Abstract
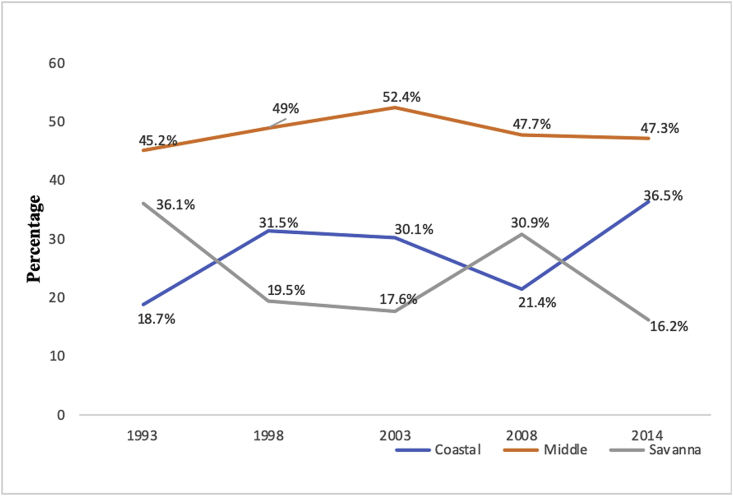
Since 1993, evidence suggest that a significant portion of Ghanaian under-five children have suffered from Acute Respiratory Infection (ARI). This study sought to examine the prevalence of ARI symptoms among under-fives across ecological zones as well as childhood and maternal factors associated with ARI between 1993 and 2014. We used data from Ghana Demographic and Health Surveys (1993–2014). The study sample included women of reproductive age who had under-five children experiencing a cough accompanied with short rapid breaths in the last two weeks preceding each of the surveys. Data were extracted and analysed using STATA version 14.2. Both bivariate and multivariate analyses were done to establish the association between the explanatory and outcome variable. Among Coastal dwellers, the prevalence of reported ARI increased from 18.7% in 1993 to 36.5% in 2014. Increment occurred between 1993 (45.2%) and 2014 (47.3%) within the Middle zone. Between 1993 and 2014, reported ARI reduced from 36.1% to 16.2% among Savanna dwellers. In addition to ecological zone, place of residence, and survey year were associated with symptoms of childhood ARIs. The multivariate results showed that children whose mothers lived in rural areas [AOR = 1.54, CI = 1.21–1.97] and those born in survey wave 1998 [AOR = 1.59, CI = 1.24–2.04] had higher odds of reporting symptoms of ARI. The study findings have demonstrated the need for public health education and sensitization on ARI to be more specific and target women with children under-five who live in the Middle zone compared to those who live in other ecological zones.
Keywords: Acute respiratory infections, Ecological zone, Under-five, Child health, Ghana
1. Introduction
Although there have been significant gains against the fight of childhood mortality over the past two decades, in 2017 alone, an estimated 6.3 million children and young adolescents died from preventable causes (UNICEF, WHO, World Bank & UN-DESA Population Division, 2018). Among the underlying factors that predispose children under five to mortality and morbidity conditions is acute respiratory infections (ARIs). It contributes significantly to deaths among under five and as a result remains a major public health concern especially in low and middle-income countries (Akinyemi & Morakinyo, 2018). ARI is an infection of any part of the respiratory tract and its related structures that affect the airways (Accinelli, Leon-Abarca & Gozal, 2017). Almost all (97%) ARI cases occur in low and middle-income countries with about 70% of those cases occurring in South Asia and sub-Saharan Africa (Harerimana, Nyirazinyoye, Thomson, & Ntaganira, 2016; UNICEF, 2016). Globally, ARI accounts for about 1.3 million deaths among children under-five annually (WHO & UNICEF, 2013). In low and middle-income countries, ARI constitutes one third of the deaths among children under five (Ujunwa & Ezeonu, 2014) and is also responsible for 60% and 30% paediatric outpatient visits and hospital admissions respectively (Nair et al., 2013; Ramani, Pattankar, & Puttahonnappa, 2016).
Previous studies have identified child factors such as age (Cardoso, Coimbra, & Werneck, 2013; Gebretsadik, Worku & Berhane 2015), sex ( Al-Sharbatti & AlJumaa, 2012), birth weight (Temani, Mayenger, & Bairwa, 2016), twin status (Thomsen et al., 2008) and birth order (Kumar et al., 2015) to be associated with ARIs. Maternal factors such as age (Cardoso et al., 2013; Gebretsadik, Worku & Berhane 2015), employment status (Cardoso et al., 2013; Gebretsadik, Worku & Berhane 2015), and educational attainment (Al-Sharbatti & AlJumaa, 2012; Kumar et al., 2015) have also shown to be associated with ARIs among under-five children. Wealth status (Cardoso et al., 2013; Savitha, Nandeeshwara, Kumar, & Raju, 2007), place of residence and household size (Kumar et al., 2015; Prajapati, Talsania, Lala, & Sonalia, 2012; Savitha et al., 2007) are also reported to predict the likelihood of acquiring ARI among children under five. Other possible factors like overcrowding, defects in immune system, overuse and misuse of antibiotics, poverty, limited ventilation and indoor air pollution also have a role to play (Harerimana et al., 2016).
Social and biological interpretations have been linked with variation in acute lower respiratory pathogens across geographic and climate zones (Akinyemi & Morakinyo, 2018; Ologunorisa & Tamuno, 2003). For example, some evidence from temperate and tropical regions found that temperature and humidity account for influenza seasonality by diminishing the host immunity. This occurs when inhalation of cold air causes vasoconstriction and reduction of blood flow while dry conditions reduce mucociliary clearance (Tamerius et al., 2013). Poor ventilated residents of colder regions may also suffer the same fate when winter conditions compel them to stay indoors (Imai et al., 2014). Conversely, Akinyemi and Morakinyo (2018) found ARI symptoms to be higher during the dry season. Since ARI pathogens are air borne and easily transmissible with dust, there is an increased childhood exposure (Akinyemi & Morakinyo, 2018; Ologunorisa & Tamuno, 2003).
In light of the foregoing study findings, we put forward that the likelihood of acquiring ARI can vary by ecological zone due to variations in geographical and climatic conditions such as variation in rainfall pattern, differences in humidity levels, closeness or otherwise to a desert, possible disparities in basic health services, level of awareness and preventive measures (Akinyemi & Morakinyo, 2018). To the best of our knowledge, no study has explored how ARI has affected children under five across the three principal ecological zones of Ghana using repeated cross sectional analytical approach on a national representative dataset. Investigation into ecological distribution of ARI symptoms over a period of two decades is of essence in light of Ghana's vision towards achieving the Sustainable Development Goal on improving health and wellbeing. The study will augment the existing efforts of governments of Ghana and policy makers focusing on prevention and control of ARIs and other WHO regions especially sub-Saharan Africa (WHO, 2006; Tazinya et al., 2018). The main objective of this study is to examine the dynamics of ARI symptoms among under-five children on the basis of ecological zones as well as childhood and maternal factors that explain the prevalence of ARI symptoms between 1993 and 2014. The study, therefore, was guided by the following research questions: What is the prevalence of ARI symptoms among children under five years? What is the trend of ALRI symptoms among children under five from 1993 to 2014? What is the association between ecological zone and symptoms of ALRI among children under five years in Ghana? What are the maternal and child characeristics that influence ARI among children under five in Ghana?
2. Methods
2.1. Data source
We used data from five rounds of the Ghana Demographic and Health Survey (GDHS) (1993, 1998, 2003, 2008 and 2014). The survey used standard DHS model questionnaire developed by the Measure DHS programme (Ghana Statistical Service, Ghana Health Service and ICF Macro,1994;1999;2004;2009;2015). The GDHS is a nationwide survey which covers all ten regions and is conducted every 5 years. The survey is done by the Ghana Statistical Service and the Ghana Health Service with ICF International giving technical support for the survey through MEASURE DHS. The GDHS concentrates on child and maternal health and is designed to offer adequate data to monitor the population and health situation in Ghana. It gathers data on various demographic and health issues including fertility, contraceptive use, child health, nutrition, malaria, HIV and AIDS, family planning, health insurance, maternal health, antenatal care, delivery care and post-natal care to monitor key maternal and child health indicators among low and middle-income countries. The survey offers reliable report and data for low and middle-income countries in monitoring maternal and child wellbeing.
The survey adopts a two-stage sampling design. The first stage involves the selection of clusters consisting of enumeration areas delineated for the Population and Housing Census preceding the surveys. These clusters were selected from the then 10 administrative regions of the country and across urban and rural areas. The second stage involved the selection of households from each cluster. Data collection was carried out using three questionnaires; household questionnaire, woman's questionnaire, and man's questionnaire. The household questionnaire was used to list all the members and visitors of the selected households with the information gathered used to identify women and men eligible for individual interviews. Eligible participants must either be permanent residents of the selected households or visitors who stayed in the household the night before the survey. The women's questionnaire was used to collect information from all eligible women aged 15–49 years. The selected women were deemed eligible to complete the women questionnaire on maternal and child health behaviour as well as their outcome. The men's questionnaire was administered to all eligible men aged 15–59 years.
We used child recode files from five out of the six GDHS conducted since the inception of the survey. We did not use the first survey (1988 GDHS) because the measure of the outcome variable was crude (it only asked whether cough occurred without asking if it was followed by short, rapid breath) making it inconsistent with measurement in all the other rounds of the survey (Ghana Statistical Service, Ghana Health Service and ICF Macro, 1994; 1999; 2004; 2009; 2015). The samples of mothers with children under five used for the study were 461, 771, 817, 600 and 744 for 1993, 1998, 2003, 2008 and 2014 respectively.
2.2. Outcome variable
The outcome variable for the study was ARI symptom which resulted from the question that asked if the child had experienced cough followed by short, rapid breath in the past two weeks preceding the survey which was posed as “When (NAME) had an illness with a cough, did he/she breathe faster than usual with short, rapid breaths or have difficulty breathing?” This question was a follow up to an initial question which asked whether the child coughed within the two weeks preceding the survey. The response to this question was a Yes or No. A dummy variable (Yes = 1 or No = 0) was generated with ‘0’ being a child who had not experienced any short rapid breath nor had difficulty breathing and ‘1’ represented a child who experienced short rapid breaths or had difficulty in breathing.
By this, the sample sizes quoted in our survey represent only children who coughed during the last two weeks preceding the survey. As earlier indicated in the preceding section of the manuscript, we could not add the 1988 survey because that survey only asked whether the child had coughed in the last two weeks without probing whether the cough was followed by short, rapid breath, which is the actual ARI symptom constituting our variable of interest.
2.3. Derivation of independent variable
In line with Ghana Statistical Service (GSS)'s (GSS, 2013; GSS et al., 2018) demarcation of Ghana into ecological strata, we divided the then ten administrative regions of the country into three ecological zones namely Coastal (comprising Western, Central and Greater Accra), Middle (made of Volta, Ashanti, Brong Ahafo and Eastern) and Savanna (constituting Northern, Upper East and Upper West). To a greater extent, these zones are distinguished by vegetation cover and atmospheric/environmental conditions making this categorization ideal for investigation into ARI symptoms. The Savannah is tropical grassland that generally has warm temperatures all year round. The rainfall is mostly seasonal and it sometimes record single pattern of rainfall. The Savannah has dispersed trees and there is enough sunlight for undergrowth which is mostly grass and shrubs. This zone is characterized with rainfall of approximately 1,000 mm annually. Middle zone is mainly made up of trees that are habitat to most wild animals. The trees are mainly hardwood and the area that surrounds the rain-forest is actually more fertile. The soils and the rains are a good combination for crop production especially roots and plantain. The middle zone receives rainfall of 2200 mm annually. The coastal zone which is made of Western, Central and Greater Accra has similar characteristics to that of the Middle zone. The annual rainfall in the coastal zone is around 1,500 mm (Nkrumah et al., 2014).
Following argument in literature (Akinyemi & Morakinyo, 2018; Biswal, Kar, Pal, & Dwibedi, 2018; Kumar et al., 2015), we included five childhood characteristics namely sex, age, size at birth, twin status and birth order as well as four maternal characteristics (residence, education, occupation and wealth quintile). In our quest to make these variables more reader-friendly and suitable for analysis, we recoded some of them. These are birth size which was recoded into larger than average = 1, average = 2 and smaller than average = 3; birth order recoded into 4 or lower = 1 and 5 or more = 2, occupation recoded into not working = 0, and not working = 1, education recoded into no none = 1, primary = 2 and secondary/higher = 3 whilst wealth was recoded into poor = 1, middle = 2 and rich = 3.
2.4. Analysis
This is a repeated cross-sectional study employing both descriptive and inferential analytical approaches. Through data exploration, all mothers who indicated that they did not know whether the cough was accompanied with short, rapid breathing were excluded because their proportion was minimal and as such did not compromise the robustness of our analysis. The excluded samples are as follows: 1993 (n = 7; 0.32%); 1998 (n = 5; 0.64%); 2003 (n = 2; 0.24%); 2008 (n = 1; 0.17%) and 2014 (n = 4; 0.53%). Following this, actual samples of mothers with children under five used for the study were 461, 771, 817, 600 and 744 for 1993, 1998, 2003, 2008 and 2014 respectively. We computed the national prevalence for each of the surveys by calculating the proportion of children who had ARI symptoms as well as distribution by childhood and maternal characteristics as indicated in Table 1. This was followed by a line graph illustrating the ecological dimension of ARI between 1993 and 2014 (See Fig. 1). Since the outcome variable was dichotomous, three Binary Logistic Regression models were estimated to examine how the Ghanaian children in the three zones have exhibited ARI symptoms at 95% confidence interval. We appended datasets for all the surveys to derive a panel data for the regression analysis. Firstly, Bivariate regression of ecological zones and ARI symptoms was conducted (Model I) after which we adjusted for child characteristics (Model II). The third model included maternal characteristics (Model III) to ascertain how both child and maternal characteristics would affect ARI as indicated in Table 2. The coefficients of the models were exponentiated to derive odds ratios (OR) and adjusted odds ratios (AOR). The adjusted OR were derived by controlling for the effect of the five aforementioned childhood and maternal characteristics (See Table 2). Sample weights were applied accordingly whereas all analyses were conducted with Stata version 14.2. This software has the advantage of directly including robust standard errors that account for the complex two stage sample design.
Table 1.
ARI symptoms by Child and Maternal Characteristics: 1993–2014.
|
Characteristics |
1993 n = 461 | 1998 n = 771 | 2003 n = 817 | 2008 n = 600 | 2014 n = 744 |
|---|---|---|---|---|---|
| ARI Prevalence | 12.6 | 26.0 | 21.1 | 18.3 | 22.0 |
| Child Characteristics | |||||
| Sex | |||||
| Male | 56.7 | 47.8 | 54.6 | 50.6 | 51.0 |
| Female | 43.7 | 52.2 | 45.4 | 49.4 | 49.0 |
| Age (In Years) | |||||
| 0 | 43.5 | 25.0 | 24.7 | 18.0 | 23.8 |
| 1 | 34.6 | 26.8 | 28.0 | 27.1 | 26.9 |
| 2 | 21.6 | 20.4 | 16.9 | 20.4 | 20.2 |
| 3 | – | 15.8 | 17.9 | 18.3 | 16.1 |
| 4 | – | 12.0 | 12.6 | 16.2 | 13.0 |
| Size at Birth | |||||
| Larger than Average | 50.0 | 54.4 | 47.5 | 60.4 | 51.9 |
| Average | 37.0 | 29.9 | 31.4 | 22.8 | 34.4 |
| Smaller than Average | 13.0 | 15.6 | 21.1 | 16.8 | 13.7 |
| Twin Status | |||||
| Single | 95.7 | 97.2 | 97.3 | 96.1 | 95.4 |
| 1st of Multiple | 1.92 | 1.35 | 1.5 | 1.7 | 2.3 |
| 2nd of Multiple | 2.4 | 1.4 | 1.1 | 2.1 | 2.3 |
| Birth Order | |||||
| 4 or lower | 68.7 | 73.3 | 72.6 | 73.4 | 76.4 |
| 5 or more | 31.3 | 26.7 | 27.4 | 26.6 | 23.6 |
| Maternal Characteristics | |||||
| Residence | |||||
| Urban | 19.3 | 20.0 | 29.6 | 36.4 | 39.8 |
| Rural | 80.8 | 80.0 | 70.4 | 63.6 | 60.2 |
| Education | |||||
| None | 49.3 | 42.6 | 39.0 | 31.4 | 27.6 |
| Primary | 47.3 | 20.3 | 24.3 | 32.8 | 20.4 |
| Secondary/Higher (3.4) | 37.1 | 36.7 | 35.8 | 52.0 | |
| Occupation | |||||
| Not working | 20.8 | 13.3 | 11.7 | 9.2 | 21.9 |
| Working | 79.2 | 86.7 | 88.3 | 90.8 | 78.1 |
| Wealth Quintile | |||||
| Poor | 50.3 | 49.4 | 51.3 | 51.0 | 41.2 |
| Middle | 31.9 | 23.4 | 19.8 | 17.0 | 26.2 |
| Rich | 18.1 | 27.2 | 28.9 | 32.0 | 32.6 |
Source: GDHS 1993–2014 || Results are in percentages || There were no observation for age 3–5 in the survey year, 1993.
Fig. 1.
Ecological distribution of ARI:1993–2014.
Sources: GDHS 1993–2014
Table 2.
Binary logistic regression results for ecological zones, child and maternal characteristics and symptoms of ARI.
| Variable | Model I |
Model II |
Model III |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Ecological Zone | ||||||
| Coastal | 1 | [1,1] | 1 | [1,1] | 1 | [1,1] |
| Middle | 1.15 | [0.98–1.36] | 1.15 | [0.92–1.42] | 1.05 | [0.88–1.26] |
| Savanna | 1.44*** | [1.20–1.72] | 1.20 | [0.94–1.50] | 1.16 | [0.94–1.43] |
| Child Characteristics | ||||||
| Sex | ||||||
| Male | 1 | [1,1] | 1 | [1,1] | ||
| Female | 0.95 | [0.80–1.13] | 0.94 | [0.82–1.30] | ||
| Age (In Years) | ||||||
| 0 | 1 | [1,1] | 1 | [1,1] | ||
| 1 | 1.11 | [0.86–1.42] | 1.06 | [0.88–1.29] | ||
| 2 | 0.95 | [0.73–1.23] | 0.94 | [0.72–1.23] | ||
| 3 | 0.93 | [0.70–1.23] | 0.92 | [0.69–1.22] | ||
| 4 | 0.91 | [0.68–1.22] | 0.95 | [0.71–1.28] | ||
| Size at Birth | ||||||
| Larger than Average | 1 | [1,1] | 1 | [1,1] | ||
| Average | 0.82* | [0.67–0.99] | 0.83 | [0.68–1.01] | ||
| Smaller than Average | 1.04 | [0.82–1.32] | 1.03 | [0.81–1.31] | ||
| Twin Status | ||||||
| Single | 1 | [1,1] | 1 | [1,1] | ||
| 1st of Multiple | 1.13 | [0.55–2.34] | 1.07 | [0.60–1.89] | ||
| 2nd of Multiple | 0.76 | [0.40–1.44] | 0.92 | [0.55–1.54] | ||
| Birth Order | ||||||
| 4 or lower | 1 | [1,1] | 1 | [1,1] | ||
| 5 or more | 1.17 | [0.96–1.43] | 1.05 | [0.89–1.25] | ||
| Maternal Characteristics | ||||||
| Residence | ||||||
| Urban | 1 | [1,1] | ||||
| Rural | 1.54** | [1.21–1.97] | ||||
| Education | ||||||
| None | 1 | [1,1] | ||||
| Primary | 0.96 | [0.80–1.17] | ||||
| Secondary/Higher | 0.92 | [0.76–1.12] | ||||
| Occupation | ||||||
| Not working | 1 | [1,1] | ||||
| Working | 0.95 | [0.77–1.16] | ||||
| Wealth Quintile | ||||||
| Poor | 1 | [1,1] | ||||
| Middle | 1.01 | [0.78–1.31] | ||||
| Rich | 0.93 | [0.68–1.27] | ||||
| Survey | ||||||
| 1993 | 1 | [1,1] | ||||
| 1998 | 1.59*** | [1.24–2.04] | ||||
| 2003 | 1.04 | [0.81–1.33] | ||||
| 2008 | 1.35* | [1.04–1.77] | ||||
| 2014 | 1.40* | [1.08–1.79] | ||||
Source: GDHS 1993–2014; OR= Odds Ratio; AOR = Adjusted Odds Ratio CI=Confidence Interval in square brackets; 1 = reference; *p < 0.05, **p < 0.01, ***p < 0.001
2.5. Ethical consideration
The authors were not directly involved in the data collection. However, Measuredhs reports that ethical clearance was obtained from the Institutional Review Board of ICF International and Ethical Review Committee of Ghana Health Service (Ghana Statistical Service, Ghana Health Service & ICF Macro, 1994; 1999; 2004; 2009; 2015). Demographic and Health Surveys (DHS) also anonymized all data before making them accessible to public. Permission to use the data set was sought from MEASURE DHS. Data set is available to the public at www.measuredhs.org.
3. Results
3.1. Descriptive results
Table 1 depicts the results of the descriptive analysis on the prevalence of ARI among children under five years in Ghana from 1993 to 2014. The samples used for the study were 461, 771, 817, 600 and 744 for 1993, 1998, 2003, 2008 and 2014 respectively. It was noted that reported ARI symptoms increased from 12.6% to 26.0% from 1993 to 1998. This declined to 18.3% in 2008 and rose to 22% in 2014. With sex, while reported ARI symptoms declined between 1993 (56.7%) and 2014 (51.0%) among the males, it increased among the females (from 43.7% to 49.0%) within that same period. Children aged one (1) recorded the highest prevalence of reported ARI symptoms (26.8%) in 1998 with the least occurring among children aged 4 (12.0%) in the same year. Similar observation was made in 2014 whereby children aged one (1) were leading (26.9%) with the least observed among those aged 4 (13.0%).
With regards to size at birth, children larger than average recorded the highest (50.0%) prevalence of reported ARI symptoms in 1993 while those smaller than average had the least (13.0%). Similar results were noted in 2014 whereby children larger than average had the highest (51.9%) cases of reported ARI symptoms with those smaller than average recording the least (13.7%) as seen in Table 1. As far as twin status is concerned, children born single had the highest (97.3%) proportion of reported ARI symptoms whilst second-of-multiple children experienced the least (1.1%) in 2003. In the same vein, children born single recorded the highest (95.4%) prevalence of reported ARI symptoms with the least occurring among second-of-multiple borns (2.3%) in 2014. The results further indicate that children at parity one registered the highest prevalence of reported ARI symptoms in 1993 (19.7%), 1998 (24.1%), 2008 (25.0%) and 2014 (22.7%) while those at parity 10 or above had the least reported ARI symptoms in 1993 (1.0%) and 2014 (0.8%).
Our study found that with maternal residence, reported ARI symptoms increased from 19.3% to almost 40.0% between 1993 and 2014 among urban dwellers. Meanwhile, it declined among rural residents from 80.8% to 60.2% between 1993 and 2014. With regards to maternal education, those with no education had the highest prevalence of reported ARI symptoms in 1993 (49%), 1998 (41.9%) and 2003 (38.8%) respectively and those with higher education had the lowest prevalence of reported ARI symptoms from 1993 to 2014. However, those with secondary education reported the highest prevalence of reported ARI symptoms in 2008 (34.5%) and 2014 (49.0%).
The analysis also revealed that children born to women who were into Agriculture or who were self-employed toped the prevalence of reported ARI symptoms from 1993 (45.9%) to 2008 (39.2%). Reported prevalence was high among the poor from 50.3% in 1993 to 41.2% in 2014. On the other hand, mothers with middle wealth quintile had the lowest prevalence of reported ARI symptoms between 1998 (23.4%) and 2014 (26.2%), although the lowest prevalence reported showed an increasing trend as seen in Table 1.
3.2. Ecological distribution of ARI: 1993–2014
Pictorial exhibition of reported prevalence of ARI symptoms across ecological zones indicates that inhabitants of Middle zone consistently reported the highest ARI symptoms from 1993 (45.2%) through 2003 (52.4%) to 2014 (47.3%) as compared to all other ecological zones. Among Coastal dwellers, the prevalence of reported ARI symptoms increased from 18.7% (1993) to 31.5% (1998) and declined to 21.4% (2008). However, there was a rise from 21.4% (2008) to 36.5% (2014). Reported ARI symptoms among Savanna inhabitants reduced from 36.1% in 1993 to 16.2% in 2014 but some fluctuations occurred within this period instead of a linear decline (See Fig. 1).
3.3. Multivariate results
Table 2 indicates the results of the binary logistic regression analysis for reported ARI symptoms across the principal ecological zones as well as child and maternal characteristics (Model I and II). As shown in Model I, ecological zone showed a statistically significant association with ARI symptoms. Specifically, Savanna residents had higher odds to experience ARI symptoms as compared to Coastal dwellers [OR = 1.44, CI = 1.20–1.72] in Model I. Meanwhile, after introducing child and maternal factors into the model (Model II), ecological zone showed no statistically significant relationship with ARI symptoms.
The analysis further revealed that residence and survey year predicted the prevalence of reported ARI symptoms. With residence, children whose mothers lived in rural areas had 1.5 higher odds to experience ARI symptoms compared to those whose mothers lived in urban areas [AOR = 1.54, CI = 1.21–1.97]. Finally, the odds of children reporting symptoms of ARI varied from survey wave years (see Model III). For instance, it was observed that the children born in survey wave year 1998 had higher odds of ARI symptoms compared to 1993 [AOR = 1.54, CI = 1.22–1.94].
4. Discussion
In this present study, our aim was to examine the trend in the prevalence of ARI symptoms among Ghanaian under-five children on the basis of ecological zones as well as childhood and maternal factors associated with the phenomenon between 1993 and 2014. With the trend in the prevalence of ARI symptoms on the basis of ecological zones, our study found that the prevalence of ARI symptoms among under-five children generally showed an increasing trend among middle zone residents. The high prevalence of ARI symptoms among middle zone dwellers in this current study is contrary to the findings of Suguna, Kumar, and Roy (2014) who carried out a study on prevalence and risk factors of acute respiratory infection among school children in coastal South India and found that more than half of them had at least one symptom of ARI in the preceding 2 weeks. This finding is also inconsistent with a community-based study in a coastal village of Karnataka, India which reported higher pneumonia prevalence among infants (Acharya, Prasanna, Nair, & Rao, 2003). Possible reason for the differences in ARI symptom prevalence for the different surveys in our study could be linked to the differences in the months in which the surveys were carried out as well as the differences in study settings. Another probable reason for the high prevalence in the middle zone could be the high rainfall pattern in the middle belt of Ghana as explained by Moura, Perdigao and Siqueira (2009) and Harerimana et al. (2016) that heavy rains increase ARI due to the fact that during heavy rains people are forced to stay indoors and those with the condition are more likely to pass it on to children under five.
The study further revealed that children whose mothers live in rural areas are more likely to experience ARI symptoms compared to those whose mothers live in urban areas. In line with the findings of the current study, Sharma, Kuppusamy, and Bhoorasamy (2013) carried out a study on the prevalence of ARI among under five children in Kancheepuram District of South India and found that most risk factors prevail in rural areas compared to urban areas. Similar findings were obtained by Kilabuko and Nakai (2007) and Rehman and Ishaq (2018) who affirmed that living in rural areas is associated with higher prevalence of ARI in relation to living in urban areas. The possible reason for the higher odds ratio of ARI symptoms for rural children has been attributed to poor access to medical care and low socioeconomic standard that exist in rural areas (Kilabuko & Nakai, 2007). On the other hand, the findings of the study contradict the findings of Kumar et al. (2015) who found that children whose mothers live in urban areas are more likely to experience ARI. The possible reasons for the variations in the study findings could be the differences in study sites and the source of data used for the various studies as well as the times the studies were conducted. Either way, it is time for government to ensure fairly equitable allocation and access to healthcare facilities and basic necessities of life. With this, a child in either rural or urban settings would have similar if not same services to meet all their health needs.
We found that children born within the year 1998 were more likely to show symptoms of ARI compared to those born in 1993. The reported high ARI symptoms in 1998 confirms the outcome of some studies on ARI which identified high prevalence of ARI within the same period (Koch et al., 2002; WHO, 1998). It must however be understood that morbidity data collected in our surveys are subjective and are based on a mothers’ perception of illness but not validated by medical diagnosis and also prevalence of ARI symptom is subject to seasonality (Ghana Statistical Service, Ghana Health Service & ICF Macro, 1999; GSS, 2016). The reported finding is largely dependent on the ability of mothers to identify symptoms of ARI and might have decreased after the 1993 survey as a result of education and sensitization on child health outcomes including ARI.
The study had its strength from the large data and high response rate. The use of nationally representative surveys (DHS) and the use of stratified two-stage sampling technique made it possible to obtain samples that are highly representative of the target populations. The large sample size and the national representative nature of the data make conclusions from our study more generalisable and valid. The limitation of the study is that data collected in the surveys are subjective that is, based on a mother's perception of illness and not validated by medical diagnosis. The study focused only on children who are coughing. Using cough as a measure of ARI has a limitation as there are numerous non-infectious causes of “cough” or even “coughs followed by short, rapid breaths”. For example, dust might contribute disproportionately to ARI symptoms in desert regions and air pollutants might also play a larger role in urban areas. Finally, due to the cross-sectional nature of the data, we were only able a measure association but not cause-effect relationship.
5. Conclusion
The study found variations in ARI symptoms across the ecological zones in Ghana. Ecological zone, place of residence, and survey year were associated with symptoms of childhood ARIs. The middle zone consistently reported the highest prevalence of ARI symptoms from 1993 to 2014 as compared to all other ecological zones. The trend identified in ARI symptoms on the basis of ecological zones points out the specific indicators to target in policy efforts, e.g. residence and ecological zone. This will facilitate curbing ARI by directing resources geared towards preventing ARI to the people who need them the most. With the high occurrence of ARI symptoms in the middle zone, public health education and sensitization on ARI need to be more specific and target women of children under five who live in middle zones compared to those who live in other ecological zones.
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board of ICF International and Ethical Review Committee of Ghana Health Service. Demographic and Health Survey (DHS) also anonymized all data before making them accessible to public. Permission to use the data set was sort from MEASURE DHS.
Acknowledgments
We are grateful to MEASURE DHS for granting us access to use the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2019.100414.
Contributor Information
Abdul-Aziz Seidu, Email: abdul-aziz.seidu@stu.ucc.edu.gh.
Edward Kwabena Ameyaw, Email: edmeyaw19@gmail.com.
Bright Opoku Ahinkorah, Email: brightahinkorah@gmail.com.
Linus Baatiema, Email: baatiemalinus@gmail.com.
Francis Appiah, Email: engman477@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Accinelli R.A., Leon‐Abarca J.A., Gozal D. Ecological study on solid fuel use and pneumonia in young children: A worldwide association. Respirology. 2017;22(1):149–156. doi: 10.1111/resp.12865. [DOI] [PubMed] [Google Scholar]
- Acharya D., Prasanna K.S., Nair S., Rao R.S. Acute respiratory infections in children: A community based longitudinal study in south India. Indian Journal of Public Health. 2003;47:7–13. [PubMed] [Google Scholar]
- Akinyemi J.O., Morakinyo O.M. Household environment and symptoms of childhood acute respiratory tract infections in Nigeria, 2003–2013: A decade of progress and stagnation. BMC Infectious Diseases. 2018;18(1):296. doi: 10.1186/s12879-018-3207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sharbatti S.S., AlJumaa L.I. Infant feeding patterns and risk of acute respiratory infections in Baghdad/Iraq. Italian Journal of Public Health. 2012;9(3) [Google Scholar]
- Biswal B., Kar S.K., Pal B.B., Dwibedi B. Bacterial and viral etiology of acute respiratory illness among children from two different geographical localities of odisha. Journal of Pure and Applied Microbiology. 2018;12(2):993–1000. [Google Scholar]
- Cardoso A.M., Coimbra C.E., Jr., Werneck G.L. Risk factors for hospital admission due to acute lower respiratory tract infection in guarani indigenous children in southern Brazil: A population‐based case‐control study. Tropical Medicine and International Health. 2013;18(5):596–607. doi: 10.1111/tmi.12081. [DOI] [PubMed] [Google Scholar]
- Gebretsadik A., Worku A., Berhane Y., Morries L., Cassano P., Henderson T. Factors associated with acute respiratory infection in children under the age of 5 years: Evidence from the 2011 Ethiopia demographic and health survey [corrigendum] Neuropsychiatric Disease and Treatment. 2015;11:2159–2175. [Google Scholar]
- Ghana Statistical Service . Ghana Statistical Service; Accra: 2016. Health of women and children in Ghana: Evidence from the demographic and health surveys. [Google Scholar]
- Ghana Statistical Service, Macro International . GSS and MI; Calverton, Maryland: 1999. Ghana demographic and health survey 1998. [Google Scholar]
- Ghana Statistical Service, Ghana Health Service, ICF Macro . GSS, GHS and ICF Macro; Accra: 2009. Ghana demographic and health survey 2008: Key indicators. [Google Scholar]
- Ghana Statistical Service, Ghana Health Service, ICF Macro . GSS, GHS and ICF Macro; Accra: 2015. Ghana demographic and health survey 2014: Key indicators. [Google Scholar]
- Ghana Statistical Service and Macro International . GSS, MI; Calverton, Maryland: 1994. Ghana demographic and health survey 1993. [Google Scholar]
- Ghana Statistical Services, Noguchi Memorial Institute for Medical Research, ORC Macro . GSS, NNMR, and ORC Macro; Calverton, Maryland: 2004. Ghana demographic and health survey 2003. [Google Scholar]
- Harerimana J.M., Nyirazinyoye L., Thomson D.R., Ntaganira J. Social, economic and environmental risk factors for acute lower respiratory infections among children under five years of age in Rwanda. Archives of Public Health. 2016;74(1):19. doi: 10.1186/s13690-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C., Brooks W.A., Chung Y., Goswami D., Anjali B.A., Dewan A. Tropical influenza and weather variability among children in an urban low-income population in Bangladesh. Global Health Action. 2014;7(1):24413. doi: 10.3402/gha.v7.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilabuko J.H., Nakai S. Effects of cooking fuels on acute respiratory infections in children in Tanzania. International Journal of Environmental Research and Public Health. 2007;4(4):283–288. doi: 10.3390/ijerph200704040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Sørensen P., Homøe P., Mølbak K., Pedersen F.K., Mortensen T. Population-based study of acute respiratory infections in children, Greenland. Emerging Infectious Diseases. 2002;8(6):586. doi: 10.3201/eid0806.010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.G., Majumdar A., Kumar V., Naik B.N., Selvaraj K., Balajee K. Prevalence of acute respiratory infection among under-five children in urban and rural areas of puducherry, India. Journal of Natural Science, Biology, and Medicine. 2015;6(1):3. doi: 10.4103/0976-9668.149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura F.E., Perdigão A.C., Siqueira M.M. Seasonality of influenza in the tropics: A distinct pattern in northeastern Brazil. The American Journal of Tropical Medicine and Hygiene. 2009;81(1):180–183. [PubMed] [Google Scholar]
- Nair H., Simões E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S.F. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. The Lancet. 2013;381(9875):1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkrumah F., Klutse N.A.B., Adukpo D.C., Owusu K., Quagraine K.A., Owusu A. Rainfall variability over Ghana: Model versus rain gauge observation. International Journal of Geosciences. 2014;5(7):673–683. [Google Scholar]
- Ologunorisa T.E., Tamuno T.T.T. Spatial and seasonal variations of sandstorms over Nigeria. Theoretical and Applied Climatology. 2003;75(1–2):55–63. [Google Scholar]
- Prajapati B., Talsania N.J., Lala M.K., Sonalia K.N. Epidemiological profile of acute respiratory infections (ARI) in under five age group of children in urban and rural communities of Ahmedabad district, Gujarat. International Journal of Medical Science and Public Health. 2012;1(2):52–59. [Google Scholar]
- Ramani V.K., Pattankar J., Puttahonnappa S.K. Acute respiratory infections among under-five age group children at urban slums of gulbarga city: A longitudinal study. Journal of Clinical and Diagnostic Research: Journal of Clinical and Diagnostic Research. 2016;10(5):LC08. doi: 10.7860/JCDR/2016/15509.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M.U., Ishaq M. Prevalence of acute respiratory infections (ARI) and its risk factors in under five children in urban and rural areas of Matta, district Swat. International Journal of Infectious Diseases. 2018;73:230. [Google Scholar]
- Savitha M.R., Nandeeshwara S.B., Kumar M.P., Raju C.K. Modifiable risk factors for acute lower respiratory tract infections. Indian Journal of Pediatrics. 2007;74(5):477–482. doi: 10.1007/s12098-007-0081-3. [DOI] [PubMed] [Google Scholar]
- Sharma D., Kuppusamy K., Bhoorasamy A. Prevalence of acute respiratory infections (ARI) and their determinants in under five children in urban and rural areas of Kancheepuram district, South India. Annals of Tropical Medicine and Public Health. 2013;6(5):513. [Google Scholar]
- Suguna E., Kumar S.G., Roy G. Prevalence and risk factors of acute respiratory infection among school children in coastal South India. Journal of Global Infectious Diseases. 2014;6(3):95. doi: 10.4103/0974-777X.138498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J.D., Shaman J., Alonso W.J., Bloom-Feshbach K., Uejio C.K., Comrie A. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathogens. 2013;9(3) doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazinya A.A., Halle-Ekane G.E., Mbuagbaw L.T., Abanda M., Atashili J., Obama M.T. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulmonary Medicine. 2018;18(1):7. doi: 10.1186/s12890-018-0579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temani K., Mayenger A., Bairwa A.L. Assessment of prevalence of acute respiratory tract infection and risk factors in under five children in anganwadi of Kota city. Indian Journal of Child Health. 2016;3(3):234–237. [Google Scholar]
- Thomsen S.F., Stensballe L.G., Skytthe A., Kyvik K.O., Backer V., Bisgaard H. Increased concordance of severe respiratory syncytial virus infection in identical twins. Pediatrics. 2008;121(3):493–496. doi: 10.1542/peds.2007-1889. [DOI] [PubMed] [Google Scholar]
- Ujunwa F.A., Ezeonu C.T. Risk factors for acute respiratory tract infections in under-five children in enugu southeast Nigeria. Annals of Medical and Health Sciences Research. 2014;4(1):95–99. doi: 10.4103/2141-9248.126610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . UNICEF; New York: 2016. One is too many: Ending child deaths from pneumonia and diarrhea. 2016. [Google Scholar]
- UNICEF, WHO, World Bank & UN-DESA Population Division Levels & trends in child mortality report 2018. 2018. Retrieved from: www.un.org/en/development/desa/.../mortality/child-mortality-report-2018.shtml.
- World Health Organization Acute respiratory infections: The forgotten pandemic. Bull WHO. 1998;76:101–103. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Vol. 27. UNICEF/WHO; Geneva: 2006. (Pneumonia, the forgotten killer of children). 2006. 10: 92-806-4048-8. [Google Scholar]
- World Health Organization, UNICEF . 2013. Ending preventable child deaths from pneumonia and diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.