Introduction
Tofacitinib is an oral small-molecule Janus kinase (JAK) inhibitor used to treat moderate or severe rheumatoid arthritis. The agent provides a new therapeutic approach against rheumatoid arthritis and was approved in Japan in 2013. Reports indicate positive effects also for the treatment of psoriatic arthritis and alopecia areata. Tofacitinib can lower the ability of the immune system to fight infections such as herpes zoster and opportunistic infections and increases cancer risks. However, we are not aware of any report of palmoplantar pustulosis–like eruptions caused by tofacitinib therapy. Here we report a case of palmoplantar pustulosis–like eruption caused by the treatment of juvenile idiopathic arthritis (JIA) with tofacitinib.
Case
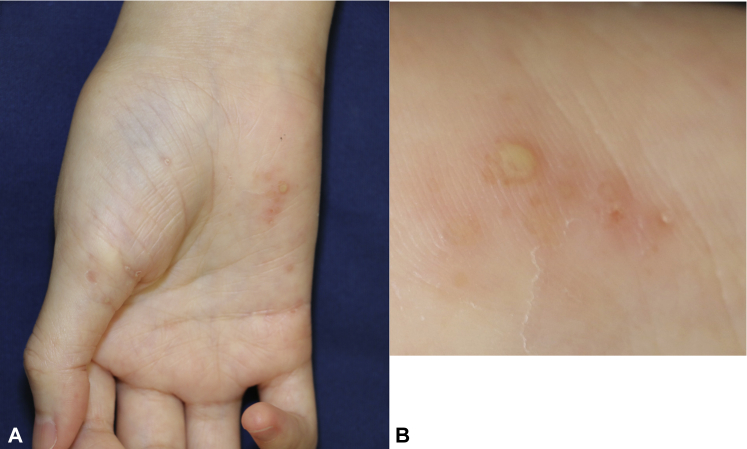
A 25-year-old Japanese woman with a history of JIA was referred to our dermatology clinic for the evaluation of pustular lesions affecting the skin of both palms and soles 14 days after initiating tofacitinib therapy. She had no personal or family history of psoriasis or atopic dermatitis. Because of the severity of the joint symptoms, she had been administered tumor necrosis factor-α (TNF-α) inhibitors such as infliximab, etanercept, and adalimumab. The patient was administered infliximab (2007-2009); was switched to etanercept (2009-2015); and was then switched to adalimumab (2015-2017). Given that the patient experienced no improvement, adalimumab was discontinued two weeks before the initiation of tofacitinib (20 mg/d) therapy. Ten days after initiating tofacitinib therapy, the eruption of pustular lesions on her palms and soles began, and she was referred to our clinic. Clinical examination found scaly erythema with pustules on her palms and soles (Fig 1, A and B). Finger deformities were also evident. Evaluation of nails found no abnormalities.
Fig 1.
A and B, Scaly erythema with pustules affecting the skin of both palms 14 days after initiating tofacitinib therapy. Finger deformities were also evident.
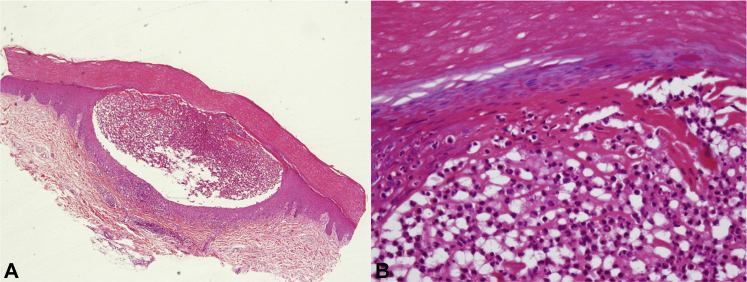
A 4-mm punch biopsy sample was obtained from the left palm, which was histopathologically examined and showed a pustule under the epidermal horny layer and infiltration by leukocytes from the basal to the top layers (Fig 2, A). High magnification showed a neutrophilic collection in the pustule (Fig 2, B). Specific bacteria were not identified from pustule samples for bacterial culture; thus, the lesions were classified as sterile. The patient underwent a trial period of tofacitinib discontinuation to rule out tofacitinib as the etiologic agent, and a betamethasone butyrate propionate ointment was also prescribed during this period. The eruption improved after tofacitinib discontinuation with near-complete clearance (Fig 3, A).
Fig 2.
Histopathology. A, Pustule under the epidermal horny layer and infiltration by leukocytes. B, High magnification shows neutrophilic collection in the pustule. (Hematoxylin-eosin stain; original magnifications: A, ×40; B, ×200.)
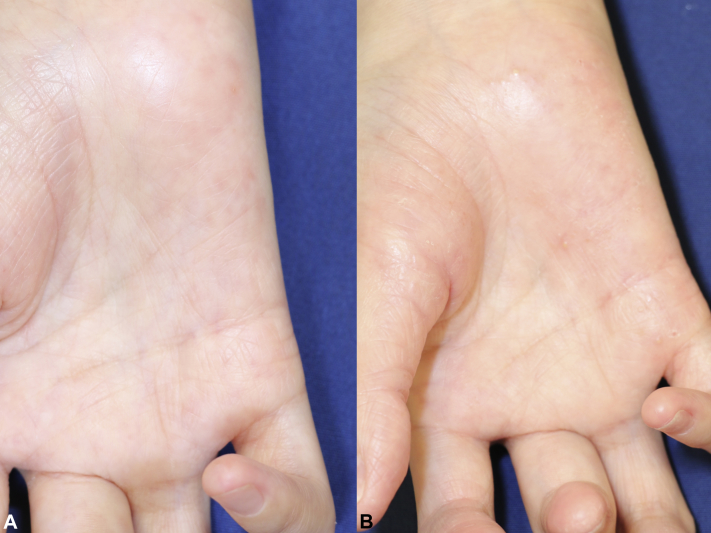
Fig 3.
A, Near-complete clearance after tofacitinib discontinuation. B, The worsening of the palmoplantar lesions after re-initiation of tofacitinib therapy.
However, because of the exacerbation of JIA (with actively swollen and tender joints), tofacitinib (10 mg/d) was re-initiated. Subsequently, her palmoplantar pustular lesions reappeared. We prescribed betamethasone butyrate propionate ointment, after which the patient experienced slight symptom improvements. However, her palmoplantar pustular lesions gradually worsened after receiving tofacitinib for the next 3 months (Fig 3, B). We diagnosed palmoplantar pustulosis–like eruption caused by tofacitinib based on the clinical course of the events and lowered the tofacitinib dosage to 5 mg every 2 days, which was adequate for her joint symptoms and rendering the palmoplantar pustulosis–like eruption tolerable for the duration of tofacitinib therapy.
Discussion
JIA is a term used to define a group of chronic immune-mediated joint diseases that develop in children younger than 16 years and lasts for at least 6 weeks. The International League of Associations for Rheumatology and the World Health Organization have classified this disease into 7 categories (systemic-onset type, persistent and extended oligoarthritis, polyarthritis with rheumatoid factor negative, polyarthritis with rheumatoid factor positive, psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis). Our case corresponded to the form of polyarthritis with rheumatoid factor positive. The outcome of patients with JIA has significantly improved because of the availability of more efficacious therapies such as biological treatments.1
Tofacitinib is an oral JAK inhibitor used for the treatment of rheumatoid arthritis with a different mechanism from that of the traditional biologic treatments. JAKs comprise 4 tyrosine kinases (JAK1, JAK2, JAK3, and TYK2), and tofacitinib selectively inhibits JAK1 and JAK3. Tofacitinib is found to be effective not only for rheumatoid arthritis but also for psoriatic arthritis and alopecia areata.2, 3 The drug can lower the ability of the immune system to fight infections such as herpes zoster; further, opportunistic infections and cancer have also been reported in users.4 Palmoplantar pustulosis is associated with chronic focal bacterial infections, such as tonsillitis, chronic sinusitis, and dental infections.5
TNF-α inhibitors can cause palmoplantar pustulosis–like eruptions as a side effect—a so-called paradoxical reaction. The mechanism for this is not yet clear, but it is thought to involve a disproportionate production of interferon-α by plasma dendritic cells after the TNF-α inhibition.6 Collamer et al7 proposed a therapeutic algorithm for paradoxical reactions. In our case, tofacitinib was the probable cause of the palmoplantar pustulosis–like eruption involving less than 5% of the body surface area, and the patient was treated with topical steroid ointments. After the gradual improvement of the eruption, tofacitinib (10 mg/d) therapy was restarted because of the refractory severe joint manifestations, and the eruption reappeared. The clinical course suggested that tofacitinib treatment facilitated the palmoplantar pustulosis–like eruption in our case; however, the mechanisms by which tofacitinib caused this symptom remain unknown. To the best of our knowledge, this is the first report of tofacitinib therapy causing palmoplantar pustulosis–like eruptions, and more reports are needed to clarify its pathogenesis.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Horneff G., Klein A., Ganser G. Protocols on classification, monitoring and therapy in children's rheumatology (PRO-KIND): results of the working group Polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78. doi: 10.1186/s12969-017-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladman D., Rigby W., Azevedo V.F. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 3.Meephansan J., Thummakriengkrai J., Ponnikorn S., Yingmema W., Deenonpoe R., Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309:729–738. doi: 10.1007/s00403-017-1777-5. [DOI] [PubMed] [Google Scholar]
- 4.Mease P., Hall S., FitzGerald O. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T. Pustulotic arthro-osteitis associated with palmoplantar pustulosis. J Dermatol. 2013;40:857–863. doi: 10.1111/1346-8138.12272. [DOI] [PubMed] [Google Scholar]
- 6.Shmidt E., Wetter D.A., Ferguson S.B., Pittelkow M.R. Psoriasis and palmoplantar pustulosis associated with tumor necrosis factor-alpha inhibitors: the Mayo Clinic experience, 1998 to 2010. J Am Acad Dermatol. 2012;67:e179–e185. doi: 10.1016/j.jaad.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Collamer A.N., Guerrero K.T., Henning J.S., Battafarano D.F. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996–1001. doi: 10.1002/art.23835. [DOI] [PubMed] [Google Scholar]