Abstract
Patient: Female, 67
Final Diagnosis: Takotsubo cardiomyopathy complicating with left ventricular thrombus
Symptoms: Acute respiratory failure
Medication: —
Clinical Procedure: Echocardiogram
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Myasthenia gravis can precipitate severe stress particularly during a myasthenic crisis episode. Takotsubo cardiomyopathy has been demonstrated in several conditions associated with emotional or physical stress. As a result, Takotsubo cardiomyopathy is not uncommon in patients with MG. The severe complications of Takotsubo cardiomyopathy include heart failure and left ventricular thrombus associated with thromboembolic risk. The concomitant myasthenic crisis and Takotsubo cardiomyopathy with apical left ventricular thrombus has never been reported.
Case Report:
A 67-year- old Thai female diagnosed with myasthenia gravis was admitted to the intensive care unit due to the myasthenic crisis. The 12-lead electrocardiogram showed marked QT interval prolongation and diffuse large T-wave inversion. Echocardiogram demonstrated basal hyperkinesia and apical akinesia with apical ballooning. Hyperechoic mass was noted in akinetic left ventricular apex. Takotsubo cardiomyopathy with apical left ventricular thrombus was diagnosed. Both conditions were successfully treated in this patient without any complications.
Conclusions:
The electrocardiogram surveillance in patients with myasthenic crisis is essential to detect the occurrence of Takotsubo cardiomyopathy and its complications. Early diagnosis and treatments may decrease mortality and morbidity related with this condition.
MeSH Keywords: Embolism and Thrombosis; Myasthenia Gravis, Autoimmune, Experimental; Takotsubo Cardiomyopathy
Background
Myasthenia gravis (MG) is a common autoimmune disorder of neuromuscular junction that causes weakness in the skeletal muscles which are responsible for breathing and moving parts of the body. Approximately 15% to 20% of patients with MG experience myasthenic crisis which develops into respiratory failure and thus need ventilator support [1].
Takotsubo cardiomyopathy (so-called stress-induced cardiomyopathy or broken heart syndrome) has been demonstrated in several conditions associated with emotional stress or physical stress. The diagnosis of Takotsubo cardiomyopathy is based on the presence of transient regional left ventricular (LV) wall abnormalities beyond the territory supplied by a single epicar-dial coronary artery. Despite the common occurrence of basal hyperkinetic segment of LV with apical hypokinesis, regional wall motion abnormality at basal or mid segment can also be seen. The presentation and imaging findings of Takotsubo cardiomyopathy can mimic acute coronary syndrome. However, this condition has a spontaneous recover mostly within few months [2]. Its serious complications include heart failure, serious cardiac arrhythmias, and LV thrombus which is associated with the increased thromboembolic risk [3].
The concomitant occurrence of these 2 conditions with serious complications, i.e., MG with myasthenic crisis and Takotsubo cardiomyopathy with apical LV thrombus, has not been reported previously.
Case Report
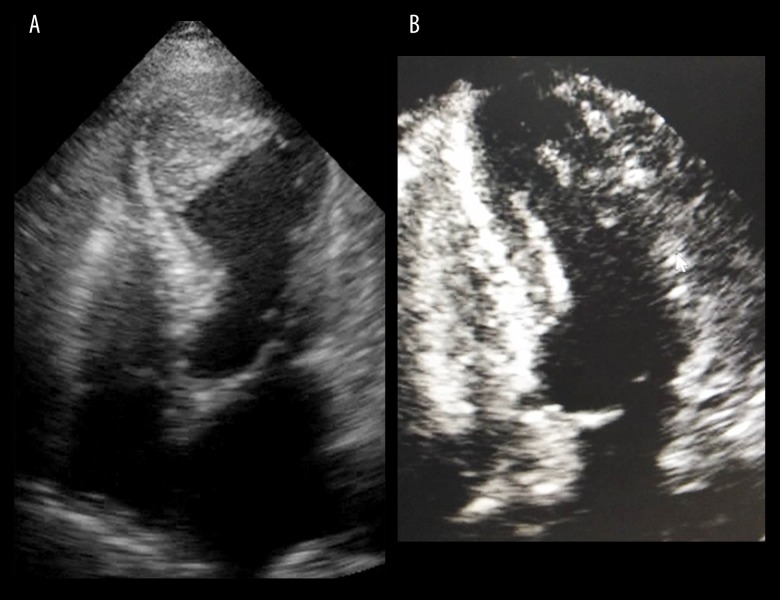
We reported a case of 67-year-old Thai female patient with MG who developed myasthenic crisis and was admitted to the intensive care unit. On admission, she presented with bilateral ophthalmoparesis and significant bulbar and respiratory muscle weakness. An endotracheal tube was inserted with respiratory support by mechanical ventilation. High dose corticosteroids and intravenous immunoglobulin were given to treat the condition. Her underlying medical conditions included type 2 diabetic mellitus, hyperlipidemia, and essential hypertension. The 12-lead electrocardiogram (ECG) on the third day after admission demonstrated normal sinus rhythm with significant prolongation of QT interval and diffuse deep T wave inversion in all leads (Figure 1A). The patient did not complain of any chest pain or other cardiac symptoms. High-sense cardiac troponin I was significantly elevated (3827 pg/mL). Echocardiogram demonstrated basal hyperkinesia and apical akinesia with apical ballooning. Moderate impairment of LV systolic function was noted with LV ejection fraction (LVEF) of 36% by Simpson’s technique. Interestingly, the hyperechoic mass attached at akinetic LV apex was observed which was compatible with large apical thrombus (Figure 2A). Coronary angiogram revealed no significant epicardial coronary artery disease. In light of these findings, Takotsubo cardiomyopathy was diagnosed.
Figure 1.
(A) The 12-lead electrocardiogram demonstrated normal sinus rhythm with significant QT prolongation and diffuse deep T wave inversion in all leads (asterisk). (B) The 12-lead electrocardiogram at 3 month after the diagnosis of Takotsubo cardiomyopathy showed significant shortening of QT interval compared to previous electrocardiogram.
Figure 2.
(A) Transthoracic echocardiogram demonstrated basal hyperkinesia and apical akinesia with apical ballooning. Large apical thrombus was noted (asterisk). (B) Transthoracic echocardiogram at 3-month follow-up revealed complete recovery of left ventricular function and no residual apical thrombus.
Regarding the treatment of Takotsubo cardiomyopathy in this patient, anticoagulant therapy with unfractionated heparin and subsequently warfarin was commenced to prevent systemic thromboembolic events. The neurohormonal modulation therapies, including beta-blocker and angiotensin-converting – enzyme inhibitor, were also initiated and titrated to improve LV function. The neurological condition gradually improved and the patient was discharged after 1 month of hospitalization. The echocardiogram before discharge showed improved LV function with LVEF of 50%. However, residual apical LV thrombus was still noted. Therefore, the patient was instructed to continue warfarin and neurohormonal modulation therapies after hospital discharge. At 3-month follow-up, repeated echocardio-gram revealed complete recovery of LV function with LVEF of 70% and no residual of LV thrombus was observed (Figure 2B).
The 12-lead ECG showed significant shortening of QT interval compared to previous ECG (Figure 1B). No serious complications of Takotsubo cardiomyopathy occurred during the 3 months of follow-up. The patient has discontinued warfarin since then.
Discussion
This reported case was a case of Takotsubo cardiomyopathy occurring in a patient with MG during myasthenic crisis, complicated by a large apical thrombus. The pathophysiology of Takotsubo cardiomyopathy is complex. Several mechanisms have been proposed. The central activation of the autonomic nervous system in response to stress may play an important role. The release of norepinephrine and stress-related neuropeptides from presynaptic terminations of the post-ganglionic sympathetic system may account for the direct toxic effect to myocardium and the impairment of microvascular function leading to ischemic stunning of myocardium. The increase in circulating catecholamines and stress hormones in response to stress has also been proposed [4–6].
Cardiac manifestation in MG is not uncommon, particularly in older age and the presence of thymoma. Takotsubo cardiomyopathy was the most commonly reported cardiomyopathy [7]. ECG abnormalities have been found in most of the patients with Takotsubo cardiomyopathy, in which marked QT prolongation and deep T wave inversion is the hallmark ECG findings. Similarly, non-specific T wave changes and QT prolongation have also been reported with MG. As a result, the presence of QT prolongation in patients with MG may render the diagnosis of Takotsubo cardiomyopathy challenging. Furthermore, Takotsubo cardiomyopathy would further lengthen QT interval in patients with MG, leading to a substrate for severe ventricular tachyarrhythmias.
Since MG has been associated with myocarditis, the exclusion of myocarditis by cardiac magnetic resonance imaging (MRI) and inflammatory markers would be very helpful in this case. However, the diagnosis of Takotsubo cardiomyopathy in our patient was made by Heart Failure Association–European Society of Cardiology Criteria [8], which included the recovery of LV function and resolution of QT prolongation at 3 months, together with normal coronary arteries. Therefore, the cardiac MRI and inflammatory markers were not obtained in our patient case.
Myasthenic crisis is a manifestation of MG in which severe skeletal muscle weakness results in respiratory distress and failure [1]. Both Takotsubo cardiomyopathy and myasthenic crisis are frequently triggered by stress conditions [9]. Respiratory tract infection was believed to be the trigger of myasthenic crisis in this case. It could not be excluded that the respiratory tract infection may also have been the trigger of Takotsubo cardiomyopathy and not the myasthenic crisis itself.
LV thrombus formation is a known serious complication of Takotsubo cardiomyopathy, which predominantly occurs in patients with apical ballooning or large areas of wall motion abnormalities [10]. It can occur at initial presentation or at any time later during the disease course. Treatment with anticoagulant therapy is strongly recommended to prevent systemic thromboembolic events. Generally, it has been suggested to continue anticoagulation therapy until the complete recovery of LV function is noted and no residual LV thrombus is seen [4]. Takotsubo cardiomyopathy has rarely been reported in patients with myasthenic crisis [9–11]. However, a case of Takotsubo cardiomyopathy with LV thrombus in a patient with myasthenic crisis has not been previously described. The diagnosis of Takotsubo cardiomyopathy in patients with myasthenic crisis is challenging. The predominant symptoms of Takotsubo cardiomyopathy, including chest pain and dyspnea, can often be undetected in intubated and sedated myasthenic crisis patients. The heart failure symptom of Takotsubo cardiomyopathy can be masked by concurrent respiratory failure due to respiratory muscle weakness. A previous study [2] showed that 22% of patients with Takotsubo cardiomyopathy developed serious in-hospital complications. The prolongation of QT interval was detectable in a considerable proportion of patients with Takotsubo cardiomyopathy. The ST-segment elevation was demonstrated in 44% of the patients. A reduced LVEF (<40%) was observed in nearly 90% of patients with Takotsubo cardiomyopathy [2].
The cardiac abnormalities in Takotsubo cardiomyopathy is usually resolved within 3 months. However, an ECG at 3-month follow-up of our patient still showed diffusion T wave inversion, even the marked shortening of QT interval. It is possible that the patient may have had pre-existing ECG abnormalities, unfortunately, we did not have prior ECG of the patients. The complete resolution of ECG abnormalities in this case might have required longer time. It has been reported that complete recovery of Takotsubo cardiomyopathy may take a longer duration, up to 1 year in some cases [6].
It has been described that the prognosis of patients with Takotsubo cardiomyopathy depends on the different stressful triggers. Emotional stress is associated with the most favorable prognosis (so-called primary form of Takotsubo cardiomyopathy) [12]. On the contrary, physical stress is associated with the worse prognosis (so-called secondary forms of Takotsubo cardiomyopathy) [12]. Of note, neurologic disease is related with the worst prognosis. The long-term therapy focusing on the MG management may have improved the prognosis in our patient who presented with Takotsubo cardiomyopathy [13,14].
This case highlighted the importance of ECG surveillance and subsequent echocardiogram in suspicious cases of Takotsubo cardiomyopathy in patients with myasthenic crisis. Angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers have been reported to improve survival at 1 year in patients with Takotsubo cardiomyopathy [2]. Anticoagulation therapy can reduce the thromboembolic risk in patients with LV thrombus. With this consideration, early detection and proper management of Takotsubo cardiomyopathy is crucial to improve short-term and long-term outcomes of the patients.
Conclusions
We reported the rare concomitance of myasthenic crisis and Takotsubo cardiomyopathy complicated with LV thrombus. Therefore, this condition should be suspected in cases of cardiac symptoms and signs because it could result in severe complications, including systemic thromboembolism. Early diagnosis and treatment can improve outcomes in this group of patients.
Footnotes
Conflict of interest
None.
References:
- 1.Roper J, Fleming ME, Long B, Koyfman A. Myasthenia gravis and crisis: Evaluation and management in the emergency department. J Emerg Med. 2017;53(6):843–53. doi: 10.1016/j.jemermed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 3.Haghi D, Papavassiliu T, Heggemann F, et al. Incidence and clinical significance of left ventricular thrombus in Takotsubo cardiomyopathy assessed with echocardiography. QJM. 2008;101(5):381–86. doi: 10.1093/qjmed/hcn017. [DOI] [PubMed] [Google Scholar]
- 4.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(16):1955–71. doi: 10.1016/j.jacc.2018.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaszczuk P, Kolodziejczyk B, Kruczek T, et al. Ischemic versus non-ischemic (neurogenic) myocardial contractility impairment in acute coronary syndromes: Prevalence and impact on left ventricular systolic function recovery. Med Sci Monit. 2018;24:3693–701. doi: 10.12659/MSM.907466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo Rivera AM, Ruiz-Bailen M, Rucabado Aguilar L. Takotsubo cardiomyopathy – a clinical review. Med Sci Monit. 2011;17(6):RA135–47. doi: 10.12659/MSM.881800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shivamurthy P, Parker MW. Cardiac manifestations of myasthenia gravis: A systematic review. IJC Metabolic & Endocrine. 2014;5:3–6. [Google Scholar]
- 8.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 9.Beydoun SR, Wang J, Levine RL, Farvid A. Emotional stress as a trigger of myasthenic crisis and concomitant Takotsubo cardiomyopathy: A case report. J Med Case Rep. 2010;4:393. doi: 10.1186/1752-1947-4-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijulal S, Harikrishnan S, Namboodiri N, et al. Takotsubo cardiomyopathy in a patient with myasthenia gravis crisis: A rare clinical association. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.06.2008.0182. pii: bcr06.2008.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai M, Ukigai H, Miyata H. [A case of transient left ventricular ballooning (“Takotsubo”-shaped cardiomyopathy) developed during plasmapheresis for treatment of myasthenic crisis] Rinsho Shinkeigaku. 2004;44(3):207–10. [in Japanese] [PubMed] [Google Scholar]
- 12.Nunez-Gil IJ, Almendro-Delia M, Andres M, et al. Secondary forms of Takotsubo cardiomyopathy: A whole different prognosis. Eur Heart J Acute Cardiovasc Care. 2016;5(4):308–16. doi: 10.1177/2048872615589512. [DOI] [PubMed] [Google Scholar]
- 13.Ghadri JR, Kato K, Cammann VL, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72(8):874–82. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Limite LR, Arcari L, Cacciotti L, et al. Cardiogenic shock in Takotsubo syndrome: A clue to unravel what hides behind the curtain? JACC Heart Fail. 2019;7(2):175–76. doi: 10.1016/j.jchf.2018.11.003. [DOI] [PubMed] [Google Scholar]