Abstract
Geptop has performed effectively in the identification of prokaryotic essential genes since its first release in 2013. It estimates gene essentiality for prokaryotes based on orthology and phylogeny. Genome-scale essentiality data of more prokaryotic species are available, and the information has been collected into public essential gene repositories such as DEG and OGEE. A faster and more accurate toolkit is needed to meet the increasing prokaryotic genome data. We updated Geptop by supplementing more validated essentiality data into reference set (from 19 to 37 species), and introducing multi-process technology to accelerate the computing speed. Compared with Geptop 1.0 and other gene essentiality prediction models, Geptop 2.0 can generate more stable predictions and finish the computation in a shorter time. The software is available both as an online server and a downloadable standalone application. We hope that the improved Geptop 2.0 will facilitate researches in gene essentiality and the development of novel antibacterial drugs. The gene essentiality prediction tool is available at http://cefg.uestc.cn/geptop.
Keywords: essential genes, prediction, prokaryotes, software, bioinformatics
Introduction
Essential genes are critical for the survival and development of organisms (Mushegian and Koonin, 1996). In dozens of prokaryotes, genome-scale essentiality data have been determined by various experimental methods and these information has been stored in online databases such as DEG (Luo et al., 2014) and OGEE (Chen et al., 2017). Studies on bacterial essential genes are helpful in understanding the essence of life (Rancati et al., 2018) and screening potential drug targets to treat pathogenic diseases (Dickerson et al., 2011).
Due to the cost and difficulties of experiments, computational identification of essential genes presents an important alternative approach (Mobegi et al., 2017). Features including evolutionary conservation (Nigatu et al., 2017; Dilucca et al., 2018), domain information (Lu et al., 2015), network topology (Jeong et al., 2001; Zhang et al., 2016; Karthik et al., 2018; Li et al., 2019), function (Lei and Yang, 2018), and expression level (Dong et al., 2018) are used in predicting gene essentiality via the approaches of bioinformatics. Based on this, many models were developed to implement gene essentiality prediction (Fan et al., 2017; Mobegi et al., 2017; Li et al., 2019; Zhang et al., 2019).
There are a few online tools to automatically predict bacterial essential genes. CEG_Match was developed to select name-known essential genes based on gene function information (Ye et al., 2013). Essential Gene Prediction (EGP) is a machine-learning-based method using only sequence compositional features (Ning et al., 2014). We utilized another machine learning algorithm (SVM) with features obtained by homology mapping to predict gene essentiality (Hua et al., 2016). The BLAST tool in DEG can be utilized to perform homolog search against essential gene set (Luo et al., 2014).
In 2013, we released Geptop to predict essential genes of sequenced bacterial genomes. It uses the reciprocal best hit (RBH) method to determine orthology, and the composition vector (CV) method to weight the contributions of each reference genome (Qi et al., 2004; Wei et al., 2013). Since the release of Geptop 1.0, it has become the most widely used computational tool for predicting bacterial essential genes, in large part due to its high accuracy and the availability as Web server (Gupta et al., 2016; Gao et al., 2017; Nigatu et al., 2017; Peng et al., 2017; Rancati et al., 2018). When using only three genomes as reference set, Geptop is competitive with other integrative methods, and its superiority becomes more pronounced when 18 genomes are used as the reference set (Wei et al., 2013). The excellent performance of Geptop depends on our intrinsic definition of orthology using RBH and the method of balancing the weights of various reference genomes according to their phylogenetic distances.
Since our release of Geptop 1.0, the number of genomes with essential gene data has increased significantly (Luo et al., 2014; Chen et al., 2017). In addition, the Python package contains a multiprocessing module that can execute multiprogramming computation, which obviously makes the process of calculating faster. In light of the progress in data and technology, we were inspired to update Geptop.
Methods
Information about gene essentiality was obtained from DEG or OGEE, and the complete protein coding sequences of all 40 bacteria were acquired from GenBank. Detailed information is displayed in Supplementary Table S1. Similar with that in Geptop 1.0 (Wei et al., 2013), each of the 40 species was used as the test set, and the other 39 proteomes being used as the reference set, separately. After obtaining the 40 area under the curve (AUC) scores calculated based on the real essentiality annotation and the predicted essentiality score, we only selected those genomes whose AUC scores were higher than 0.60 as the final reference set. And three genomes got the AUC scores lower than 0.60. Indeed, essentiality data of the three eliminated genomes may have significant biases (Wei et al., 2013; Cheng et al., 2014).
Geptop 1.0 used the method of geometric mean to combine the contribution of each reference genome to obtain the essentiality score of inquiry gene. When the orthologous gene in one reference proteome is non-essential or the inquiry gene has no ortholog, we need to neglect the contribution of this reference genome and only consider those reference genomes that contain essential orthologs. This manner of dealing with this issue generates reasonable essentiality score; however, the original equation for implementing this operation in Geptop 1.0 may be difficult to understand.
In the new implementation of Geptop 2.0, to define the gene essentiality score Si for gene i, we changed the cumulative formula as follows:
| (1) |
where j is the jth proteome in the reference set, N is the count of all reference proteomes, M is the mapping score. M is two-value-variables “1” and “0.” “1” means the ortholog of a query gene is essential in the reference genome whereas “0” means that a query gene has not any orthologs or the ortholog is non-essential. Dj is the evolutionary distance between the query proteome and the jth reference proteome. It can be calculated by the CV method (Qi et al., 2004). When D = 0, which means that the query genome and reference genome are the same, we set D to 0.01 to avoid division by zero. After obtaining the essentiality scores for all genes in the query genome, we use the following formula to normalize the result:
| (2) |
where Sfinal is the final S score of gene i, Min is the minimum of S over all genes in the query proteome, and Max is the maximum. Sfinal ranges from 0 to 1, and the more essential a gene is, the larger the value of Sfinal will be.
In addition, we utilized the multiprocessing module in Python to increase computational efficiency.
Performance Assessment
The following indexes were used to assess the performance of the predictor:
Here TP, FN, FP, and TN denote the true positives, false negatives, false positives, and true negatives, respectively. The sensitivity index represents the proportion of essential genes that have been correctly identified, and the specificity index represents the proportion of non-essential genes that have been correctly identified, and the precision index is the probability that the predicted essential genes were indeed essential. The F-measure represents the harmonic mean of precision and sensitivity. The Mathew Correlation Coefficient (MCC) index represents the reliability of the algorithm which ranges from -1 to 1. When FP and FN are both equal to 0, MCC is equal to 1, meaning that the result of prediction is totally right; conversely, if FP and FN are both equal to 1, MCC is equal to -1, meaning that the result of prediction is totally wrong.
Results
Improved Performance of Geptop 2.0 Than Geptop 1.0 by Cross-Species-Validation
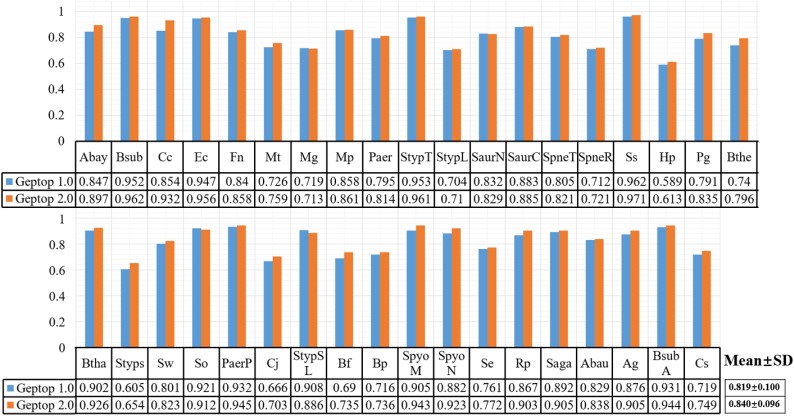
After choosing 37 genomes with reliable essentiality information and aligning them as reference set in Geptop 2.0, we performed further computation to compare the prediction performance between Geptop 1.0 and 2.0. For this purpose, we ran both versions for each of the 37 genomes and obtained 37 pairs of AUC scores by cross-validations. The results are shown in Figure 1, in which each genome is represented by one column. The average AUC value of Geptop 1.0 among the 37 genomes was about 0.82, whereas that of Geptop 2.0 was higher than 0.84, indicating an improvement of over 2.0%. And the variance in Geptop 2.0 was less than that in Geptop 1.0 among the 37 AUC values. In the case of Caulobacter crescentus NA1000 (Cc), the AUC score of Geptop 2.0 was 8.0% higher.
FIGURE 1.
The AUC result of 37 genomes among Geptop 1.0, Geptop 2.0. And the abbreviation of 37 bacteria please refers to Supplementary Table S1.
After changing the scoring method as shown in formulae (1) and (2), we reset the default threshold value of the essentiality score to 0.24, meaning that if the essentiality score of one gene is higher than 0.24, then the gene should be predicted as essential gene. Moreover, users may also change this threshold according to their specific requirements to ensure fewer false positives or higher precision.
Besides AUC evaluation, we also utilized the sensitivity, specificity, MCC and F-measure indexes to assess the prediction performance for both versions of Geptop. The complete results are shown in Supplementary Table S2. Except that the average specificity index among 37 species is about 0.9% lower in Geptop 2.0 than that in Geptop 1.0, other averages for indexes in Geptop 2.0 are higher ranging from 1.5 to 4.3%.
The Advantage of Geptop 2.0 Compared With Other Essential Gene Prediction Models
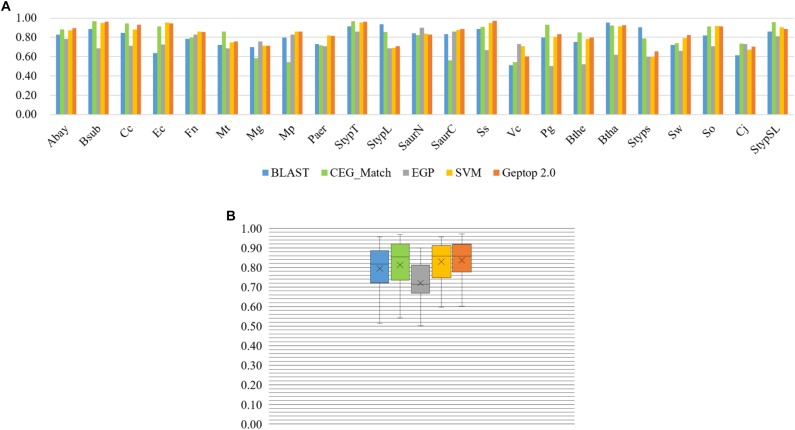
By AUC evaluation only, Geptop 2.0 is competitive with other models. We performed prediction in four models introduced above for 23 organisms (Hua et al., 2016; Peng et al., 2017). The result was exhibited in Figure 2. Except EGP, all these models performed well in the 23 organisms. The lowest AUC score among 23 organisms of Geptop 2.0 is still higher than 0.60, and the highest is 0.97, while all other models have some AUC scores lower than 0.60, indicating that the prediction of Geptop 2.0 is reliable enough. Considering the average AUC score of 23 organisms, Geptop 2.0 has the value of 0.84, which is the highest among the five models.
FIGURE 2.
Prediction performance of BLAST tool, CEG_Match, EGP, SVM, and Geptop 2.0 in the 23 genomes. (A) AUC scores of the gene essentiality prediction by BLAST tool, CEG_Match, EGP, SVM, and Geptop 2.0, respectively, for each genome. (B) Box plot of AUC scores from the prediction of the five tools for the 23 genomes.
Discussion
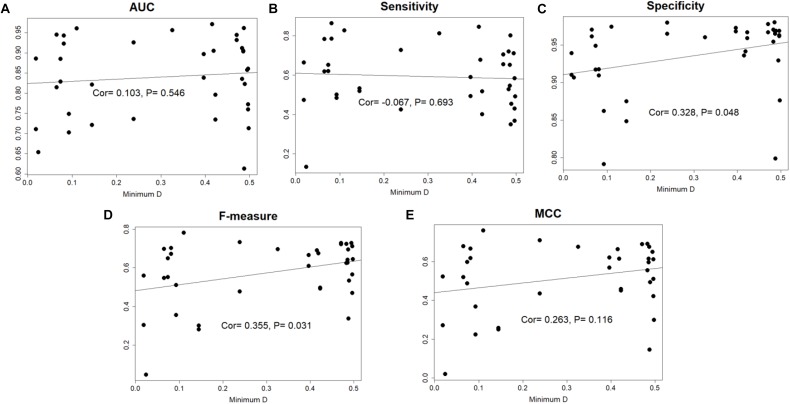
The species distance of query genome with reference genomes can influence the prediction performance, so we calculated the minimal distance between query genome and the 36 reference genomes, then the closest genomes would be removed from reference set. We performed prediction again for the query genome using 35 reference genomes. The 14 species whose AUC scores higher than 0.90 in Geptop 2.0 were selected. Loss of closest species in reference genomes would indeed decrease the performance in most case. However, the difference is slight and the average decrease of AUC is only about 1%. There is even 0.1% increment of AUC for Salmonella enterica serovar Typhi Ty2.
In the total 37 genomes, 18 genomes obtained a minimal distance higher than 0.41 and this is quite far species distance. Among these 18 genomes, 8 genomes got an AUC score higher than 0.90, while only one genome got an AUC score lower than 0.70, indicating that the performance of Geptop 2.0 is stable and reliable in the essential genes identification without close reference genomes. If we directly calculate the correlation coefficient between minimal distance and each of the five indexes for the 37 species, it will find there is not significant association (Figure 3). Hence, Geptop’s performance generally relies on the scale of reference genomes and a few special genomes will have trivial influence on it. When there are fewer reference genomes, the prediction accuracy will depend significantly on the reference genome with the smallest distance. However, if we have numerous reference genomes, the effect of smallest evolution distance on prediction accuracy will be significantly weaken and all reference genomes will play a collective effect on the prediction. In fact, a larger number of genomes could also weak the quality bias caused by one or two special reference genomes. Therefore, updating Geptop into the version 2.0 could generate more stable prediction.
FIGURE 3.
The correlation between minimal species distance and prediction performance among 37 genomes. In each sub-picture, the horizontal axis represents the minimal distance between the query genome and 36 reference genomes. The vertical axis represents the prediction performance estimated by AUC, Sensitivity, Specificity, F-measure and MCC indexes. The “Cor” value in figure is “Pearson correlation coefficient.” (A) The correlation between AUC score and minimal species distance. (B) The correlation between the sensitivity index and minimal species distance. (C) The correlation between the specificity index and minimal species distance. (D) The correlation between the F-measure index and minimal species distance. (E) The correlation between MCC index and minimal species distance.
Conclusion
With more reference genomes, Geptop 2.0 can get better performance than Geptop 1.0, and it’s competitive with other gene essentiality prediction models. Despite the limitation in some species, its performance is reliable enough in most species. We are confident that Geptop 2.0 would generate more stable predictions with larger-scale reference set. Besides, we used the multiprocessing module to achieve the multiprogramming computation using a Linux 4 CPU system, ultimately increasing the computation efficiency by more than fourfold. For example, for Escherichia coli K12 MG 1655 (4100 genes in total), our server returned prediction results in less than 35 min. The web server and standalone version of Geptop are available at http://cefg.uestc.cn/geptop.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: http://origin.tubic.org/deg/public/index.php.
Author Contributions
F-BG designed and coordinated this project and revised the manuscript. Q-FW the programmed Geptop 2.0 and revised both the website and software. SL checked the AUC results. Q-FW drafted the manuscript. CD and SL checked the Geptop algorithm. H-XG and Y-ZG took part in the AUC computation. All authors read and approved this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Wen Wei, Sen Luo, and Hong-Li Hua for their help in understanding Geptop 1.0 and Chong Peng for kindly giving us the original data in her review “A Comprehensive Overview of Online Resources to Identify and Predict Bacterial Essential Genes.”
Footnotes
Funding. This work has been supported by the National Natural Science Foundation of China (31871335) and the Science Strength Promotion Program of UESTC.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01236/full#supplementary-material
References
- Chen W. H., Lu G., Chen X., Zhao X. M., Bork P. (2017). OGEE v2: an update of the online gene essentiality database with special focus on differentially essential genes in human cancer cell lines. Nucleic Acids Res. 45 D940–D944. 10.1093/nar/gkw1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Xu Z., Wu W., Zhao L., Li X., Liu Y., et al. (2014). Training set selection for the prediction of essential genes. PLoS One 9:e86805. 10.1371/journal.pone.0086805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson J. E., Zhu A., Robertson D. L., Hentges K. E. (2011). Defining the role of essential genes in human disease. PLoS One 6:e27368. 10.1371/journal.pone.0027368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilucca M., Cimini G., Giansanti A. (2018). Essentiality, conservation, evolutionary pressure and codon bias in bacterial genomes. Gene 663 178–188. 10.1016/j.gene.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Dong C., Jin Y. T., Hua H. L., Wen Q. F., Luo S., Zheng W. X., et al. (2018). Comprehensive review of the identification of essential genes using computational methods: focusing on feature implementation and assessment. Brief. Bioinform. 10.1093/bib/bby116 [DOI] [PubMed] [Google Scholar]
- Fan Y., Tang X., Hu X., Wu W., Ping Q. (2017). Prediction of essential proteins based on subcellular localization and gene expression correlation. BMC Bioinformatics 18:470. 10.1186/s12859-017-1876-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., Lu G., Lercher M. J., Chen W. H. (2017). Selection for energy efficiency drives strand-biased gene distribution in prokaryotes. Sci. Rep. 7:10572. 10.1038/s41598-017-11159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gross R., Dandekar T. (2016). An antibiotic target ranking and prioritization pipeline combining sequence, structure and network-based approaches exemplified for Serratia marcescens. Gene 591 268–278. 10.1016/j.gene.2016.07.030 [DOI] [PubMed] [Google Scholar]
- Hua H. L., Zhang F. Z., Labena A. A., Dong C., Jin Y. T., Guo F. B. (2016). An approach for predicting essential genes using multiple homology mapping and machine learning algorithms. Biomed. Res. Int. 2016:7639397. 10.1155/2016/7639397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Mason S. P., Barabasi A. L., Oltvai Z. N. (2001). Lethality and centrality in protein networks. Nature 411 41–42. 10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- Karthik A., Balaraman R., Karthik R. (2018). Network-based features enable prediction of essential genes across diverse organisms. PLoS One 13:e0208722. 10.1371/journal.pone.0208722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X. J., Yang X. Q. (2018) A new method for predicting essential proteins based on participation degree in protein complex and subgraph density. PLoS One 13:e0198998. 10.1371/journal.pone.0198998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li W., Zeng M., Zheng R., Li M. (2019). Network-based methods for predicting essential genes or proteins: a survey. Brief. Bioinform. 10.1093/bib/bbz017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lu Y., Lu Y., Deng J., Peng H., Lu H., Lu L. J. (2015). A novel essential domain perspective for exploring gene essentiality. Bioinformatics 31 2921–2929. 10.1093/bioinformatics/btv312 [DOI] [PubMed] [Google Scholar]
- Luo H., Lin Y., Gao F., Zhang C. T., Zhang R. (2014). DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 42 D574–D580. 10.1093/nar/gkt1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobegi F. M., Zomer A., de Jonge M. I., van Hijum S. A. (2017). Advances and perspectives in computational prediction of microbial gene essentiality. Brief. Funct. Genomics 16 70–79. 10.1093/bfgp/elv063 [DOI] [PubMed] [Google Scholar]
- Mushegian A. R., Koonin E. V. (1996). A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc. Natl. Acad. Sci. U.S.A. 93 10268–10273. 10.1073/pnas.93.19.10268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigatu D., Sobetzko P., Yousef M., Henkel W. (2017). Sequence-based information-theoretic features for gene essentiality prediction. BMC Bioinformatics 18:473. 10.1186/s12859-017-1884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning L. W., Lin H., Ding H., Huang J., Rao N., Guo F. B. (2014). Predicting bacterial essential genes using only sequence composition information. Genet. Mol. Res. 13 4564–4572. 10.4238/2014.June.17.8 [DOI] [PubMed] [Google Scholar]
- Peng C., Lin Y., Luo H., Gao F. (2017). A comprehensive overview of online resources to identify and predict bacterial essential genes. Front. Microbiol. 8:2331. 10.3389/fmicb.2017.02331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Luo H., Hao B. (2004). CVTree: a phylogenetic tree reconstruction tool based on whole genomes. Nucleic Acids Res. 32 W45–W47. 10.1093/nar/gkh362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati G., Moffat J., Typas A., Pavelka N. (2018). Emerging and evolving concepts in gene essentiality. Nat. Rev. Genet. 19 34–49. 10.1038/nrg.2017.74 [DOI] [PubMed] [Google Scholar]
- Wei W., Ning L. W., Ye Y. N., Guo F. B. (2013). Geptop: a gene essentiality prediction tool for sequenced bacterial genomes based on orthology and phylogeny. PLoS One 8:e72343. 10.1371/journal.pone.0072343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y. N., Hua Z. G., Huang J., Rao N., Guo F. B. (2013). CEG: a database of essential gene clusters. BMC Genomics 14:769. 10.1186/1471-2164-14-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Peng W., Yang Y., Dai W., Song J. (2019). A novel method for identifying essential genes by fusing dynamic protein(-)protein interactive networks. Genes 10:E31. 10.3390/genes10010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Acencio M. L., Lemke N. (2016). Predicting essential genes and proteins based on machine learning and network topological features: a comprehensive review. Front. Physiol. 7:75 10.3389/fphys.2016.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://origin.tubic.org/deg/public/index.php.