Summary
Effectiveness and side-effect profile data on pharmacotherapy for daytime sleepiness in central hypersomnias are largely based upon randomised controlled trials. Evidence regarding the use of combination therapy is scant. The aim of this study was to examine the effectiveness and occurence of drug-related side-effects of these drugs in routine clinical practice. Adult patients diagnosed with a central hypersomnia over a 54-month period at a tertiary sleep disorders centre were identified retrospectively. Side-effects were recorded at every follow-up visit. 126 patients, with a total of 3,275 patient-months of drug exposure, were categorised into narcolepsy type 1 (n=70), narcolepsy type 2 (n=47) and idiopathic hypersomnia (n=9). Modafinil was the most common drug used as a first-line treatment (93%) and in combination therapy (70%). 39% of the patients demonstrated a complete, 25% partial and 36% a poor response to treatment. Combination treatment improved daytime sleepiness in 55% of the patients with residual symptoms despite monotherapy. 60% of patients reported side-effects, and 30% reported treatment-limiting side-effects. Drugs had similar side-effect incidence (p=0.363) and their side-effect profile met those reported in the literature. 27% of the patients received combination treatment and had fewer side-effects compared to monotherapy (29.4% versus 60% respectively, p=0.001). Monotherapy appears to achieve satisfactory symptom control in most patients with central hypersomnia, but significant side-effects are common. Combination therapy appears to be a useful and safe option in patients with refractory symptoms.
Keywords: pharmacotherapy, therapeutic schemes, treatment outcome, NT1, NT2, IH
Introduction
The principal central hypersomnias are narcolepsy, affecting approximately one in every 3000 people (Longstreth et al., 2007), and idiopathic hypersomnia (IH), which is encountered approximately ten times less frequently than narcolepsy in sleep centre cohorts (Billiard and Dauvilliers, 2001).
The current mainstay treatments for daytime sleepiness in central hypersomnias are the wake promoter modafinil, stimulant therapy, and sodium oxybate, the sodium salt of gamma-hydroxybutyrate (GHB). Although lifestyle changes such as addressing problems with sleep hygiene and taking scheduled naps have been shown to be of some benefit, pharmacotherapy is required to overcome hypersomnolence in most cases (Billiard et al., 2006; Morgenthaler et al., 2007).
The current first-line drug of choice in patients with narcolepsy is modafinil (Billiard et al., 2006; Morgenthaler et al., 2007) supported by the results of well-designed, randomized trials (Moldofsky et al., 2000; Schwartz et al., 2004; Schwartz et al., 2003; Schwartz et al., 2005; Ivanenko et al., 2003; Black and Houghton, 2006; Saletu et al., 2004). In IH, while evidence is significantly more limited, modafinil is still considered the first line option (Bastuji and Jouvet, 1988; Morgenthaler et al., 2007; Philip et al., 2014; Mayer et al., 2015). Modafinil is a non-amphetamine wake-promoting agent, thought to act through dopaminergic, adrenergic, serotonergic, and gamma-aminobutyric acid pathways, although its exact mechanism of action is unknown. Major reported side effects (SEs) include headache, nausea, rash, dry mouth, anxiety and anorexia (Littner et al., 2001; Billiard et al., 2006; Broughton et al., 1997; U.S.M.N.M.S.G., 1998; U.S.M.N.M.S.G., 2000).
Due to lack of evidence on benefit-to-risk ratios from existing studies, amphetamines and methylphenidate are considered second line treatments for narcolepsy and IH (Billiard et al., 2006; Morgenthaler et al., 2007), except in paediatric populations, where methylphenidate is used as a first-line treatment due to concerns regarding hypersensitivity reactions with modafinil. Major SEs of these agents include palpitations, tachycardia, hypertension, insomnia, diarrhoea, constipation, anorexia and infrequently psychotic episodes. In comparison to amphetamines, methylphenidate is less frequently associated with loss of appetite and increased blood pressure but it is equally associated with insomnia, tachycardia, headache, dizziness and nervousness (Mitler et al., 1994; Littner et al., 2001; Guilleminault et al., 1974). Scant data exist examining the utility and safety of these drugs used in combination.
Sodium oxybate is currently authorized by the European Medicines Agency to treat narcolepsy with cataplexy in adults, and by the US Food and Drug Administration (FDA) to treat cataplexy in patients with narcolepsy, with an “expanded indication” for the treatment of excessive daytime sleepiness (EDS)(Billiard et al., 2006; Morgenthaler et al., 2007). In routine European clinical practice, and particularly in the UK, sodium oxybate is reserved for a sub-group of patients with narcolepsy with cataplexy, patients more likely to be on multiple other therapies with a more severe phenotype (Drakatos et al.). Enuresis, nausea, dizziness and headache may commonly develop while on sodium oxybate treatment, especially on higher doses, while dizziness and gait problems may be experienced if patient wakes while the drug is still effective (X.I.S.G., 2002; X.I.S.G., 2003; X.I.S.G., 2004; X.I.S.G., 2005; Mamelak et al., 2015; European et al., 2011). The abuse potential of the drug is an additional concern (Billiard et al., 2006). None of the aforementioned drugs are considered safe for administration during pregnancy (Littner et al., 2001).
Except for a retrospective study comparing the efficacy of sodium oxybate and modafinil in patients with narcolepsy and cataplexy, no direct comparison data exist with the traditional stimulants, and the latter tend to be reserved for those patients who do not benefit from or are unable to tolerate modafinil (Black et al., 2016; Billiard et al., 2006; Morgenthaler et al., 2007).
The primary aim of this study was to provide a snapshot into current clinical practice and estimate the extent and distribution of drug-related side effects in patients with narcolepsy and IH. Effectiveness of the drugs as monotherapy or combination treatment is discussed.
Methods
Patient selection
Drug-naive patients with a diagnosis of narcolepsy or IH were identified retrospectively from the medical records, on the basis of nocturnal polysomnography (NPSG) followed by multiple sleep latency test (MSLT), in combination with the clinical presentation at a tertiary referral sleep disorders centre between June 2009 and November 2013. All patients had been evaluated by a sleep physician and had completed a sleep diary and/or two weeks of actigraphy prior to their sleep studies. A standardised pharmacological approach was shared between clinicians, with modafinil offered as first option followed by methylphenidate, amphetamines and sodium oxybate. The criteria to add another drug to monotherapy or combined therapy based on the above pharmacological approach, was if partial response was observed and no intolerable SEs have occurred. Methylphenidate was not combined with amphetamines. Approval for this study was obtained from Guy's and St Thomas' Hospital review board on human research (project number 4262).
The NSPG and MSLT assessment was performed as per current recommendations (Berry R. et al., 2012.; Littner et al., 2005).
Cases were subsequently reviewed and diagnoses of narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and IH were made according to the International Classification of Sleep Disorders, 3rd edition (ICSD-3) criteria (A.A.S.M., 2014.). Patients with diagnostic uncertainty, such as the presence of psychiatric disease, incomplete clinical information, less than 6 hours of sleep during the NPSG, technical issues, failure to stop sleep-interfering medications and lack of compliance with the treatment of other sleep disorders, were excluded. The presence of additional sleep disorders, such as periodic limb movement during sleep (PLMS) or sleep disordered breathing, was not automatically a basis for exclusion if patients were already treated or the conditions were deemed to have no clinical significance, e.g. where an NT1 diagnosis was based on the presence of clear cataplexy coupled with pathognomonic sleep studies, or when repeated polysomnographic assessment did not confirm the previously seen increased number of periodic limb movements during sleep, but ICSD-3 criteria were fulfilled for NT2 diagnosis. Only patients with a follow-up (FU) period of at least six months were included.
Data collection
A full medical history, Epworth Sleepiness Scale (ESS) scores, sleep history, sleep study results and demographics were recorded for all patients. Treatment response regarding EDS and at the last FU, was assessed qualitatively by considering a three-group response categorisation, in line with two previously published studies (Drakatos et al., 2016; Ali et al., 2009). These three categories consisted of: ‘complete response’, which correlated with adjectives such as ‘excellent’, ‘great’ or ‘entirely satisfactory’, provided there was no change in pharmacotherapy; ‘partial response’, which correlated with phrases such as ‘doing better’ or ‘improved’, but in the setting of increased dose adjustment of stimulant medication or adding another drug; and ‘no or poor response’, which associated with phrases such as ‘still sleepy’ or ‘discontinued due to development of SEs’ and/or with a subsequent switch to another medication. In order to assess the validity of the outcome measurement scale, an inter-rater agreement analysis was performed on scoring of two blinded independent raters (KP, PD) for 20 patients. The agreement was found to be high (Cronbach’s a = 0.895)(Cronbach, 1951), and the residual disagreements between raters were discussed further on a patient by patient basis until consensus was reached.
Safety and tolerability were evaluated based on the reported SEs, without a pre-established list of SEs, during every FU visit per monotherapy and combined therapeutic regimes and per diagnosis group. Patients were informed of possible drug-related SEs and were advised of gradual dose increases followed by monthly FUs until a stable dose had been achieved for each initiated drug, depending on treatment response and potential side effects. Subsequently patients were followed up routinely on an annual basis unless a need occurred for an earlier appointment. Sleep-specialised pharmacist emergency appointments were also offered to patients to assist with occurring SEs at any point and during dose escalation. SEs were categorized into groups: namely infections, psychiatric, neurological, gastrointestinal, general, sleep disorders and cardiological SEs in keeping with those listed in the summary of the products characteristics (XYREM.; Modafinil.; Methylphenidate.). Reports from patients' notes of mood changes throughout the day, which could vary from elevated mood to anger to sadness within a few hours, and changes in mood clearly out of proportion to circumstances which could also cause impairment in functioning, were grouped under the term mood swings. Psychotic symptoms were confirmed by formal psychiatric assessment and were made according to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-V) criteria (A.P.A., 2013.).
Patients exposed to sodium oxybate were limited due to restrictions that apply in UK and thus precluded valuable conclusions. To avoid selection bias these were included in the cohort and overall analysis, but drug-related analysis is limited to modafinil and stimulants.
Statistical Analysis
SPSS statistical analysis program (IBM, V20.0, Chicago, IL/US) was used for statistical analysis. Data is reported as mean ± standard deviation (SD), unless stated otherwise. The association of categorical variables was analysed using Fisher-Freeman-Halton test and Chi-square test as appropriate. Following testing for normality, the similarity of the means for two groups was compared by using Mann-Whitney U test and for three groups the Kruskal–Wallis test with Dunn’s Multiple Comparison Test as and when needed. Ordinal regression analysis was applied to evaluate potential predictors of treatment outcome. A value of p<0.05 was considered as statistically significant.
Results
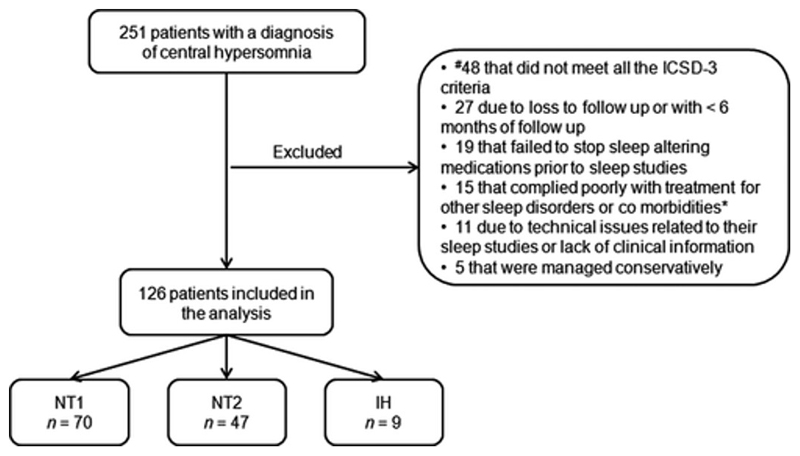
Retrospective data collection over a four and a half-year period identified 251 previously treatment naïve patients with an initial diagnosis of central hypersomnia. 125 patients were finally excluded from the analysis as outlined in figure 1.
Fig. 1. Flow chart of the patients included in the analysis.
ICSD-3, International Classification of Sleep Disorders 3d edition 27; #, 21 patients with less than 6 hours of sleep time on the nocturnal polysomnography; 17 NT2 patients who had less than 2 sleep-onset REM periods on repeated polysomnographic assessments; 10 IH patients with a total 24-hour sleep shorter than 660 minutes and sleep efficiency significantly less than 90%*, psychiatric disorders; NT1, narcolepsy type 1; NT2, narcolepsy type 2; IH, idiopathic hypersomnia.
The 126 patients (37% male) were classified into NT1 (n = 70), NT2 (n = 47), and IH (n = 9). Hypocretin values were limited to 10 patients. 39 patients had been HLA typed for the genetic locus DQB1 *0602, of whom 13 were positive and diagnosed with NT1 and 26 negative and belonged in the NT2 group. One patient with IH exhibited a mean sleep latency of >8min and the final diagnosis was based on an increased 24-hour sleep time combined with the presence of clinical manifestations associated with IH, such as morning sleep drunkenness and unrefreshing long daily naps.
There were no significant differences in the age, gender distribution or body mass index between the diagnostic categories. However, the NT1 group was subjectively sleepier, as measured by the ESS, than the NT2 (p = 0.021) and both NT1 and NT2 groups were objectively sleepier compared to IH group based on the mean sleep latency (MSL) (p=0.001 and p=0.024 respectively). With regard to sleep architecture, the NT1 group had significantly lower sleep efficiency (p=0.002) with higher percentage of sleep stage 1 (N1) when compared to the NT2 group, (p = 0.001). (Table 1)
Table 1. Diagnostic groups, demographics and sleep parameters of the cohort.
| Diagnosis | |||||
|---|---|---|---|---|---|
| All (n = 126) | NT1 (n = 70) | NT2 (n = 47) | IH (n = 9) | P-value | |
| Demographics | |||||
| Gender (male/female) | 47/79 | 28/42 | 16/31 | 3/6 | 0.782* |
| Age (years) | 37.9 ± 13.1 | 38.3 ± 13.9 | 38. ± 12.4 | 33.4 ± 9.1 | 0.687 |
| BMI (kg m2) | 29.3 ± 7.3 | 30.4 ± 7.5 | 27.9 ± 7.1 | 26.0 ± 4.6 | 0.135 |
| ESS | 17.5 ± 4.1 | 18.6 ± 3.5 | 16.2 ± 4.4 | 16.2 ± 4.7 | 0.021† |
| Sleep parameters | |||||
| TST (min) | 387.4 ± 72.5 | 373.8 ± 73.8 | 406.4 ± 69.5 | 393.8 ± 28.1 | 0.223 |
| Sleep efficiency (%) | 82.3 ± 14.5 | 78.6 ± 16.3 | 87.1 ± 10.2 | 87.8 ± 4.4 | 0.002† |
| Sleep onset (min) | 10.3 ± 20.5 | 10.6 ± 24.6 | 9.5 ± 13.6 | 16.0 ± 3.0 | 0.106 |
| REM latency (min) | 62.0 ± 61.9 | 59.8 ± 66.9 | 62.8 ± 55.9 | 74.0 ± 57.0 | 0.486 |
| AHI | 3.4 ± 6.1 | 3.4 ± 5.4 | 3.6 ± 7.3 | 0.8 ± 1.0 | 0.711 |
| PLMI | 15.5 ± 29.3 | 18.7 ± 34.6 | 9.92 ± 17.9 | 3.3 ± 5.0 | 0.678 |
| REM duration (min) | 79.9 ± 35.4 | 75.3 ± 34.7 | 86.5 ± 36.5 | 78.5 ± 28.1 | 0.571 |
| N1 (% of TST) | 7.1 ± 4.2 | 8.4 ± 4.4 | 5.5 ± 3.2 | 4.93 ± 1.7 | 0.001† |
| N2% | 36.4 ± 10.4 | 35.1 ± 10.0 | 37.9 ± 10.5 | 41.3 ± 15.4 | 0.302 |
| M3% | 23.3 ± 8.6 | 21.4 ± 8.4 | 25.6 ± 8.3 | 26.6 ± 11.2 | 0.078 |
| REM% | 17.5 ± 6.8 | 16.5 ± 7.2 | 18.8 ± 6.1 | 18.0 ± 5.8 | 0.311 |
| Arousal index | 20.1 ± 11.8 | 21.8 ± 13.2 | 17.7 ± 9.5 | 20.2 ± 1.0 | 0.252 |
| MSL (min) | 4.2 ± 3.1 | 3.5 ± 2.9 | 4.2 ± 2.5 | 8.4 ± 4.7 | 0.001† |
NT1, narcolepsy type 1; NT2, narcolepsy type 2; IH, idiopathic hypersomnia; BMI, body mass index; ESS, Epworth Sleepiness Score; REM; rapid eye movement; AHI, apnea–hypopnea index; PLMI, Periodic Limb Movement Index; N1, Stage 1 sleep; N2, Stage 2 sleep; N3, Stage 3 sleep; TST, total sleep time; MSL, mean sleep latency.
Analysis was performed using chi-square test for the diagnostic categories.
Post-hoc test showed significant difference between diagnostic categories: ESS of NT1 versus NT2, P = 0.021; sleep efficiency (%) of NT1 versus NT2, P = 0.002; N1 (% of TST) of NT1 versus NT2, P = 0.001; MSL of NT1 versus IH, P = 0.001; MSL of NT2 versus IH, P = 0.024.
Effectiveness
The FU time for our cohort had a median 25.4 months (interquartile range 12.8-38.2), with a total of 3,275 patient-months of drug exposure. At last FU, 49 (39%) patients demonstrated a complete response to treatment, 32 (25%) partial and 45 (36%) a poor response to treatment. The change in ESS (ΔESS) between these response groups was statistically significant (Table 2).
Table 2. Three-group response to treatment categorization.
| Required criteria |
|||||
|---|---|---|---|---|---|
| Response | Comments from notes | Changes to medication | On monotherapy at last FU, n = 92 (%)* | On combined treatment at last FU, n = 34 (%) | ΔESS (no. of patients available)† |
| Complete response | ‘Great, excellent, entirely satisfactory, very well indeed’ | None | 43 (47) | 6 (18) | −9.83 ± 4.13 (18) |
| Partial response | ‘Reasonably well controlled, doing better, better overall’ | Dose increase or drug added | 19 (21) | 13 (38) | −4.38 ± 4.91 (16) |
| Poor response | ‘Still sleepy, has not done well, intolerable SEs’ | Medication changed | 30 (33) | 15 (44) | −3.95 ± 5.16 (20) |
ESS, Epworth Sleepiness score; SEs, side effects; FU, follow-up.
P = 0.009. Comparison of treatment outcome between monotherapy and combined treatment using chi-square wilh Cramer’s V product.
P = 0.001. Analysis was performed using the Kruskal–Wallis lest with Dunn’s multiple comparison lest. Complete response versus poor response, P = 0.002. Complete response versus partial response, P = 0.005. Δ: delta. Dala are presented as mean ± standard deviation (SD).
The mean daily doses of prescribed monotherapy at the last FU are shown in table 3. Modafinil, as per current recommendations, was the most common first-line treatment used in our cohort (93%) (Billiard et al., 2006; Morgenthaler et al., 2007). Of those, 63% remained on the drug and 80% of these were receiving it as a monotherapy at their last FU. Methylphenidate was the next most commonly prescribed drug (39%) for our patients and, as with modafinil, the majority of those (59%) remained on the drug at their last FU. Only 20% of the patients received amphetamines and 14% sodium oxybate, with the majority remaining on these drugs on their last FU (92% and 67% respectively). Comparison of treatment responses between monotherapies, excluding sodium oxybate, did not reveal any significant difference (p=0.109). (Table 3)
Table 3. Distribution of patients on drugs used and trealmenl responses.
| Medication | Pt exposed to drug at any time, no. (% total) | Pts' initial treatment, no. (% total) | Pt on drug at last visit, as in combination no. (% total exposed) | Pt on drug at last visit, as monotherapy no. (% total exposed) | Pt on drug at last visit on. (% total exposed) | Pt with complete/partial/poor response, as monotherapy no. (% total exposed as monotherapy)* | ΔESS as monotherapy in last visit (no. with ESS) | Dose (mg) |
|---|---|---|---|---|---|---|---|---|
| Modafinil | 118/126 (94) | 117/126 (93) | 15/118 (13) | 59/118 (50) | 74/118 (63) | 39 (33) | 6.0 ± 5.4 (51) | 278.9 ± 111.0 |
| 9(8) | ||||||||
| 69 (59) | ||||||||
| Methylphenidate ER/IR | 49/126 (39) | 5/126 (4) | 9/49 (18) | 20/49 (41) | 29/49 (59) | 8 (20) | 3.1 ± 4.7 (23) | 19.1 ± 9.5 |
| 8 (20) | ||||||||
| 24 (60) | ||||||||
| Amphetamines | 25/126 (20) | 1/126 (1) | 8/25 (32) | 15/25 (60) | 23/25 (92) | 3 (19) | 3.3 ± 3.4 (9) | 19.2 ± 12.9 |
| 3(19) | ||||||||
| 10 (62) | ||||||||
| Sodium oxybate | 18/70 (26) | 0/70 (0) | 9/18 (50) | 3/18 (17) | 12/18 (67) | 2(25) | − | 6.1 ± 2.7 (g) |
| 0(0) | ||||||||
| 6(75) |
Data as presented as mean ± standard deviation (SD). Poor response includes the patients who tired and discontinued the drug at any point either because of lack of efficacy or development of limiting side effects.
The denominator used for the first two columns for patients exposed to sodium oxybate reflects the number of patients with narcolepsy type 1.
ESS, Epworth sleepinees scale; no., number; Pt, patients; ER, extended release; IR, immediate release.
Fisher–Freeman–Halton test did not reveal significant differences in treatment response between modafinil and stimulants (P = 0.109).
92 (73%) of our patients were on monotherapy and 34 (27%) on a combination treatment at their last FU. The two groups did not differ significantly in age, BMI, gender and diagnoses (p>0.05). Comparison of treatment outcome between monotherapy and combination treatment at last FU showed better symptom control in patients receiving monotherapy (p=0.009). (Table 2) Combination therapy was offered to patients that had a partial response to monotherapy and a transition to complete response was seen in 17.6% of these cases. Another 38.2% reported improvement in EDS compared to monotherapy but did not fulfill the criteria to enter the complete response group. Treatment outcome could not be predicted by demographics, diagnoses or drug choice (p=0.276). Comorbidities and concomitant medication were not associated with treatment outcome (p=0.303 and p=0.0.439 respectively), or with the number of drugs patients were receiving for EDS treatment at the last FU (p=0.913 and p=0.899 respectively).
52.5% (37/70) of NT1 patients were receiving antidepressants as part of their cataplexy treatment, and there was no association with treatment outcome (p=0.276), or with the number of medications required for EDS treatment at last FU (p=0.054).
Safety
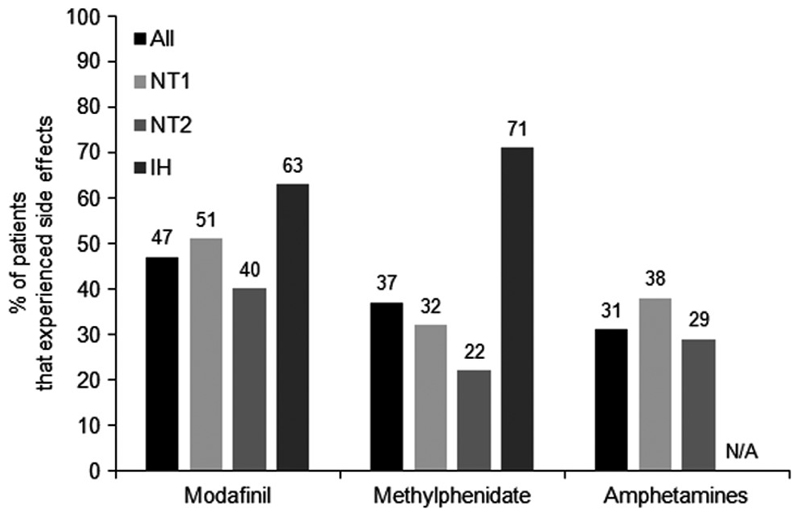
Almost 2/3 of patients (76/126) reported drug-related SEs when receiving monotherapy. As a proportion of those exposed to the respective monotherapy, (47%) of patients on modafinil reported SEs followed by methylphenidate (37%), and amphetamines (31%) (p=0.363). Patients that experienced SEs while on any monotherapy were marginally younger compared to those that did not (35.5±12.6 versus 40.0±13.1, p=0.048) and did not differ significantly in BMI (p=0.225), gender distribution (p=0.171) and drug doses (p>0.05, table 4). SEs incidence was not dose-dependent for modafinil, methylphenidate and amphetamines (p=0.559, p=0.511 and p=0.702 respectively) nor associated with the ESS as recorded in the first appointment, or the diagnoses (p=0.587 and p=0.121 respectively). (Fig. 2).. A weak inverse association between age and SEs was found (r=-0.177, p=0.048). Further analysis showed that comorbidities and concomitant medications were not associated with SEs incidence (p=0.569 and p=0.310 respectively) nor with antidepressants prescribed for cataplexy in NT1 patients (p=0.632). Insertion of Fig. 2.
Table 4. Recorded side effecls per monotherapy.
| Side effects | Monotherapy |
||
|---|---|---|---|
| Modafinil (n = 117) |
Methylphenidate (n = 40) |
Amphetamines (n = 16) |
|
| Cardiac | |||
| Hypertension | 3 (2.7%) | 0 (0%) | 0 (0%) |
| Palpitations | 10 (8.9%) | 3 (7.5%) | 1 (6.2%) |
| Gastrointestinal | |||
| Constipation | 1 (0.89%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 4 (3.6%) | 1 (2.5%) | 2 (12.5%) |
| Faecal incontinence | 1 (0.89%) | 0 (0%) | 0 (0%) |
| Nausea | 4 (3.6%) | 1 (2.5%) | 1 (6.2%) |
| Vomit | 1 (0.89%) | 0 (0%) | 0 (0%) |
| General | |||
| Asthaenia (fatigue) | 1 (0.89%) | 0 (0%) | 0 (0%) |
| Neurological | |||
| Dizziness | 0 (0%) | 4 (10%) | 0 (0%) |
| Headache | 17 (15.2%) | 1 (2.5%) | 2 (12.5%) |
| Impaired concentration | 3 (2.7%) | 0 (0%) | 0 (0%) |
| Muscle cramps | 2 (1.8%) | 0 (0%) | 0 (0%) |
| Psychiatric | |||
| Anxiety | 5 (4.4%) | 6 (15%) | 0 (0%) |
| Hallucinations | 0 (0%) | 0 (0%) | 0 (0%) |
| Mood swings | 12 (10.7%) | 5 (12.5%) | 1 (6.2%) |
| Psychosis | 0 (0%) | 0 (0%) | 1 (6.2%) |
| Suicidal ideation | 1 (0.89%) | 0 (0%) | 0 (0%) |
| Shin | |||
| Skin rash | 3 (2.7%) | 0 (0%) | 0 (0%) |
| Sleep disorders | |||
| Enuresis | 0 (0%) | 0 (0%) | 0 (0%) |
| Insomnia | 4 (3.6%) | 1 (2.5%) | 0 (0%) |
| Sleepwalking | 0 (0%) | 0 (0%) | 0 (0%) |
| Dose (mg)* | |||
| Side effect occurred | 285.8 ± 102.5 | 22.4 ± 12.6 | 25.0 ± 16.1 |
| No side effect | 283.3 ± 122.8 | 21.3 ± 12.3 | 18.0 ± 12.3 |
Patients could have presented with more than one side effect for each drug.
Data are presented as mean ± standard deviation (SD). Comparison between drug doses based on the occurrence or not of a side effect: modafinil, P = 0.800; methylphenidale, P = 0.826; amphetamines, P = 0.675.
Fig. 2. Proportion of patients on each monotherapy that experienced any side effect and per diagnosis.
NT1: narcolepsy type 1, NT2: narcolepsy type 2, IH: idiopathic hypersomnia; N/A, not applicable. Amphetamines were not prescribed in patients with IH..
Headache (15.2%), mood swings (10.7%) and palpitations (8.9%) were the most commonly reported SEs from patients receiving modafinil. Patients on methylphenidate were primarily affected by induced anxiety (15%) and mood swings (12.5%), and patients on amphetamines by dyspepsia (12.5%) and headache (12.5%). (Table 4)
30% of our patients experienced SEs that failed to resolve, or were severe or intolerable while receiving monotherapy, leading to a change of their medication. Based on our experience, the timing of the drug discontinuation was primarily affected by patient's subjective experience of SE intensity along with the severity of the SE, namely palpitations and psychosis. Almost one third of the patients receiving modafinil or amphetamines had to discontinue the drug, as did 20% of patients that received methylphenidate. When the above discontinuation rates are expressed as a proportion of those that experienced SE for each drug, then all patients onamphetamines had finally to stop the drug, while the respective percentages for modafinil and methylphenidate were 70% and 53%. The use of modafinil was most commonly ceased due to headache (21%), mood swings (15%) and cardiovascular incidents (12.5%), including palpitations and hypertension; methylphenidate most commonly caused dizziness/disorientation (37.5%) and mood swings (37.5%); amphetamines were primarily discontinued due to gastrointestinal (GI) problems such as nausea and dyspepsia (40% of the reported causes). One incident of psychosis and one of suicidal ideation were reported in patients on dexamphetamine and modafinil respectively, none of whom had a previous relevant psychiatric history. Importantly, these symptoms resolved after discontinuation of the responsible drug in both patients.
Patients reporting intolerable SEs were compared with patients with no SEs, and those that experienced mild or resolving SEs. The former group consisted of younger patients compared to those with no SEs (33.86±12.56 versus 40.04±12.89 years, p=0.006), but did not reach the level of significance against those with mild or resolving SEs (33.86±12.57 versus 39.94±12.05, p=0.055). BMI and gender distribution did not differ significantly between groups (p=0.591 and p=0.376 respectively). Drug doses for modafinil, methylphenidate and amphetamines also did not differ significantly between groups (p=0.194, p=0.513 and p=0.186 respectively) and no dose-dependent effect was found for the occurrence of intolerable SEs in the cohort (p=0.764, p=0.649 and p=0.299 respectively). Age was inversely correlated with the presence of intolerable SEs (r=-0.245, p=0.006).
With regard to combination therapy, 27% (34/126) of our patients were exposed and 10 (29.4%) of those reported SEs, which led to treatment discontinuation in 8/34 (23.5%) of the patients. The most commonly combined therapeutics were modafinil/methylphenidate (35%), modafinil/amphetamines (23.5%) and methylphenidate/sodium oxybate (17.6%), with similar treatment outcome and SEs incidence (p=0.320 and p=0.111 respectively). 70% of the combination therapies included modafinil and accounted for 79% of the complete and partial response seen in patients receiving combination therapy. The majority of the patients exposed to combination therapy were diagnosed with NT1 (82%). Combined treatment was associated with lower SEs incidence compared to monotherapy (29.4% versus 60% respectively, p=0.001)
Discussion
In this study, we analysed drug-related SEs and effectiveness in clinical practice following current recommendations on EDS treatment approach in hypersomnias of central origin. The exposure to monotherapy was associated with frequently reported SEs and a high discontinuation rate. Recorded SEs were consistent with previously reported profiles for the specific drugs. Patients that were exposed to combination therapy (27%), reported a lower incidence of SEs (29.4%, p=0.001). Combination therapy improved EDS in 55% of the patients previously receiving monotherapy, and offered a complete or partial resolution of symptoms in a quarter of our cohort. These findings should however be interpreted through the prism of clinical practice standards and guidelines application rather than as a direct comparison of drug safety and effectiveness.
In this study, current recommendations on pharmacotherapy were followed but were influenced by the restrictions on sodium oxybate administration that apply in UK, with the majority of the patients being initiated on modafinil (93%), leaving the traditional stimulants as a second line treatment when modafinil usage was limited either by lack of effectiveness or limiting SEs. Nevertheless the recorded SEs meet the existing safety profiles for modafinil and stimulants (Littner et al., 2001; Billiard et al., 2006; Ali et al., 2009) and no dose-dependent association was found, as seen in the literature (Mitler et al., 2000). In our study there was a weak association of SEs with younger age, in contrast with prior data from our group examining the use of sodium oxybate (Drakatos et al.).
Interestingly, while insomnia is considered a significant side effect associated with wake promoters and stimulant usage, in our study insomnia very rarely led to drug discontinuation as sleep problems were usually resolved with better drug timing administration or/and improved sleep hygiene. Cardiovascular related SEs were primarily associated with modafinil administration, with fewer seen with methylphenidate use, and in contrast to the existing literature, none with amphetamines (Billiard et al., 2006; Littner et al., 2001). Since SEs were not dose-dependent, a possible explanation could be that the worse safety profile of amphetamines might have led to a more cautious approach with regard to dose escalation as evidenced from table 3 compared to modafinil. Furthermore, amphetamines, due to their rapid boost effect on alertness and the awareness of its worse safety profile, may be more commonly used by patients on an as needed basis rather than on a regular basis. In our study the standard approach and recommendation for each drug administered was to be used on a regular basis. It has been noticed though, that patients could at times and depending on their personal preference, daily commitments, sensitivity to drugs and ability to cope with sleepiness, take drugs on as needed basis for a few days a month which may have impacted our results.
In line with existing data, mood swings, nervousness, anxiety and emotional lability were common causes of drug discontinuation (Littner et al., 2001; Billiard et al., 2006). Despite the close monitoring of the patients at the initiation of each drug and until stable dose had been achieved, drug discontinuation due to SEs remained an individual decision. This means that in cases where the drug was stopped after only a few doses, not enough time would have passed to possibly let SEs to diminish or resolve, and will potentially have affected our results. Only 14% of our patients were exposed to sodium oxybate, reflecting the licensing and financial restrictions that apply in the UK, limiting the evaluation capacity of its safety profile.
As mentioned above, there is little published evidence examining the safety of combination therapy in this area (Black et al., 2016). 27% of our patients were exposed to combinations of drugs. The SE rate of combination therapy compared to monotherapy appears to be lower (p=0.001). These data contradict with those reported by Black et al. where 23 patients with unknown response to stable doses of modafinil received sodium oxybate starting at 6g/night for four weeks and then 9g/night for the second four week period. This study reported a marginally higher incidence of SEs rate compared to monotherapy and placebo (p=0.040) (Black et al., 2016). In our study 34 patients received combination therapy after partial response to monotherapy was seen, and our data for that specific combination of drugs, i.e. modafinil and sodium oxybate, is limited to 6 patients, who had sodium oxybate gradually up titrated starting from 4.5g/night. Thus direct comparison of the two studies is problematic. Our results once again reflect prescribing practices, since this cohort would not include patients vulnerable to intolerable SEs on monotherapy in the first instance. Nevertheless, these data do lend support to the use of combination therapy of modafinil plus another drug as a reasonable strategy in routine clinical practice.
Effectiveness data need to be interpreted with caution. At first glance these data imply that modafinil is more effective than other therapies, but once again, due to prescribing practices, this likely illustrates that patients refractory to treatment with wake promoters may still benefit from stimulant medication. Thus, it does support the current strategy of escalating pharmacotherapy if there is little or no response to modafinil, suggesting that methylphenidate, amphetamines and sodium oxybate provide additional benefit above and beyond modafinil in the treatment of EDS (Billiard, 2008; Black and Houghton, 2006). Furthermore, combination of these drugs when a partial response to monotherapy is seen is also supported by our data. While monotherapy was found to be more effective compared to combination therapy, this likely reflects that the combination therapy was reserved for patients with EDS refractory to monotherapy. In 55% of these patients combination therapy offered a complete or improved partial response which combined with the lower SE incidence for combination therapy, clinically justified the continuation of the drugs.
Limitations
Due to the retrospective nature of the study, we were unable to comment on the compliance of patients to their pharmacotherapy which could alter the frequency of SEs experienced. Our findings reflect on 50% of the actual clinical cohort, due to strict exclusion criteria detailed in Figure 1, and also cannot reflect reliably on patients with IH due to their small number. Similar limitation applies to conclusions for amphetamines safety and effectiveness, and to a lesser extent to sodium oxybate. Furthermore, the use of the ESS and the three-response categorisation assessment rely on subjective rather than objective assessment of EDS. The retrospective nature of the study has also resulted in the incomplete capture of ESS scores, as seen in table 2. Nonetheless our results support previous studies (Ali et al., 2009; Drakatos et al., 2016). While drawing this sample from a single institution signifies the utilisation of a consistent approach in data collection, a pre-established side effects list would have offered a more standardised assesstment.
Clinical practice in the management of hypersomnias of central origin is not expected to be universal. There remain discrepancies between European Medicines Agency (EMA) and US Food and Drug Administration (FDA) drugs licensing. In addition to the extended indication of sodium oxybate for EDS in US compared to Europe, armodafinil has also received approval for narcolepsy in US but not in Europe, while pitolisant was recently approved in Europe only. As previously discussed, country-specific policies also apply as with sodium oxybate in UK and mazindol which is only easily available in France (Thorpy and Dauvilliers, 2015). This study provides potentially valuable information based on daily clinical experience in a UK based tertiary sleep centre, and prospective multi-centre international studies would be required to add credence to our findings and explore treatment in a holistic approach.
Antidepressants have not received approval for NT1, either from the EMA and FDA, and are used as off label. In this study we focused on EDS control and safety profile of the drugs, irrespective of cataplexy control.
Conclusion
Following current recommendations on pharmacotherapy and based on clinical practice, monotherapy is a relatively safe and effective option for patients with central hypersomnia and combination therapy should be considered as an alternative in patients with refractory daytime sleepiness.
Acknowledgements
Dr Steier’s contribution was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Dr Rosenzweig is supported by the Wellcome Trust [103952/Z/14/Z]. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
This is not a financially supported or off-label or investigational use, research study.
Footnotes
CT, KP, PD, GD, BK, AN, HS, IR, JS report no disclosures.
Prof. Williams reports previous fee from UCB Pharma as a speaker. Prof. Williams reports no conflicts of interest.
Dr Leschziner reports a received honorarium from UCB Pharma and Somnomed for an advisory board. Dr Leschziner reports no conflicts of interest.
Author contributorship
Data acquisition, analysis, writing and discussion: KP, CT
Concept, writing and interpretation: GD, AN, HS, JS, IR, AW
Analysis, concept, writing, design and interpretation: BK, GL, PD
References
- A.A.S.M. International classification of sleep disorders 3rd edn. American Academy of Sleep Medicine; Darien, IL: 2014. [Google Scholar]
- A.P.A. Diagnostic and Statistical Manual of Mental Disorders 5th edn. American Psychiatric Association; Washingcton, DC: 2013. [Google Scholar]
- Ali M, Auger RR, Slocumb NL, Morgenthaler TI. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med. 2009;5:562–568. [PMC free article] [PubMed] [Google Scholar]
- Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:695–700. doi: 10.1016/0278-5846(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Berry R, Brooks R, Camaldo CE, Harding SM, Marcus CL, V BV for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012. www.aasmnet.org. [Google Scholar]
- Billiard M. Narcolepsy: current treatment options and future approaches. Neuropsychiatr Dis Treat. 2008;4:557–566. [PMC free article] [PubMed] [Google Scholar]
- Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- Billiard M, Dauvilliers Y. Idiopathic Hypersomnia. Sleep Med Rev. 2001;5:349–358. doi: 10.1053/smrv.2001.0168. [DOI] [PubMed] [Google Scholar]
- Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29:939–946. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- Black J, Swick T, Bogan R, Lai C, Carter LP. Impact of sodium oxybate, modafinil, and combination treatment on excessive daytime sleepiness in patients who have narcolepsy with or without cataplexy. Sleep Med. 2016;24:57–62. doi: 10.1016/j.sleep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Broughton RJ, Fleming JA, George CF, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 1997;49:444–451. doi: 10.1212/wnl.49.2.444. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Drakatos P, Lykouras D, D’ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. doi: 10.1016/j.sleep.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakatos P, Patel K, Thakrar C, Williams AJ, Kent BD, Leschziner GD. Sleep-stage sequencing of sleep-onset REM periods in MSLT predicts treatment response in patients with narcolepsy. J Sleep Res. 2016;25:203–210. doi: 10.1111/jsr.12363. [DOI] [PubMed] [Google Scholar]
- Gilhus NE, Barnes MP, Brainin M, editors. European Handbook of Neurological Management: Volume 1. 2nd. Blackwell Publishing Ltd; 2011. [Google Scholar]
- Guilleminault C, Carskadon M, Dement WC. On the treatment of rapid eye movement narcolepsy. Arch Neurol. 1974;30:90–93. doi: 10.1001/archneur.1974.00490310092014. [DOI] [PubMed] [Google Scholar]
- Ivanenko A, Tauman R, Gozal D. Modafinil in the treatment of excessive daytime sleepiness in children. Sleep Med. 2003;4:579–582. doi: 10.1016/s1389-9457(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Littner M, Johnson SF, Mccall WV, et al. Practice parameters for the treatment of narcolepsy: an update for 2000. Sleep. 2001;24:451–466. [PubMed] [Google Scholar]
- Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, Van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Swick T, Emsellem H, Montplaisir J, Lai C, Black J. A 12-week open-label, multicenter study evaluating the safety and patient-reported efficacy of sodium oxybate in patients with narcolepsy and cataplexy. Sleep Med. 2015;16:52–58. doi: 10.1016/j.sleep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Mayer G, Benes H, Young P, Bitterlich M, Rodenbeck A. Modafinil in the treatment of idiopathic hypersomnia without long sleep time--a randomized, double-blind, placebo-controlled study. J Sleep Res. 2015;24:74–81. doi: 10.1111/jsr.12201. [DOI] [PubMed] [Google Scholar]
- Methylphenidate. Methylphenidate. Summary of product characteristics. European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Methylphenidate_31/WC500011138.pdf. [Google Scholar]
- Mitler MM, Aldrich MS, Koob GF, Zarcone VP. Narcolepsy and its treatment with stimulants. ASDA standards of practice. Sleep. 1994;17:352–371. [PubMed] [Google Scholar]
- Mitler MM, Harsh J, Hirshkowitz M, Guilleminault C. Long-term efficacy and safety of modafinil (PROVIGIL((R))) for the treatment of excessive daytime sleepiness associated with narcolepsy. Sleep Med. 2000;1:231–243. doi: 10.1016/s1389-9457(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Modafinil. Modafinil. Summary of product charactersitics. European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Modafinil_31/WC500099178.pdf. [Google Scholar]
- Moldofsky H, Broughton RJ, Hill JD. A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy. Sleep Med. 2000;1:109–116. doi: 10.1016/s1389-9457(99)00014-3. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P, Chaufton C, Taillard J, et al. Modafinil improves real driving performance in patients with hypersomnia: a randomized double-blind placebo-controlled crossover clinical trial. Sleep. 2014;37:483–487. doi: 10.5665/sleep.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Saletu-Zyhlarz GM, et al. EEG-tomographic studies with LORETA on vigilance differences between narcolepsy patients and controls and subsequent double-blind, placebo-controlled studies with modafinil. J Neurol. 2004;251:1354–1363. doi: 10.1007/s00415-004-0543-8. [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Feldman NT, Bogan RK. Dose effects of modafinil in sustaining wakefulness in narcolepsy patients with residual evening sleepiness. J Neuropsychiatry Clin Neurosci. 2005;17:405–412. doi: 10.1176/jnp.17.3.405. [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Feldman NT, Bogan RK, Nelson MT, Hughes RJ. Dosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy. Clin Neuropharmacol. 2003;26:252–257. doi: 10.1097/00002826-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Nelson MT, Schwartz ER, Hughes RJ. Effects of modafinil on wakefulness and executive function in patients with narcolepsy experiencing late-day sleepiness. Clin Neuropharmacol. 2004;27:74–79. doi: 10.1097/00002826-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16:9–18. doi: 10.1016/j.sleep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- U.S.M.N.M.S.G. Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. US Modafinil in Narcolepsy Multicenter Study Group. Ann Neurol. 1998;43:88–97. doi: 10.1002/ana.410430115. [DOI] [PubMed] [Google Scholar]
- U.S.M.N.M.S.G. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology. 2000;54:1166–1175. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- X.I.S.G. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Xyrem International Study Group. Sleep. 2002;25:42–49. [PubMed] [Google Scholar]
- X.I.S.G. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Xyrem International Study Group. Sleep. 2003;26:31–35. [PubMed] [Google Scholar]
- X.I.S.G. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy.Xyrem International Study Group. Sleep Med. 2004;5:119–123. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- X.I.S.G. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Xyrem International Study Group. Sleep Med. 2005;6:415–421. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Xyrem. XYREM. Summary of product characteristics. European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000593/WC500057103.pdf.