Abstract
Background
Spontaneous intracerebral hemorrhage (ICH) is a devastating disease with high mortality rate. This study aimed to predict hematoma expansion in spontaneous ICH from routinely available variables by using support vector machine (SVM) method.
Methods
We retrospectively reviewed 1157 patients with spontaneous ICH who underwent initial computed tomography (CT) scan within 6 h and follow-up CT scan within 72 h from symptom onset in our hospital between September 2013 and August 2018. Hematoma region was manually segmented at each slice to guarantee the measurement accuracy of hematoma volume. Hematoma expansion was defined as a proportional increase of hematoma volume > 33% or an absolute growth of hematoma volume > 6 mL from initial CT scan to follow-up CT scan. Univariate and multivariate analyses were performed to assess the association between clinical variables and hematoma expansion. SVM machine learning model was developed to predict hematoma expansion.
Findings
246 of 1157 (21.3%) patients experienced hematoma expansion. Multivariate analyses revealed the following 6 independent factors associated with hematoma expansion: male patient (odds ratio [OR] = 1.82), time to initial CT scan (OR = 0.73), Glasgow Coma Scale (OR = 0.86), fibrinogen level (OR = 0.72), black hole sign (OR = 2.52), and blend sign (OR = 4.03). The SVM model achieved a mean sensitivity of 81.3%, specificity of 84.8%, overall accuracy of 83.3%, and area under receiver operating characteristic curve (AUC) of 0.89 in prediction of hematoma expansion.
Interpretation
The designed SVM model presented good performance in predicting hematoma expansion from routinely available variables.
Fund
This work was supported by Health Foundation for Creative Talents in Zhejiang Province, China, Natural Science Foundation of Zhejiang Province, China (LQ15H180002), the Science and Technology Planning Projects of Wenzhou, China (Y20180112), Scientific Research Staring Foundation for the Returned Overseas Chinese Scholars of Ministry of Education of China, and Project Foundation for the College Young and Middle-aged Academic Leader of Zhejiang Province, China. The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Keywords: Spontaneous intracerebral hemorrhage, Hematoma, CT, Stroke, Support vector machine
Research in context.
Evidence before this study
Spontaneous intracerebral hemorrhage is a devastating disease with high mortality rate, and it accounts for 10–15% of all cases of stroke. Previous research shows that hematoma volume is a powerful and easy-to-use determinant of 30-day mortality, and hematoma expansion is associated with poor outcomes. Reducing hematoma growth and mortality is the current focus of clinical trials. Therefore, successfully predicting hematoma expansion is necessary and valuable for therapeutic intervention in patients with ICH. Many clinical variables have been used for the prediction of hematoma expansion; however, the prediction performance is generally not good.
Added value of this study
By combining different variables, including patients' demographic parameters, clinical status, laboratory test parameters, and image signs, we developed a prediction model by using support vector machine method. Our model demonstrated a good performance in prediction of hematoma expansion.
Implications of all the available evidence
Support vector machine may provide a valuable tool in prediction of hematoma expansion for patients with spontaneous intracerebral hemorrhage. Our prediction model may be practical and widely applicable, especially in many institutions where CTA is not readily available or routinely performed for spontaneous intracerebral hemorrhage patients.
Alt-text: Unlabelled Box
1. Introduction
Spontaneous intracerebral hemorrhage (ICH) refers to bleeding within the brain parenchyma that may extend into the ventricles or subarachnoid space [1]. As a devastating disease with high mortality rate, it accounts for 10–15% of all cases of stroke [1]. Although modern medical science and technology have been developing fast, a safe and effective therapeutic method for ICH is elusive, and the overall mortality of ICH remains high. Hematoma volume is a powerful and easy-to-use determinant of 30-day mortality [2], and hematoma expansion is associated with poor outcomes [[3], [4], [5]]. Reducing hematoma growth and mortality is the current focus of clinical trials, such as INTERACT [6], ATACH-II [7], STOP-AUST [8], FAST [9], STOP-IT [10], and SPOTLIGHT [10]. Because hematoma expansion is a treatment target of clinical interventions in patients with ICH, it is meaningful to predict hematoma expansion.
Various factors of hematoma expansion have been determined in recent decades, including initial hematoma volumes [2], anticoagulation use [11], alcohol consumption [12], time interval between onset and admission [13], computed tomography (CT) imaging findings such as black hole sign [5] and spot sign [8], and laboratory parameters such as platelet counts and fibrinogen levels [12]. However, accurately predicting hematoma expansion remains challenging because of the complex association between these risk factors and hematoma expansion [14]. Machine learning technique may be used to solve this problem. As a promising supervised machine learning technique, support vector machine (SVM) is capable of learning from the observing data sets, devising complex models to capture the intrinsic relationships between input and output variables, and making data-driven predictions or decisions, and it has been successfully applied in multiple biomedicine fields and demonstrated great performance [15,16].
In this study, we aimed to predict hematoma expansion in spontaneous ICH from routinely available variables using SVM method.
2. Materials and methods
2.1. Patients and clinic variables
This study was approved by our institutional ethics committee, and written informed consent was waived. We retrospectively reviewed patients aged >18 years who had spontaneous ICH in our hospital between September 2013 and August 2018. A total of 8722 patients were identified from the radiological information system by use of the search key words “hemorrhage” and “hematoma”, and they were eligible for the study if the initial and follow-up CT scans were performed within 6 and 72 h after symptom onset, respectively. Patients were excluded from the study if they had hemorrhage due to cerebral aneurysm, arteriovenous malformation, brain tumor, traumatic brain injury, or hemorrhagic infarction. Those receiving anticoagulation or antiplatelet therapy or those underwent surgical intervention before the follow-up CT scan were also excluded from the study. Finally, a total of 1157 patients were enrolled in this study. For the purpose of model validation, additional independent dataset for testing was collected from a different affiliated hospital of our university between March 2017 and August 2018. After exclusion from 1960 patients, 105 patients were included as the additional test dataset.
After admission, patients' age, sex, and neurological findings were recorded. Parameters of laboratory blood test, including glucose, white blood cell count, platelet count, hemoglobin, red blood cell, albumin, alanine transaminase, aspartate transaminase, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, international normalized ratio, and fibrinogen etc., were collected. In addition, Glasgow Coma Score, history of hemorrhage, history of hypertension, history of infarction, history of diabetes mellitus, and smoking and drinking habits were documented.
2.2. Image acquisition
Noncontrast CT scan images were acquired by using a 64-channel multidetector CT scanner (LightSpeed VCT 64; GE Medical Systems, Milwaukee, WI, USA) with a slice thickness of 5 mm and a reconstruction interval of 5 mm, or a 16-channel multidetector CT scanner (BrightSpeed; GE Medical Systems, Milwaukee, WI, USA) with a slice thickness of 5 mm and a reconstruction interval of 5 mm. Patient orientation was head first. Reconstruction algorithm was ‘Stnd’. The image matrix size was 512 × 512.
2.3. Radiological characterization and definition of hematoma expansion
Hematoma region in each slice was manually segmented, and corresponding hematoma volume was measured on initial and follow-up CT scans using GE Healthcare Advantage Workstation 4.6. Hematoma expansion was defined as a proportional increase of hematoma volume > 33% or an absolute growth of hematoma volume > 6 mL from initial CT scan to follow-up CT scan [14,17,18].
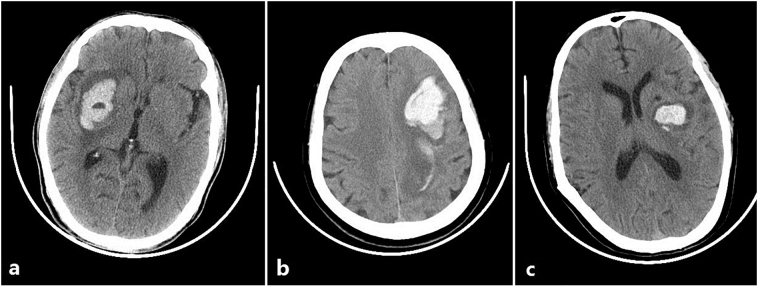
Radiological characteristics were independently evaluated by two experienced radiologists who were blinded to the clinical information of patients. Midline shift [19] in the CT slice with the maximum hematoma area was measured on GE Healthcare Advantage Workstation 4.6. CT image findings, including intraventricular extension [20], black hole sign [5], blend sign [21], and satellite sign [22] were assessed on initial CT scan. Fig. 1 shows typical examples of black hole sign, blend sign, and satellite sign.
Fig. 1.
Illustration of CT image findings: (a) Black hole sign; (b) Blend sign; (c) Satellite sign.
2.4. Support vector machine
In recent years, SVM has been introduced to solve various biomedicine problems [23,24]. As a supervised machine learning method, SVM aims to classify data points by maximizing the margin between classes. It can be used for non-linear classification using kernel trick [23], implicitly mapping the inputs into high-dimension feature spaces using different kernel function. In this study, the radial basis function (RBF) kernel function was adopted. Of the included 1157 patients, 925 random patients (80%) were selected for training and the remaining 232 patients (20%) for testing. The recursive feature elimination method was applied for feature selection using the training dataset. The input features can be recorded in the formats of ‘xlsx’ or ‘cvs’ and uploaded into the SVM model. Randomized search strategy was implemented for tuning parameters of our classifier, and a 10-fold cross-validation approach was used during the training. Parameters C and γ were determined to be 9.222 and 0.009, respectively. An interpreted, high-level, and open-source programming language Python 2.7 and an efficient data mining tool Scikit-learn 0.20.1 were used for machine learning coding [25].
The performance of SVM was evaluated by sensitivity, specificity, overall accuracy, and area under the receiver operating characteristic curve (AUC). Overall accuracy is the ratio of correctly predicted patients.
2.5. Statistical analysis
Statistical analysis was performed using the software package IBM SPSS 22.0 (IBM SPSS, Armonk, New York, USA). Continuous variables were presented as means ± standard deviations and categorical variables as frequency (percentage). Student t-tests or Mann-Whitney U tests were applied for comparison of continuous variables, and χ2 tests or Fisher exact tests were used for comparison of categorical variables, as appropriate. It was of great significance to understand the relationship between independent variables and the dependent variable in clinics. The multivariate logistic analysis could be used to determine the independent risk factors and obtain odds ratio in the presence of more than one explanatory variable [26]. Multivariate logistic regression model with a forward method was performed to identify factors that were independently associated with hematoma expansion. Baseline variables clinically relevant with outcome with a P-value < .05 on univariate analysis were considered in the multivariate logistic regression analysis. Variables with >5% missing values were not involved in the multivariate logistic regression analysis. Given the number of events available, the variables for inclusion were determined to ensure parsimony of the final logistic regression model. Note that feature selection was applied to select a subset of relevant feature for use in the machine learning model for shorting training time, avoiding the curse of dimensionality, and simplifying models [27]. The selected features were not necessary to be exactly the same as the independent risk factors from multivariate analysis.
3. Results
Of the included 1157 patients with ICH, 246 (21.3%) experienced hematoma expansion. The mean age was 61.6 ± 12.8 years (range 19–95) and 760 patients (65.7%) were male. The incidence of hematoma expansion in female was lower than that in male patients (59 of 397 [14.9%] vs 187 of 760 [24.6%]). Of the validation patients, there were 67 men and 38 women; their mean age was 60.4 ± 14.0 years; 19 patients had hematoma expansion.
A comparison of variables between expanders and nonexpanders is provided in Table 1. Hematoma expansion occurred more commonly in male patients (P < .001), and it was associated with shorter time from symptom onset to initial CT scan (2.6 ± 1.3 vs. 3.2 ± 1.4 h, P < .001) and larger mean hematoma volume on the initial CT scan (26.83 ± 18.64 vs. 19.84 ± 15.03 mL, P < .001). Laboratory examine parameters, including platelet, hemoglobin, red blood cell count, albumin, fibrinogen, total cholesterol, and LDL-C were statistically different between expanders and nonexpanders. CT imaging findings, including black hole sign, blend sign, and satellite sign, were more commonly found in expanders than in nonexpanders (all P < .001). Midline shift was larger in patients with hematoma expansion than those without (P < .001). The multivariate logistic regression analysis (Table 2) revealed that male patient, time to initial CT, Glasgow Coma Scale, fibrinogen level, black hole sign, and blend sign were independently associated with hematoma expansion.
Table 1.
Comparison of variables between expanders and nonexpanders.
| Clinical variables | Expander (246) | Nonexpander (911) | P Value |
|---|---|---|---|
| Men | 187 (76.0%) | 573 (62.9%) | <.001 |
| Age (years) | 61.0 ± 12.9 | 61.7 ± 12.8 | .408 |
| Glasgow Coma Score a | 10.9 ± 3.5 | 12.4 ± 3.1 | <.001 |
| Time to initial CT scan (h) | 2.6 ± 1.3 | 3.2 ± 1.4 | <.001 |
| Reexamine time (h) | 16.0 ± 14.4 | 23.5 ± 15.9 | <.001 |
| Platelet (×109/L) b | 197 ± 64 | 209 ± 76 | .026 |
| Hemoglobin (g/L) b | 142 ± 19 | 138 ± 16 | .001 |
| White blood cell (×109/L) b | 9.49 ± 4.01 | 9.94 ± 3.70 | .066 |
| Red blood cell (×1012/L) b | 4.61 ± 0.57 | 4.50 ± 0.56 | .005 |
| Glucose (mmol/L) b | 7.74 ± 2.65 | 7.77 ± 2.68 | .860 |
| International normalized ratio c | 1.05 ± 0.27 | 1.01 ± 0.15 | .014 |
| Fibrinogen (g/L) c | 3.15 ± 0.81 | 3.50 ± 1.03 | <.001 |
| Albumin (g/L) d | 39.1 ± 5.3 | 40.0 ± 5.5 | .043 |
| Alanine transaminase (U/L) e | 31 ± 20 | 29 ± 32 | .365 |
| Aspartate transaminase (U/L) f | 33 ± 19 | 30 ± 22 | .130 |
| Total cholesterol (mmol/L) g | 4.95 ± 1.48 | 5.29 ± 1.28 | .002 |
| Triglycerides (mmol/L) g | 1.57 ± 1.34 | 1.67 ± 1.44 | .346 |
| HDL-C (mmol/L) g | 1.26 ± 0.35 | 1.29 ± 0.59 | .476 |
| LDL-C (mmol/L) g | 2.90 ± 1.14 | 3.17 ± 0.99 | .001 |
| Baseline hematoma volume (mL) | 26.83 ± 18.64 | 19.84 ± 15.03 | <.001 |
| Location of hemorrhage | .279 | ||
| Deep gray matter | 205 (83.3%) | 745 (81.8%) | |
| Lobar regions | 20 (8.1%) | 78 (8.6%) | |
| Cerebellum | 5 (2.0%) | 44 (4.8%) | |
| Brain stem | 6 (2.4%) | 20 (2.2%) | |
| Multiple locations | 10 (4.1%) | 24 (2.6%) | |
| Intraventricular extension | 101 (41.1%) | 328 (36.0%) | .145 |
| Black hole sign | 68 (27.6%) | 111 (12.2%) | <.001 |
| Blend sign | 88 (35.8%) | 115 (12.6%) | <.001 |
| Satellite sign | 147 (59.8%) | 429 (47.1%) | <.001 |
| Midline shift (mm) h | 4.17 ± 2.95 | 3.38 ± 2.57 | <.001 |
| History of hemorrhage | 15 (6.1%) | 31 (3.4%) | .055 |
| History of infarction | 8 (3.3%) | 42 (4.5%) | .353 |
| History of diabetes mellitus | 23 (9.3%) | 100 (11.0%) | .586 |
| History of hypertension | 176 (71.5%) | 728 (79.9%) | .005 |
| Smoking | 71 (28.9%) | 253 (27.8) | .735 |
| Drinking | 76 (30.9%) | 248 (27.2%) | .255 |
Note: HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol.
30/1157 (2.6%) missing values.
4/1157 (<1.0%) missing values.
7/1157 (<1.0%) missing values.
146/1157 (12.6%) missing values.
88/1157 (7.6%) missing values.
112/1157 (9.7%) missing values.
185/1157 (16.0%) missing values.
28/1157 (2.4%) missing values.
Table 2.
Results of multivariate logistic regression analysis.
| Variable | β coefficient† | OR | 95% CI | P-value |
|---|---|---|---|---|
| Men | 0.60 ± 0.19 | 1.82 | 1.26–2.63 | .001 |
| Time to initial CT scan | −0.32 ± 0.06 | 0.73 | 0.65–0.83 | <.001 |
| Glasgow Coma Score | −0.15 ± 0.03 | 0.86 | 0.82–0.90 | <.001 |
| Fibrinogen level | −0.33 ± 0.10 | 0.72 | 0.59–0.87 | .001 |
| Black Hole sign | 0.93 ± 0.21 | 2.52 | 1.69–3.78 | <.001 |
| Blend sign | 1.39 ± 0.19 | 4.03 | 2.77–5.85 | <.001 |
Note: OR = odds ratio, CI = confidence interval.
Values are means ± standard errors.
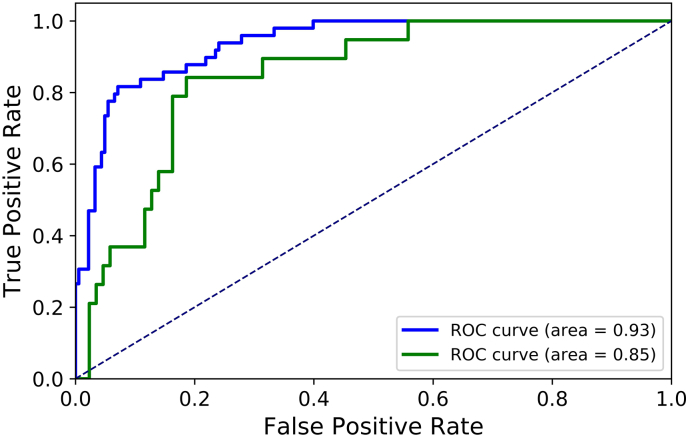
Nine features were selected as the input of SVM model, including fibrinogen level, sex, Glasgow Coma Score, time to initial CT scan, black hole sign, blend sign, satellite sign, midline shift, and baseline hematoma volume. Table 3 demonstrates the prediction results for the two independent test datasets using the developed SVM model. For the first independent test dataset, the sensitivity was 83.7%, the specificity was 85.8%, and the overall accuracy was 85.3%, respectively. For the additional independent test dataset, the sensitivity was 78.9%, the specificity was 83.7%, and the overall accuracy was 81.3%. Fig. 2 illustrates the ROC curves for the two independent test datasets. The AUC values for the internal and external test datasets were 0.93 and 0.85, respectively. Thus, the mean values of sensitivity, specificity, overall accuracy, AUC for the two test datasets were 81.3%, 84.8%, 83.3%, and 0.89, respectively.
Table 3.
Prediction results for two independent test datasets.
| Actual class | Predicted class |
||
|---|---|---|---|
| Expander | Nonexpander | % correct | |
| (a) Test dataset 1 | |||
| Expander | 41 | 8 | 83.7% |
| Nonexpander | 26 | 157 | 85.8% |
| Overall | 85.3% | ||
| (b)Test dataset 2 | |||
| Expander | 15 | 4 | 78.9% |
| Nonexpander | 14 | 72 | 83.7% |
| Overall | 81.3% | ||
Fig. 2.
Receive operating characteristic curves for two independent test datasets.
4. Discussion
In the present study, the association between many clinical variables and hematoma expansion were assessed and the independent predictors were determined by multivariate logistic regression analysis from 1157 patients with spontaneous ICH. A SVM model was developed and applied to predict hematoma expansion, and a good performance was achieved.
The incidence of hematoma expansion varies from 13% to 34% across different studies [3,14,28], most likely because of different definitions of hematoma expansion, time to initial CT scan, and hematoma volume measurement. In our study hematoma expansion occurred in 21.3% patients with ICH. Using same definition of hematoma expansion and time to initial CT scan as ours, 33.7% patients with ICH had hematoma expansion in the study of Li et al. [28]. Accuracy of hematoma volume measurement is also crucial for hematoma expansion analysis. Methods of ABC/2 and modified ABC/2 have been developed for hematoma volume measurement [2,5,29]; however, these techniques should be used with caution, especially in calculation of irregular and discontinuous hematomas [30]. Hematoma region in our study was manually drawn slice by slice, and corresponding volume was calculated after 3-dimensional reconstruction. Although our volume measurement is relatively time-consuming, hematoma volume measurement may be more accurate.
This study revealed that 6 factors were independently associated with hematoma expansion. Male patient was an independent predictor. Hematoma expansion occurred more often in male patients. Our study included a Chinese population, and only a few of the women had smoking and drinking habits because of the Chinese tradition. Smoking and drinking were potential risk factors of hematoma expansion [12,31]. The sex difference of hematoma expansion may be caused by the difference of living habits between men and women. The time interval from onset to baseline CT was confirmed as a significant predictor of hematoma expansion in our study. Previous research reported that the incidence of hematoma expansion decreased as the time interval from onset to baseline CT increased. Patients admitted earlier after symptom onset might have a relatively high possibility of undergoing a CT scan before the stabilization of hematoma growth [12]. Glasgow Coma Score provides a reliable and objective way of recording the conscious state of a patient for initial as well as subsequent assessment. Patients with disturbed consciousness had a likelihood of having hematoma expansion [12]. Our study demonstrated that expanders had lower Glasgow Coma Score than nonexpanders. Fibrinogen plays a significant role in platelet aggregation and glycoprotein IIa/IIIb on the surface of platelets requires fibrinogen for aggregation, and low levels of fibrinogen might be associated with an impairment of hemostasis [12]. Black hole sign and blend sign have been proved to be significantly correlated with hematoma expansion recently [5,21]. Our study further confirmed these findings. Both black hole sign and blend density sign imply the heterogeneity of hematoma. The presence of the two signs within hematoma may reflect different age of bleeding; fresh blood shows hypoattenuating on nonenhanced CT scan; after clot retraction, serum sequesters out of hematoma, which make the bleed hyperintense on CT scan [32].
Various variables have been used to predict hematoma expansion. Quantitative CT densitometry values of ICH density distribution were adopted for predicting intracerebral hemorrhage growth, and AUC of 0.73 was obtained with a conventional binary logistic regression model incorporating baseline hematoma volume, time-to-scan, and coefficient of variation of ICH attenuation [33]. A novel imaging sign, black hole, was found to be independent predictor, based on which a sensitivity of 32% was achieved in predicting early hematoma growth [5]. More recently, island sign was proposed for predicting early hematoma expansion and poor outcome in patients with ICH; corresponding prediction sensitivity of the island sign was 45% [28]. The spot sign of computed tomography angiography (CTA) was well-established imaging marker of hematoma expansion; a recent prospective observational study [34] reported that sensitivity of the spot sign in predicting hematoma expansion was 51%. In addition to CTA spot sign, leakage sign on delayed-phase CT after CTA was valuable to predict hematoma expansion, and corresponding sensitivity was even as high as 93% [35]. However, delayed-phase CT after CTA is not a routine examination; an additional CT scan was required to confirm the leakage sign and patient would suffer greater radiation exposure [35]. Moreover, contrast administration may be not applicable for patients with kidney disease or severe diabetes because X-ray contrast medium can further harm kidney function [5,36]. Machine learning is capable of effectively learning from training data to make accurate predictions or decisions on new data, and has been successfully applied for hemorrhage detection in CT scans [37], outcome prediction of patients with subarachnoid hemorrhage [38], and mortality prediction of spontaneous ICH [39]. The novelty of our work was the use of SVM machine learning technique to predict hematoma expansion from routinely available variables. Our model achieved an average sensitivity of 81.3%, a specificity of 84.8%, and an AUC of 0.89, which indicated that our model presented good performance in prediction of hematoma expansion and might be widely applied in clinics.
There are several limitations in our study. First, our study data were collected from two affiliated hospital of our university in the same city; the population may not represent the general ICH populations. Second, as a retrospectively study, timing of the follow-up CT scan was not standardized, which may limit our model in other institutions. Third, as a machine learning technique, SVM required hardware and software configuration, and each institution would need the technical knowledge to perform the analysis. Finally, our model require different features of patients' demographic factor, neurological status, parameter from laboratory test, time to initial CT scan, CT imaging characteristics. To use the model, same features of a patient should be provided; however, these features may not be readily available in all hospitals.
In summary, we conducted multivariate analysis of predictors of hematoma expansion in spontaneous ICH and predicted hematoma expansion by using SVM approach. The SVM model demonstrated good performance in predicting hematoma expansion. Moreover, the developed SVM model was based on simple routinely available variables and corresponding clinical examinations to acquire these variables were safe to patients. Those who are predicted to suffer hematoma expansion using SVM model may benefit from the intervention. Therefore, our prediction model may be practical and widely applicable, especially in many institutions where CTA is not readily available or routinely performed for spontaneous ICH patients.
Funding sources
This work was supported by Health Foundation for Creative Talents in Zhejiang Province, China, Natural Science Foundation of Zhejiang Province, China (LQ15H180002), the Science and Technology Planning Projects of Wenzhou, China (Y20180112), Scientific Research Staring Foundation for the Returned Overseas Chinese Scholars of Ministry of Education of China, and Project Foundation for the College Young and Middle-aged Academic Leader of Zhejiang Province, China. The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Declaration of interests
The authors declare no conflict of interests.
Author contributions
Y.J.Y. and W.J.C. were the principal investigator; Y.J.Y., W.J.C., and J.J.L. designed the research. J.J.L. drafted the manuscript. H.L.X., Q.C., T.T.Z., W.S.S., Q.H., J.W.S., D.P.H., L.L., and Y.X.L. collected data. J.J.L. analyzed the data. All authors reviewed and approved the manuscript.
Contributor Information
Weijian Chen, Email: wyyycwj@163.com.
Yunjun Yang, Email: yyjunjim@163.com.
References
- 1.Qureshi A.I., Tuhrim S., Broderick J.P., Batjer H.H., Hondo H., Hanley D.F. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J.P., Brott T.G., Duldner J.E., Tomsick T., Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 3.Dowlatshahi D., Demchuk A.M., Flaherty M.L., Ali M., Lyden P.L., Smith E.E. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Huang Y.J., Zhang G. Intraventricular hemorrhage and early hematoma expansion in patients with intracerebral hemorrhage. Sci Rep. 2015;5:11357. doi: 10.1038/srep11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Zhang G., Xiong X. Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. 2016;47(7):1777. doi: 10.1161/STROKEAHA.116.013186. [DOI] [PubMed] [Google Scholar]
- 6.Anderson C.S., Huang Y., Wang J.G. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi A.I., Palesch Y.Y. Antihypertensive treatment of acute cerebral hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meretoja A., Churilov L., Campbell B.C. The spot sign and tranexamic acid on preventing ICH growth—AUStralasia trial (STOP-AUST): protocol of a phase II randomized, placebo-controlled, double-blind, multicenter trial. Int J Stroke Off J Int Stroke Soc. 2014;9(4):519–524. doi: 10.1111/ijs.12132. [DOI] [PubMed] [Google Scholar]
- 9.Mayer S.A., Brun N.C., Begtrup K. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 10.Aviv R.I., Gladstone D., Goldstein J., Flaherty M., Broderick J., Demchuk A. Contrast extravasation predicts hematoma growth: where to now? AJNR Am J Neuroradiol. 2008;29(9):E80. doi: 10.3174/ajnr.A1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty M.L., Tao H., Haverbusch M. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71(14):1084. doi: 10.1212/01.wnl.0000326895.58992.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii Y., Takeuchi S., Sasaki O., Minakawa T., Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke; A J Cereb Circ. 1998;29(6):1160. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers H.B., Falcone G.J., Mcnamara K.A. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care. 2012;17(3):421. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwers H.B., Greenberg S.M. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35(3):195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furey T.S., Cristianini N., Duffy N., Bednarski D.W., Schummer M., Haussler D. Support vector machine classification and validation of cancer tissue samples using microarray expression data. Bioinformatics. 2000;16(10):906–914. doi: 10.1093/bioinformatics/16.10.906. [DOI] [PubMed] [Google Scholar]
- 16.Denis M., Wan L., Fatemi M., Alizad A. Ultrasound characterization of bone demineralization using a support vector machine. Ultrasound Med Biol. 2018;44(3):714. doi: 10.1016/j.ultrasmedbio.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delcourt C., Huang Y., Wang J. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2) Int J Stroke. 2010;5(2):110–116. doi: 10.1111/j.1747-4949.2010.00415.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Luna D., Coscojuela P., Rodriguez-Villatoro N. Multiphase CT angiography improves prediction of intracerebral hemorrhage expansion. Radiology. 2017;285(3) doi: 10.1148/radiol.2017162839. [DOI] [PubMed] [Google Scholar]
- 19.Zafonte R., Kurowski B. Encyclopedia of clinical neuropsychology. 2011. Midline Shift; p. 1599. [Google Scholar]
- 20.Tuhrim S., Horowitz D.R., Sacher M., Godbold J.H. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27(3):617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 21.Li Q., Zhang G., Huang Y.J. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46(8):2119. doi: 10.1161/STROKEAHA.115.009185. [DOI] [PubMed] [Google Scholar]
- 22.Shimoda Y., Ohtomo S., Arai H., Okada K., Tominaga T. Satellite sign: a poor outcome predictor in intracerebral hemorrhage. Cerebrovasc Dis. 2017;44(3–4):105–112. doi: 10.1159/000477179. [DOI] [PubMed] [Google Scholar]
- 23.Statnikov A., Aliferis C.F., Hardin D.P., Guyon I. 2013. A gentle introduction to support vector machines in biomedicine. [Google Scholar]
- 24.Vanitha C.D.A., Devaraj D., Venkatesulu M. Gene expression data classification using support vector machine and mutual information-based gene selection. Procedia Comput Sci. 2015;47:13–21. [Google Scholar]
- 25.Pedregosa F., Varoquaux G., Gramfort A. Scikit-learn: machine learning in Python. J Mach Learn Res. 2013;12(10):2825–2830. [Google Scholar]
- 26.Sperandei S. Understanding logistic regression analysis. Biochem Med. 2014;24(1):12–18. doi: 10.11613/BM.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ian Witten, Eibe Frank. 2nd ed. Morgan Kaufmann Series: Morgan Kaufmann Publishers Inc.; 2011. Data mining: Practical machine learning tools and techniques. [Google Scholar]
- 28.Li Q., Liu Q.J., Yang W.S. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. 2017;48(11):3019. doi: 10.1161/STROKEAHA.117.017985. [DOI] [PubMed] [Google Scholar]
- 29.Petersen O.F., Espersen J.O. Extradural hematomas: measurement of size by volume summation on CT scanning. Neuroradiology. 1984;26(5):363–367. doi: 10.1007/BF00327488. [DOI] [PubMed] [Google Scholar]
- 30.Divani A.A., Majidi S., Luo X. The ABCs of accurate volumetric measurement of cerebral hematoma. Stroke. 2011;42(6):1569. doi: 10.1161/STROKEAHA.110.607861. [DOI] [PubMed] [Google Scholar]
- 31.Kurth T., Kase C.S., Berger K., Gaziano J.M., Cook N.R., Buring J.E. Smoking and risk of hemorrhagic stroke in women. Stroke; A J Cereb Circ. 2003;34(12):2792–2795. doi: 10.1161/01.STR.0000100165.36466.95. [DOI] [PubMed] [Google Scholar]
- 32.Schlunk F., Greenberg S.M. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res. 2015;6(4):257–263. doi: 10.1007/s12975-015-0410-1. [DOI] [PubMed] [Google Scholar]
- 33.Barras C.D., Tress B.M., Christensen S. Quantitative CT densitometry for predicting intracerebral hemorrhage growth. AJNR Am J Neuroradiol. 2013;34(6):1139–1144. doi: 10.3174/ajnr.A3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demchuk A.M., Dowlatshahi D., Rodriguez-Luna D. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 35.Orito K., Hirohata M., Nakamura Y. Leakage sign for primary intracerebral hemorrhage. Stroke; A J Cereb Circ. 2016;47(4):958–963. doi: 10.1161/STROKEAHA.115.011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blacquiere D., Demchuk A.M., Alhazzaa M. Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke. 2015;46(11):3111. doi: 10.1161/STROKEAHA.115.010566. [DOI] [PubMed] [Google Scholar]
- 37.Grewal M., Srivastava M.M., Kumar P., Varadarajan S. 2018. RADnet: Radiologist level accuracy using deep learning for hemorrhage detection in CT scans. [Google Scholar]
- 38.Paula D.T., Rios P.M., Agapito L., Araceli S., Alen J.F., Alfonso L. Predicting the outcome of patients with subarachnoid hemorrhage using machine learning techniques. IEEE Trans Inform Technol Biomed A Publ IEEE Eng Med Biol Soc. 2009;13(5):794. doi: 10.1109/TITB.2009.2020434. [DOI] [PubMed] [Google Scholar]
- 39.Peng S.Y., Chuang Y.C., Kang T.W., Tseng K.H. Random forest can predict 30-day mortality of spontaneous intracerebral hemorrhage with remarkable discrimination. Eur J Neurol. 2010;17(7):945–950. doi: 10.1111/j.1468-1331.2010.02955.x. [DOI] [PubMed] [Google Scholar]