nanoscopic scale, the mechanosignal input through focal adhesions in the adhered interfaces would be almost averaged within the adhered area of a single cell. The cell-scale heterogeneity is expected to provide large fluctuation and oscillation of mechanosignal input between the conditions of growth and destruction of the mechanical dose.
Design of a heterogeneous matrix elasticity to induce nomadic migration of MSCs
To realize the above-mentioned nomadic culture of MSC on a heterogeneous matrix elasticity, there is a critical requirement for the substrate design. In general, adherent cells show directional movement toward stiffer regions, so-called durotaxis (Lo et al. 2000). MSCs are a typical type of adherent cell and exhibit durotaxis. However, durotactic migration is an obstacle for the induction of nomadic culture, because the cells will accumulate in a stiff region according to durotactic motility if the stiff region is larger than the adhered area of a single cell.
To address this issue, cellular durotaxis should be controlled through appropriate design of the culture matrix. To satisfy this requirement, we have developed the photolithographic micro elasticity pattern of cell culture hydrogels of photocurable gelatin (Kidoaki and Matsuda 2008; Kawano and Kidoaki 2011; Kidoaki and Sakashita 2013; Kuboki et al. 2014; Ueki and Kidoaki 2015; Moriyama and Kidoaki 2018). Based on this methodology, we previously determined the threshold gradient strength of elasticity to induce durotaxis (Kawano and Kidoaki 2011; Moriyama and Kidoaki 2018), constructed a gel to rectify durotaxis (Kidoaki and Sakashita 2013), established the condition of durotactic repulsion from a soft narrow band (Kuboki et al. 2014), and found the curvature condition of the elasticity boundary to induce inverse durotaxis (Ueki and Kidoaki 2015). By applying these design criteria, nomadic migration of MSCs on a heterogeneous matrix elasticity can be possible with optimization of the size and the shape of stiff regions. If the area of the stiff region is set to be smaller than the spread area of a single cell, MSCs will fail to completely spread in the stiff region and return to the soft region. The returned MSCs will try to re-enter the stiff region according to their durotactic responses, and thus will continuously move between stiff and soft regions on the heterogeneous matrix elasticity. Such nomadic movement of MSCs between regions with different levels of elasticity is expected to induce frustrated differentiation.
Demonstration of a proof of concept of frustrated differentiation
To realize the nomadic culture of MSCs on a heterogenous matrix elasticity, we actually fabricated microelastically striped-patterned gel with optimized widths of soft and stiff bands (Fig. 2a). Young’s modulus for the soft and stiff regions was about 10 kPa and 300 kPa, respectively (Fig. 2b), according to similar preparation conditions reported previously (Kawano and Kidoaki 2011; Kidoaki and Sakashita 2013; Kuboki et al. 2014). The width of each region was designed to be 50 μm, which is smaller than the size of a single MSC (long-axis length of ca. 70–100 μm) to inhibit complete spreading. The migration behaviors of MSCs on the prepared gels were observed, and the cell trajectory was analyzed for 24 h at 1 day, 4 days, and 7 days after seeding. As the cell trajectory graph shows (Fig. 2c), cells certainly moved around and over the 100-μm-wide unit patterns, indicating the induction of nomadic movement. The time course plot of how long each MSC stayed in soft and stiff regions indicates random nomadic movement between the two regions during the 7 days of culture (Fig. 2d). From this plot, the number of times cells crossed a boundary and the time that cells stayed in each region were determined to be several hours (Fig. 2e). By designing the microelastically stripe-patterned gels, nomadic movement of MSCs between soft and stiff regions can be successfully maintained during 1 week of culture.
Fig. 2.
a Phase contrast microscopic image for elastically stipe-patterned gelatinous gel. Scale bar shows 100 μm. b Distribution of Young’s modulus in a unit stipe pattern measured with microindentation test using an atomic force microscope. c Moving trajectory of MSCs cultured on the elastically stripe-patterned gel for 24 h at 7th day culture, obtained from time lapse observation with 15-min interval. d Time course plot of staying period of each cell for stiff and soft region of the elastically stipe-patterned gel. Upper and lower lines show the period when the cells were in stiff and soft regions, respectively. e Mean staying period of the cells in stiff and soft regions of elastically strip-patterned gel. (Kidoaki and Jinnouchi 2012)
Figure 3 shows the results regarding the expression of differentiation markers. Interestingly, differentiation markers for neuro-, myo-, and osteogenic lineages were confirmed to be simultaneously suppressed, which suggests the emergence of the frustrated differentiation of MSCs.
Fig. 3.

Immunofluorescence observations for expression markers of neuro (β3 tublin), myo (MyoD), and osteogenic (CBFα1) lineages in MSCs culture on the elastically stipe-patterned gel and elastically plain gels with Young’s modulus of 10, 100, and 300 kPa. Positive and negative expressions are highlighted with red and blue boxes, respectively. Scale bar shows 100 μm. (Kidoaki and Jinnouchi 2012)
Conclusion and future works
In this minireview, I introduced a methodology for maintaining the stemness of MSCs through nomadic culture on a heterogeneous matrix elasticity. Though the concept of frustrated differentiation and its basic proof have been demonstrated, comprehensive characterization of an MSC population and rigorous analysis of gene expression of the undifferentiated phenotype must be completed. To accomplish all of these goals, the unsteady nature of MSC samples as mentioned in the “Introduction” section may hinder precise characterization of the undifferentiated state of MSCs. We are exploring these issues and will report complete results in the near future.
Acknowledgments
This research was supported by AMED-CREST under Grant Number JP19gm0810002.
Compliance with ethical standards
Conflict of interest
Satoru Kidoaki declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bennett M, Cantinia M, Rebouda J, Coopera JM, Roca-Cusachs P, Salmeron-Sancheza M. Molecular clutch drives cell response to surface viscosity. PNAS. 2018;115:1192–1197. doi: 10.1073/pnas.1710653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calpan A. Mesenchymal stem cells: time to change the name! Stem Cell Translat Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2015;15:326–334. doi: 10.1038/NMAT4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343:42–53. doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Digabel JL, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- Freeman BT, Jung JP, Ogle BM. Single-cell RNA-seq of bone marrow-derived mesenchymal stem cells reveals unique profiles of lineage priming. PLoS One. 2015;10:e0136199. doi: 10.1371/journal.pone.0136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Kidoaki S. Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels. Biomaterials. 2011;32:2725–2733. doi: 10.1016/j.biomaterials.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Kidoaki S, Jinnouchi S. Frustrated differentiation of mesenchymal stem cell cultured on microelastically-patterned photocurable gelatinous gels. Biophys J. 2012;102(suppl.1):716a. doi: 10.1016/j.bpj.2011.11.3885. [DOI] [Google Scholar]
- Kidoaki S, Matsuda T. Microelastic gradient gelatinous gels to induce cellular mechanotaxis. J Biotechnol. 2008;133:225–230. doi: 10.1016/j.jbiotec.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kidoaki S, Sakashita H. Rectified cell migration on saw-like micro-elastically patterned hydrogels with asymmetric gradient ratchet teeth. PLoS One. 2013;8:e78067. doi: 10.1371/journal.pone.0078067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidoaki S, Ebata H, Sawada R, Moriyama K, Kuboki T, Tsuji Y, Kono K, Tanaka K, Sasaki S. Characterization of the frustrated differentiation of mesenchymal stem cells induced by normadic migration between stiff and soft region of gel matrix. Biophys J. 2017;112(suppl.1):436a. doi: 10.1016/j.bpj.2016.11.2328. [DOI] [Google Scholar]
- Kuboki T, Chen W, Kidoaki S. Time-dependent migratory behaviors in the long-term studies of fibroblast durotaxis on a hydrogel substrate fabricated with a soft band. Langmuir. 2014;30:6187–6196. doi: 10.1021/la501058j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Dezawa M. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp. 2011;59:369–378. doi: 10.1007/s00005-011-0139-9. [DOI] [PubMed] [Google Scholar]
- Li L, Eyckmans J, Chen CS. Designer biomaterials for mechanobiology. Nat Mater. 2017;16:1164–1168. doi: 10.1038/nmat5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Stroncek DF, Zhao Y, Chen V, Shi R, Chen J, Ren J, Liu H, Bae HJ, Highfill SL, Jin P. Single cell sequencing reveals gene expression signatures associated with bone marrow stromal cell subpopulations and time in culture. J Transl Med. 2019;17:23. doi: 10.1186/s12967-018-1766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C-M, Wang H-B, Dembo M, Wang Y-L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- Martin I, Galipeau J, Kessler C, Le Blanc K, Dazzi F. Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019;11:eaat2189. doi: 10.1126/scitranslmed.aat2189. [DOI] [PubMed] [Google Scholar]
- McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217–231. doi: 10.22203/eCM.v034a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Moriyama Kousuke, Kidoaki Satoru. Cellular Durotaxis Revisited: Initial-Position-Dependent Determination of the Threshold Stiffness Gradient to Induce Durotaxis. Langmuir. 2018;35(23):7478–7486. doi: 10.1021/acs.langmuir.8b02529. [DOI] [PubMed] [Google Scholar]
- Nardone G, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, Nakamura T, Kiyono T, Yoguchida J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. BBRC. 2002;295:354–361. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Stricter standards sought to curb stem-cell confusion. Nature. 2013;499:389. doi: 10.1038/499389a. [DOI] [PubMed] [Google Scholar]
- Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561:455–457. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- Smith L, Cho S, Discher DE. Mechanosensing of matrix by stem cells: from matrix heterogeneity, contractility, and the nucleus in pore-migration to cardiogenesis and muscle stem cells in vivo. Semin Cell Dev Biol. 2017;71:84–98. doi: 10.1016/j.semcdb.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki A, Kidoaki S. Manipulation of cell mechanotaxis by designing curvature of the elasticity boundary on hydrogel matrix. Biomaterials. 2015;41:45–52. doi: 10.1016/j.biomaterials.2014.11.030. [DOI] [PubMed] [Google Scholar]
- Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Ohta M, Itokazu Y, Matsumoto N, Dezawa M, Suzuki Y, Taguchi A, Watanabe Y, Adachi Y, Ikehara S, Sugimoto H, Ide C. Neuroprotective effect of bone marrow-derived mononuclear cells promoting functional recovery from spinal cord injury. J Neurotrauma. 2007;24:1026–1036. doi: 10.1089/neu.2007.132R. [DOI] [PubMed] [Google Scholar]