Abstract
Gastrointestinal toxicity is a frequently observed adverse event during cancer treatment with traditional chemotherapeutics. Currently, traditional chemotherapeutics are often combined with targeted biologic agents. These biologics, however, possess a distinct toxicity profile, and they may also exacerbate the adverse effects of traditional chemotherapeutics. In this study, we aimed to characterize the gastrointestinal and metabolic changes after a 2-week treatment period with aflibercept, an antiangiogenic VEGFR decoy, and with erlotinib, a tyrosine-kinase inhibitor. Male rats were treated either with aflibercept or erlotinib for 2 weeks. During the 2-week treatment period, the animals in the aflibercept group received two subcutaneous doses of 25 mg/kg aflibercept. The erlotinib group got 10 mg/kg of erlotinib by oral gavage every other day. The control groups were treated similarly but received either saline injections or oral gavage of water. Intestinal toxicity was assessed by measuring intestinal permeability and by histological analyses of intestinal tissues. Metabolic changes were measured with 1H nuclear magnetic resonance in serum and urine. Neither aflibercept nor erlotinib induced changes in intestinal permeability or intestinal tissue morphology. However, aflibercept treatment resulted in stunted body weight gain and altered choline, amino acid, and lipid metabolism. Two-week treatment with aflibercept or erlotinib alone does not induce observable changes in gastrointestinal morphology and function. However, observed aflibercept-treatment related metabolic changes suggest alterations in intestinal microbiota, nutrient intake, and adipose tissue function. The metabolic changes are also interesting in respect to the systemic effects of aflibercept and their possible associations with adverse events caused by aflibercept administration.
Introduction
Gastrointestinal (GI) toxicity is a common and well-known adverse effect of chemotherapy [1]. Chemotherapeutic drugs such as 5-fluorouracil (5-FU) and irinotecan are associated with a variety of GI symptoms such as diarrhea, abdominal pain, weight loss, and infections. Overall, these symptoms may significantly affect treatment outcomes [1]. Recently, the clinical outcomes of cancer treatment have improved with the introduction of targeted biologic agents, which, however, possess a distinct profile of adverse events that can differ from those of traditional cytotoxic agents [2]. In addition, combined with chemotherapy, biologics can also exacerbate the adverse effects associated with traditional chemotherapeutics [2].
Aflibercept is an antiangiogenic biologic agent that inhibits tumor growth by blocking the formation of new blood vessels [3]. New blood vessel formation requires several circulating growth factors such as vascular endothelial growth factors (VEGFs) and placental growth factors (PIGFs) that initiate angiogenesis by binding to their receptors (VEGFRs). Aflibercept acts as a soluble VEGFR decoy that binds VEGF-A, VEGF-B, PIGF-1, and PIGF-2 and thus blocks them from activating the angiogenesis cascade [3]. Aflibercept (as ziv-aflibercept, trade name Zaltrap) is approved in combination with 5-FU, leucovorin, and irinotecan (FOLFIRI) for the treatment of metastatic colon cancer (mCRC) that is resistant to or has progressed following an oxaliplatin-containing regimen [4]. Clinical studies have shown that combining aflibercept with FOLFIRI-regimen improves overall survival in patients with mCRC compared to FOLFIRI alone [5]. However, in a phase III study by Van Cutsem et al. (2012), better clinical outcomes were also accompanied by a higher incidence of grade III and IV diarrhea in the aflibercept arm compared to the placebo arm [6]. Folprecht et al. (2016) also observed an increased incidence of grade III and IV diarrhea but not any increases in efficacy when aflibercept was added to first-line treatment with oxaliplatin and 5-FU/folinic acid (mFOLFOX6) [7]. These observations suggest that aflibercept can exacerbate the GI toxicities associated with chemotherapy.
Epidermal growth factor receptor (EGFR) pathway mediates cell proliferation and replication, and EGFR is widely expressed throughout the body. Tumors frequently overexpress EGFR, making the inhibition of the EGFR pathway an important mechanism in the treatment of several different cancers such as colorectal, lung, and pancreatic cancer [8], [9], [10], [11]. Cancer treatment regimens with EGFR pathway inhibition are based on either monoclonal antibodies against EGFR (panitumumab; a biologic agent) or on tyrosine-kinase inhibitors that selectively block EGFR activity (erlotinib; a small molecule drug). However, treatment with these agents is associated with adverse effects such as skin rash and diarrhea which may lead to dose reductions and treatment cessations [9], [12].
The primary aim of this study was to investigate the GI effects of the antiangiogenic biologic agent aflibercept and the small molecule EGFR pathway inhibitor erlotinib in rats by measuring possible changes in intestinal permeability and performing a histological examination of the intestinal tissues after a 2-week drug treatment period. We hypothesized that any alterations in GI function and possible toxicities might also be associated with changes in the global metabolome. Thus, to further assess the potential adverse effects of these drugs, we conducted a metabolic profiling of urine and serum by 1H nuclear magnetic resonance (NMR) spectroscopy.
Materials and Methods
Ethical Statement
The animal experiments conformed to the European (Directive 2010/63/EU) and Finnish (Act 2013/497 and Decree 2013/564) regulations on the protection of animals used for scientific purposes and were approved by the National Ethics Committee for Animal Procedures in Finland (project license ESAVI/114/04.10.07/2015).
Animals
A total of 48 male Hsd:Sprague–Dawley (SD) rats (Rattus norvegicus; Envigo, Udine, Italy) aged 6 weeks were used in this study. The animals were obtained and acclimatized for 18 days before the start of the experimental protocol. Health reports from the animal supplier indicated that the rats were free of known viral, bacterial, and parasitic pathogens. Upon arrival, the rats were housed under specific pathogen-free laboratory conditions using artificial lightening with a 12-hour light/dark cycle with lights on at 6 am in a temperature- (22°C ± 2°C) and humidity- (55% ± 15%) controlled room. The animals were housed in in social groups of four rats and kept in stainless-steel open cages (59.5 × 38.0 × 20 cm) with solid bottoms and filled with Aspen chips as bedding (Tapvei, Harjumaa, Estonia) and a cardboard tube for environmental enrichment. All rats were allowed free access to drinking tap water delivered in polycarbonate bottles and to a maintenance diet given ad libitum consisting of a rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories, Madison, WI). The rat colony's health status was monitored by a health monitoring program in the animal's holding room according to the Federation of European Laboratory Animal Science Associations guidelines.
Experimental Protocol
At the beginning of the protocol, the rats were 9 weeks old and their average body weight was 282 ± 14 g. The animals were block randomized to treatments and were divided into four experimental groups: 1) aflibercept control, 2) aflibercept, 3) erlotinib control, and 4) erlotinib (n = 12 per group). Baseline intestinal permeability was assessed in vivo (Measurement of Intestinal Permeability) after which the animals were administered with the experimental drugs (Drug Administration). Intestinal permeability was measured again, and urine was collected for metabolic profiling after a 14-day observation period before euthanasia. For euthanasia, the rats were fully anesthetized using isoflurane (Vetflurane 1000 mg/g, Virbac, Suffolk, UK) and subsequently exsanguinated by cardiac puncture (blood sampling for metabolic profiling) and by severing of the aorta. During the 2-week period after the first drug administrations, the animals were weighted and checked for diarrhea every other day.
Drug Administration
Rats in the aflibercept group received two subcutaneous doses of 25 mg/kg (injection volume approx. 0.3 ml) aflibercept on days 0 and 7. Vehicle for aflibercept contained sodium phosphate, sodium citrate, sodium chloride, 200 mg/ml sucrose and 1 mg/ml polysorbate 20 (provided by Sanofi-Aventis, France). Saline solution (0.9% NaCl; 1 ml/kg, injection volume approx. 0.3 ml) was used for the aflibercept control group. All injections were administered under isoflurane anesthesia. Rats in the erlotinib group were administered every other day (starting on day 0; seven doses in total) with 10 mg/kg erlotinib (Tarceva 25 mg, provided by Roche, UK) dissolved in tap water by oral gavage. The erlotinib control group was gavaged with tap water.
Measurement of Intestinal Permeability
The intestinal permeability was assessed with iohexol (Omnipaque 300, 647 mg iohexol/ml, GE Healthcare, Oslo, Norway). The animals were weighed and gavaged with 1 ml of solution containing 647 mg iohexol. After iohexol administration, the animals were immediately placed in individual metabolic cages for urine collection. After 24 hours, the amount of collected urine was measured and stored in −80°C for later analysis. Samples were discarded if fecal contamination or incomplete urine collection was observed.
Analysis of Iohexol
The urine concentration of iohexol was measured by enzyme-linked immunosorbent assay according to the manufacturer's instructions (BioPAL Inc., Worcester, MA). The percentage of excreted iohexol was calculated using the following equation:
Blood Sampling
The blood samples from the heart were collected in serum separation tubes [VenoSafe Clot Act. (Z), Terumo Europe, Leuven, Belgium] and centrifuged at 1500g for 10 minutes at 4°C. The separated serum was collected and stored in −80°C for later analysis.
Metabolic Profiling of Serum and Urine
For 1-mm proton nuclear magnetic resonance (1H NMR) analysis, 20 μl of serum was mixed with 2.5 μl of sodium-3′-trimethylsilylpropionate-2,2,3,3-d4 (TSP, 2.5 mM) in deuterium oxide (D2O). For urine samples, 2 μl of a phosphate buffer solution (0.06 M Na2HPO4/ 0.04 M NaH2PO4, pH 7) and TSP 2.5 mM were added to overcome the pH variation problem. A total of 20 μl of the mixture of each sample was then transferred into a 1-mm high-quality NMR tube individually. NMR spectra were recorded, at 310 K, on a Bruker Avance 600 spectrometer operating at 600.13-MHz with a 1-mm 1H/13C/15N TXI probe. All spectra were acquired using a standard one-dimensional pulse sequence with water suppression. Water presaturation was used during 1 second along the recycling delay for solvent signal suppression. Each free induction decay was zero-filled to 64 k points and multiplied by a 0.3-Hz exponential line broadening function before Fourier transformation. All spectra were manually phased and baseline corrected, and chemical shifts were referenced internally to alanine (at δ = 1.478 ppm) using MestReNova 8.1 (Mestrelab Research S.L., Spain). The spectra were binned into 0.01-ppm buckets between 0.5 and 9.5 ppm and mean centered for multivariate analysis and normalized to total aliphatic spectral area (0.5-4.3 ppm) to eliminate differences in metabolite total concentration. The relative concentrations of 66 and 111 regions for serum and urine, respectively, based on its metabolite's enrichment were exported to MATLAB (MathWorks, 2013a) for semiautomated in-house integration and peak-fitting routines. Using literature data and the Chenomx Profiler module (Chenomx NMR 7.6), the NMR peaks were assigned to their corresponding metabolites. Multivariable analysis was assessed for spectral regions containing contributions of a single or at most two metabolites. Data were pareto-scaled before analysis using orthogonal projection to latent structures-discriminant analysis (OPLS-DA). Score plots were used to visualize the separation of the groups, and variable importance in projection (VIP) values of OPLS-DA models >1.0 were used to determine which spectral variables most significantly contributed to the separation of the groups on the score plot.
Tissue Collection
Following euthanasia, tissue samples (1 cm) of jejunum and colon were harvested and flushed with cold PBS to remove any intestinal content. Tissue samples were then fixed in 10% neutral buffered formaldehyde (Sigma-Aldrich, St. Louis, MO) for 24-48 hours, embedded in paraffin, sectioned at 4-μm thickness, and stained with hematoxylin–eosin.
Histological Analysis
Jejunum and colon samples were evaluated, and mucosal lesions graded separately. Histological analysis was performed as previously described [13]. Briefly, a total of six change categories were assessed from jejunal samples: villous stunting, villous epithelial injury, crypt hyperplasia, crypt epithelial injury, Paneth cell injury, and leucocyte infiltration in lamina propria. Comparable six change categories were analyzed from the colon: surface epithelial injury, crypt hyperplasia, crypt dilatation and distortion, crypt epithelial injury, crypt atrophy (destruction), and leucocyte infiltration in lamina propria. Each change category was graded using a four-tier scale: minimal (1), mild (2), moderate (3), and marked (4). Histopathological assessment was done in a partly blinded manner. The reader of the slides (J.L.) was aware of the experimental design and which animals were in the same group but was unaware of the group identities.
Data Analysis
Normality of the data sets was tested with Kolmogorov–Smirnov test, and based on this analysis, statistical differences between treatment groups and their respective control groups were tested with independent-samples t test (SPSS Statistics 22.0, IBM, Armonk, NY). All data are expressed as means ± standard deviations. Differences between groups were deemed significant when P values < .05.
Results
Drug Response
Aflibercept treatment stunted body weight gain during the 2-week experiment (Figure 1A). At the end of the experiment, rats that received aflibercept had gained weight 12.3% ± 3.1% of their initial body weight, which was significantly (P = .03) less than the rats in the aflibercept control group (16.2% ± 5.1%). There were no differences in body weight gain between rats that received erlotinib and their respective controls (erlotinib control: 15.4% ± 1.7%; erlotinib: 15.4% ± 1.2%) (Figure 1B). In both treatment groups, none of the animals showed any signs of diarrhea during the experiment.
Figure 1.
Intestinal permeability to iohexol (% of administered iohexol) after a 2-week treatment with 25 mg/kg aflibercept i.p. (A) or with 10 mg/kg p.o. erlotinib (B). Aflibercept treatment induced significant stunting of body weight gain (%) compared to the control group (C). Aflibercept was administered on day 0 and day 7. There were no differences in body weight gain between erlotinib treatment group and their respective control group (D). Erlotinib was administered every other day starting from day 0. Box plots show mean with upper and lower quartiles. Whiskers show minimum and maximum. Line graphs show mean with standard deviations. (**P < .01, *P < .05).
Intestinal Permeability to Iohexol
Aflibercept treatment did not cause any changes in intestinal permeability to iohexol compared to the aflibercept control group (0.36% ± 0.09% vs. 0.44% ± 0.18%, respectively) (Figure 1C). There were no differences in iohexol permeability between erlotinib control group and erlotinib treatment group (0.46% ± 0.15% vs. 0.56% ± 0.25%, respectively) (Figure 1D). There were also no differences in baseline permeability (data not shown).
Global Metabolome Variations
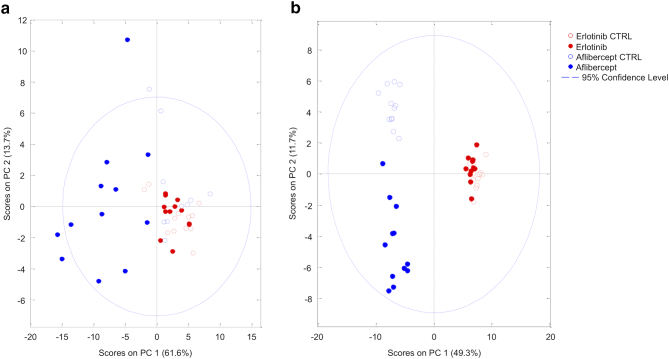
Aflibercept serum PCA showed two separate clusters (aflibercept treatment group and the control group), indicating that aflibercept induced metabolic shift in the rats' sera (Figure 2A). The erlotinib groups clustered together, suggesting no metabolic differences between the treatment and control group. Urine PCA revealed three distinct clusters (aflibercept, aflibercept control, and both erlotinib treatment and control group), suggesting that aflibercept, but not erlotinib, also caused changes in the urinary metabolome (Figure 2B). OPLS-DA discriminated well between the treatment groups and their respective controls (Figure 3, Figure 4). Relevant metabolites that contributed the most to the observed discrimination and the metabolites' levels compared to the control groups are visualized in Figure 3, Figure 4. Based on this analysis, the metabolic differences in the aflibercept group were mostly driven by decreased serum levels of multiple amino acids (tryptophan, phenylalanine, arginine, methionine, tyrosine, glutamine, threonine, and valine) and increased serum levels of very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and fatty acids moieties -CH3, =CH-CH2-CH=, and CH2-CH=C. In addition, aflibercept induced significant changes in methylamine metabolism, increasing the serum levels of N(CH3)3 (a choline moiety) while decreasing the serum levels of methylamines. In the urine, the metabolic differences between the aflibercept treatment and the control group were characterized by significant changes in tryptophan metabolism (tryptophan and its metabolites 3-indoxylsulfate and 5-hydroxyindole-3-acetate), niacin metabolism (increased excretion of trigonelline), and increased excretion of branch-chained amino acids (isoleucine, leucine, and valine) and their metabolite 2-hydroxyisovalerate. In the erlotinib treatment group, the metabolic changes were minor compared to the control group, with only a few metabolites showing significantly different resonances. Also, despite statistical significance, the biological significance of these changes is questionable considering that the observed metabolite resonances show only minor differences between the two groups (Table S2, Table S4). All identified metabolites and their resonances are listed in the Supplementary Material.
Figure 2.
Principal component analyses revealed that animals in the aflibercept group form a separate cluster in both serum (A) and urine (B) analysis, indicating aflibercept-induced alterations in serum and urine metabolome. In serum analysis, both control groups (empty shapes) and the erlotinib group (red circles) clustered together, suggesting metabolic similarity between groups. Similarly, in the urine analysis, animals in the Erlotinib group clustered together with their respective controls.
Figure 3.
OPLS-DA of serum (A) and urine (C) metabolome showed discrimination between the aflibercept group and the aflibercept control group. The OPLS-DA models were used to calculate VIP scores for each individual metabolite. VIP scores revealed the individual metabolites that were the most relevant for discrimination (VIP score >1) between the aflibercept group and the aflibercept control group (B and D). The color of the bars indicates the direction of the metabolic change relative to the control group (green = significantly increased resonances relative to control group, red = significantly decreased resonances relative to control group).
Figure 4.
OPLS-DA of serum (A) and urine (C) metabolome showed discrimination between the erlotinib group and the erlotinib control group. The OPLS-DA models were used to calculate VIP scores for each individual metabolite. VIP scores revealed the individual metabolites that were the most relevant for discrimination (VIP score >1) between the erlotinib group and the erlotinib control group (B and D). The color of the bars indicates the direction of the metabolic change relative to the control group (green = significantly increased resonances relative to control group, red = significantly decreased resonances relative to control group, gray = no significant differences in resonances between groups).
Histological Analysis
Histological analyses of the jejunum and colon showed that neither treatment impacted intestinal tissue morphology (data not shown).
Discussion
Our results show that a 2-week aflibercept treatment caused stunted body weight gain but no other clinical side effects, increased intestinal permeability, or observable changes in the histology of intestinal tissues. These findings indicate that aflibercept treatment itself does not cause GI toxicity. Thus, it seems that the reported increases in GI symptoms observed in clinical studies featuring aflibercept in combination with other chemotherapeutics [6], [7] may result from a synergic effect of angiogenesis blockade and cytotoxic insult. Also, adding aflibercept to chemotherapeutic treatment increases the risk of GI perforation [14], suggesting that aflibercept can indeed disturb normal GI function when combined with other chemotherapeutics. The exact mechanisms behind these effects are unknown, but Kamba et al. (2006) showed previously that VEGF inhibition regresses capillaries in the small intestinal villi in adult mice [15]. In addition, blocking VEGF activity decreases nitric oxide release [16], which subsequently may reduce intestinal blood flow. Hence, it is possible that aflibercept makes the intestine more susceptible to the toxic effects of traditional chemotherapeutics by decreasing the flow of oxygen and nutrients to the enterocytes. Additionally, our metabolomic analysis revealed that aflibercept treatment induced an increase in serum levels of N(CH3)3 (a choline moiety) and concomitantly decreased the levels of its degradation product trimethylamine. The formation of trimethylamine from choline is a microbial metabolism pathway [17] which would suggest treatment-induced changes in intestinal microbiota. Whether these alterations contribute to GI toxicity during cancer treatment is an interesting question warranting future studies.
Interestingly, aflibercept administration resulted in a significant stunting of body weight gain and significant alterations in amino acid and lipid metabolism. Specifically, the aflibercept-treated group exhibited decreased serum levels of multiple amino acids and increased serum levels of lipoproteins VLDL and LDL, as well as several lipid moieties, such as LDL-like lipid particles (-CH3) and polyunsaturated fatty acids (=CH-CH2-CH=). Overall, these findings are to be interpreted in the context of the systemic effects of aflibercept. Firstly, aflibercept as well as other antiangiogenetic treatments have been associated with reduced appetite in experimental animals [18], [19], [20]. In our data, this effect could be reflected in the decreased serum levels of several essential amino acids (e.g., tryptophan, phenylalanine, methionine, threonine) and in the elevated urinary levels of branched-chain amino acids and 2-hydroxyisovalerate indicating skeletal muscle protein breakdown and ketoacidosis [21], respectively. Additionally, the detected increase in serum levels of lipids moieties may result from increased fatty acid utilization under nutrient depletion. However, although decreased feed intake might explain part of our findings, in mice, decreased body weight gain seems to accompany antiangiogenic treatment independent of caloric intake [18], [19]. This suggests the induction of some additional metabolic mechanisms during antiangiogenic treatment.
The aflibercept-induced changes in serum lipids are especially interesting considering that angiogenesis is a highly active process during adipogenesis coupling angiogenic factors to lipid metabolism. For example, angiopoietin-like protein 4 (ANGPLT4) modulates angiogenesis independent of VEGF but also inhibits the activity of lipoprotein lipase (LPL), an enzyme that cleaves plasma triglycerides from VLDL and chylomicrons [22]. The inhibition of LPL results in reduced uptake of fatty acids into the adipose tissue and elevated serum levels of VLDL and fatty acids [23] similarly to our observations. Aflibercept treatment has been shown to affect adipose tissue vasculature [20], and adipose tissue hypoxia is one of the driving factors for ANGPLT4 induction [24]. Thus, possibly, by suppressing adipose tissue angiogenesis, aflibercept treatment increases the concentration of circulating fatty acids which subsequently may result in decreased appetite.
Different chemotherapy regimens have been shown to exert alterations in normal lipid metabolism possibly via mechanisms involving oxidative stress and inflammation [25], [26], [27]. Regarding the effects of anti-VEGF agents, Joerger (2010) described previously in a case report a breast cancer patient whose chemotherapy-induced hyperlipoproteinemia persisted until the cessation of monoclonal VEGF-inhibitor bevacizumab [28]. In addition, Jobard et al. (2015) have shown that a 2-week combination treatment with bevacizumab and temrolimus (an mTOR inhibitor) increases the levels of serum lipoproteins and lipids [29]. Although the authors attributed this effect to temrolimus, our results suggest that VEGF inhibition alone can increase serum lipid concentrations. In vitro studies with different glioma cell lines have also demonstrated increased levels of fatty acids and changes in choline metabolism after pharmacological treatment with VEGF receptor 2 inhibitor or with bevacizumab [30], [31]. Overall, similar metabolic alterations have also been associated with increased apoptosis in several cell lines [30], [31], [32], [33], [34]. Although aflibercept does not appear to overtly induce apoptosis in cell models [35], [36], [37], [38], the metabolic shifts observed in our study could reflect aflibercept-mediated in vivo tissue hypoxia and oxidative stress that drive apoptotic processes. In addition, aflibercept has also been shown to enhance the generation of reactive oxygen species during oxidative stress [39] as well as promote the expression of inflammatory mediators in vascular endothelial cells [40]. Clinically, these findings suggest that aflibercept might exacerbate the oxidative stress of chemotherapy which contributes to the increased incidence of adverse effects observed during cancer treatment with antiangiogenic agents.
We did not observe any physiological changes in the erlotinib group. Additionally, the metabolic alterations were also minimal. Previously, Fan et al. (2014) reported that daily oral dosing of 18 mg/kg erlotinib stunts body weight gain in C57BL/6J mice as well as induces histological changes and inflammatory signals in murine intestine [12]. On the other hand, Higgins et al. (2004) administered tumor-bearing nu/nu-nuBR nude mice with 25 mg/kg of erlotinib daily for 3 weeks and reported no changes in body weight or other toxicities [41]. Similarly, even daily erlotinib doses up to 100 mg/kg for 2 weeks seem well tolerated in mice [42]. Thus, it is possible that the erlotinib dose and treatment duration in our study were not sufficient to induce any observable toxicities. Nevertheless, more research is needed to elucidate the pathophysiological mechanisms behind erlotinib-induced GI toxicity.
In conclusion, our results show that a 2-week aflibercept or erlotinib administration does not cause changes in intestinal permeability or induce notable histological damage in the intestine. However, aflibercept treatment stunted body weight gain and caused significant alterations in choline, amino acid, and lipid metabolism. These findings are interesting in respect to the systemic effects of aflibercept and their possible associations with adverse events associated with aflibercept administration.
The following are the supplementary data related to this article.
All Identified Serum Metabolites with Their Respective Chemical Shift Regions.
All Identified Serum Metabolites with Their Respective Chemical Shift Regions.
All Identified Urine Metabolites with Their Respective Chemical Shift Regions.
All Identified Urine Metabolites with Their Respective Chemical Shift Regions.
Acknowledgments
Acknowledgements
The study received funding from the Cancer Society of Finland, Finska Läkaresällskapet, Foundation of Clinical Chemistry Research, and Biomedicum Helsinki Foundation. We gratefully acknowledge the Ministry of Economy and Competitiveness of Spain (SAF2014-52875R) and Instituto de Salud Carlos III and FEDER (grant number PIE15/00013) for co-funding the study. We also gratefully acknowledge the Ministry of Science, Innovation and Universities (PCIN-2017-117) and the EU Joint Programming Initiative ‘A Healthy Diet for a Healthy Life’ (JPI HDHL INTIMIC-085). M. C. C. would like to acknowledge the support of the Spanish Government of Economy and Competitiveness (MINECO, ref. AGL2015-707487-P). We are most grateful to Sari Laakkonen, Päivi Leinikka, Hanne Salmenkari, and Aino Siltari for their skillful assistance with the animal work. We also thank Kaisa Aaltonen, Kirsi Laukkanen, and Professor Satu Sankari for their expertise with the technical analyses. We also thank Sanofi and Roche for providing us the drugs used in this study.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Richard A. Forsgård, Email: richard.forsgard@helsinki.fi.
Vannina G Marrachelli, Email: vannina.gonzalez@uv.es.
Jere Lindén, Email: jere.linden@helsinki.fi.
Rafael Frias, Email: rafael.frias@ki.se.
Maria Carmen Collado, Email: mcolam@iata.csic.es.
Riitta Korpela, Email: riitta.korpela@helsinki.fi.
Daniel Monleon, Email: daniel.monleon@uv.es.
Thomas Spillmann, Email: thomas.spillmann@helsinki.fi.
Pia Österlund, Email: pia.osterlund@helsinki.fi.
References
- 1.Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179–192. doi: 10.1136/gutjnl-2011-300563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grenon NN. Managing toxicities associated with antiangiogenic biologic agents in combination with chemotherapy for metastatic colorectal cancer. Clin J Oncol Nurs. 2013;17:425–433. doi: 10.1188/13.CJON.425-433. [DOI] [PubMed] [Google Scholar]
- 3.Ciombor KK, Berlin J, Chan E. Aflibercept. Clin Cancer Res. 2013;19:1920–1925. doi: 10.1158/1078-0432.CCR-12-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regeneron Pharmaceuticals/Sanofi-Aventis U.S. (2012) ZALTRAP (ziv-aflibercept) injection for intravenous infusion prescribing information. http://products.sanofi.us/zaltrap/zaltrap.html. Accessed 2 Aug 2016
- 5.Scartozzi M, Vincent L, Chiron M, Cascinu S. Aflibercept, a new way to target angiogenesis in the second line treatment of metastatic colorectal cancer (mCRC) Target Oncol. 2016;11:489–500. doi: 10.1007/s11523-016-0447-4. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 7.Folprecht G, Pericay C, Saunders MP, Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim J-S, Hofheinz R-D. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol. 2016;27:1273–1279. doi: 10.1093/annonc/mdw176. [DOI] [PubMed] [Google Scholar]
- 8.Bareschino MA, Schettino C, Troiani T, Martinelli E, Morgillo F, Ciardiello F. Erlotinib in cancer treatment. Ann Oncol. 2007;18(Suppl 6):vi35–41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- 9.You B, Chen EX. Anti-EGFR monoclonal antibodies for treatment of colorectal cancers: development of cetuximab and panitumumab. J Clin Pharmacol. 2012;52:128–155. doi: 10.1177/0091270010395940. [DOI] [PubMed] [Google Scholar]
- 10.Tournigand C, Chibaudel B, Samson B, Scheithauer W, Vernerey D, Mésange P, Lledo G, Viret F, Ramée J-F, Tubiana-Mathieu N. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2015;16:1493–1505. doi: 10.1016/S1470-2045(15)00216-8. [DOI] [PubMed] [Google Scholar]
- 11.Weickhardt AJ, Price TJ, Chong G, Gebski V, Pavlakis N, Johns TG, Azad A, Skrinos E, Fluck K, Dobrovic A. Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the phase II DUX study in chemotherapy-refractory, advanced colorectal cancer. J Clin Oncol. 2012;30:1505–1512. doi: 10.1200/JCO.2011.38.6599. [DOI] [PubMed] [Google Scholar]
- 12.L Fan L Hu, Yang B, Fang X, Gao Z, Li W, Sun Y, Y Shen X Wu, Shu Y. Erlotinib promotes endoplasmic reticulum stress-mediated injury in the intestinal epithelium. Toxicol Appl Pharmacol. 2014;278:45–52. doi: 10.1016/j.taap.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Forsgård RA, Korpela R, Holma R, Lindén J, Frias R, Spillmann T, Österlund P. Intestinal permeability to iohexol as an in vivo marker of chemotherapy-induced gastrointestinal toxicity in Sprague-Dawley rats. Cancer Chemother Pharmacol. 2016;78:863–874. doi: 10.1007/s00280-016-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi W-X, Shen F, Qing Z, Xiao-Mao G. Risk of gastrointestinal perforation in cancer patients treated with aflibercept: a systematic review and meta-analysis. Tumour Biol. 2014;35:10715–10722. doi: 10.1007/s13277-014-2369-z. [DOI] [PubMed] [Google Scholar]
- 15.Kamba T, Tam BYY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 17.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 19.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(2012) Assessment report: Zaltrap
- 21.Landaas S, Jakobs C. The occurrence of 2-hydroxyisovaleric acid in patients with lactic acidosis and ketoacidosis. Clin Chim Acta. 1977;78:489–493. doi: 10.1016/0009-8981(77)90082-1. [DOI] [PubMed] [Google Scholar]
- 22.La Paglia L, Listì A, Caruso S, Amodeo V, Passiglia F, Bazan V, Fanale D. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. 2017. https://www.hindawi.com/journals/ppar/2017/8187235/ PPAR Res; In. [DOI] [PMC free article] [PubMed]
- 23.Dijk W, Kersten S. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 2014;25:146–155. doi: 10.1016/j.tem.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 24.González-Muniesa P, de Oliveira C, Pérez de Heredia F, Thompson MP, Trayhurn P. Fatty acids and hypoxia stimulate the expression and secretion of the adipokine ANGPTL4 (angiopoietin-like protein 4/ fasting-induced adipose factor) by human adipocytes. J Nutr Nutr. 2011;4:146–153. doi: 10.1159/000327774. [DOI] [PubMed] [Google Scholar]
- 25.Madssen TS, Thune I, Flote VG, Lundgren S, Bertheussen GF, Frydenberg H, Wist E, Schlichting E, Schäfer H, Fjøsne HE. Metabolite and lipoprotein responses and prediction of weight gain during breast cancer treatment. Br J Cancer. 2018;119:1144–1154. doi: 10.1038/s41416-018-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma M, Tuaine J, McLaren B, Waters DL, Black K, Jones LM, McCormick SPA. Chemotherapy agents alter plasma lipids in breast cancer patients and show differential effects on lipid metabolism genes in liver cells. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Wang Z, Wang F, Lei X, Yan S, Wang D, Zhang F, Xu R, Wang L, Li Y. Predictive value of chemotherapy-related high-density lipoprotein cholesterol (HDL) elevation in patients with colorectal cancer receiving adjuvant chemotherapy: an exploratory analysis of 851 cases. Oncotarget. 2016;7:57290–57300. doi: 10.18632/oncotarget.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joerger M, Riesen WF, Thürlimann B. Bevacizumab-associated hyperlipoproteinemia type IIb in a patient with advanced invasive-ductal breast cancer. J Oncol Pharm Pract. 2011;17:292–294. doi: 10.1177/1078155210378912. [DOI] [PubMed] [Google Scholar]
- 29.Jobard E, Blanc E, Négrier S, Escudier B, Gravis G, Chevreau C, Elena-Herrmann B, Trédan O. A serum metabolomic fingerprint of bevacizumab and temsirolimus combination as first-line treatment of metastatic renal cell carcinoma. Br J Cancer. 2015;113:1148–1157. doi: 10.1038/bjc.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesti T, Savarin P, Triba MN, Le Moyec L, Ocvirk J, Banissi C, Carpentier AF. Metabolic impact of anti-angiogenic agents on U87 glioma cells. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0099198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesti T, Bouchemal N, Banissi C, Triba MN, Marbeuf-Gueye C, Cemazar M, Moyec LL, Carpentier AF, Savarin P, Ocvirk J. Nuclear magnetic resonance metabolic fingerprint of bevacizumab in mutant IDH1 glioma cells. Radiol Oncol. 2018;52:392–398. doi: 10.2478/raon-2018-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankenberg FG, Storrs RW, Naumovski L, Goralski T, Spielman D. Detection of apoptotic cell death by proton nuclear magnetic resonance spectroscopy. Blood. 1996;87:1951–1956. [PubMed] [Google Scholar]
- 33.Delikatny EJ, Cooper WA, Brammah S, Sathasivam N, Rideout DC. Nuclear magnetic resonance-visible lipids induced by cationic lipophilic chemotherapeutic agents are accompanied by increased lipid droplet formation and damaged mitochondria. Cancer Res. 2002;62:1394–1400. [PubMed] [Google Scholar]
- 34.Maurmann L, Belkacemi L, Adams NR, Majmudar PM, Moghaddas S, Bose RN. A novel cisplatin mediated apoptosis pathway is associated with acid sphingomyelinase and FAS proapoptotic protein activation in ovarian cancer. Apoptosis. 2015;20:960–974. doi: 10.1007/s10495-015-1124-2. [DOI] [PubMed] [Google Scholar]
- 35.Schnichels S, Hagemann U, Januschowski K, Hofmann J, Bartz-Schmidt K-U, Szurman P, Spitzer MS, Aisenbrey S. Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br J Ophthalmol. 2013;97:917–923. doi: 10.1136/bjophthalmol-2013-303130. [DOI] [PubMed] [Google Scholar]
- 36.Saenz-de-Viteri M, Fernández-Robredo P, Hernández M, Bezunartea J, Reiter N, Recalde S, García-Layana A. Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochem Pharmacol. 2016;103:129–139. doi: 10.1016/j.bcp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Costa de Andrade G, Wertheimer C, Eibl K, Wolf A, Kampik A, Buchele Rodrigues E, Farah ME, Haritoglou C. Viability of primary human pigment epithelium cells and Muller-glia cells after intravitreal ziv-aflibercept and aflibercept. Ophthalmology. 2016;236:223–227. doi: 10.1159/000452677. [DOI] [PubMed] [Google Scholar]
- 38.Ammar DA, Mandava N, Kahook MY. The effects of aflibercept on the viability and metabolism of ocular cells in vitro. Retina. 2013;33:1056–1061. doi: 10.1097/IAE.0b013e31827b646d. [DOI] [PubMed] [Google Scholar]
- 39.Ranjbar M, Brinkmann MP, Zapf D, Miura Y, Rudolf M, Grisanti S. Fc receptor inhibition reduces susceptibility to oxidative stress in human RPE cells treated with bevacizumab, but not aflibercept. Cell Physiol Biochem. 2016;38:737–747. doi: 10.1159/000443030. [DOI] [PubMed] [Google Scholar]
- 40.Arnott C, Punnia-Moorthy G, Tan J, Sadeghipour S, Bursill C, Patel S. The vascular endothelial growth factor inhibitors ranibizumab and aflibercept markedly increase expression of atherosclerosis-associated inflammatory mediators on vascular endothelial cells. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins B, Kolinsky K, Smith M, Beck G, Rashed M, Adames V, Linn M, Wheeldon E, Gand L, Birnboeck H. Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in combination in human non-small cell lung cancer tumor xenograft models. Anti-Cancer Drugs. 2004;15:503–512. doi: 10.1097/01.cad.0000127664.66472.60. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Takayama K, Wang S, Shiraishi Y, Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74:1297–1305. doi: 10.1007/s00280-014-2610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All Identified Serum Metabolites with Their Respective Chemical Shift Regions.
All Identified Serum Metabolites with Their Respective Chemical Shift Regions.
All Identified Urine Metabolites with Their Respective Chemical Shift Regions.
All Identified Urine Metabolites with Their Respective Chemical Shift Regions.