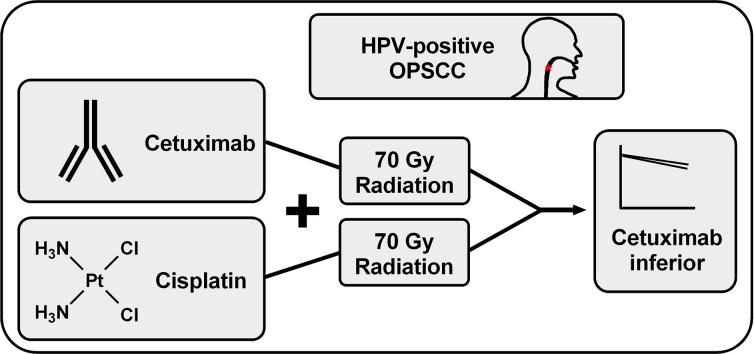
Graphical abstract
Keywords: HNSCC, HPV, Cetuximab, EGFR, Deintensification
Highlights
-
•
Recent trial results show inferiority of cetuximab- to cisplatin-radiotherapy in HPV+ OPSCC.
-
•
Previous data also question the benefit of cetuximab in HNSCC in the curative setting.
-
•
The data provide guidance for research on radiosensitization through molecular targeting.
Abstract
Human Papillomavirus-positive oropharyngeal cancer is a rising tumor entity with unique characteristics and favorable prognosis. Because current multimodal therapies are associated with severe toxicity, different strategies for treatment de-intensification are being tested in clinical trials. In this context two phase 3 studies, which examined the potential of the monoclonal anti-EGFR antibody cetuximab to replace concomitant cisplatin-based chemotherapy, have concordantly reported inferiority of this de-intensification approach. In this opinion article we discuss these recent negative results in the light of previous clinical and preclinical research on the combination of EGFR-inhibition and irradiation. Collectively these data question the effectiveness of EGFR-inhibition in the curative treatment of both HPV-positive and HPV-negative head and neck cancer but provide guidance for future translational research.
1. HPV-positive head and neck cancer
High risk types of the Human Papillomavirus (HPV) are the cause for a growing fraction of head and neck squamous cell carcinoma (HNSCC), especially of the oropharynx (OPSCC). HPV-positive HNSCC represent a biologically distinct disease as tumorigenesis is largely driven through viral oncoproteins. Patients are often younger, in an otherwise healthy state and the typical risk factors heavy tobacco and alcohol consumption can be completely missing [1]. A striking and well recognized feature is their highly favorable prognosis, which has resulted in a separate TNM-stage classification for HPV-positive OPSCC [2]. Standard treatment does not differentiate between HPV-positive and HPV-negative tumors and for locally advanced disease consists of either primary cisplatin-based chemoradiation or adjuvant chemoradiation after surgery. The high cure rates and the often severe and partly irreversible toxicities have fueled the desire for de-intensified, less toxic regimes and a number of clinical trials have been initiated [3]. Without such data it is impossible to judge whether at present HPV-positive tumors are in fact overtreated or whether the current regimes deliver just what is necessary to obtain these high cure rates. So far results have been published for irradiation dose reduction, either in primary chemoradiation [4] or in studies using the response to induction chemotherapy for risk stratification with the assumption that radiotherapy (RT) can be reduced in good responders [5], [6]. The results observed so far suggest that deintensification may be feasible for those patients with the best prognosis. Since January 2019, data are available for the exchange of cisplatin through the monoclonal anti-epidermal growth factor receptor (EGFR) antibody cetuximab. With three phase 3 trials initiated this strategy represents the clinically most advanced approach. Since RT was not de-escalated, the rational for de-intensification is entirely based on cetuximab being less toxic than cisplatin. Despite this rather cautious de-intensification strategy, two of the three phase 3 trials have now unanimously reported inferiority of cetuximab, which defines cisplatin-based chemoradiation as the current standard of care [7], [8].
2. The failure of cetuximab-based de-intensification
In De-ESCALaTE HPV Mehanna and colleagues recruited 334 patients with locally advanced HPV-positive OPSCC in an international randomised controlled trial. Recruitment was limited to low-risk patients defined through a smoking status of less than 10 pack years following the concept introduced by Ang et al. [1]. The primary endpoint was overall severe toxicity after 24 month, secondary endpoints included overall survival (OS) and time to recurrence. With regard to the primary endpoint, the treatment arms did not differ in the rates of severe and overall acute and late toxicities, although the spectrum varied with skin toxicity and infusion reactions more prevalent in the cetuximab arm and haematological, metabolic, and renal toxicity as well as otological symptoms more prevalent in the cisplatin arm. The major drawback, however, was not the failure in reducing toxicity, but the observation of a significant inferiority of the cetuximab arm. Two year OS was 97.5% vs. 89.4% in favor of cisplatin (p = 0.0012; HR = 5), two year recurrence rate was 6 vs. 16.1% (p = 0.0007; HR 3.4). Patients were retrospectively assorted according to the current, 8th TNM staging system. Even in stage I/II disease (276 patients) the impairment in overall survival in the cetuximab arm reached significance (OS: 98.4% vs. 93.2%: p = 0.048). In stage III disease (58 patients), defined by T4 or N3 tumors, the difference was quite drastic with an OS of 93.3% in the cisplatin arm vs. 67.1% with cetuximab (p = 0.0304), underscoring the importance of tumor size and nodal status for the definition of low-risk tumors in addition to smoking status.
In RTOG-1016 Gillison et al. compared cetuximab and cisplatin in a randomized, multicenter non-inferiority trial. Since cetuximab-radiotherapy is an approved treatment option in HNSCC and since radiation dose was not reduced the authors considered both arms as standard treatment. Differing from De-ESCALaTE HPV recruitment was not restricted to low-risk patients by smoking status and the primary end point was overall survival. 849 patients were randomized to both arms. After a median follow up of 4.5 years the cetuximab arm showed inferior 5-year OS (77.9% vs. 84.6%; p = 0.0163; HR 1.45). Accordingly, progression free survival (PFS) was reduced and locoregional failure (LRF) enhanced with cetuximab (PFS: 67.3% vs 78.4%; p = 0.0002; HR 1.72 / LRF: 17.3% vs. 9.9%; p = 0.0005; HR 2.05). Moderate to severe toxicity was slightly reduced in the cetuximab arm but overall similar and differences did not reach statistical significance.
So despite a number of differences in trial design, highly concordant results were obtained in the two studies, which provide very strong evidence for cetuximab being in fact inferior to cisplatin when combined with RT in HPV-positive OPSCC.
3. EGFR-targeting in HNSCC in the curative setting
The rationale behind both studies are the data obtained by Bonner et al. who had reported enhanced survival rates after addition of cetuximab to RT as compared to RT alone in the IMCL-9815 trial, which led to the approval of cetuximab for the curative treatment of HNSCC [9]. At the time IMCL-9815 was initiated, chemoradiation was not yet the standard of care and the contribution of HPV to HNSCC was not yet recognized. Approval was and since then is granted regardless of HPV-status, although, since the trial was not restricted to OPSCC, the majority of tumors was likely HPV-negative. However, in subgroup analyses it was shown that the benefit conferred through cetuximab was most evident in OPSCC, in younger patients and in those with a better Karnofsky performance score, which pointed towards effectiveness in HPV-positive tumors [10]. An unplanned retrospective analysis of the HPV-status through p16 IHC-staining of available OPSCC specimen could not proof a significant interaction between treatment group and p16 status but strongly suggested a meaningful benefit in the HPV-positive fraction (HRs for cetuximab addition: 0.38 (OS); 0.46 (PFS); 0.31 (LRC) for HPV-positive and 0.93; 0.76; 0.78 for HPV-negative OPSCC) [11]. Nevertheless, the rationale of EGFR-inhibition in HPV-positive HNSCC has been controversially discussed, especially since these tumors may be less dependent on altered signaling pathways due to the oncogenic properties of the HPV-oncoproteins E6 and E7. In fact, HPV-positive HNSCC harbor less driver mutations than HPV-negative with the exception of activating PI3K-mutations [12]. These may, however, partly override effects of upstream EGFR-inhibition. Furthermore, expression or gene amplification of EGFR is actually negatively associated with HPV status [13]. The recent reports of cetuximab being inferior to cisplatin now confirm the skepticism. Taking into account that in De-ESCALaTE HPV and RTOG-1016 the survival difference between chemoradiation and cetuximab-RT is in the range of the 8% survival benefit that can be expected from adding chemotherapy to RT in HNSCC [14], [15], there is actually little room for any benefit attributable to cetuximab.
One question that comes into mind is whether this lack of effectiveness is specific for the HPV-positive fraction of OPSCC/HNSCC tumors, which clearly have a different biology than HPV-negative. Focusing on prospective trials with mostly HPV-negative tumors, cetuximab failed to enhance survival when added to chemoradiation in RTOG-0522 and when added to induction chemotherapy and subsequent RT in DeLOS-II [16], [17]. Furthermore, the alternative anti-EGFR antibodies panitumumab (CONCERT-1&2) and zalutumumab (DAHANCA 19) also failed to compete with cisplatin when added to RT or to enhance survival when added to chemoradiation, as well as the EGFR-inhibitor erlotinib [18], [19], [20], [21]. Collectively, these data show that the addition of EGFR-inhibiting agents to RT-based treatment is also not effective in HPV-negative HNSCC in otherwise unselected patient cohorts. Even in case small subgroups may exist that would benefit from EGFR-inhibition, to date there are no predictive biomarkers for patient selection, and also EGFR expression itself was shown not to be predictive [22]. Together with De-ESCALaTE HPV and RTOG-1016, the above mentioned prospective trials have now recruited more than 3300 HNSCC patients into studies combining EGFR-inhibition with radiation without benefit.
4. Preclinical studies of EGFR-targeting in HNSCC
Keeping the mostly negative clinical data in mind, what is the status of preclinical data regarding the combination of cetuximab with radiation and are there lessons to be learned? An obvious finding is that there is a big difference in the amount of data for the two HNSCC entities with a plethora of publications analyzing HPV-negative HNSCC cell lines or tumor models but very few data for HPV-positive. For the latter until now, which means several years after the initiation of the cetuximab-based de-intensification trials, to the best of our knowledge, there are no convincing preclinical data that would show a radiosensitizing or otherwise promising effect of cetuximab when added to radiation. We had tested the combined treatment in 5 HPV-positive HNSCC cell lines and none of these showed a radiosensitizing effect in colony formation assays (in contrast to the inhibition of PARP1 ± Chk1) [23]. So far, these experiments represent the only colony formation data for EGFR-inhibition and irradiation specifically performed in HPV-positive HNSCC cells. Combined treatment with cetuximab and irradiation was further independently performed in two xenograft models of the HPV-positive HNSCC cell lines UM-SCC-47 and UT-SCC-45, which also did not result in an enhanced efficacy as compared to radiation alone [24], [25]. Therefore, current preclinical data regarding cetuximab-RT in HPV-positive HNSCC are both, sparse and negative. In our hands HPV-positive cell lines were equally sensitive towards cisplatin as HPV-negative [26] and another study reported an even enhanced sensitivity [27]. So together, the rare preclinical data presently existing basically suggest that in RTOG-1016 and De-ESCALaTE HPV an at least partially effective compound was exchanged by an ineffective.
For HPV-negative HNSCC, although this stratification was not established in these days, first preclinical studies had shown radiosensitization and other antitumoral activity, including growth inhibition and induction of apoptosis [28]. After the publication of the IMCL-9815 in 2006, many additional studies aimed to explain the reported survival benefit. Most of these, including some of our own, reported enhanced cellular radiation sensitivity primarily caused by a block of DNA double-strand break (DSB) repair and cell cycle effects [29], [30], [31], [32]. Interestingly, we and others have shown that an impaired DSB repair after cetuximab treatment, as analyzed by surrogate markers such as 53BP1 or γH2AX foci, does not necessarily result in radiosensitization [33], [34]. This indicates that the interplay between EGFR and DNA damage detection, which in the case of DSB repair foci not only depends on repair but also on marking and marker release, is more complex than generally assumed. In contrast to DSB repair, the effects on cell cycle could potentially explain the discrepancies between the pre-clinically observed cellular radiosensitization and the lack of clinical benefit. Our results strongly suggest that cellular radiosensitization is mainly dependent on seemingly permanent but in the end reversible cell cycle arrests, because their induction and release correlated well with the presence or lack of radiosensitization in HNSCC cells when varying the experimental conditions [33]. Such release of cells from a reversible cell cycle arrest might be caused by tumor cell repopulation during fractionated RT. This would offer an explanation for the failure of cetuximab in the curative setting, while in recurrent/metastatic disease there is an observable (though transient) benefit that has made cetuximab part of standard first line treatment for the last decade [35]. While cetuximab was further described to exert effects on the tumor microenvironment, i.e. the induction of antibody-derived cellular cytotoxicity (ADCC) [36], in view of the negative clinical results described above, these mechanisms obviously also don‘t have a meaningful impact when cetuximab is combined with RT or chemoradiation.
5. Lessons for translational research
In the light of the aforementioned clinical and preclinical studies, we believe that there are several important lesson to be learned for translational research: i) intensive experimental studies preceding and accompanying clinical trials are essential to really understand the mechanisms leading to radio- or chemosensitization; ii) extensive preclinical studies have to be performed testing a variety of relevant cell lines, tumor models or alternative techniques to enable, to a reasonable extent, an estimation of the abundance and clinical relevance of a given positive effect; iii) negative results are also important and their publication is necessary to avoid a selective-publication bias towards cell lines and models that show the presumed or desired effect, iv) more physiological model systems have to be established and used, such as ex-vivo tumor tissue cultures or patient-derived xenograft tumors, since it is well accepted that cell lines do not sufficiently reflect the tumor situation and v) all novel treatment options should be thoroughly and critically tested to offer the best possible advice for clinical trial design and decision making and to overcome these recent clinical drawbacks for the approach of molecular targeting to enhance the effectiveness of RT in the curative setting. Although EGFR targeting has failed to show a benefit in unselected HPV-positive and, to our opinion, also HPV-negative HNSCC, we are convinced molecular targeting can effectively sensitize tumors to RT and chemoradiation but upcoming concepts should undergo intensive preclinical testing and ideally should implement predictive biomarkers to enable individualized treatment. HPV-positivity in OPSCC may well represent such a biomarker and the reported impairment in DSB repair [37], [38] may represent a weakness that could be exploited by molecular targeting approaches, e.g. through inhibition of the functional components of the DNA damage response [23], [39], which, however, remains to be further elaborated.
With all the lessons learned over the last 10–15 years and a continuously growing number of specific targeting therapeutics, we are convinced that, despite the recent drawbacks, molecular targeting for the radiosensitization of HNSCC and other solid malignancies has the potential to play an important role in future therapeutic regimes.
Declaration of Competing Interest
None.
References
- 1.Ang K.K. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22(2):128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]; Ang, K.K., et al., Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol, 2012. 22(2): p. 128-42. [DOI] [PubMed]
- 2.Huang S.H. Overview of the 8th Edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18(7):40. doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]; Huang, S.H., et al., Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr Treat Options Oncol, 2017. 18(7): p. 40. [DOI] [PubMed]
- 3.Mirghani H. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin Transl Radiat Oncol. 2018;8:4–11. doi: 10.1016/j.ctro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mirghani, H., et al., Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin Transl Radiat Oncol, 2018. 8: p. 4-11. [DOI] [PMC free article] [PubMed]
- 4.Chera B.S. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2018;124(11):2347–2354. doi: 10.1002/cncr.31338. [DOI] [PubMed] [Google Scholar]; Chera, B.S., et al., Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer, 2018. 124(11): p. 2347-2354. [DOI] [PubMed]
- 5.Chen A.M. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18(6):803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, A.M., et al., Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol, 2017. 18(6): p. 803-811. [DOI] [PMC free article] [PubMed]
- 6.Marur S. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN cancer research group. J Clin Oncol. 2017;35(5):490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marur, S., et al., E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol, 2017. 35(5): p. 490-497. [DOI] [PMC free article] [PubMed]
- 7.Gillison M.L. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gillison, M.L., et al., Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet, 2019. 393(10166): p. 40-50. [DOI] [PMC free article] [PubMed]
- 8.Mehanna H. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mehanna, H., et al., Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet, 2019. 393(10166): p. 51-60. [DOI] [PMC free article] [PubMed]
- 9.Bonner J.A. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]; Bonner, J.A., et al., Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med, 2006. 354(6): p. 567-78. [DOI] [PubMed]
- 10.Bonner J.A. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]; Bonner, J.A., et al., Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol, 2010. 11(1): p. 21-8. [DOI] [PubMed]
- 11.Rosenthal D.I. Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol. 2016;34(12):1300–1308. doi: 10.1200/JCO.2015.62.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rosenthal, D.I., et al., Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol, 2016. 34(12): p. 1300-8. [DOI] [PMC free article] [PubMed]
- 12.Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cancer Genome Atlas, N., Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 2015. 517(7536): p. 576-82. [DOI] [PMC free article] [PubMed]
- 13.Mirghani H. Oropharyngeal cancers: relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer. 2014;50(6):1100–1111. doi: 10.1016/j.ejca.2013.12.018. [DOI] [PubMed] [Google Scholar]; Mirghani, H., et al., Oropharyngeal cancers: relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer, 2014. 50(6): p. 1100-11. [DOI] [PubMed]
- 14.Blanchard P. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33–40. doi: 10.1016/j.radonc.2011.05.036. [DOI] [PubMed] [Google Scholar]; Blanchard, P., et al., Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol, 2011. 100(1): p. 33-40. [DOI] [PubMed]
- 15.Pignon J.P. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]; Pignon, J.P., et al., Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol, 2009. 92(1): p. 4-14. [DOI] [PubMed]
- 16.Ang K.K. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ang, K.K., et al., Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol, 2014. 32(27): p. 2940-50. [DOI] [PMC free article] [PubMed]
- 17.Dietz A. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann Oncol. 2018;29(10):2105–2114. doi: 10.1093/annonc/mdy332. [DOI] [PubMed] [Google Scholar]; Dietz, A., et al., Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann Oncol, 2018. 29(10): p. 2105-2114. [DOI] [PubMed]
- 18.Eriksen J.G. Update of the randomised phase III trial DAHANCA 19: primary C-RT or RT and zalutumumab for squamous cell carcinomas of head and neck. Radiother Oncol. 2015;114(Supplement I):10. [Google Scholar]; Eriksen, J.G., et al., Update of the randomised phase III trial DAHANCA 19: Primary C-RT or RT and zalutumumab for squamous cell carcinomas of head and neck. Radiother and Oncol, 2015. 114(Supplement I): p. 10, DOI: http://dx.doi.org/10.1016/S0167-8140(15)34769-1.
- 19.Giralt J. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):221–232. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]; Giralt, J., et al., Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol, 2015. 16(2): p. 221-32. [DOI] [PubMed]
- 20.Martins R.G. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31(11):1415–1421. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]; Martins, R.G., et al., Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol, 2013. 31(11): p. 1415-21. [DOI] [PubMed]
- 21.Mesia R. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):208–220. doi: 10.1016/S1470-2045(14)71198-2. [DOI] [PubMed] [Google Scholar]; Mesia, R., et al., Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol, 2015. 16(2): p. 208-20. [DOI] [PubMed]
- 22.Bossi P. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;7(45):74362–74379. doi: 10.18632/oncotarget.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bossi, P., et al., Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget, 2016. 7(45): p. 74362-74379. [DOI] [PMC free article] [PubMed]
- 23.Guster J.D. The inhibition of PARP but not EGFR results in the radiosensitization of HPV/p16-positive HNSCC cell lines. Radiother Oncol. 2014;113(3):345–351. doi: 10.1016/j.radonc.2014.10.011. [DOI] [PubMed] [Google Scholar]; Guster, J.D., et al., The inhibition of PARP but not EGFR results in the radiosensitization of HPV/p16-positive HNSCC cell lines. Radiother Oncol, 2014. 113(3): p. 345-51. [DOI] [PubMed]
- 24.Koi L. EGFR-amplification plus gene expression profiling predicts response to combined radiotherapy with EGFR-inhibition: a preclinical trial in 10 HNSCC-tumour-xenograft models. Radiother Oncol. 2017;124(3):496–503. doi: 10.1016/j.radonc.2017.07.009. [DOI] [PubMed] [Google Scholar]; Koi, L., et al., EGFR-amplification plus gene expression profiling predicts response to combined radiotherapy with EGFR-inhibition: A preclinical trial in 10 HNSCC-tumour-xenograft models. Radiother Oncol, 2017. 124(3): p. 496-503. [DOI] [PubMed]
- 25.Zeng L. Combining Chk1/2 inhibition with cetuximab and radiation enhances in vitro and in vivo cytotoxicity in head and neck squamous cell carcinoma. Mol Cancer Ther. 2017;16(4):591–600. doi: 10.1158/1535-7163.MCT-16-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zeng, L., et al., Combining Chk1/2 Inhibition with Cetuximab and Radiation Enhances In Vitro and In Vivo Cytotoxicity in Head and Neck Squamous Cell Carcinoma. Mol Cancer Ther, 2017. 16(4): p. 591-600. [DOI] [PMC free article] [PubMed]
- 26.Busch C.J. Similar cisplatin sensitivity of HPV-positive and -negative HNSCC cell lines. Oncotarget. 2016;7(24):35832–35842. doi: 10.18632/oncotarget.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Busch, C.J., et al., Similar cisplatin sensitivity of HPV-positive and -negative HNSCC cell lines. Oncotarget, 2016. 7(24): p. 35832-35842. [DOI] [PMC free article] [PubMed]
- 27.Ziemann F. Increased sensitivity of HPV-positive head and neck cancer cell lines to x-irradiation +/ Cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis. Am J Cancer Res. 2015;5(3):1017–1031. [PMC free article] [PubMed] [Google Scholar]; Ziemann, F., et al., Increased sensitivity of HPV-positive head and neck cancer cell lines to x-irradiation +/- Cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis. Am J Cancer Res, 2015. 5(3): p. 1017-31. [PMC free article] [PubMed]
- 28.Huang S.M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59(8):1935–1940. [PubMed] [Google Scholar]; Huang, S.M., et al., Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res, 1999. 59(8): p. 1935-40. [PubMed]
- 29.Baumann M. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007;83(3):238–248. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]; Baumann, M., et al., EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol, 2007. 83(3): p. 238-48. [DOI] [PubMed]
- 30.Meyn R.E. Receptor signaling as a regulatory mechanism of DNA repair. Radiother Oncol. 2009;92(3):316–322. doi: 10.1016/j.radonc.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meyn, R.E., et al., Receptor signaling as a regulatory mechanism of DNA repair. Radiother Oncol, 2009. 92(3): p. 316-22. [DOI] [PMC free article] [PubMed]
- 31.Mukherjee B. Targeting nonhomologous end-joining through epidermal growth factor receptor inhibition: rationale and strategies for radiosensitization. Semin Radiat Oncol. 2010;20(4):250–257. doi: 10.1016/j.semradonc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mukherjee, B., et al., Targeting nonhomologous end-joining through epidermal growth factor receptor inhibition: rationale and strategies for radiosensitization. Semin Radiat Oncol, 2010. 20(4): p. 250-7. [DOI] [PMC free article] [PubMed]
- 32.Rodemann H.P. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83(11–12):781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]; Rodemann, H.P., et al., Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol, 2007. 83(11-12): p. 781-91. [DOI] [PubMed]
- 33.Kriegs M. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget. 2016;7(29):45122–45133. doi: 10.18632/oncotarget.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kriegs, M., et al., Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget, 2016. 7(29): p. 45122-45133. [DOI] [PMC free article] [PubMed]
- 34.Stegeman H. EGFR-inhibition enhances apoptosis in irradiated human head and neck xenograft tumors independent of effects on DNA repair. Radiat Res. 2013;180(4):414–421. doi: 10.1667/RR3349.2. [DOI] [PubMed] [Google Scholar]; Stegeman, H., et al., EGFR-inhibition enhances apoptosis in irradiated human head and neck xenograft tumors independent of effects on DNA repair. Radiat Res, 2013. 180(4): p. 414-21. [DOI] [PubMed]
- 35.Vermorken J.B. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]; Vermorken, J.B., et al., Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med, 2008. 359(11): p. 1116-27. [DOI] [PubMed]
- 36.Srivastava R.M. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19(7):1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]; Srivastava, R.M., et al., Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res, 2013. 19(7): p. 1858-72. [DOI] [PMC free article] [PubMed]
- 37.Dok R. p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res. 2014;74(6):1739–1751. doi: 10.1158/0008-5472.CAN-13-2479. [DOI] [PubMed] [Google Scholar]; Dok, R., et al., p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res, 2014. 74(6): p. 1739-51. [DOI] [PubMed]
- 38.Rieckmann T. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107(2):242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]; Rieckmann, T., et al., HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol, 2013. 107(2): p. 242-6. [DOI] [PubMed]
- 39.Busch C.J. G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiother Oncol. 2017;122(2):260–266. doi: 10.1016/j.radonc.2016.11.017. [DOI] [PubMed] [Google Scholar]; Busch, C.J., et al., G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiother Oncol, 2017. 122(2): p. 260-266. [DOI] [PubMed]