Abstract
Background
Bone destruction is one of many severe complications that occurs in patients with rheumatoid arthritis (RA) and current therapies are unable to cure this manifestation. This study here aims to determine whether GMSC can directly inhibit osteoclast formation and eventually attenuate osteoclastogenesis and bone erosion in an inflammatory milieu.
Method
GMSC were co-cultured with osteoclast precursors with or without CD39 inhibitor, CD73 inhibitor or adenosine receptors inhibitors pretreatment and osteoclast formation were evaluated in vitro. 2×10^6 GMSC per mouse were transferred to CIA mice and pathology scores, the frequency of osteoclasts, bone erosion in joints were assessed in vivo.
Finding
GMSC but not control cells, markedly suppressed human or mice osteoclastogenesis in vitro. GMSC treatment also resulted in a dramatically decreased level of NF-κB p65/p50 in osteoclasts in vitro. Infusion of GMSC to CIA significantly attenuated the severity of arthritis, pathology scores, frequency of osteoclasts, particularly bone erosion, as well as a decreased expression of RANKL in synovial tissues in vivo. Blockade of CD39/CD73 or adenosine receptors has significantly abrogated the suppressive ability of GMSC in vitro and therapeutic effect of GMSC on bone erosion during CIA in vivo.
Interpretation
GMSC inhibit osteoclast formation in vitro and in vivo partially via CD39-CD73-adenosine signals. Manipulation of GMSC may have a therapeutic implication on rheumatoid arthritis and other bone erosion related diseases.
Fund
This study was supported by grants from the National Key R&D Program of China (2017YFA0105801 to F.H); the Zhujiang Innovative and Entrepreneurial Talent Team Award of Guangdong Province (2016 ZT 06S 252 to F·H) and National Institutes of Health (R01 AR059103, R61 AR073409 and NIH Star Award to S.G.Z).
Keywords: Rheumatoid arthritis, Mesenchymal stem cells, CD39, Adenosine
Research in context.
Evidence before this study
Accumulating studies found that the mesenchymal stem cells (MSC) by virtue of their tissue recovery and immunoregulatory properties have shown broad prospects in various autoimmune and degenerative diseases. Our previous studies showed that transplantation of gingiva-derived MSC ameliorates collagen-induced arthritis, but the underlying mechanism still remains to be explored. Additionally, it is unknown if GMSC can modulate osteoclastogenesis and eventually prevent bone erosion in the inflammation.
Added value of this study
In the study, we found that GMSC suppressed osteoclastogenesis in vitro and bone erosion in vivo. The function was partially dependent on the expression of CD39/CD73 on GMSC, which promotes the production of adenosine in osteoclasts. Blockade of CD39/CD73 expression on GMSC or adenosine receptors expression on pre-osteoclasts significantly abrogated the suppressive ability of GMSC to inhibit the osteoclastogenesis and the therapeutic effect of GMSC on bone erosion during CIA.
Implications of all the available evidence
This article shows a novel observation that human gingiva-derived MSC (GMSC) can play a remarkably suppressive role in the development of osteoclastogenesis, even attenuating the bone destruction in autoimmune-mediated systemic arthritis. Thus, manipulation of GMSC may have a therapeutic implication on patients with rheumatoid arthritis and other bone erosion related diseases.
Alt-text: Unlabelled Box
1. Introduction
Rheumatoid arthritis (RA) is the most common chronic autoimmune disorder associated with significant mortality and morbidity [1,2]. It is a symmetric polyarticular arthritis, progressively exacerbated by inflammatory synovitis, resulting in the destruction of articular cartilage and marginal bone, which is generally thought to be irreversible [3]. Clinical drug development for rheumatologists has progressed slowly. RA patients are normally treated with disease-modifying anti-rheumatic drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs) [1,3], TNF inhibitors [4,5], IL-1 antagonists [6], and/or IL-6 receptor antibody [7,8]. Cumulatively, among the therapeutic approaches, none is curative for RA patients, leading investigators to search novel approaches for this common autoimmune disorder [9].
Bone damage is one of the most severe complications in patients with RA [10]. Osteoclasts are the principal bone resorptive cells that are derived from hematopoietic cells of the myeloid lineage [11]. Osteoclast precursors (OCPs) can differentiate into mature osteoclasts under the stimulation of macrophage colony stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) [12,13]. Thus, any approaches that can control the differentiation of osteoclasts will be attractive in preventing and treating the bone loss in patients with RA.
It has recently been demonstrated that mesenchymal stem cells (MSC) inhibit T-cell proliferation and have a potential role in controlling autoimmune arthritis and other inflammatory diseases [[14], [15], [16]]. Gingival tissue-derived MSC (GMSC), a population of mesenchymal stem cells isolated from human gingiva, has a profound set of immunomodulatory capacities [17]. Recent studies have revealed that GMSC have several advantages over bone marrow-derived mesenchymal stem cells (BMSC). Most importantly, they are homogenous and proliferate more rapidly than BMSC, with stable morphological and functional characteristics at higher passage numbers and are not tumorigenic [18,19].
We have recently observed that GMSC have a potent immune regulatory ability to suppress innate and adaptive immune responses and eventually attenuate the development and progression of inflammatory diseases in several animal models and humanized animal models [[19], [20], [21], [22]]. Additionally, GMSC also enhanced the differentiation of Foxp3+ regulatory T cells and type 2 macrophages [20,21], both are known to be important immune regulators [23,24], suggesting GMSC have broad prospects in controlling autoimmune diseases. Nonetheless, the ability of GMSC to directly suppress the formation of osteoclasts under diseases and inflammation conditions in vitro and in vivo, and their relative importance in bone protection in CIA, has yet to be elucidated.
In the present study, we employed in vitro and in vivo experiments to investigate the role of GMSC in regulating the differentiation and formation of osteoclasts during inflammation. We demonstrate that GMSC directly inhibit the osteoclasts differentiation by reducing the activities of NF-κB. We also observed that GMSC attenuate the disease severities, pathological scores, and bone erosion in CIA. These effects are related to the reduction of osteoclasts in CIA. Moreover, the effects of GMSC on osteoclast and bone protection are associated with the reduction of osteoclasts in vivo during CIA. At the molecular level, GMSC exert their immunological suppression through CD39/CD73/adenosine signal pathway. Collectively, our data suggests that application of GMSC represents a potential therapeutic approach for patients with rheumatoid arthritis and other bone erosions related autoimmune diseases.
2. Materials and methods
2.1. Mice
DBA/1 J mice (female, 8–10 week old) were obtained from Jackson Laboratory (Bar Harbor, ME), bred and kept under standard laboratory conditions in the animal laboratory center of Sun Yat-sen University, Penn State Hershey Medical Center and Ohio State Wexner Medical Center’ Central Animal facility. All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees at three institutes.
2.2. Cell culture
Human gingiva tissue samples were collected following routine dental procedures at the clinic of oral and maxillofacial surgery in the College of Dental Medicine at Nova Southeastern University, the School of Dentistry of USC, and the Division of Dentistry in the Third Hospital at Sun Yat-sen University, which were approved by the medical ethics committees of Institutional Review Boards (IRB) at the Nova Southeastern University, the Third Hospital at the Sun Yat-sen University, the Keck School of Medicine at the University of Southern California and at the Penn State University Hershey Medical Center. The written informed consent was obtained from the donors. The transfer of gingival tissues from the Nova Southeastern University and University of Southern California to the Penn State University Hershey Medical Center was approved by both institutions. Human GMSC were prepared from these samples as previously described [21,25]. Briefly, gingival tissues were obtained from discarded tissues of patients who undergone routine dental procedures. GMSC were isolated within 4 h in medium or within 18 h in medium in wet ice (overnight shipping) after routine dental procedures. Tissues were treated aseptically and incubated with dispase II (2 mg/mL) at 4 °C overnight followed with digestion by collagenase IV (4 mg/mL) at 37 °C for 2 h after being minced into 1–3 mm2 fragments. Then the dissociated cell suspension was filtered through a 40-μm cell strainer (Falcon) and centrifuged to get cell deposition. The cell deposition was re-suspended and plated on a 10 cm petri dish with MEM alpha (Gibco) medium supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin/100 μg/mL streptomycin (Gibco) and cultured at 37 °C in an incubator with 5% CO2 and 95% O2. After cultured for 72 h, the non-adherent cells were removed. The plastic-adherent cells were treated with 0.25% trypsin containing 1 mM EDTA and passaged when they reached about 90% confluent density and sub-cultured continuously. For GMSC characterization markers detection, GMSC from the third passage to the sixth passages were stained with mAbs for human CD90, CD73, CD29, CD105, CD45, HLA-DR and CD31 and assessed by flow cytometry. Cells at second passage were stored at liquid nitrogen no longer than 6 months before use. Fresh cells or stored cells that had been resuscitated from the third to the fifth passages were used in the experiments.
A human dermal fibroblast cell line (ATCC, #PCS-201-012) was obtained from ATCC (https://www.atcc.org/). The primary cultured human dermal fibroblasts were also used as a control of GMSC. Primary human dermal fibroblasts were isolated from the foreskin dermis of children aged between 6 and 8 years who underwent surgery in the Third Affiliated Hospital at the Sun Yat-sen University, which was approved by the medical ethics committee of IRB in the institute. Informed consents were obtained from all guardians of the donor participants. The dermal tissue was washed with PBS for three times, minced into small pieces with sterile scissors, and allowed to adhere to tissue culture flasks for 30 min in a CO2 incubator at 37 °C. Then Dulbecco's modification of Eagle's medium (Corning) supplemented with 10% fetal bovine serum (Gibco) was then added to the flasks. Fibroblasts were allowed to grow out of the tissue pieces and attached to the tissue culture flask. Cells were subcultured when they reached 80–90% confluence. Fibroblasts from the third to the fifth passages were used in the experiments.
2.3. Induction of collagen-induced arthritis
Complete Freund's adjuvant (CFA) was prepared by re-suspending 4 mg of heat-denatured mycobacterium (Chondrex, LLC, Seattle, WA) in 1 mL of incomplete Freund's adjuvant (BD Biosciences). Bovine type II collagen (CII, 2 mg/mL) was emulsified with CFA at a ratio of 1:1 and then injected intradermally into the tail (1.5 cm from the base) of DBA1/J mice to induce collagen-induced arthritis. To determine the intervention effects, mice were divided into different groups randomly, with or without a single intravenous injection of 2 × 10^6 GMSC or 2 × 10^6 CD4 + T cells that had been activated in vitro with anti-CD3/CD28 antibodies on day 14 after immunization. Alternatively, a similar dose of human dermal fibroblast cell line (ATCC, #PCS-201-012) or dermal fibroblasts were injected intravenously as a control of GMSC.
2.4. Evaluation of clinical arthritis
Clinical signs of arthritis were assessed to determine arthritis incidence every 2–3 days. Paws' swelling was measured, evaluated and scored by examiners blinded to the group conditions, using a 0 to 4 scoring system. Scores were summed to yield an individual mouse score, with a maximum score of 16 for each animal. Each paw score was judged as follows: 0, no evidence of erythema and swelling; 1, mild swelling confined to the tarsal bones or ankle joint; 2, mild swelling extending from the ankle to the tarsal bones; 3, moderate swelling extending from the ankle to the metatarsal joints; and 4, severe swelling encompassing the ankle, foot and digits, or ankylosis of the limb.
2.5. CT analysis
Mice were anesthetized with 2% isoflurane. The high-resolution micro-computed tomography (micro-CT) system (Viva CT 40, Scanco, Switzerland) was used to acquire in vivo imaging of the three-dimensional bone. The scans were performed with 3.6 mm length including entire single mouse foot with the following parameters: 17.5 μm voxel size at 55 kV, 145 μA, 200 ms integration time, 211 image slices. The micro-CT images were converted to 8-bit, imported into Mimics software (Materialise, Belgium), then filtered using discrete Gaussian filtering (variance = 1; max kernel width = 1). Volumes of interest at the metatarsophalangeal joint were used to quantify bone erosion as follows. Second through fourth metatarsal and phalangeal bones were segmented from surrounding soft tissue using a consistent image intensity threshold. Three volumes of interest were set with +/− 1 mm length in the distal and proximal direction from the center of each metatarsophalangeal joint. These volumes of interest were oriented consistently based on the 3D longitudinal axis of the third metatarsal. The bone volumes of the three metatarsophalangeal joints were then calculated.
2.6. Osteoclastogenesis
For mouse osteoclastogenesis, CD11b + cells were isolated from bone marrow of WT DBA1/J mice by autoMACS with biotin anti-mouse CD11b antibody (BioLegend) and anti-biotin microBeads (Miltenyi Biotec), purity >95%. Then CD11b + cells were suspended and cultured in 24 well (1.5 × 10^6 cells/well) in α-MEM culture medium (containing 10% FBS) in the presence of mouse M-CSF (50 ng/mL) for 3 days, and the adherent cells were used as osteoclast precursors (OCPs). OCPs were then stimulated with mouse RANKL (50 ng/mL) and mouse M-CSF (50 ng/mL) (R&D systems, Minneapolis, MN, USA) for another 3 days to induce osteoclast formation. For human osteoclastogenesis, human CD14 + cells were isolated from peripheral blood mononuclear cells (PBMC) of healthy subjects by autoMACS with biotin anti-human CD14 antibody (BioLegend) and anti-biotin microbeads (Miltenyi Biotec), purity >95%. Then CD14 + cells were suspended and cultured in 24 well (1 × 10^6 cells/well) in α-MEM culture medium (containing 10% FBS) in the presence of human M-CSF (25 ng/mL) for 7 days and used as human osteoclast precursors (hOCPs). hOCPs were then stimulated for an additional 14 days with human RANKL (50 ng/mL) and human M-CSF (25 ng/mL) to induce human osteoclast formation. To evaluate osteoclast formation, cells were stained with tartrate-resistant acid phosphatase (TRAP) (Sigma, St Louis, MO, USA) according to the manufacturer's instructions, and TRAP+ cells were enumerated by microscopy.
In some experiments, GMSC pretreated with JNK inhibitor (SP600125, Sigma-Aldrich, 25uM), CD39 inhibitor (Sodium polyoxotungstate [POM1]; Tocris Bioscience; 100 μM), CD73 inhibitor (α,β-methylene ADP [APCP]; Sigma-Aldrich; 200 μM), indoleamine-2,3-dioxygenase (IDO) inhibitor (1-methyl-L-tryptophan [1-MT]; Sigma-Aldrich; 500 μM), anti-TGF-β (BD PharMingen; 10 μg/mL), ERK2 inhibitor (5Z-7-Oxozeaenol, Sigma-Aldrich, 5 μM), p38 MAPK inhibitor (SB203580, Sigma-Aldrich, 10 μM), TNF-a antibody (anti-TNF-a,10 μg/mL), overnight were co-cultured with OCPs in the presence of M-CSF and RANKL. As well as OCPs pretreated with selective A2A adenosine receptor competitive antagonist (SCH58261; Tocris Bioscience; 25 μM), selective A2B adenosine receptor antagonist (Alloxazine; Sigma-Aldrich; 10 μM) overnight were co-cultured with GMSC in the presence of M-CSF and RANKL.
2.7. Quantitative real-time PCR
RNA was extracted from harvested cells using RNA kit according to manufacturer's instructions (Tiangen Biotech), cDNA was synthesized using RT-Master Mix (TaKaRa). The cDNA product was amplified by qRT-PCR in ABI prism 7700 sequence-detection system (Applied Biosystem, Foster City, CA) with relevant primers for mice or human. Data were analyzed using the relative gene expression method and were normalized with GAPDH levels in the samples. The measurement of each sample was performed in triplicate. For real-time PCR, the following primers were synthesized by Applied Biosystems: mGAPDH forward, ACC ACA GTC CAT GCC ATC AC, reverse, TCC ACC ACC CTG TTG CTG TA; mRANKL forward, CCA TCG GGT TCC CAT AAA G, reverse, TGA AGC AAA TGT TGG CGTA; mTRAP forward, GCT GGA AAC CAT GAT CAC CT, reverse, GAG TTG CCA CAC AGC ATC AC; mCathepsin K forward, CTT CCA ATA CGT GCA GCA GA, reverse, TCT TCA GGG CTT TCT CGT TC; mNFATc1 forward, TGG AGA AGC AGA GCA CAG AC, reverse, GCG GAA AGG TGG TAT CTC AA; hGAPDH forward, AGG TCG GTG TGA ACG GAT TTG, reverse, GGG GTC GTT GAT GGC AAC A; hENTPD1 forward, CTC AGG AAA AGG TGA CTG AGA T, reverse, CTC CTT TAC TCC AGC GTA AGA T; hNT5E forward, AGC AGC ATT CCT GAA GAT CCA AGC, reverse, GCA TGA TTG AGA GGA GCC ATC CAG; hADORA2A forward, CAA CTA CTT TGT GGT GTC ACT G, reverse, GAC GAA GCA GGC AAT GAA G; hADORA2B forward, CAG CTC AGG GTA AAA ATA AGC C, reverse, TTT GTG AAA AGT GTA GCG GAA G.
2.8. RNA-seq
GMSC and fibroblast cells were prepared as described above, and RNA was extracted using RNA kit according to the manufacturer's instructions (Tiangen Biotech), cDNA was synthesized using RT-Master Mix (TaKaRa). cDNA library construction and Illumina sequencing were completed by Beijing Novogene Bioinformatics Technology Co., Ltd. Briefly, sequencing libraries were generated using NEBNext Ultra RNA Library Prep Kit following manufacturer's recommendations. The products were sequenced on an Illumina HiSeq platform using a 125 bp/150 bp paired-end mode.
2.9. Western blot analysis
Proteins were extracted from harvested cells and their concentration was determined using a BCA assay (Pierce). Protein samples (30 μg) were resolved by SDS-PAGE and transferred to a PVDF membrane. The following antibodies were used: anti-mouse RANKL (Santa Cruz, FL-317), NF-κB P50 (Abcam, ab32360) and NF-κB P65 (Cell signaling, #3033). The results were visualized with Kodak autoradiography film (Kodak XAR film).
2.10. Statistical analysis
For comparison of treatment groups, we performed unpaired t-tests (Mann-Whitney), paired t-tests, and one-way or two-way ANOVA (where appropriate) methods. Percent comparisons were done using the chi-square test. All statistical analyses were performed by GraphPad Prism Software (version 7.00). p < .05 was considered as statistically significant.
2.11. Data availability
RNA-seq data used in this study is available at the NCBI BioProject database under accession number PRJNA540091.
3. Results
3.1. GMSC but not fibroblast cells suppress osteoclastogenesis in vitro
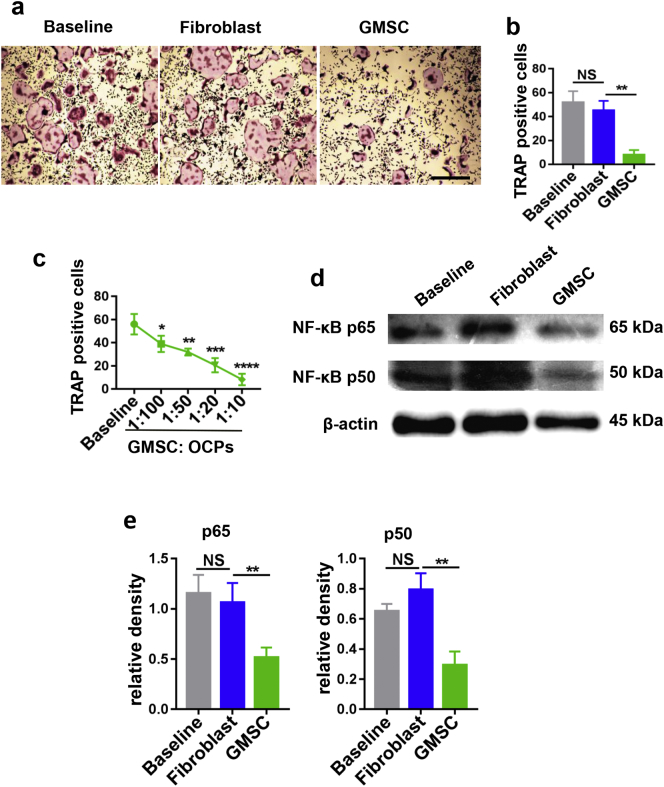
GMSC were verified to express the stem cell phenotypic markers of CD90, CD73, CD29, CD105 and negative for CD45, HLA-DR and CD31 (Supplementary Fig. 1) as previously reported [25]. To determine whether GMSC can suppress osteoclastogenesis, the suppressive effects of GMSC on osteoclastogenesis were explored. OCPs isolated from bone marrow can be differentiated into typical osteoclasts following stimulation with M-CSF and RANKL, which then can be confirmed and identified with tartrate-resistant acid phosphatase (TRAP) staining [26]. Addition of GMSC to cultures of OCPs (GMSC to OCPs = 1:5) stimulated with rm-M-CSF and rm-RANKL almost completely suppressed the generation of TRAP + cells, whereas addition of identical numbers of fibroblast cells cell line had no such effect (Fig. 1a, b). We also used primary dermal fibroblasts isolated from patients who had underwent a surgery as control cells, like cell line of fibroblasts, primary dermal fibroblasts failed to suppress the formation of osteoclast (supplementary Fig. 2). We used primary fibroblasts or cell line as control cells since they share similar morphology but have completely different functional characteristics. Additionally, addition of control cells also helped to exclude any possible non-specific role of GMSC in osteoclast differentiation. This suppression seems to be dose-dependent and potent since addition of GMSC to OCPs at a 1:100 ratio still significantly suppressed osteoclast formation (Fig. 1c). This data demonstrates that GMSC display an ability to directly suppress osteoclastogenesis in vitro.
Fig. 1.
GMSC inhibit osteoclastogenesis in vitro. OCPs were co-cultured with GMSC, fibroblast cells or alone (Baseline) in the presence of M-CSF (50 ng/mL) and RANKL (50 ng/mL) for 3 days, followed with TRAP staining. TRAP+ cells were counted under microscopy. (a) Representative images of osteoclast generation under different conditions, scale bar 500 μm. (b) TRAP-positive osteoclast numbers of per area (under 4x microscope) under different conditions were quantified. Results are mean ± SD, n = 6 independent experiments. (c) GMSC were co-cultured with OCPs in different ratios in the present of M-CSF and RANKL, TRAP-positive osteoclast numbers of per area (under 4x microscope) were quantified. Results are mean ± SD, n = 4 independent experiments. (d) p65/50 expression on OCPs after being co-cultured with GMSC, fibroblast cells or alone (baseline) in the present of M-CSF and RANKL were analyzed by western blot. (e) relative density of p65 and p50 to β-actin are shown. One-way ANOVA was used to compare different groups. Data are mean ± SD, n = 4 independent experiments. *, p < .05; **, p < .01; ***, p < .001.
3.2. GMSC inhibit osteoclastogenesis via RANK/RANKL-initiated NF-κB pathway
It has been well known that the RANK/RANKL-initiated NF-κB pathway plays a key role in the early stage of osteoclast formation [12]. Thus, we hypothesize that GMSC inhibit osteoclastogenesis through modulation of these molecular pathways. To address this possibility, OCPs were co-cultured with GMSC or fibroblast cells at 1:20 ratio for 3 days in transwell system (with GMSC cultured in the insert well, separate from OCPs), after which, OCPs were carefully harvested. Protein was prepared and purified from harvested cells and then subjected to western blot analysis. As expected, NF-κB P65/P50 protein expression on OCPs was highly detectable when these cells are stimulated with M-CSF and RANKL, while the expression of NF-κB P65/P50 was markedly decreased in OCPs that had been co-cultured with GMSC compared with OCPs cultured alone or co-cultured with fibroblast cell groups (Fig. 1d, e). We previously reported that a Transwell system did not interfere with the suppressive function of GMSC [20], this experiment indeed ensures purified OCPs were tested.
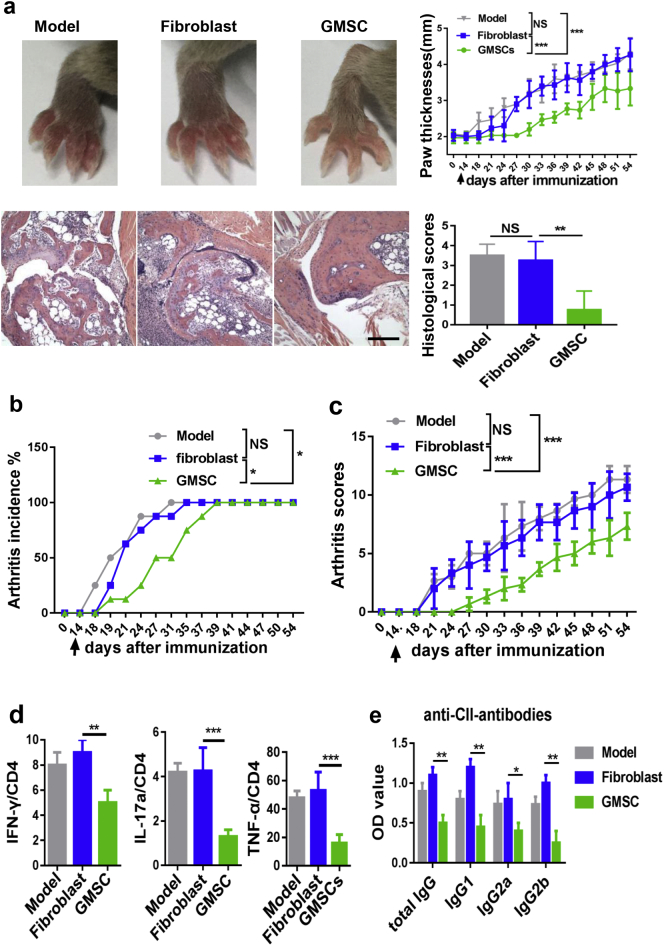
3.3. GMSC attenuate CIA and prevent bone erosion in autoimmune arthritis
Bone destruction is a severe condition and increased frequency and activity of osteoclasts contribute to bone loss in patients with RA [12,27]. To further determine whether the immunosuppressive potential of GMSC on the formation of osteoclasts in vitro can extend to the inflammation-induced bone loss in vivo, we employed the CIA model, which shares many pathological features of human RA, including synovial hyperplasia, joint swelling, as well as bone and cartilage destruction [10,28]. We observed an evident reduction of joint swelling (Fig. 2a), a significant delay in disease onset (Fig. 2b) and markedly less severity of arthritis scores (Fig. 2c) following a single injection of 2 million GMSC on day 14 after CII/CFA immunization. Although disease onset does not occur, substantial amounts of pro-inflammatory cytokines and auto-antibodies are significantly elevated on 14 days after CII/CFA [23,26]. The infusion of GMSC during this time point has a therapeutic implication. Mice were sacrificed on day 54 after CII/CFA immunization for histologic and quantitative analysis of whole ankle joints, a significant decrease in pannus formation, and in destruction of bone and cartilage was observed in GMSC-treated mice compared with model or fibroblast cells-treated mice (Fig. 2a). We also conducted an additional experiment where activated CD4 + T cells were adoptively transferred into CIA and results showed these cells had no significant influence on CIA disease severities and the frequency of osteoclast in CIA (supplementary Fig. 3). This experiment further excludes the possibility that infusion of GMSC treats CIA and bone change due to their non-specific role.
Fig. 2.
Adoptive transfer of GMSC alleviates CIA in vivo. CIA was induced in DBA/1 mice, after 14 days of immunization, mice were treated with GMSC, fibroblast cells (2 × 106 per mouse, iv) or PBS (model) and euthanized on day 54. (a) Paw thicknesses changed with time were measured, representative images of gross appearance and histological images of arthritic joints on day 54 were showed, scale bar: 200 μm. Data are mean ± SD, n = 6 mice. (b and c) Incidences and clinical scores of CIA mice changed with time. Data are mean ± SD, n = 6 mice. (d) The expression of cytokines IFN-γ, IL-17, and TNF-α by CD4+ T cells in draining lymph nodes in CIA were determined by flow cytometry, Data are mean ± SD, n = 6 mice. (e) Serum levels of anti-collagen II antibodies of CIA mice received different treatment were detected by ELISA. Bar graphs show the Mean ± SD, n = 6 mice. *, p < .05; **, p < .01; ***, p < .001. Experiments were repeated twice with similar results.
Additionally, the imbalance between pro-inflammatory and anti-inflammatory cytokines also contributes to the pathogenesis and development of CIA. We systematically examined cytokine levels and sources of cytokines producers and found that CD4 + IFN-γ +, IL-17+, and TNF-α + cells were significantly decreased in the spleen cells of GMSC-treated CIA compared to CIA model and fibroblast-treated CIA (Fig. 2d). Other cytokines, such as IL-5 and IL-13 were undetectable (data not shown). In addition, the serum levels of various antibodies including anti-CII IgG, IgG1, IgG2a, and IgG2b were also significantly decreased in GMSC-treated CIA than that of model or fibroblast-treated CIA (Fig. 2e).
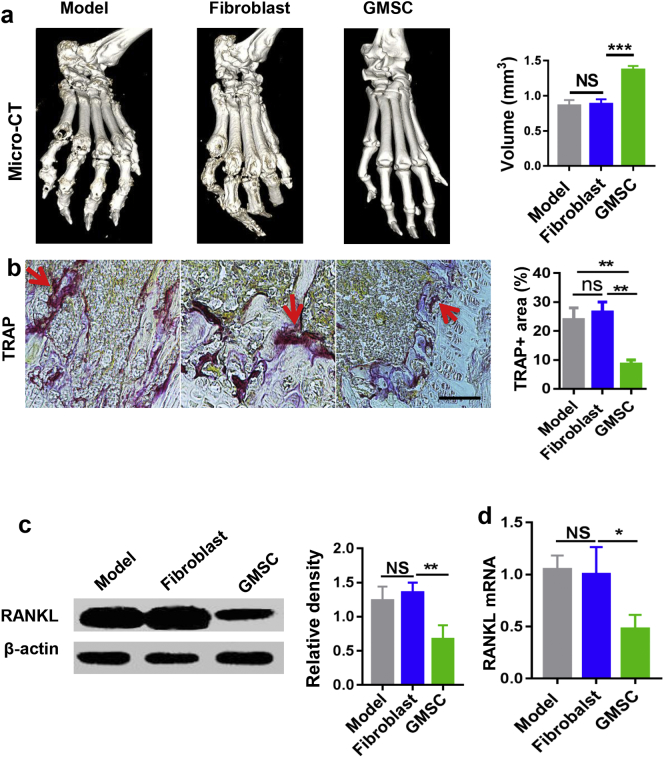
Moreover, we revealed that infusion of GMSC markedly prevented the collagen-immunized mice from severe bone destruction as seen in representative high-resolution micro-computed tomography (micro-CT) (Fig. 3a). Micro-CT is better than histologic analysis to identify bone erosion. Erosions were particularly evident around the metatarsophalangeal and ankle joints in CIA mice and CIA mice that received the infusion of control fibroblast cells (Fig. 3a). The metatarsophalangeal joint volumes from GMSC-treated mice were significantly larger than that from model or control cells-treated mice (Fig. 3a).
Fig. 3.
GMSC suppress bone erosion, partly via inhibiting RANKL expression on synovial cells in vivo. (a) Representative micro-CT imaging of ankle joints and bone volumes of the metatarsophalangeal joints from CIA mice received different treatment. Data are mean ± SD, n = 4 mice. (b) Representative images of TRAP staining of mouse ankle joint sections, red arrow: TRAP positive sites. Scale bar: 200 μm. Data are mean ± SD, n = 6 mice. (c and d) Synovial tissues from ankle joints were isolated, protein and mRNA level of RANKL expression were determined. Bar graphs show the Mean ± SD, n = 3 mice. *, p < .05; **, p < .01; ***, p < . 001. Experiments were repeated twice with similar results. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. GMSC diminish osteoclast formation and RANKL expression in vivo during autoimmune arthritis
In line with the observations we made from in vitro experiments, we also demonstrated that GMSC treatment produced significantly fewer numbers of TRAP+ cells in vivo during CIA when compared to disease alone and fibroblast treatment groups (Fig. 3b). It is likely that GMSC treatment protects bone damage partly through suppressing the numbers and function of osteoclasts in CIA. It has been well recognized that inflammatory cytokines produced from innate and adaptive immune cells lead to systemic inflammation, which subsequently drives bone destruction. RANKL produced by inflamed synovial tissues is a key component and main source among these pro-inflammation triggers in promoting and enhancing osteoclastogenesis [27]. We noted that GMSC treatment significantly reduced the expression of RANKL expression on isolated synovial tissues in CIA, conversely, fibroblast treatment did not result in such effect (Fig. 3c, d). Thus, GMSC not only directly suppress the formation of osteoclasts, but also stifle immune and inflammatory responses that indirectly attenuate the osteoclast, eventually contributing to bone protection in CIA.
3.5. GMSC curb osteoclasts and protect bone loss through CD39-adenosine signal pathway
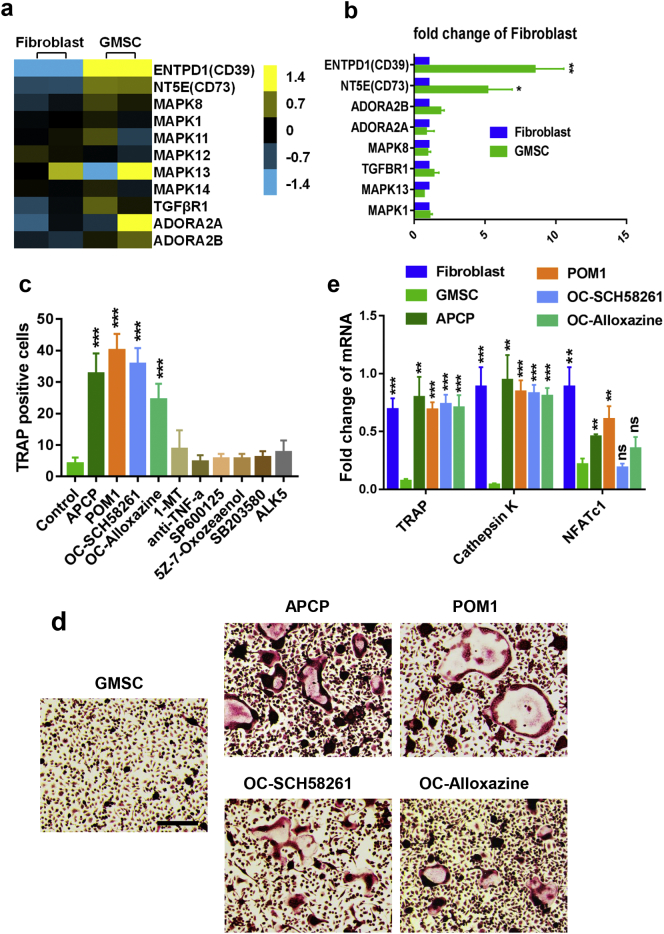
Previous studies have demonstrated that MSC suppress immune responses through multiple immune suppression molecules [14,16,29,30]. To determine the relative molecule(s) that are responsible for immune suppression on osteoclasts and inflammation in CIA, we used RNA-seq to methodically analyze the profiles of GMSC vs fibroblast cells (NCBI BioProject, accession number PRJNA540091). As shown in Fig. 4a, although the gene expression profile of GMSC in each individual is not completely similar, the expression levels of CD39, CD73, TGFβR1 and MAPK family were consistently greater in GMSC than in fibroblast cells, we also validated that expression of CD39 and CD73 was dramatically greater in GMSC than in fibroblast via qPCR and flow cytometry in both gene and protein levels (Fig. 4b and supplementary Fig. 1). In addition, GMSC hardly expressed HLA-DR, this is a likely reason why human GMSC can survive and function in the mouse. We recently systemically studied the dynamic distribution and fates of GMSC through various routes of injection and found GMSC can distribute in lymph nodes, spleen and many important organs (Zhao J, MS under revision).
Fig. 4.
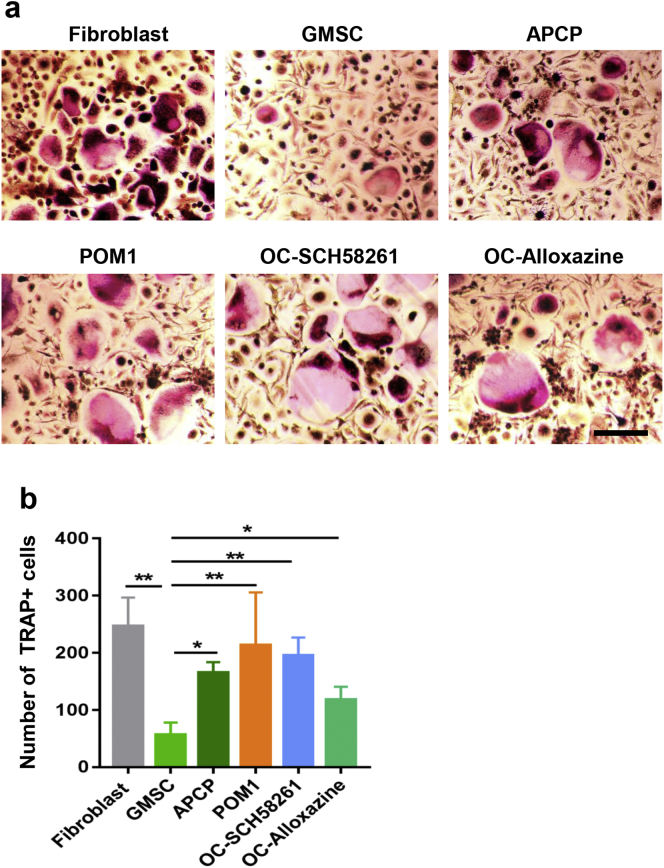
GMSC inhibit osteoclast induction partly via CD39/CD73/adenosine signals in vitro. (a) Heat map showing the relative gene expression of osteoclastogenesis regulated genes between fibroblast cells (two cell lines) and GMSC (two donors) by RNA-seq. (b) qPCR results showing the expression of osteoclastogenesis regulated genes between fibroblast cells and GMSC. Data are Mean ± SD, n = 3 independent experiments. (c) GMSC were pretreated with JNK inhibitor (SP600125, 25 μM), ERK2 inhibitor (5Z-7-Oxozeaenol, 5 μM), p38 MAPK inhibitor (SB203580, 10 μM), IDO inhibitor (1-MT, 500 μM), CD73 inhibitor (APCP, 200 μM), CD39 inhibitor (POM1, 100 μM), TNF-a antibody (anti-TNF-a,10 μg/mL) and ALK5 (10 μg/mL), or OCPs were pretreated with A2A adenosine receptor antagonist (OC-SCH58261, 25 μM), A2B adenosine receptor antagonist (OC-Alloxazine, 10 μM) overnight, then OCPs and GMSC were co-cultured together in the presence of M-CSF and RANKL for 3 days followed with TRAP staining. TRAP-positive cells were counted under microscopy (under 4x microscope). Data are Mean ± SD, n = 3 independent experiments. (d) Representative images from some groups of (c) were showed, scale bar: 200 μm. (e) qPCR result showing osteoclast related genes TRAP, Cathepsin K and NFATc1 expression in the different groups, each group was compared to GMSC-treated group using one-way ANOVA. Data are Mean ± SD, n = 3 independent experiments. *, p < .05; **, p < .01; ***, p < .001.
We added corresponding inhibitors to pretreat GMSC or adenosine receptors inhibitors to pretreat OCPs, then they were added to co-cultures to determine underlying molecular mechanisms. We observed that pretreatment of GMSC with CD39 inhibitor (POM1) or CD73 inhibitor (APCP) but not others almost completely abolished their suppressive effects on osteoclast formation (Fig. 4c, d). We found that pretreatment of CD39 and CD73 inhibitors in the doses we used did not change the GMSC viability including apoptosis and cell proliferation ability (supplementary Fig. 4), excluding the possibility that these inhibitors changed biological viability and then altered the functional activity of GMSC. Interestingly, pretreatment of OCPs with adenosine A2A receptor inhibitor (SCH58261) or adenosine A2B receptor inhibitor (Alloxazine) also significantly reverted the suppressive effects of GMSC on osteoclast formation in vitro (Fig. 4c, d). We also examined the expression of genes related to osteoclast formation and development, including TRAP, Cathepsin K and NFATc1. Addition of GMSC but not fibroblast cells significantly suppressed the expression of these genes, while the blockade of CD39/CD73 signal on GMSC or blockade of adenosine receptor signal on OCPs dramatically abolished the suppressive effects of GMSC on these gene levels (Fig. 4e).
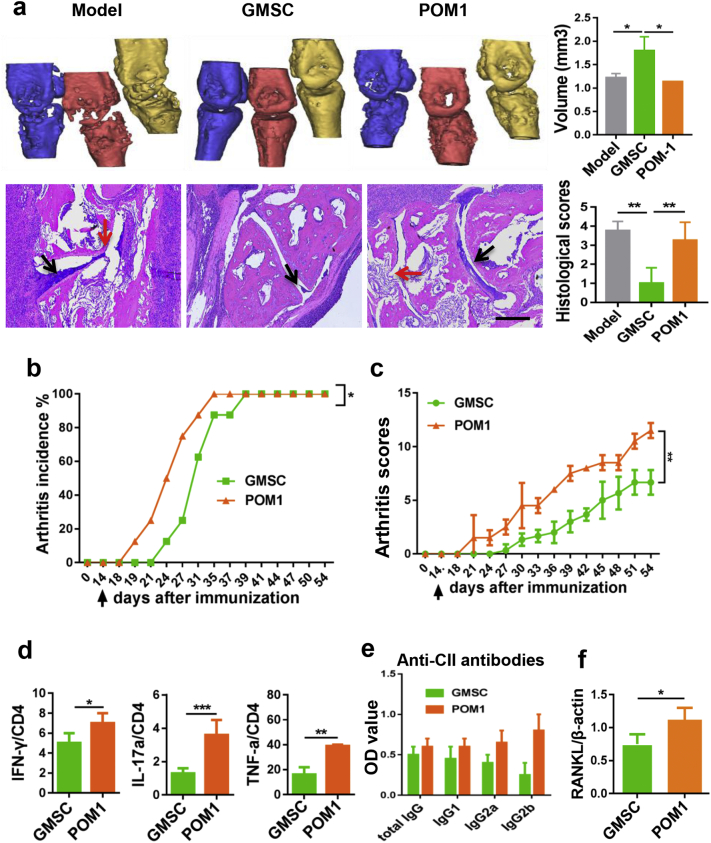
We further determine whether these mechanisms also involve in the protective effects of GMSC on bone damage and disease development during CIA in vivo. As CD39 is a key molecule of CD39/CD73/adenosine signal pathway, we choose CD39 inhibitor to pretreat GMSC and then transferred these GMSC or un-pretreated GMSC into CIA mice. As shown in Fig. 5, GMSC significantly protected bone damage, reduced disease pathology including cartilage erosion and joint structure change, delayed disease onset and severities, and decreased the production of inflammatory CD4 T helper cells and autoantibodies, blockade of CD39 signal on GMSC almost completely abrogated their effects on these manifestations. Additionally, GMSC suppressed RANKL mRNA expression on inflamed synovial tissues in CIA, and this effect is also dependent upon CD39 signal (Fig. 5f).
Fig. 5.
Adoptive transfer of GMSC alleviates CIA partly via CD39 signal in vivo. CIA mice received 2 × 106 per mouse of GMSC or GMSC pretreated with POM1 overnight (POM1) on day 14 after CII/CFA immunization were euthanized on day 54. (a) Representative Micro-CT and Histological images, black arrow: synovial hyperplasia; red arrow: cartilage erosion. Bar graphs show the Mean ± SD, scale bar 200 μm, n = 4 mice. (b and c) Incidences and clinical scores of CIA mice changed with time, Data are Mean ± SD, n = 6 independent experiments. (d) The expression of cytokines IFN-γ, IL-17, and TNF-α by CD4+ T cells in draining lymph nodes were determined by flow cytometry. Data are Mean ± SD, n = 3 independent experiments. (e) Serum levels of anti-collagen II antibodies of CIA mice received different treatment were detected by an ELISA. Bar graphs show the Mean ± SD, n = 6 mice. F, Synovial tissues from ankle joints were isolated, mRNA level of RANKL expression were determined, Data are Mean ± SD, n = 3 mice. *, p < .05; **, p < .01; ***, p < .001. Experiments were repeated twice with similar results. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. GMSC display a similar suppressive function and mechanism in human osteoclastogenesis in vitro
To consider the clinical translational value, we extended our study from animal osteoclasts to human osteoclasts. Similarly, human CD14 + cells isolated from peripheral blood mononuclear cells (PBMCs) can differentiate into osteoclasts in the presence of rh-M-CSF and rh-RANKL for 14–21 days [31,32]. We observed a marked reduction of TRAP+ cells in human CD14+ cells when co-cultured with GMSC. Conversely, addition of the same number of fibroblast cells had no such effect on human osteoclast formation (Fig. 6a, b). Interestingly, blockade of CD73 or CD39 signal on GMSC, or blockade of adenosine receptor signal on hOCPs all significantly eliminated the inhibitory effects of GMSC on human osteoclastogenesis (Fig. 6a, b), suggesting that GMSC have a potential therapeutic role for bone protection in patients with RA and other relative bone loss diseases.
Fig. 6.
GMSC inhibit human osteoclast generation partially via CD39/CD73/adenosine signals. GMSC were pretreated with or without CD73 inhibitor (APCP, 200 μM) or CD39 inhibitor (POM1, 100 μM) overnight or hOCPs were pretreated with A2A adenosine receptor antagonist (OC-SCH58261, 25 μM) or A2B adenosine receptor antagonist (OC-Alloxazine, 10 μM) overnight, then GMSC and hOCPs were co-cultured in transwell system (with GMSC cultured in the insert well) in the presence of M-CSF and RANKL for 14 days followed with TRAP staining. (a) Representative images from different groups were showed, scale bar: 200 μm. Results are showed as mean ± SEM, n = 3 independent experiments. (b) Total TRAP-positive cells in 24 well under different condition were counted under microscopy. Results are showed as mean ± SEM, n = 3 independent experiments. *, p < .05; **, p < .01; ***, p < .001.
4. Discussion
We undertook this study to test our chief hypothesis that GMSC can play a remarkably suppressive role in the development of osteoclastogenesis, even in immune-mediated systemic arthritis. Increased osteoclastic bone resorption is a central link in the pathogenesis of many bone diseases including rheumatoid arthritis [4]. Inhibiting osteoclast differentiation and function becomes an important part of the treatment regimen when bone destruction is involved in the disease pathogenesis. The immunosuppressive function of MSC is ascribed to their inhibitory effects on T cells and other immune cells including DCs and NK cells [33,34]. We previously reported that GMSC not only suppress Th1 and Th17 but also promote Treg cells [20]. In the current study, we gained new insights into how GMSC regulate osteoclast formation.
Using a series of in vitro and in vivo experiments, we now demonstrate that GMSC have a potent suppressive ability to control osteoclastogenesis. This ability is consistent in animal and human cells. Interestingly, the infusion of GMSC into CIA mice not only suppresses arthritis severities and pathology, but also bone erosion. These conclusions, which combine our recent study results using a humanized model, lead to an important implication: manipulation of GMSC could be clinically therapeutic in the near future.
It is likely that GMSC directly and/or indirectly suppress the frequency and/or function of osteoclasts. The experimental results in vitro provide evidence that GMSC directly suppress the formation of osteoclast, nonetheless, GMSC may also exert their anti-inflammation properties thereby, indirectly reducing the osteoclast formation. Development of osteoclasts involves two essential osteoclastogenic mediators, macrophage colony-stimulating factor 1 (M-CSF) and RANKL [35]. RANKL is a key mediator of osteoclastogenesis and directly induces osteoclast development and bone resorption. The RANKL–RANK–OPG system, is crucial to bone homeostasis through regulation of osteoclasts [36]. RANKL global knockout mice or RANKL inhibitor-treated mice were protected against bone destruction, yet with equivalent inflammation compared to RANKL-sufficient mice [37,38]. RANKL-RANK pathway interacts with tumor necrosis factor receptor-associated factors (TRAFs), especially TRAF6, activating mitogen-activated protein kinases (MAPKs) p38 and JNK, as well as a cascade of transcription factors, including NF-κB, c-Fos, Fra-1, and nuclear factor of activated T cells cytoplasmic 1 (NFATc1), controlling osteoclast differentiation, activation and a series of pathologic conditions characterized by increased bone turnover [35,36,39]. Our data on the suppressive effect of GMSC on the expression and activities of NF-κB, NFATc1 and RANKL leads to the conclusion that GMSC prevent bone loss through restraining osteoclasts.
It has been previously reported that MSC exert their immune regulation through releasing suppressive molecules, such as IL-10, NO, IDO, PGE2 and TGFβ1 [17,[40], [41], [42]]. Using RNA-seq, we indeed found that GMSC had an increased expression of TGFRI, CD39, CD73 and adenosine receptors. However, the suppressive function is mainly related to CD39-CD73-adnosine axis since pretreating GMSC with CD73 and CD39 inhibitors and pretreating OCP with adenosine receptors inhibitors distinctly abolished their functional activities. The surface molecule CD39 is co-expressed in concert with CD73 on GMSC and other regulatory T cells, catalyzing the generation of adenosine that triggers the elevation of cytoplasmatic cAMP, a strong immunosuppressive agent, which promotes various immunoregulatory activities and reduces inflammation [11,43,44]. Other studies have also showed that blocking of the adenosine pathway either by the A2A receptor antagonist or the CD39 inhibitor almost completely blocked MSC-mediated suppression of T-cell proliferation [43,44]. Additionally, CD39 can stabilize Foxp3+ Treg cells, boost Tr1 cell differentiation and function and contributes to their suppressive function [11,45], adding additional mechanisms to promote anti-inflammation effects.
The limitations of this study may include no direct study of function of GMSC derived from patients with RA. We plan to conduct a separate project to study and compare the biological characteristics of GMSC derived from RA and other patients with autoimmune diseases.
In conclusion, our data first offers convincing evidence that GMSC attenuate bone destruction by preventing RANKL-induced osteoclastogenesis via CD39/CD73/adenosine signal. These results suggest that manipulation of GMSC or targeting their signal axis has a therapeutic potential on RA and other bone damage diseases.
Authors' contributions
SGZ initiated the study, designed the experiments and wrote the paper. YL, WW, JG, XZ, JD, YX, YZ performed the experiments and statistical analyses. FH, JY, UK provided gingival tissues. JW helped with animal experiments. JC, UK, QF edited MS.
Declaration of interests
The authors declare no conflict of interests.
Funding sources
This study was supported by grants from the National Key R&D Program of China (2017YFA0105801 to F.H); the Zhujiang Innovative and Entrepreneurial Talent Team Award of Guangdong Province (2016 ZT 06S 252 to F·H) and National Institutes of Health (R01 AR059103, R61 AR073409 and NIH Star Award to S.G.Z).
Acknowledgments
We thank all the patients and clinicians who participated in providing us with gingival tissue. We thank animal breeders who help us to take care of animals used in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.04.058.
Appendix A. Supplementary data
Supplementary material
References
- 1.Halenius A., Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int. 2014;2014 doi: 10.1155/2014/472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou Y., Xu S., Xiao Y. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J Clin Invest. 2018;128:4510–4524. doi: 10.1172/JCI97965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamas M.M., Filippucci E., Becciolini A. Bone erosions in rheumatoid arthritis: ultrasound findings in the early stage of the disease. Rheumatology (Oxford) 2014;53:1100–1107. doi: 10.1093/rheumatology/ket484. [DOI] [PubMed] [Google Scholar]
- 4.Gashi A.A., Rexhepi S., Berisha I. Treatment of rheumatoid arthritis with biologic DMARDS (rituximab and Etanercept) Med Arch. 2014;68:51–53. doi: 10.5455/medarh.2014.68.51-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruoff G. Rheumatoid arthritis: early treatment with corticosteroids and nonsteroidal anti-inflammatory drugs. J Fam Pract. 2014;63:S27–S30. [PubMed] [Google Scholar]
- 6.Bubonja-Sonje M., Rubinic D., Anic F. Salmonella enterica arthritis in a patient with rheumatoid arthritis receiving anti-tumour necrosis factor therapy. West Indian Med J. 2013;62:270–272. [PubMed] [Google Scholar]
- 7.Chatzidionysiou K., Askling J., Eriksson J. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890–896. doi: 10.1136/annrheumdis-2013-204714. [DOI] [PubMed] [Google Scholar]
- 8.Ikonomidis I., Tzortzis S., Andreadou I. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 9.Smolen J.S., Weinblatt M.E., Sheng S. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;73:1616–1625. doi: 10.1136/annrheumdis-2013-205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W., Wang J., Xu Z. Apremilast ameliorates experimental arthritis. Front Immunol. 2018;9:1662. doi: 10.3389/fimmu.2018.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiolat A., Semerano L., Pers Y.M. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014;66:273–283. doi: 10.1002/art.38246. [DOI] [PubMed] [Google Scholar]
- 12.Sucur A., Katavic V., Kelava T. Induction of osteoclast progenitors in inflammatory conditions: key to bone destruction in arthritis. Int Orthop. 2014;38:1893–1903. doi: 10.1007/s00264-014-2386-y. [DOI] [PubMed] [Google Scholar]
- 13.Zheng T., Wang X., Yim M. Miconazole inhibits receptor activator of nuclear factor-kappaB ligand-mediated osteoclast formation and function. Eur J Pharmacol. 2014;737:185–193. doi: 10.1016/j.ejphar.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Ansboro S., Roelofs A.J., De Bari C. Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr Opin Rheumatol. 2017;29:201–207. doi: 10.1097/BOR.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulou A., Yiangou M., Athanasiou E. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Ann Rheum Dis. 2012;71:1733–1740. doi: 10.1136/annrheumdis-2011-200985. [DOI] [PubMed] [Google Scholar]
- 16.Su W., Wan Q., Huang J. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136 doi: 10.1016/j.jaci.2014.12.1926. [423-32.e8] [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Shi S., Liu Y. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F., Battiwalla M., Ito S. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014;32:1278–1288. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang F., Chen M., Chen W. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39-CD73-adenosine and IDO signals. Front Immunol. 2017;8:68. doi: 10.3389/fimmu.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M., Su W., Lin X. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Huang F., Li W. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. doi: 10.3389/fimmu.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W., Zhou L., Dang J. Human gingiva-derived mesenchymal stem cells ameliorate streptozoticin-induced T1DM in mice via suppression of T effector cells and up-regulating Treg subsets. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong N., Lan Q., Chen M. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galani I.E., Wendel M., Stojanovic A. Regulatory T cells control macrophage accumulation and activation in lymphoma. Int J Cancer. 2010;127:1131–1140. doi: 10.1002/ijc.25132. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Shi S., Liu Y. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol (Baltimore, Md: 1950) 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong N., Lan Q., Chen M. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann Rheum Dis. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravallese E.M., Manning C., Tsay A. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Brand D.D., Latham K.A., Rosloniec E.F. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 29.Uccelli A., de Rosbo N.K. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114–126. doi: 10.1111/nyas.12815. [DOI] [PubMed] [Google Scholar]
- 30.Su W., Li Z., Jia Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J Mol Cell Biol. 2017;9:289–301. doi: 10.1093/jmcb/mjx022. [DOI] [PubMed] [Google Scholar]
- 31.Maria S., Samsonraj R.M., Munmun F. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res. 2018;64 doi: 10.1111/jpi.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colucci S., Brunetti G., Rizzi R. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood. 2004;104:3722–3730. doi: 10.1182/blood-2004-02-0474. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W., Ge W., Li C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 34.Patel S.A., Meyer J.R., Greco S.J. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 35.Asagiri M., Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Walsh M.C., Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettit A.R., Ji H., von Stechow D. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamijo S., Nakajima A., Ikeda K. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem Biophys Res Commun. 2006;347:124–132. doi: 10.1016/j.bbrc.2006.06.098. [DOI] [PubMed] [Google Scholar]
- 39.Kang I.S., Kim C. NADPH oxidase gp91phox contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci Rep. 2016;6 doi: 10.1038/srep38014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q.Z., Su W.R., Shi S.H. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Mu R., Wang S. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang F., Liu Z.M., Zheng S.G. Updates on GMSCs treatment for autoimmune diseases. Curr Stem Cell Res Ther. 2018;13:345–349. doi: 10.2174/1574888X13666180220141114. [DOI] [PubMed] [Google Scholar]
- 43.Katebi M., Soleimani M., Cronstein B.N. Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J Leukoc Biol. 2009;85:438–444. doi: 10.1189/jlb.0908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattler C., Steinsdoerfer M., Offers M. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplant. 2011;20:1221–1230. doi: 10.3727/096368910X546553. [DOI] [PubMed] [Google Scholar]
- 45.Schuler P.J., Saze Z., Hong C.S. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
RNA-seq data used in this study is available at the NCBI BioProject database under accession number PRJNA540091.