Introduction
Acne fulminans (AF) and hidradenitis suppurativa (HS) are 2 severe conditions with no consensus on medical treatment and no clear pathophysiology.1, 2 We report the remission of long-lasting and severe AF with HS that was unresponsive to many previous treatments.
Case report
A 19-year-old man suffered from concomitant AF and HS for 3.5 years. His mother and aunt had HS; his maternal grandmother had rheumatoid arthritis. AF appeared 1 month after isotretinoin treatment prescribed for doxycycline-resistant acne. HS appeared 1 month later, after oral steroid initiation. The following treatments, prescribed for AF and HS, failed: isotretinoin; oral steroids for 4 months combined first with isotretinoin for 2 months then with tetracycline for 2 months; zinc; nonsteroidal anti-inflammatory drugs; and rifampin-clindamycin combination, alone for 5 months, then associated with infliximab for 7 months and with adalimumab for 5 months.
At first examination, the patient had lost 11 kg and had a low-grade fever. He could not sit or walk because of pain in HS lesions, shoulders, elbows, and ankles (8/10 on a visual analogic scale). He presented with erosions and crusts over his face and back (Fig 1), Hurley stage 3 HS lesions in 1 axilla, and Hurley 2 lesions in all other HS areas (Fig 2).
Fig 1.
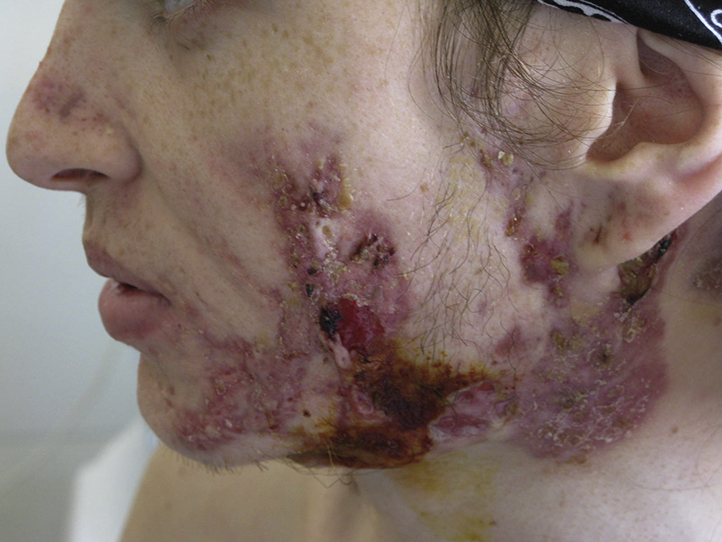
Chronic AF lasting for 3.5 years. Aspect of the lesions before treatment.
Fig 2.
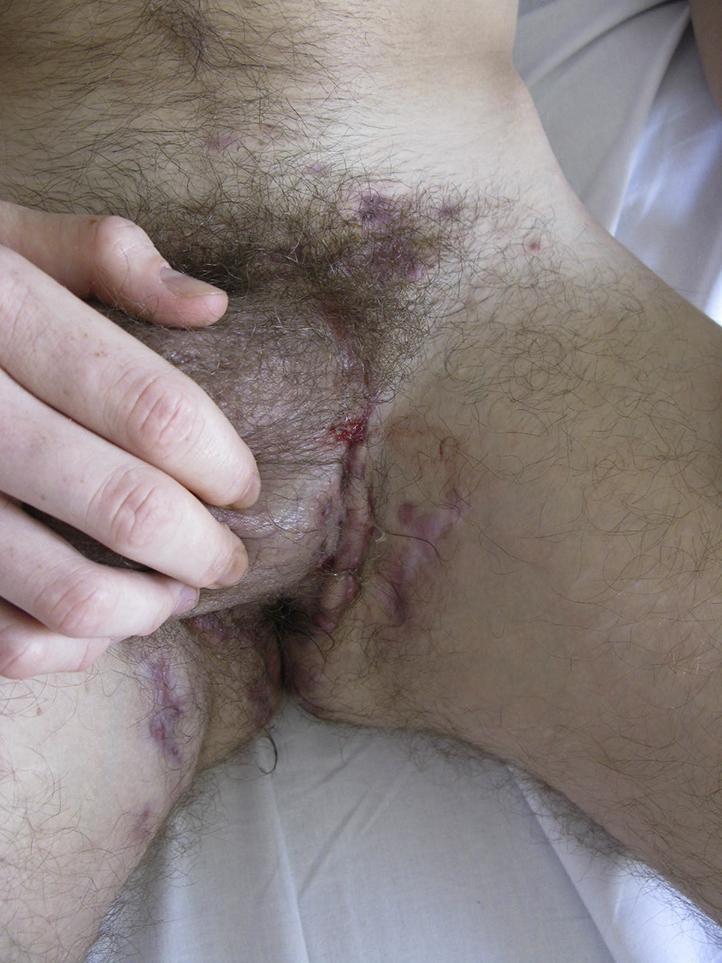
HS lesions in left groin before treatment. Hurley stage 2 lesions.
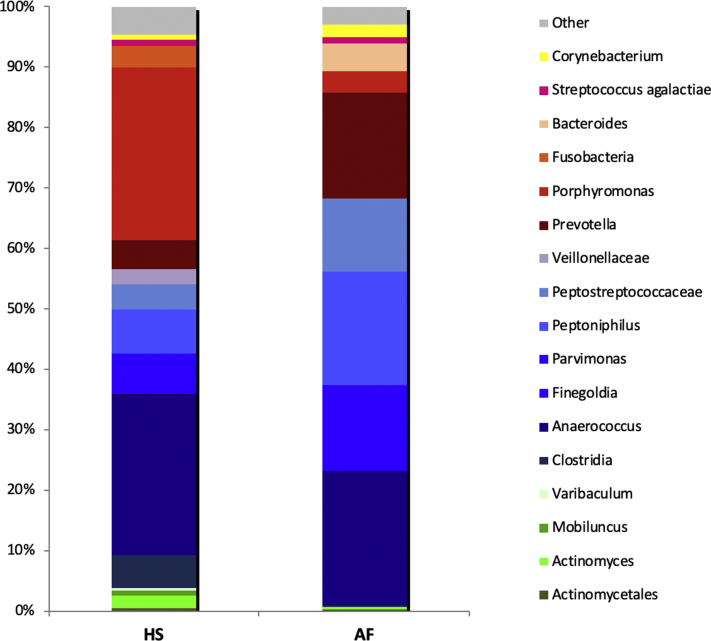
Before treatment, biology showed increased levels of the following blood tests: C-reactive protein (140 mg/L), erythrocyte sedimentation rate (102 mm), white blood cell and neutrophil count (11800 and 8300/mm3, respectively), tumor necrosis factor (TNF)-α (33 pg/mL for N<20 pg/mL), interleukin (IL)-6 (25 pg/mL for N<8 pg/mL), with normal serum IL-1β and negative human leukocyte antigen-B27. Normal 17α-OH-progesterone level excluded adrenal hyperplasia. Bacterial cultures revealed after 7 days a polymorphous anaerobic flora (predominantly Prevotella and Porphyromonas spp) with actinomycetes and Streptococcus agalactiae from his HS lesions as previously reported,3 but also from AF lesions. A 16S ribosomal RNA gene high throughput sequencing identified similar microbiological profiles in both lesion types (Fig 3). The search for γ-secretase, PSTPIP1 and FGFR2 gene mutations, performed by targeted next-generation sequencing, was negative.
Fig 3.
Bacterial metagenomics of HS and AF lesions before treatment. For each lesion, DNA was extracted from pus samples, followed by 16S ribosomal gene amplification by polymerase chain reaction.6 Sequencing was performed using the 454 Roche technology. A predominant and similar anaerobic flora was associated with both lesional types.
We treated this severe case according to our protocol for Hurley stage 3 HS patients4, 5 based on microbiology data,3, 6 with ertapenem for 10 weeks (plus linezolid for the last month) through a peripherally inserted central catheter, followed by oral rifampin plus moxifloxacin plus metronidazole for 6 weeks, then rifampin plus moxifloxacin for 8 weeks. Maintenance treatment with cotrimoxazole was initiated after remission.
All AF with HS areas progressively stopped draining, softened, and became paler (Fig 4). Joint pain disappeared in 1 month and HS pain in 3 months. The patient regained 8 kg in 4 months. AF face and back lesions and HS buttocks and groin lesions were in remission at month 4. In parallel, TNF-α serum levels normalized in 3 weeks, erythrocyte sedimentation rate and C-reactive protein, in 3 months, and IL-6 in 4 months. Persistent active HS in both axillae at month 6 required localized excisions performed under ertapenem for 3 weeks at month 12, which allowed complete remission. Because of 3 inguino-anal HS relapses after remission, these scars were surgically removed, and no relapse occurred under cotrimoxazole after surgery. No AF relapse occurred for 6 years.
Fig 4.
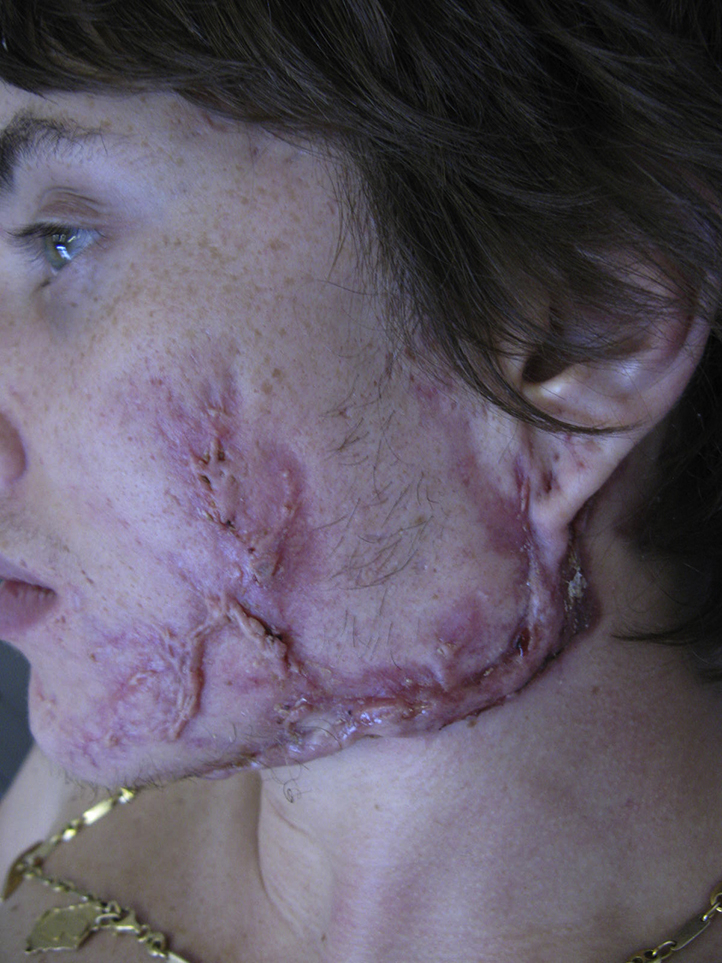
AF lesions after 6 weeks of ertapenem, left cheek.
Discussion
AF and HS are therapeutic challenges. Currently recommended medical therapies for HS only allow for improvement. The only treatment obtaining definitive HS cure is wide surgery, but this does not prevent relapses in new locations or in removed areas, thus demonstrating the need for better therapeutic options.2
AF is a rare but severe form of acne, with currently no established therapeutic consensus.1 This lack of consensus is well reflected by multiple previous treatment failures in this patient. Usually AF treatment consists of a steroid and isotretinoin combination.1 However, this strategy did not succeed, neither with antiacne antibiotics nor 2 different biotherapies. Previous treatment failures, continuous severe AF with HS for the last 3.5 years, identification of a similar flora susceptible to ertapenem in both AF and HS lesions, and dramatic improvement within 6 weeks of ertapenem (Fig 1, Fig 2, Fig 3) plead against a placebo effect or a spontaneous remission. Some authors argue about anti-inflammatory action of antibiotics in HS and acne. However, ertapenem has a recognized pro-inflammatory activity through bacterial lysis,7 suggesting an antimicrobial activity in our patient.
Because most AF patients are teenagers, treatments without steroids during growth are needed. Several reports demonstrated efficacy of cephalosporins,8 minocycline, and dapsone in AF. Steroids are recommended for AF but are almost never prescribed without antibiotics, which questions their true efficacy without antibiotics. Of note, although this patient had an increased serum TNF-α level, anti–TNF-α biotherapies did not improve his condition, whereas targeted antibiotics alone induced remission and normalization of TNF-α level.
AF microbiology was never studied by prolonged cultures and metagenomics. The presence of a similar commensal flora in the patient's HS3, 6 and AF lesions identified by bacterial cultures and confirmed by metagenomics, the dramatic efficacy of targeted antibiotics on both conditions, and the association and parallel evolution of both AF and HS strongly suggest an infectious participation with an abnormal reaction against the host's microbiota. As suggested by some authors, AF could be a facial form of HS.9
HS is currently considered a skin innate immunity deficiency. This defect could allow abnormal proliferation of pathogenic/opportunistic bacteria from microbiota due to an inappropriate host response, both responsible for chronic skin inflammation.3
Our case is a chronic AF, whereas AF usually lasts for a few months. Only one other AF patient in the literature lasted more than a year.10 Like our patient, who was treated continuously with steroids or biotherapies for 3 years, this case persisted under prolonged immune modulators. Another AF patient also presented under steroid treatment8 and several HS cases under biotherapies.11 These cases question whether immune modulators may unmask an infectious component in AF and in HS.
Conclusion
AF and HS microbiota composition were similar in our patient and may explain the efficacy of targeted antibiotherapy on both AF and HS and on inflammatory markers. This finding raises the question of a common pathogenic mechanism of host microbiome disease for AF and HS and suggests further investigation of AF microbiology to test these new HS therapeutic options adapted to Hurley staging in controlled studies.
Acknowledgments
We thank the Fondation pour la Recherche Médicale through Roxane funding, the French Society of Dermatology, and the French Patients' Association for Research in HS for their support.
Footnotes
Funding sources: Fondation pour la Recherche Médicale, Paris, France (Roxane Project grant number: LMV20100519581).
Conflicts of interest: None disclosed.
Previously presented as a poster at Journées Dermatologiques de Paris (JDP), December 10-14, 2014, Paris and European Academy of Dermatology-Venereology (EADV) Meeting, October 9-12, 2014, Copenhagen.
References
- 1.Zaba R., Schwartz R., Jarmuda S., Czarnecka-Operacz M., Silny W. Acne fulminans: explosive systemic form of acne. J Eur Acad Dermatol Venereol. 2011;25(5):501–507. doi: 10.1111/j.1468-3083.2010.03855.x. [DOI] [PubMed] [Google Scholar]; Zaba R, Schwartz R, Jarmuda S, Czarnecka-Operacz M, Silny W. Acne fulminans: explosive systemic form of acne. J Eur Acad Dermatol Venereol 2011 May; 25(5): 501-507. [DOI] [PubMed]
- 2.Zouboulis C.C., Desai N., Emtestam L. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]; Zouboulis CC, Desai N, Emtestam L et al: European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015, Apr ;29(4):619-644. [DOI] [PubMed]
- 3.Guet-Revillet H., Coignard-Biehler H., Jais J.P. Bacterial pathogens are associated with Hidradenitis suppurativa. Emerg Infect Dis. 2014;20(12):1990–1998. doi: 10.3201/eid2012.140064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Guet-Revillet H, Coignard-Biehler H, Jais JP et al. Bacterial pathogens are associated with Hidradenitis suppurativa. Emerg Infect Dis 2014, 20(12): 1990-1998. [DOI] [PMC free article] [PubMed]
- 4.Join-Lambert O., Coignard H., Jais J.P. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222(1):49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]; Join-Lambert O, Coignard H, Jais JP et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology 2011 Feb; 222(1): 49-58. [DOI] [PubMed]
- 5.Join-Lambert O., Coignard-Biehler H., Jais J.P. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71(2):513–520. doi: 10.1093/jac/dkv361. [DOI] [PubMed] [Google Scholar]; Join-Lambert O, Coignard-Biehler H, Jais JP et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016 Feb; 71(2):513-520. [DOI] [PubMed]
- 6.Guet-Revillet H., Jais J.P., Ungeheuer M.N. The microbiological landscape of anaerobic infections in Hidradenitis Suppurativa: a prospective metagenomic study. Clin Infect Dis. 2017;65(2):282–291. doi: 10.1093/cid/cix285. [DOI] [PubMed] [Google Scholar]; Guet-Revillet H, Jais JP, Ungeheuer MN et al. The microbiological landscape of anaerobic infections in Hidradenitis Suppurativa: a prospective metagenomic study. 2017. Clin Infect Dis. Apr 1. doi: 10.1093/cid/cix285. [DOI] [PubMed]
- 7.Van Vlem B., Vanholder R., De Paepe P. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]; Van Vlem B, Vanholder R, De Paepe P et al. Immunomodulating effects of antibiotics: literature review. Infection 1996; 24: 275-291. [DOI] [PubMed]
- 8.Palleschi G.M., Bruscino N., Cristofaro G. A case of acne fulminans presenting with systemic symptoms resembling acute appendicitis with maculopapular purpura-like eruption exacerbated by systemic steroids. Dermatol Ther. 2013;26(4):367–369. doi: 10.1111/dth.12001. [DOI] [PubMed] [Google Scholar]; Palleschi GM, Bruscino N, Cristofaro G. A case of acne fulminans presenting with systemic symptoms resembling acute appendicitis with maculopapular purpura-like eruption exacerbated by systemic steroids. Dermatol Ther. 2013 Jul-Aug; 26(4): 367-369. [DOI] [PubMed]
- 9.Seukeran D.C., Cunliffe W.J. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141(2):307–309. doi: 10.1046/j.1365-2133.1999.02982.x. [DOI] [PubMed] [Google Scholar]; Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol 1999; 141(2): 307-309. [DOI] [PubMed]
- 10.Poli F., Revuz J. Acne flare on isotretinoin: a pointer to diagnosis of hidradenitis suppurativa. Ann Dermatol Venereol. 2019;146(1):4–8. doi: 10.1016/j.annder.2018.07.020. [DOI] [PubMed] [Google Scholar]; Poli F, Revuz J. Acne flare on isotretinoin: a pointer to diagnosis of hidradenitis suppurativa. Ann Dermatol Venereol. 2019 Jan;146(1):4-8. doi: 10.1016/j.annder.2018.07.020. [DOI] [PubMed]
- 11.Faivre C., Villani A.P., Aubin F. French Society of Dermatology and Club Rheumatisms and Inflammation. Hidradenitis suppurativa (HS): An unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74(6):1153–1159. doi: 10.1016/j.jaad.2016.01.018. [DOI] [PubMed] [Google Scholar]; Faivre C, Villani AP, Aubin F et al. French Society of Dermatology and Club Rheumatisms and Inflammation. Hidradenitis suppurativa (HS): An unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016 Jun ;74(6) :1153-1159. doi: 10.1016/j.jaad.2016.01.018. Epub 2016 Mar 8. [DOI] [PubMed]