Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is a complex hypersensitivity reaction that is associated with an allergic immunological response to Aspergillus species via Th2-related inflammation. The long-term use of a systemic corticosteroid is often needed for the treatment of ABPA. However, systemic corticosteroid treatment imposes a risk of the onset of a nontuberculous mycobacterial infection. Here we report the case of a patient with ABPA who required the long-term use of an oral corticosteroid because her repeated asthmatic attacks were successfully treated with mepolizumab, an anti-interleukin-5 monoclonal antibody. The patient, a 60-year-old Japanese female, had been treated with an oral corticoid and itraconazole. Despite the success of the initial treatment for ABPA, it was difficult to discontinue the use of the oral corticosteroid. In addition, Mycobacterium avium was detected from her bronchial lavage. We initiated mepolizumab treatment to taper the amount of corticosteroid and control the asthma condition. The patient's number of blood eosinophils, serum IgE level, fractional exhaled nitric oxide level, dosage of oral prednisolone, and need for inhaled budesonide/formoterol all improved, without an exacerbation of her asthma attacks. Although further research regarding mepolizumab treatment is needed, we believe that mepolizumab could be considered one of the agents for treating refractory ABPA.
Keywords: Allergic bronchopulmonary aspergillosis, Nontuberculous mycobacterial infection, Mepolizumab, Asthma
1. Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is one of the most severe manifestations of asthma, and it is associated with an allergic immunological response to airway Aspergillus fumigatus (A. fumigatus). ABPA is clinically characterized by peripheral blood eosinophilia, increased levels of serum IgE, and radiographic findings including pulmonary infiltrations, central bronchiectasis, and mucous plugs [1]. Delays in the diagnosis and treatment of ABPA lead to the irreversible destruction of lungs and fibrosis, which may cause respiratory failure. It is important to control the disease progression by recommended treatment, such as systemic corticosteroid and concomitant anti-fungal agents [2,3]. Although systemic corticosteroids can control the symptoms and disease progression of most ABPA patients, it is better to reduce or discontinue the systemic corticosteroid use after the symptoms and clinical findings are improved, because of the corticosteroid's adverse effect on disturbance of the immune system which predisposes the patient to infection. However, there are some ABPA patients who require the continued use of a corticosteroid because of their asthma attacks. Notably, nontuberculous mycobacterial (NTM) infection is associated with pulmonary deterioration in patients with ABPA who have a history of a long-term systemic corticosteroid treatment [4].
Molecular target drugs have recently been used to treat systemic corticosteroid-dependent ABPA. Omalizumab, a monoclonal anti-immunoglobulin E (IgE) antibody, was reported to be useful for reducing the required amount of corticosteroid [5]. However, there is a limit regarding the serum IgE level in the use of omalizumab; not a few ABPA patients cannot be treated with omalizumab because of the overlimit of serum IgE levels. Mepolizumab, a monoclonal antibody against interleukin (IL)-5, has been used for refractory asthma, as has omalizumab, which suppresses Th2 cell-related inflammation [6]. Mepolizumab is thought to contribute to the reduction of the rate of exacerbation and the steroid requirement, and to an improvement of pulmonary function and symptoms. The effectiveness of mepolizumab for refractory asthma is established, but the efficacy of mepolizumab for ABPA is not yet known.
Here, we describe the case with ABPA and NTM infection for whom the required corticosteroid amount was safely reduced and whose asthmatic symptoms (productive cough and dyspnea on exertion) and radiological findings (mucous plugs and pulmonary infiltrate shadows) were improved after the administration of mepolizumab.
2. Clinical report
In 2013, a 60-year-old Japanese female was admitted to our hospital because of the symptoms of productive cough and dyspnea on exertion, which appeared 3 months prior to this admission. She had been diagnosed with asthma 1 year before, and she was using inhaled budesonide/formoterol (BUD/FM), four puffs per day (BUD 480 μg and FM 18 μg/day) and montelukast 10 mg/day. At her admission, her laboratory data and pulmonary function test results showed a white blood cell count 7,900/mm3 with eosinophils 7.1% on differential count; hemoglobin, 11.4 g/dL; platelet count, 214,000/mm3; C-reactive protein (CRP), 0.05 mg/dL; serum total IgE, 8,327 IU/mL; forced vital capacity (FVC), 2.09 L (85.0% of predicted); forced expiratory volume in 1 second (FEV1), 1.59 L (79.3% of predicted); and FEV1/FVC ratio, 76.3%. The specific IgE and immediate cutaneous reaction to A. fumigatus and serum precipitation antibodies against Aspergillus were positive.
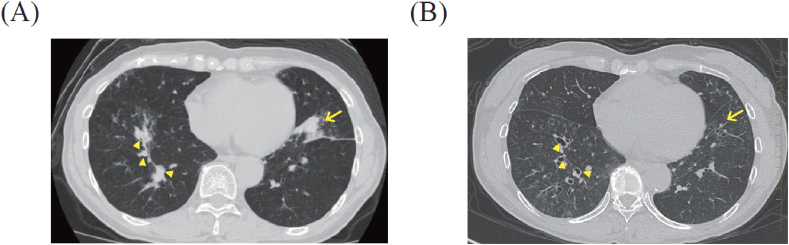
No acid-fast bacilli were detected by Ziehl-Neelsen staining and culture of sputum. High-resolution computed tomography (HRCT) of the chest revealed bilateral infiltrative shadows and central bronchiectasis with mucous plugs (Fig. 1). Bronchoscopy showed mucous plugs in the anterior basal segmental bronchus of the left lung (Fig. 2). Sputum and bronchial lavage cultures revealed A. fumigatus, and the cytological examination of the bronchial lavage showed branching fungal hyphae. Since these data fulfilled the seven major criteria and two secondary criteria proposed by Rosenberg [7], we treated the patient with a systemic corticosteroid (prednisolone 30 mg/day) and itraconazole (200 mg/day) for ABPA. Then her symptoms improved and the bilateral infiltrative shadows and mucous plugging had disappeared at 6 months after the start of this treatment (Fig. 1).
Fig. 1.
Chest CT at the diagnosis of ABPA and 6 months after the start of the systemic corticosteroid and itraconazole. A: Chest CT at the diagnosis of ABPA (at admission) in the patient, a 60-year-old Japanese female. The chest CT shows the bilateral infiltrative shadows (arrow) and central bronchiectasis with mucous plugs (arrowheads). B: Chest CT at 6 months after treatment. The chest CT shows the disappearance of the bilateral infiltrative shadows and mucous plugs.
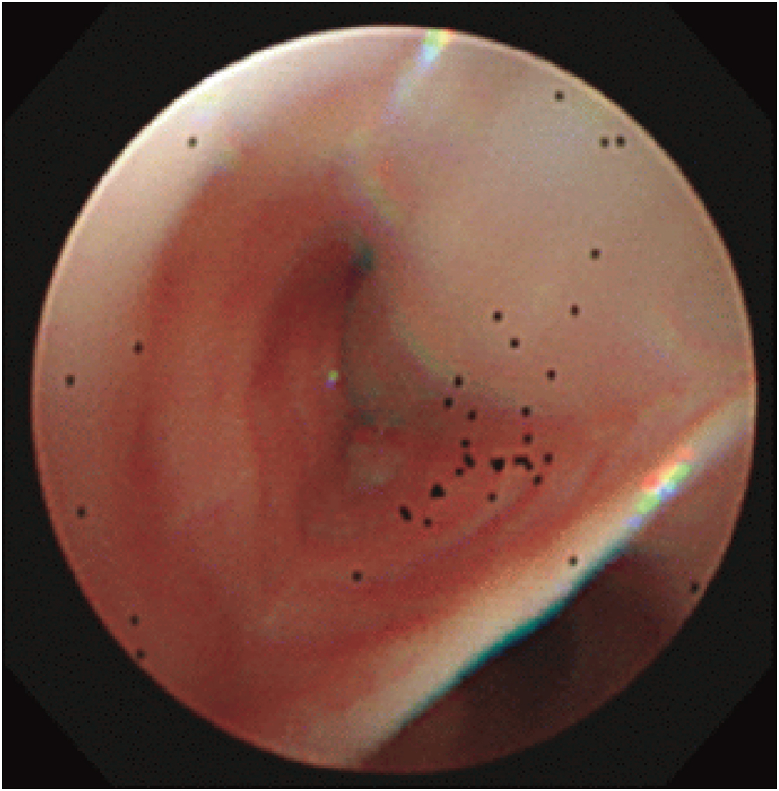
Fig. 2.
Bronchoscopy findings at the diagnosis of ABPA. Mucous plugs filled the anterior basal segmental bronchus of the left lung.
The prednisolone was gradually tapered to 5 mg/day by 5 months after admission, and we continued the systemic corticosteroid and antifungal treatment. However, the patient experienced asthma attacks when she was taking 5 mg/day of prednisolone in December 2015. She thus continuously used 10 mg/day of prednisolone. The Ziehl-Neelsen staining and culture test of sputum were negative in February 2016 and March 2017.
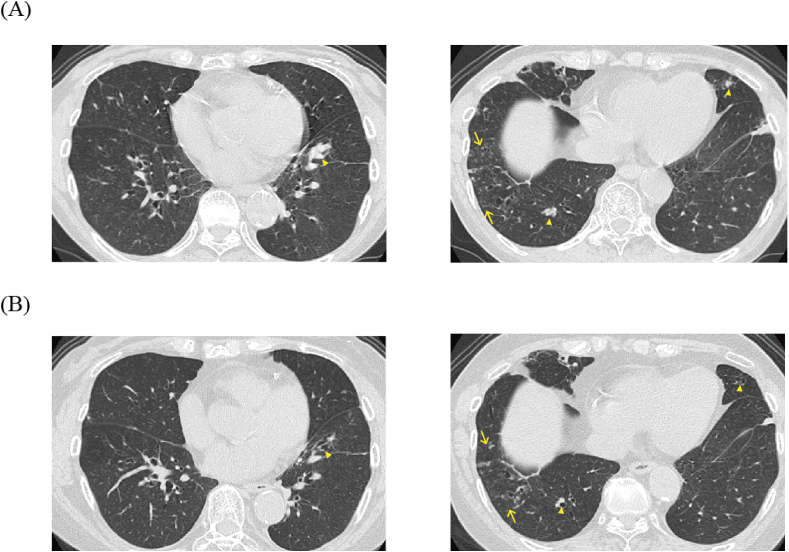
In April 2018, the patient reported often re-experiencing asthma attacks and dyspnea on exertion, despite increasing of the dose of prednisolone. A chest CT examination revealed new mucous plugs in the posterior basal segment bronchus of the right lung and the anterior basal segment bronchus of the left lung and small nodular shadows in the anterior basal segment of the right lower lobe (Fig. 3A). The culture test and polymerase chain reaction test of bronchial lavage from the anterior basal segment bronchus of the right lung revealed Mycobacterium avium (M. avium).
Fig. 3.
Chest CT at 4 months before and 5 months after the mepolizumab treatment. A: Chest CT at 4 months before the administration of mepolizumab. The chest CT shows mucous plugs in the posterior basal segment bronchus of the right lung (arrowhead) and the anterior basal segment bronchus of the left lung and small nodular shadows in the anterior basal segment of the right lower lobe (arrow). B: Chest CT at 5 months after the mepolizumab treatment. The chest CT shows the disappearance of mucous plugs (arrowhead) and no change in the small nodular shadows in the anterior basal segment of the right lower lobe (arrow). The bilateral infiltrative shadows and central bronchiectasis with mucous plug. B: Chest CT at 6 months after treatment, showing the disappearance of bilateral infiltrative shadows and mucous plugs.
In August 2018, to control asthma with the minimum dose of predonisolone, mepolizumab (100 mg/4 weeks) was started, which improved the patient's coughs and dyspnea and reduced the frequency of her asthma attacks. Her fractional exhaled nitric oxide (FeNO), serum total IgE level, and peripheral blood eosinophils were decreased. The daily doses of prednisolone and BUD/FM were also reduced without the exacerbation of asthma symptoms (Fig. 4). At the final visit, 5 months after starting mepolizumab, the patient was free from respiratory symptoms, the mucous plugs had disappeared and the small nodular shadows were comparable with the CT findings of 5 months before (Fig. 3B). Although the patient's M. avium treatment was extended because of a left femoral neck fracture due to an injury that needed surgical treatment and the subsequent muscle rehabilitation, the patient is scheduled to receive a clarithromycin-based regimen (clarithromycin, rifampicin, and ethambutol) for the M. avium infection.
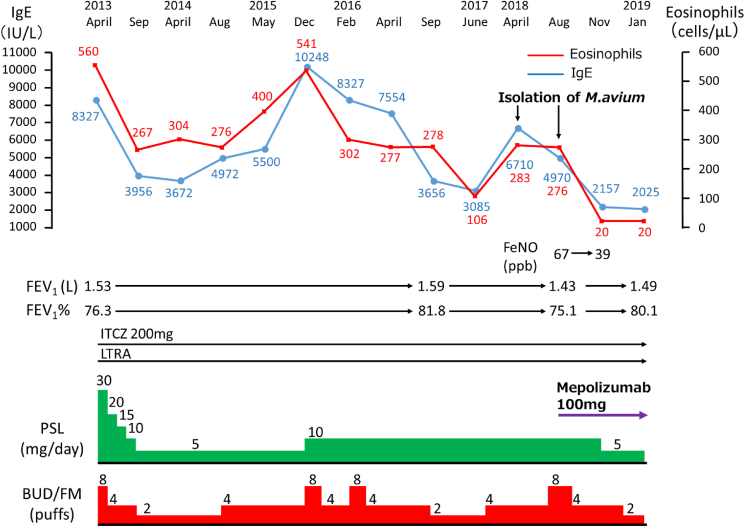
Fig. 4.
The patient's clinical course, before and after mepolizumab treatment. Changes in the patient's blood eosinophil count, serum IgE level, dose of oral prednisolone and inhalation of budesonide/formoterol (BUD/FM), forced expiratory volume in one second (FEV1), forced expiratory volume % in one second (FEV1%), and fractional exhaled nitric oxide (FeNO) level. After the mepolizumab treatment (100 mg/4 weeks), the blood eosinophil count dropped from 276/μL to 20/μL, the serum IgE level dropped from 4,970 IU/L to 2157 IU/L, and the FeNO level dropped from 67 ppb to 39 ppb.
3. Discussion
This is the first report of the clinical presentation and course of treatment with mepolizumab in a patient with ABPA and M. avium infection. Mepolizumab therapy improved her respiratory symptoms (cough and dyspnea), resolved the mucous plugs, and enabled to reduce the daily amount of oral corticosteroid without recurring asthma attacks.
The underlying cause of ABPA is thought to be a complex hypersensitivity reaction to colonized A. fumigatus in the airway, which occurs in individuals with asthma or cystic fibrosis [8]. For the treatment of ABPA, systemic corticosteroid therapy is recommended based on the results of clinical case series [9,10]. In an unblinded randomized trial, Agarwal et al. [4] reported that a medium dose (0.5 mg/kg/day) and a high dose (0.75 mg/kg/day) of oral corticosteroids were both effective but the medium dose was safer than the high dose. Antifungal therapy with itraconazole is thought to be effective in ABPA cases in which it is difficult to discontinue or taper an oral corticosteroid [2,11].
For our patient, we selected the medium dose of oral corticosteroid combined with itraconazole as the initial treatment. Although this treatment lead to an improvement of her respiratory symptoms and radiological findings, the 10 mg/day dose of corticosteroid was necessary from 2015 to 2018 because of her frequent asthma attacks. The reported odds ratios of a low dose (<5 mg/day), medium dose (5– <10 mg/day), and high dose (≥10 mg/day) of an oral corticosteroid for NTM disease in patients with rheumatoid arthritis were 1.97, 2.51, and 4.87, respectively compared to the non-current use of an oral corticosteroid [12]. Since the long-term use of a systemic corticosteroid is known to one of the risk factors for nontuberculous mycobacterial infection, the dose and duration of use of a corticosteroid may affect the establishment of an NTM infection.
Our patient had pulmonary symptoms; M. avium was isolated from the sputum and bronchial lavage 5 years after the start of oral corticosteroid use, and HRCT revealed small nodular shadows. These findings met the American Thoracic Society/Infectious Diseases Society of America statement regarding the diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [13]. Kobayashi et al. reported that the sputum-negative conversion rate and the clinical improvement rate in patients with pulmonary M. avium complex (MAC) disease who continued oral corticosteroid treatment were 33.3% and 11.1%, respectively [14]. Since the sputum-negative conversion rate was 60%–71.8% in previous reports [15,16], systemic corticosteroid use is thought to contribute to the poor prognosis of treatment of nontuberculous mycobacterial infections. Regarding the treatment for pulmonary MAC disease, it is recommended to reduce the amount of corticosteroid as much as possible. For ABPA patients, the tapering and discontinuation of a corticosteroid remains a challenge in clinical practice. In the present case, reduction of the corticosteroid despite the presence of M. avium infection was difficult because of the poor control of the patient's asthma. In order to reduce the dose of the corticosteroid and improve the patient's asthma control, we administered mepolizumab.
The underlying mechanisms of ABPA have begun to be elucidated. Since IL-5 is known to play a central role in eosinophil differentiation, maturation, and survival [17], IL-5 appears to be involved in the promotion of the progression of ABPA. In support of this concept, the following findings were reported. In lungs in an A. fumigatus antigen induced-murine ABPA model, the anti-IL-5 antibody reduced the peripheral eosinophil number and the expression level of eosinophil peroxidase [18]. In addition, the Th2/Th1 ratio in ABPA patients was shifted toward to Th2 [19], and A. fumigatus-induced IL-5 production of peripheral blood mononuclear cells in ABPA patients was accelerated compared to those of healthy controls [19].
Few reports are available on the efficacy of mepolizumab in patients with ABPA, but four case reports are shown in the English literature [[20], [21], [22], [23]]. All of the patients with the co-existence of ABPA and steroid-dependent asthma and our present patients achieved improved clinical symptoms, pulmonary function, and radiological findings by mepolizumab treatment. Our patient's pulmonary symptoms and mucous plugs in the lower lobes improved and the number of eosinophils, IgE value, FeNO value, and daily required number of BUD/FM inhalations were decreased by mepolizumab treatment. The monoclonal antibody omalizumab binds to free serum IgE, which downregulates the cell-surface high-affinity IgE receptor (FcεR1) on basophils and mast cells, and omalizumab is considered an effective treatment for patients with ABPA; however, there are ABPA cases that are refractory for omalizumab treatment [20,24].
Our patient was treated with mepolizumab rather than omalizumab because of her excessive IgE values. Hirota et al. reported that radiological findings (e.g., bronchial wall thickness and infiltration) and asthmatic symptoms were improved after a change of treatment from omalizumab to mepolizumab [20]. Since there is a case of a patient with uncontrolled asthma who was successfully treated by a change of treatment from mepolizumab to benralizumab [25], comparative trials are needed to evaluate the effectiveness of biological therapies targeting Th2-pathway molecules or cells for ABPA, including omalizumab, mepolizumab, benralizumab, reslizumab and dupilumab. In conclusion, our ABPA patient with an M. avium infection was treated successfully with mepolizumab. Although there are few reports of ABPA patients for whom mepolizumab was effective, the use of mepolizumab should be considered for patients with ABPA and steroid-dependent asthma.
Conflicts of interest
The authors declare that there were no conflicts of interest.
References
- 1.Ueki S., Hebisawa A., Kitani M., Asano K., Neves J.S. Allergic bronchopulmonary aspergillosis-A luminal hypereosinophilic disease with extracellular trap cell death. Front. Immunol. 2018;9:2346. doi: 10.3389/fimmu.2018.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens D.A., Schwartz H.J., Lee J.Y., Moskovitz B.L., Jerome D.C., Catanzaro A., Bamberger D.M., Weinmann A.J., Tuazon C.U., Judson M.A., Platts-Mills T.A., DeGraff A.C., Jr. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N. Engl. J. Med. 2000;342:756–762. doi: 10.1056/NEJM200003163421102. [DOI] [PubMed] [Google Scholar]
- 3.Moss R.B. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur. Respir. J. 2014;43:1487–1500. doi: 10.1183/09031936.00139513. [DOI] [PubMed] [Google Scholar]
- 4.Mussaffi H., Rivlin J., Shalit I., Ephros M., Blau H. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur. Respir. J. 2005;25:324–328. doi: 10.1183/09031936.05.00058604. [DOI] [PubMed] [Google Scholar]
- 5.Li J.X., Fan L.C., Li M.H., Cao W.J., Xu J.F. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir. Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Jeimy S., Tsoulis M.W., Hachey J., Kim H. Eligibility of monoclonal antibody-based therapy for patients with severe asthma: a Canadian cross-sectional perspective. Allergy Asthma Clin. Immunol. 2018;14:68. doi: 10.1186/s13223-018-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg M., Patterson R., Mintzer R., Cooper B.J., Roberts M., Harris K.E. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann. Intern. Med. 1977;86:405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 8.Greenberger P.A. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 2002;110:685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 9.Patterson R., Greenberger P.A., Halwig J.M., Liotta J.L., Roberts M. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch. Intern. Med. 1986;146:916–918. doi: 10.1001/archinte.146.5.916. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R., Gupta D., Aggarwal A.N., Behera D., Jindal S.K. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–448. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 11.Salez F., Brichet A., Desurmont S., Grosbois J.M., Wallaert B., Tonnel A.B. Effects of itraconazole therapy in allergic bronchopulmonary aspergillosis. Chest. 1999;116:1665–1668. doi: 10.1378/chest.116.6.1665. [DOI] [PubMed] [Google Scholar]
- 12.Liao T.L., Lin C.F., Chen Y.M., Liu H.J., Chen D.Y. Risk factors and outcomes of nontuberculous mycobacterial disease among rheumatoid arthritis patients: a case-control study in a TB endemic area. Sci. Rep. 2016;6:29443. doi: 10.1038/srep29443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., Holland S.M., Horsburgh R., Huitt G., Iademarco M.F., Iseman M., Olivier K., Ruoss S., von Reyn C.F., Wallace R.J., Jr., Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 14.Kobashi Y., Matsushima T. Clinical analysis of pulmonary Mycobacterium avium complex disease in association with corticosteroid treatment. J. Infect. Chemother. 2003;9:68–74. doi: 10.1007/s10156-002-0216-4. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka E., Kimoto T., Tsuyuguchi K., Watanabe I., Matsumoto H., Niimi A., Suzuki K., Murayama T., Amitani R., Kuze F. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am. J. Respir. Crit. Care Med. 1999;160:866–872. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 16.Kwak N., Park J., Kim E., Lee C.H., Han S.K., Yim J.J. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin. Infect. Dis. 2017;65:1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 17.Moss R.B. The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann. N. Y. Acad. Sci. 2012;1272:49–57. doi: 10.1111/j.1749-6632.2012.06810.x. [DOI] [PubMed] [Google Scholar]
- 18.Murali P.S., Kumar A., Choi H., Banasal N.K., Fink J.N., Kurup V.P. Aspergillus fumigatus antigen induced eosinophilia in mice is abrogated by anti-IL-5 antibody. J. Leukoc. Biol. 1993;53:264–267. doi: 10.1002/jlb.53.3.264. [DOI] [PubMed] [Google Scholar]
- 19.Becker K.L., Gresnigt M.S., Smeekens S.P., Jacobs C.W., Magis-Escurra C., Jaeger M., Wang X., Lubbers R., Oosting M., Joosten L.A., Netea M.G., Reijers M.H., van de Veerdonk F.L. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin. Exp. Allergy. 2015;45:423–437. doi: 10.1111/cea.12354. [DOI] [PubMed] [Google Scholar]
- 20.Hirota S., Kobayashi Y., Ishiguro T., Nishida T., Kagiyama N., Shimizu Y., Takayanagi N. Allergic bronchopulmonary aspergillosis successfully treated with mepolizumab: case report and review of the literature. Respir. Med. Case Rep. 2019;26:59–62. doi: 10.1016/j.rmcr.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda N., Miyahara N., Senoo S., Itano J., Taniguchi A., Morichika D., Fujii U., Maeda Y., Kiura K., Kanehiro A. Severe asthma concomitant with allergic bronchopulmonary aspergillosis successfully treated with mepolizumab. Allergol. Int. 2018;67:521–523. doi: 10.1016/j.alit.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Altman M.C., Lenington J., Bronson S., Ayars A.G. Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. Pract. 2017;5:1137–1139. doi: 10.1016/j.jaip.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Terashima T., Shinozaki T., Iwami E., Nakajima T., Matsuzaki T. A case of allergic bronchopulmonary aspergillosis successfully treated with mepolizumab. BMC Pulm. Med. 2018;18:53. doi: 10.1186/s12890-018-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashkenazi M., Sity S., Sarouk I., Bar Aluma B.E., Dagan A., Bezalel Y., Bentur L., De Boeck K., Efrati O. Omalizumab in allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J. Asthma Allergy. 2018;11:101–107. doi: 10.2147/JAA.S156049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurosawa M., Sutoh E. Severe uncontrolled eosinophilic asthma, which responded to benralizumab after failure to respond to mepolizumab. Ann. Allergy Asthma Immunol. 2019;122:431–433. doi: 10.1016/j.anai.2018.12.014. [DOI] [PubMed] [Google Scholar]