Abstract
Rationale
Geographic clusters of low vaccination uptake reduce the population-level efficacy of vaccination programs. However, little is known about the mechanisms that drive geographic patterns in vaccination rates. Traditional economic theory considers vaccination as a classic public good and suggests that free riding—individuals taking advantage of public goods by relying on others’ immunization behavior without contributing toward them—is a primary cause of low vaccination rates. However, behavioral economics suggests that free riding does not fully explain observed individual behavior, and the presence of both high and low clusters of vaccination rates suggest that this theory alone does not fully explain geographic patterns of vaccination.
Objective
We assessed geographic clustering of HPV vaccination uptake and examined the evidence for or against free riding in HPV vaccination decisions.
Methods
We analyzed HPV vaccination decisions of low-income adolescent females (N = 601) residing in urban neighborhoods in Dallas, Texas, USA during 2011–2012. Spatial econometric models were estimated to assess the relationship between neighborhood vaccination rates and individual vaccination decisions.
Results
We found a positive and significant relationship between individual HPV vaccination choices and the average neighborhood vaccination rate at the time parents were making vaccine decisions for their adolescent daughters while controlling for neighborhood sorting and other confounders.
Conclusion
Individuals were more likely to complete the HPV vaccination series when others in their neighborhood had already completed the series. We do not find evidence for free riding in HPV vaccination decisions.
Keywords: Public goods, Health, Cancer, Inequality, Vaccination
Highlights
-
•
Free riding describes individual's ability to benefit from vaccination decisions of others while remaining unvaccinated.
-
•
The dominant theory explaining free riding is the public goods model developed by economists.
-
•
We find no empirical support for free riding in HPV vaccination decisions.
-
•
HPV vaccination decisions are complex and produce both social- and health-related externalities.
1. Introduction
Due to the method of transmitting communicable diseases, gains from effective vaccination programs are inherently geographic in nature and are not necessarily distributed evenly across space. For example, geographic clustering of individuals refusing to vaccinate despite governmental mandates has been linked to outbreaks of measles, a disease once considered eradicated in developed countries (Ferris, 1/28/15). The potential importance of spatial patterns in vaccination uptake in understanding how policy might influence vaccination rates for non-mandatory vaccines, such as the human papillomavirus (HPV) vaccine, are largely unaddressed by prevailing theories of vaccine uptake.
Because vaccination is important both at individual and community levels, there have been many efforts to understand an individual's vaccination decisions and the resulting population-level patterns of vaccination. Health behavior researchers have proposed theories that focused on individual's intention, beliefs and motivations (e.g., Brewer & Fazekas, 2007). However, these theories typically do not consider the role of community-level vaccination rates in changing individuals' incentives to become vaccinated. In contrast, economists have focused on the role of community-level vaccination rates (Boulier, Datta, & Goldfarb, 2007; Geoffard & Philipson, 1997), but have typically overlooked the role of individual beliefs and social influences.
The current prevailing theory for understanding spatial patterns of vaccination comes from economics and focuses on the role of local vaccination rates. Vaccinations are considered a public good because they provide non-rival benefits (i.e. the benefits from Maria having been vaccinated do not deplete as additional people come in contact with Maria, also benefitting from her vaccination) and non-exclusive benefits (i.e. once Maria has been vaccinated, she cannot exclude others from benefitting from her decision) to all community members. The free-rider problem is key to economic theories explaining public good provision. For vaccinations, the free rider problem arises when increasing local vaccination rates reduce disease risk and, if unvaccinated individuals are aware of the local vaccination rate, then they may have less incentive to decide to vaccinate (Boulier et al., 2007). Further, awareness of the local rate and its impact on risk, is not a necessary condition for free riding to occur (Cornes & Sandler, 1996). Free riding may occur simply because vaccination reduces feedback that would otherwise signal risks incentivizing individuals to become vaccinated. We undertake one of the first empirical studies examining evidence for or against free riding behavior in HPV vaccination uptake. Results inform whether the broad assumption of free riding as a significant behavioral influence applies to HPV vaccination uptake.
Free riding theory is agnostic to the underlying parental intentions and motivations for vaccination decisions. Rather, the theory posits that a variety of motivations/intentions may be explained by free riding simply because externalities associated with high local vaccinations rates inhibit the incentives for individuals to vaccinate—regardless of whether individuals are conscious of the influence caused by the externalities. For example, parents may state that they refuse vaccination because of religious or personal belief; but if we observe that these refusals are more likely to be sought when disease risk is low, then economists would posit that this is evidence for free riding. Researchers have documented that among church members reporting religious, safety or philosophical objections to vaccination, vaccine hesitancy and intention improved following a local outbreak of Measles (Kennedy, 2008). This change in intention despite self-reported motivations unrelated to local vaccination rates is consistent with free riding.
Free riding as a theory explaining vaccination uptake has been very broadly applied to both new and established vaccines. Economic experiments have shown that free riding behavior in vaccination decisions was indifferent to changes in the risk and severity of the disease being targeted by the vaccine (Ibuka, Li, Vietri, Chapman, & Galvani, 2014). This is consistent with qualitative work, showing that immigrant women reported willingness to receive the HPV vaccine if recommended by their physician despite having a low perceived risk of infection (McComb, Ramsden, Olatunbosun, & Williams-Roberts, 2018). Health economics textbooks attribute vaccination programs’ failure to achieve herd immunity to the free rider problems—without differentiation between types of vaccinations and the diseases prevented (Bhattacharya, Hyde, & Tu, 2013). In prior economic research, free riding was simply assumed as a hurdle to be overcome when developing new government public health strategies to increase vaccination (Berezin & Eads, 2016; Hendrix, Sturm, Zimet, & Meslin, 2016; Klepac, Megiddo, Grenfell, & Laxminarayan, 2016). Free riding behavior has been assumed in studies: (1) documenting declining vaccination rates for established vaccines, such as measles (Browne, 2016), and (2) examining “new” pre-emptive vaccination strategies (Molina & Earn, 2015). Others suggest that public health campaigns should highlight the benefits of herd immunity to combat free-riding motives (Betsch, Böhm, & Korn, 2013; Quadri-Sheriff et al., 2012).
Despite the wide-spread application of free-riding theory in a large body of work aimed at informing policy, few empirical studies have measured the actual extent of free riding. In fact, recent experimental work has suggested that peer influence or conformity, rather than free riding, informs vaccination behavior (Verelst, Willem, Kessels, & Beutels, 2018). In the case of other public goods, the empirical evidence is mixed and suggests that free riding behavior is context dependent (Andreoni, 1988; Fischbacher, Gächter, & Fehr, 2001; Isaac, Walker, & Thomas, 1984; Marwell & Ames, 1981). Our prior empirical studies have attempted to test whether evidence for free riding exists by assessing whether neighbors' public good contributions are statistically significant predictors of own-public good contributions (Beron, Murdoch, & Vijverberg, 2003; Kotchen & Moore, 2007; Murdoch & Sandler, 1984; Sandler & Murdoch, 1990). This work has advanced economists' and other social scientists' understanding of alternative public good models, such as the impure public goods theoretical framework (Cornes & Sandler, 1994; Vicary, 1997). Free-riding theory is viewed to only be consistent with a negative relationship between neighbor's public good contributions and own-contributions, while the impure public goods model is consistent with either a positive or negative relationship. Collectively, these results call for a broader discussion about the context of the public good contribution decision, rather than a general application of the free riding assumption (Cornes & Sandler, 1984; Leonard, 2016).
Studies have documented geographic clustering of low HPV vaccination uptake (Pruitt & Schootman, 2010; Wei, Moore, & Green, 2013), and researchers have attempted to explain this clustering by examining correlates of vaccination decisions (e.g. Bartlett & Peterson, 2011; Brewer & Fazekas, 2007; Garcini, Galvan, & Barnack-Tavlaris, 2012; Holman et al., 2014). This extant literature suggests HPV vaccination is correlated with factors related to the individual costs and benefits from vaccination; this is consistent with the economic theory of public good provision, which then posits that this focus on individual costs and benefits will result in free riding. However, no studies have assessed whether free riding explains observed spatial patterns in vaccination rates. We fill this gap by testing the free riding hypothesis in the context of low-income parents’ decisions to obtain the HPV vaccine for their daughters.
Specifically, we conducted a secondary analysis of a longitudinal intervention study promoting HPV vaccination behavior among adolescent females attending safety-net pediatric clinics in Dallas, Texas. Studying this issue in Dallas is important because the area has both a significant cervical cancer burden as well as suboptimal vaccination rates when compared to other cities in Texas and across the US (Tiro et al., 2012). Our analysis exploits temporal variation in the clinic's invitation to obtain HPV vaccinations to test the robustness of our empirical results. Based on our empirical framework, free riding behavior would be evidenced by an inverse relationship between the average neighborhood HPV vaccination rate at the time of the vaccination decision and the likelihood of individuals becoming vaccinated.
2. Methods
2.1. Source of study data
The longitudinal intervention study of 815 parent-daughter dyads was conducted between 2011 and 2012. All study participants were randomized to one of two intervention arms. The outcome, HPV vaccination, was measured via the electronic medical record (EMR). All vaccines are provided for free through Vaccines for Children (VFC), a federally funded program to improve vaccination access and remove cost barriers (Centers for Disease Control and Prevention [CDC], 2016b). Because our analytic goal was to investigate geographic clustering of vaccination decisions, we treated data as observational, and consider intervention status as a model covariate.
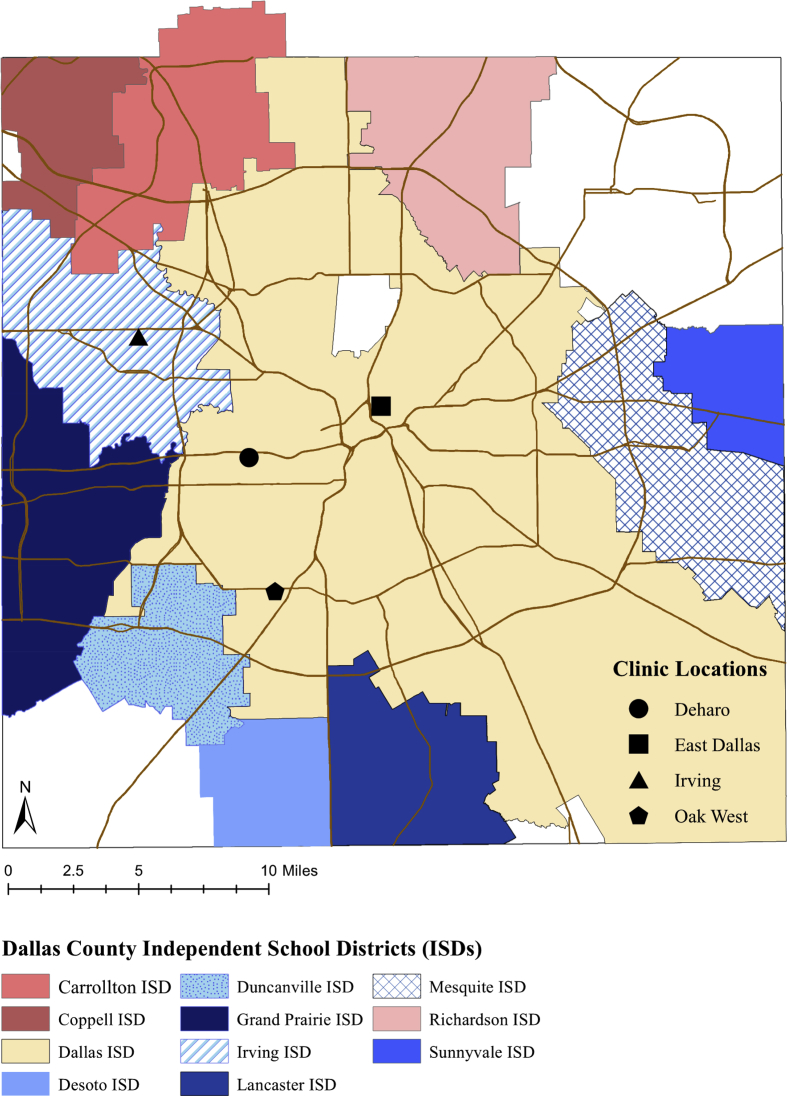
The study setting, Dallas, has the highest population density in Texas and has substantial residential segregation by race/ethnicity, income, and health insurance status. Participants were recruited from the county tax-supported Parkland Health and Hospital System, a safety-net healthcare system with pediatric ambulatory clinics in low-income neighborhoods with high uninsurance rates. These neighborhoods are clustered in the southern sections of the county (see Fig. 1).
Fig. 1.
Dallas country: Independent School Districts (ISDs) and Clinic locations.
The study population was parent-daughter dyads. The daughters were female patients at the clinic who were aged 11–18, had not started the HPV vaccine series, and had an upcoming clinic appointment at one of the four largest Parkland clinics (10 total). Participants were randomized to receive by mail 2 weeks before their clinic visit either an HPV vaccine-specific brochure (“HPV Brochure” group) or a general brochure (“General Brochure” group) about all four recommended adolescent vaccines (HPV, tetanus diphtheria acellular pertussis [TDAP], meningococcal, and influenza). A few days after the brochure mailing, participants who did not opt out of the study were invited to meet with a research assistant immediately prior to their daughter's clinic visit to provide consent for additional contact and complete a survey about the HPV vaccine. Participants' vaccine status was extracted from the EMR at the end of a 12-month study window, timed to begin with the daughter's clinic visit. The majority of the study windows did not completely overlap between participants. See (Tiro et al., 2015) for more detailed description of the main intervention study.
2.2. Analytic approach: spatial econometric modeling
To test for the presence and nature of spatial dependency, we applied a spatial econometric model that followed the empirical methodology employed by Murdoch and Sandler (1984), among others.
2.2.1. Moran's I test for spatial autocorrelation
Prior to estimating spatial econometric models, the degree of spatial autocorrelation in the outcomes was assessed. If no spatial autocorrelation was found, then the rationale for a spatial econometric approach would be less clear. We conducted a modified Moran's I test of spatial autocorrelation using an approach for non-continuous outcome variables (LeSage & Pace, 2009; Lin & Zhang, 2007).
2.2.2. Spatial autoregressive probit model
Next, we estimated a spatial autoregressive (SAR) probit model (Equation (1)) (LeSage & Pace, 2009). The latent variable, represents the underlying continuous vaccination decision process where high values of indicate an increased likelihood of vaccination.
| (1) |
Average neighborhood vaccination rate, , is the key exposure variable of interest. is a matrix of covariates measuring patient demographics, preventive health behaviors (including receipt of other vaccines), healthcare access, intervention group assignment and consent for additional contact. To account for residential sorting, we controlled for many features in X along which households might be likely to sort: race/ethnicity, school districts, and clinic attended. is the key predictor variable of interest, and will be used to test the free rider hypothesis.
The weight matrix, , specifies the neighbors for each individual. Neighbors were weighted equally and was row-standardized. Neighbors were included in the weight matrix if they lived within 0.5 miles of an individual's home address. Geographic proximity was used to define neighbors because adolescents who lived near to each other often attended the same schools, used the same bus, and participated in the same sports and other activities. Likewise, parents often interact when attending school events, and learn about the decisions of other parents through their child. Tiro et al. (2012) describe a more detailed conceptual model of the HPV decision process. Alternative definitions for neighbors (i.e., weight by inverse distance, nearest 5 neighbors) were also used to assess the robustness of our results.
We calculated direct and indirect average marginal effects from our spatial model (Pace & LeSage, 2009). In general, the direct marginal effect measures how a change in an explanatory variable affected the participant's own likelihood of vaccination. The indirect marginal effect measures the impact of a change in the explanatory variable on neighbors' vaccination decisions. The total effects capture both the direct impact on the individual and the indirect or spatial spillover impact on neighbors (Pace & LeSage, 2009). The SAR probit model was estimated using the Econometrics Toolbox Matlab code provided by LeSage (LeSage, 2010). In addition, we estimated a non-spatial probit model (where the restriction ρ = 0 is imposed), the approach traditionally taken in most public health research; since the results for the other covariates were consistent with the spatial model, they are not presented.
2.2.3. Robustness tests: neighborhood definition and neighborhood sorting
To test for sensitivity to the specification of the weights matrix, we estimated additional models using 2 alternative specifications: inverse distance up to 0.5 miles and nearest 5 neighbors. Next, it is possible that our estimates of may be a result of spurious spatial correlation. To test for the possibility of spurious spatial correlation, we included more distant neighbors in the weight matrix: neighbors who were also participants that lived between 0.5-1 mile and 1–1.5 miles away. Estimates for are expected to decay and/or become statistically insignificant with weight matrices including increasingly distant neighbors.
Another potential source of bias in our estimates for is related to residential sorting: if individuals chose residential neighborhoods based on characteristics that also made them more/less likely to obtain the HPV vaccine for their children (e.g., religious affiliation, social norms, or shared culture) then confounding could occur. While we controlled for multiple features upon which people could have sorted into neighborhoods (e.g., race/ethnicity) and examined the timing of vaccine decisions, this bias may still persist in the data. To account for this we utilized the time variation in our data. In the modified SAR probit model, we estimated , where captured the average neighborhood vaccination rate at the precise time that the reference individual was vaccinated, and an additional term () was included to control for the average vaccination rate among neighbors who had not initiated, yet (i.e. those who will ultimately decide to vaccinate after the reference individual or never):
| (2) |
Because the timing of clinic visits was independent of vaccination likelihood, the average vaccination rate among neighbors who vaccinated after the reference individual () provides an exogenous control for any spatially varying factors associated with vaccination rates. Thus the estimate of in the modified SAR probit model will not suffer from endogeneity associated with failing to control for a spatially varying unobserved neighborhood or individual characteristics. Both and are weights matrices constructed by modifying W in equation (1). For , the elements of W that corresponded to a neighbor who had not yet initiated were set to 0; likewise, for , the elements of W that corresponded to a neighbor who had already initiated at time t, were set to 0. For example, suppose individual i had 5 neighbors, then the non-zero elements of W were equal to 0.2. If 2 of these neighbors had initiated at time t, then only those elements associated with those neighbors remained 0.2 in and the other elements were set to 0. Thus, , indicating that 40% of the neighbors had already initiated at the time that individual i was making their decision.
2.2.4. Study sample
Study data on the adolescent-parent dyads were extracted from the EMR. In addition to the main study's eligibility criteria (N = 815), we further restricted our sample to those who: 1) had a residential address and complete data for all covariates needed in the analysis (76 observations dropped), 2) lived within a 20 mile buffer of one of the four clinics (9 observations dropped), and 3) were not geographically isolated (i.e., had at least one neighboring participant within 0.5 miles; 129 observations dropped). In all, 214 observations were dropped for one or more of these reasons. It was not possible to tell if observations with missing data were random. We did not impute missing observations because it would likely induce error as the analysis is dependent on knowing both participant characteristics and geographic location. Robust spatial imputation methods necessary for our application would require more observations than we had available (LeSage & Pace, 2009).
2.2.5. Outcome measure
The 12-month study period limited our ability to observe full 3-dose completion without censure because participants receiving the second dose late in the study period may have obtained the third dose after data collection ended. Therefore, we analyzed the likelihood of participants receiving at least 2 doses; this decision corresponds with 2016 guidelines that recommended only 2 doses for those receiving Dose 1 before age 15 (Centers for Disease Control and Prevention [CDC], 2016a). The outcome variable Complete was coded 1 for participants receiving at least 2 doses.
To test for the possibility of censoring, we divided the sample of patients who received at least 2 doses (N = 147) into three groups: Censored (received Dose 2 < 4 months before study end), Semi-Censored (received Dose 2 4–6 months before study end), and Uncensored (received Dose 2 with >6 months before study end). For each group, we computed the percentage of patients who continued on and received Dose 3. Approximately, 62.5%, 50.0%, and 76.3% of the Censored, Semi-Censored, and Regular groups, respectively, went on to receive Dose 3.
2.2.6. Covariates
Sociodemographic characteristics were defined as follows— race/ethnicity (African American, Hispanic/non-Hispanic White), age (dummy variables for age groups 11–12, 13–14, 15–16, 17–18, based on age at the study visit), and health insurance (private, public, or none). Measures for preventive health and previous vaccine behavior included missed appointments (average number of missed appointments at any point prior to the study visit), past HPV vaccine refusal (equal to 1 if parent ever refused, 0 otherwise), and receipt of other adolescent vaccinations (Influenza in past year, tetanus, diphtheria, pertussis [TDAP] and meningococcal [MCV] prior to randomization). Intervention status was captured with two variables, type of brochure (equal to 1 for the HPV-specific, and 0 for the general brochure) and parental consent for telephone intervention (equal to 1 if prior to the study visit, the parent completed the optional survey and gave consent for reminders from study staff).
Finally, clinic attended (dummy variables for each of the four clinics) and independent school district (ISD) zones were included as healthcare access and geographic variables. To determine the GIS-derived ISD measures, study participants were assigned to one of twelve school districts based on their residential location reported in the EMR (geocoded to State Plane projected coordinates using ArcMap 10.1) and Dallas-area school district boundaries. These districts were then grouped together into four independent school zones, either based on attendance or geographic proximity: (1) the Dallas ISD, which had the largest attendance in the sample; (2) the Irving ISD, which had the second largest attendance; (3) the Above I-30 ISDs, containing three school zones in the more affluent northern Dallas County; and (4) the Below I-30 ISDs, containing the seven school zones in the less affluent southern Dallas County.
3. Results
3.1. Summary characteristics
Summary statistics for the sample (N = 601) are shown in Table 1. 54 percent of daughters were 11–12 years old and the fraction of the sample in the remaining age groups decreased with age, presumably due to the fact that younger girls were more likely to be unvaccinated (an enrollment criterion for the original study). Almost all of the sample either had public insurance (e.g., Medicaid, 73.5 percent) or no insurance (24.6 percent). The average number of past missed appointments was slightly under one, 10 percent of parents had previously refused the HPV vaccine, and between 40-50 percent of the daughters had received one or more of the other recommended adolescent vaccines (i.e., flu, TDAP, meningococcal) prior to randomization.
Table 1.
Summary characteristics (N = 601)a.
| Variable | Mean (%) |
|---|---|
| Outcome | |
| Completed at Least 2 HPV Vaccine Doses | 24.6 |
| Race/Ethnicity | |
| African American, non-Hispanic | 26.1 |
| (Hispanic/White, non-Hispanic) | 73.9 |
| Child Age at Randomization (years) | 13.1 |
| Insurance Status | |
| Private Insurance | 1.8 |
| Public Insurance | 73.5 |
| (No Insurance) | 24.7 |
| Avg. Number of Missed Appointments (count) | 0.83 |
| Refused HPV Vaccine in Past | 10.2 |
| Prior Vaccine History | |
| Influenza | 48.4 |
| TDAP | 44.6 |
| MCV | 41.3 |
| Mailed Brochure | |
| Received HPV-Specific Brochure | 48.8 |
| (Received General Vaccine Brochure) | 51.3 |
| Parental Consent for Telephone Intervention | 46.8 |
| Clinic Attended | |
| (Clinic 1) | 22.5 |
| Clinic 2 | 15.3 |
| Clinic 3 | 30.8 |
| Clinic 4 | 31.4 |
| Independent School District (ISD) Zone | |
| ISD1 [Dallas] | 71.5 |
| ISD2 [Irving] | 16.8 |
| ISD3 [Above I-30] | 3.0 |
| (ISD4 [Below I-30]) | 8.7 |
All summary statistics are shown as percentages unless otherwise specified. Variables in parentheses represent reference groups.
Fig. 1 displays the Dallas county road system, the school zones, and clinic locations. Considering the geographic and healthcare access variables, the average distance traveled to attend one of the four geographically-based clinics was ∼4.4 miles (measured as the Euclidean or straight-line distance between residential address and clinic location), and over three-quarters were within one standard deviation of the mean travel distance. Over 62 percent of participants attended either Clinic 3 or 4, while only 15% attended Clinic 2 (Clinic 1 was the reference group). Similarly, 71% participants lived within Dallas ISD boundaries (ISD1), while only 3 percent resided within one of the northern Dallas County school districts (ISD2).
3.2. Moran's I results
The modified Moran's I test for the presence of spatial autocorrelation in HPV vaccine uptake indicated positive spatial autocorrelation for the outcome measure Complete ( = 2.84, < 0.01)(Lin & Zhang, 2007). Thus, geographic clustering in HPV uptake was evident in our sample.
3.3. SAR probit model results
The estimated results for the SAR probit model (1) are shown in Table 2, and are reported as marginal effects, except for the parameter, which is reported as a coefficient estimate (see LeSage, Kelley Pace, Lam, Campanella, & Liu, 2011 for a detailed discussion of marginal effects in the context of spatial probit models) (LeSage et al., 2011).
Table 2.
Direct, Indirect, and Total Marginal Effects for the SAR probit model on likelihood of completion of the HPV series (N = 601).
| ρ (Substitution Effect)± | 0.1643†† |
||
|---|---|---|---|
| Direct Effects | Indirect Effects | Total Effects | |
| HPV-Specific Brochure | 0.0385 | 0.0074 | 0.0459 |
| [-0.0238, 0.1002] | [-0.0043, 0.0264] | [-0.0288, 0.1175] | |
| Parental Consent for Telephone Intervention | 0.1546†† | 0.0303†† | 0.1849†† |
| [0.0876, 0.2239] | [0.0048, 0.0671] | [0.0996, 0.2741] | |
| African American | −0.1409†† | −0.0272†† | −0.1682†† |
| [-0.2382, −0.0500] | [-0.0688, −0.0039] | [-0.2836, −0.0599] | |
| Age: 13-14 | −0.1060† | −0.0206† | −0.1266† |
| [-0.2023, −0.0040] | [-0.0556, −0.0004] | [-0.2432, −0.0046] | |
| Age: 15-16 | −0.1288†† | −0.0248†† | −0.1536†† |
| [-0.2258, −0.0379] | [-0.0581, −0.0033] | [-0.2699, −0.0448] | |
| Age: 17-18 | −0.1390 | −0.0271 | −0.1661 |
| [-0.3030, 0.0050] | [-0.0789, 0.0007] | [-0.3577, 0.0060] | |
| Private Insurance | −0.0821 | −0.0155 | −0.0976 |
| [-0.3613, 0.1558] | [-0.0782, 0.0323] | [-0.4251, 0.1844] | |
| Public Insurance | −0.0397 | −0.0080 | −0.0477 |
| [-0.1141, 0.0347] | [-0.0282, 0.0063] | [-0.1376, 0.0417] | |
| Avg. Number Missed Appts. | −0.0325† | −0.0062† | −0.0388† |
| [-0.059, −0.0064] | [-0.0160, −0.0005] | [-0.0720, −0.0073] | |
| Refused HPV Vaccine | −0.0716 | −0.0137 | −0.0853 |
| [-0.1721, 0.0416] | [-0.0451, 0.0085] | [-0.2070, 0.0495] | |
| Influenza Vaccine | 0.0328 | 0.0065 | 0.0394 |
| [-0.0392, 0.1072] | [-0.0085, 0.0280] | [-0.0447, 0.1347] | |
| TDAP Vaccine | −0.0823 | −0.0158 | −0.0982 |
| [-0.2598, 0.0730] | [-0.0610, 0.0154] | [-0.3118, 0.0897] | |
| MCV Vaccine | 0.1781† | 0.0347† | 0.2128† |
| [0.0260, 0.3485] | [0.0021, 0.0928] | [0.0319, 0.4251] | |
| Clinic 2 | 0.0494 | 0.0093 | 0.0588 |
| [-0.1068, 0.2219] | [-0.0242, 0.0507] | [-0.1319, 0.2644] | |
| Clinic 3 | −0.0486 | −0.0095 | −0.0581 |
| [-0.1232, 0.0329] | [-0.0311, 0.0051] | [-0.1488, 0.0390] | |
| Clinic 4 | −0.0049 | −0.0008 | −0.0057 |
| [-0.1013, 0.0924] | [-0.0229, 0.0204] | [-0.1265, 0.1101] | |
| ISD1 | −0.1553†† | −0.0296†† | −0.1850†† |
| [-0.2324, −0.0767] | [-0.0680, −0.0057] | [-0.2779, −0.0926] | |
| ISD2 | −0.2306†† | −0.0439†† | −0.2746†† |
| [-0.4166, −0.0603] | [-0.1096, −0.0047] | [-0.4879, −0.0714] | |
| ISD3 | −0.2026† | −0.0389† | −0.2415† |
| [-0.4096, −0.0139] | [-0.1059, −0.0014] | [-0.4844, −0.0171] |
Note: Significance displayed as typical for estimation using spatial models. Confidence intervals are shown in brackets. † indicates significance at 0.05 and 0.95 CI; †† indicates significance at 0.01 and 0.99 CI.
± Estimate for ρ is a parameter estimate (marginal effects not estimable). The standard deviation of ρ is 0.0703.
The coefficient estimate for was highly significant and indicated a positive association between HPV vaccination uptake and the average neighborhood vaccination rate ( = 0.1634, p < .01). In other words, individuals were more likely to become vaccinated when the average neighborhood vaccination rate was higher. Considering the free riding hypothesis, we found no evidence for free riding. Adolescent females who were African American were less likely to complete the series, compared to Hispanics, and we observed a clear age effect: older girls were less likely to complete compared to the reference group of 11–12 year olds (the youngest age-group). The only recommended vaccine that was significantly related to HPV vaccination was the MCV vaccine, where receipt was positively related to completion of the HPV series. Finally, residing in the Dallas, Irving or Above I-30 ISDs were all negatively related to the likelihood of vaccination completion, as compared to the Below I-30 ISDs.
3.4. Robustness tests: neighborhood definition
Additionally, we tested the extent to which results were sensitive to the definition of neighborhood. In our main specification, 65% of study participants had >3 neighbors; however, this also means that 35% of study participants had only 1 or 2 neighbors. Thus, we tested the extent to which results were sensitive to the definition of neighborhood. The results for the direction, significance and relative magnitude of the direct and indirect average marginal effects, and total effects were insensitive to alternative weights matrix specifications. In particular, results were insensitive to the 5-nearest neighbors weight matrix specification, which overcomes a limitation of the primary specification by assigning all study participants a minimum of 5 neighbors. Next, we tested for spatial decay in the estimated by using successive iterations of more distant neighbors (distance rings of 0.5 miles). Estimates of decreased in magnitude and significance as neighbors became more distant.
3.5. Robustness tests: control for timing of vaccination decision (modified SAR probit model)
The estimated results for the modified SAR probit model (2) are shown in Table 3. The endogenous coefficient for the average vaccination rate of neighbors who had not yet initiated was positive but small ( = 0.0314), and significant at the 0.05 level. Thus, it appears that unobservable spatially varying factors were causing endogeneity in the SAR probit model, and failing to control for them may have consequently biased results. However, in the modified SAR probit model, while controlling for potential endogeneity, we also observed a statistically significant positive association between the average neighborhood-level initiation rate of the HPV vaccine () and individual vaccination uptake: there was a statistically significant direct effect of a 38.6 percent increase in the probability of completing the HPV vaccine series for each 1 unit increase in the average neighborhood initiation rate, while the cumulative indirect (or spillover) effects were positive (indirect effect of 0.04 percent) but insignificant. In our sample the average study participant had 4.5 neighbors. If 1 additional neighbor becomes vaccinated, then the average vaccination rate (assuming all neighbors are equal distance) increases by ¼.5. Thus if 1 additional neighbor becomes vaccinated, then the reference individual's vaccination likelihood increases by approximately 8.6% (y = 38.6*(1/4.5)). Again, we find no evidence in support of the free rider hypothesis.
Table 3.
Direct, Indirect, and Total Marginal Effects for the modified SAR probit model on likelihood of completion of the HPV series (N = 601).
| ρNI (Effect of “Not Initiated” Neighbors)± | 0.0314† |
|||
|---|---|---|---|---|
| Direct Effects | Indirect Effects | Total Effects | ||
| ρI (Substitution Effect)± | 0.3863†† | 0.0004 | 0.3868†† | |
| [0.1684, 0.6247] | [-0.0064, 0.0140] | [0.1702, 0.6231] | ||
| HPV-Specific Brochure | 0.0300 | 0.0000 | 0.0300 | |
| [-0.0348, 0.0900] | [-0.0009, 0.0015] | [-0.0343, 0.0904] | ||
| Parental Consent for Telephone Intervention | 0.1477†† | 0.0002 | 0.1479†† | |
| [0.0815, 0.2099] | [-0.0023, 0.0053] | [0.0811, 0.2115] | ||
| African American | −0.1297†† | −0.0001 | −0.1298†† | |
| [-0.2309, −0.0375] | [-0.0050, 0.0022] | [-0.2328, −0.0377] | ||
| Age: 13-14 | −0.0979† | −0.0001 | −0.0980† | |
| [-0.1918, −0.0031] | [-0.0039, 0.0018] | [-0.1915, −0.0031] | ||
| Age: 15-16 | −0.1201† | −0.0001 | −0.1202† | |
| [-0.2149, −0.0248] | [-0.0049, 0.0023] | [-0.2136, −0.0248] | ||
| Age: 17-18 | −0.1184 | −0.0001 | −0.1186 | |
| [-0.2655, 0.0118] | [-0.0051, 0.0021] | [-0.2648, 0.0119] | ||
| Private Insurance | −0.0974 | −0.0001 | −0.0975 | |
| [-0.3912, 0.1646] | [-0.0059, 0.0034] | [-0.3872, 0.1676] | ||
| Public Insurance | −0.0461 | −0.0000 | −0.0462 | |
| [-0.1250, 0.0277] | [-0.0022, 0.0011] | [-0.1245, 0.0275] | ||
| Avg. Number Missed Appts. | −0.0372†† | −0.0000 | −0.0373†† | |
| [-0.0675, −0.0093] | [-0.0016, 0.0006] | [-0.0671, −0.0093] | ||
| Refused HPV Vaccine | −0.0708 | −0.0001 | −0.0710 | |
| [-0.1922, 0.0401] | [-0.0041, 0.0017] | [-0.1918, 0.0398] | ||
| Influenza Vaccine | 0.0273 | 0.0000 | 0.0273 | |
| [-0.0457, 0.1010] | [-0.0009, 0.0017] | [-0.0452, 0.1018] | ||
| TDAP Vaccine | −0.0739 | −0.0001 | −0.0741 | |
| [-0.2424, 0.0954] | [-0.0038, 0.0020] | [-0.2421, 0.0947] | ||
| MCV Vaccine | 0.1638 | 0.0002 | 0.1640 | |
| [-0.0220, 0.3271] | [-0.0031, 0.0067] | [-0.0217, 0.3249] | ||
| Clinic 2 | 0.0477 | 0.0000 | 0.0477 | |
| [-0.1193, 0.2133] | [-0.0022, 0.0038] | [-0.1197, 0.2114] | ||
| Clinic 3 | −0.0325 | −0.0000 | −0.0326 | |
| [-0.1288, 0.0565] | [-0.0021, 0.0012] | [-0.129, 0.0566] | ||
| Clinic 4 | −0.0101 | −0.0000 | −0.0102 | |
| [-0.1084, 0.0952] | [-0.0016, 0.0011] | [-0.1076, 0.0952] | ||
| ISD1 | −0.1913†† | −0.0002 | −0.1916†† | |
| [-0.2728, −0.1097] | [-0.0076, 0.0031] | [-0.2710, −0.1085] | ||
| ISD2 | −0.2680†† | −0.0003 | −0.2684†† | |
| [-0.4349, −0.0941] | [-0.0106, 0.0044] | [-0.4383, −0.0954] | ||
| ISD3 | −0.2788†† | −0.0004 | −0.2793†† | |
| [-0.5036, −0.0547] | [-0.0120, 0.0048] | [-0.5069, −0.0548] | ||
Note: Significance displayed as typical for estimation using spatial models. Confidence intervals are shown in brackets. † indicates significance at 0.05 and 0.95 CI; †† indicates significance at 0.01 and 0.99 CI.
± Estimate for ρNI is a parameter estimate (marginal effects not estimable). The standard deviation of ρNI is 0.0288.
4. Discussion
Individuals were more likely to receive the HPV vaccine when the neighborhood vaccination rate was higher at the time they were making the vaccination decision. Importantly, our results were robust to inclusion of the vaccination uptake that occurred after the vaccination decision as an important control for unobserved norms that may have generated higher/lower vaccination rates in some neighborhoods. We found that individuals were more likely to receive the HPV vaccine when the neighborhood vaccination rate was higher, and this result remained after controlling for the possibility of spatially varying shared norms and beliefs. We found no evidence that free riding behavior in HPV vaccination decisions among people living near to each other was driving the observed geographic clustering of HPV vaccination rates. Notably, our results are consistent with: 1) more recent work done by behavioral economists studying free riding exceptions (e.g., Verelst et al., 2018); and 2) qualitative studies investigating parent-reported motivation for vaccinating (e.g., Sobo, 2015; Sobo, 2016). Our findings also help explain documented geographic disparities in HPV vaccination. Nevertheless, our study only examined geographic patterns in HPV vaccination uptake. We concluded that we observed spatial patterns that are inconsistent with what would be expected if free riding were occurring and thus our lack of evidence for free-riding in HPV vaccination behavior is limited to spatial patterns that are inconsistent with what would be expected if free riding were occurring.
Our results are based on HPV uptake data from 2011 to 2012. While some have commented that the determinants of vaccine hesitancy have evolved (Gowda & Dempsey, 2013), much of the work examining vaccine hesitancy was conducted in Europe and consisted of cross-sectional observational studies (Karafillakis et al., 2019). Interpretation of our work would benefit from similar studies conducted using longitudinal electronic health record data in the U.S.
4.1. Free riding and economic theory
Our study is the first to empirically examine free riding in HPV vaccination, and is consistent with evidence from many experimental studies of decisions about contributing toward public goods that suggest free riding does not occur as frequently as posited by economic theory (Fischbacher et al., 2001; Isaac et al., 1984; Marwell & Ames, 1981).
Behavioral economists have shown that individuals frequently deal with complex decision-making through use of heuristics (simplified decision rules) or obtaining cues about the “correct” answer (Bertrand, Mullainathan, & Shafir, 2006; Mullainathan & Shafir, 2014). The cost-benefit assessment required to rationally consider the HPV vaccine decision is quite complex involving consideration of future health behaviors and risks. In the case of a socially divisive vaccine such as HPV, cues and heuristics are likely to come from the behaviors of others in one's peer group (Akerlof, 1997). For instance, in communities with high vaccination rates, it may be socially unacceptable to remain unvaccinated because unvaccinated individuals risk passing the HPV infection on to others. In this case, vaccination is a signal of personal and social responsibility: parents acknowledge and accept that their child may engage in sexual activity, and act in a way to both protect their own child as well as their child's future sexual partners. In contrast, in communities with low vaccination rates, it may be socially unacceptable to have one's child vaccinated, since vaccination may signal acceptance of youth sexual promiscuity or a deviation from social norms questioning the value of vaccines.
Our results suggest that the impure public goods model applied to the case of HPV vaccination behavior may provide a means for modeling the complex decisions associated with preventive health behaviors that produce both social- and health-related externalities. Under this framework, vaccination produces two “joint products”: (1) individual immunity to HPV (a private consumption good) and (2) lower community-level HPV risk (a public good) (Cornes & Sandler, 1984, 1994; Leonard, 2016). Free riding would not be observed when complementarities exist between the public and private benefits created by the joint products because the private consumption good (individual immunity) is perceived as having more value when the public consumption good (lower community risk because others are also vaccinated) is more abundant (Cornes & Sandler, 1994; Murdoch & Sandler, 1984; Vicary, 1997). For HPV vaccination decisions, these complementarities may occur because perceived risk of HPV infection is likely driven by social factors (i.e. risk is high if my friends are vaccinating their kids) rather than by objective information (i.e. risk is lowered when more kids are vaccinated) (Allen et al., 2010; Leader, Weiner, Kelly, Hornik, & Cappella, 2009). Alternatively, complementarities might occur if the positive feelings emerging from vaccination decisions made as a form of social responsibility or self-love are heightened when shared with others possessing the same motives. This occurs when individuals’ vaccination decisions are primarily motivated by the social consequences of their decisions, rather than by risk of contracting the disease.
4.2. Geographic disparities in HPV vaccine uptake
Our modified SAR probit model showed a positive and statistically significant direct effect, meaning that individuals had a higher probability of completing the HPV vaccine series when the average neighborhood initiation rate was higher at the time they were making their decisions. Interestingly, while the total effect was also positive and highly significant, the indirect or spatial spillover effect was close to zero and insignificant. This indirect spillover effect may be interpreted as in Wang, Kockelman, and Damien (2014). If some type of social mechanism (e.g., conformity) is driving the direct effect, the insignificant indirect effect suggests that there is a limit to the degree of influence conformism has on individual decisions. There are likely local clusters of varying cultural or social perceptions of the vaccine. These clusters may generate local complementarities in the joint products, but these local complementarities may not be strong enough to overcome clusters of opposing beliefs.
Our results suggest that policies aimed directly at publicly held beliefs may be effective at ameliorating geographic disparities in HPV vaccination rates. This is supported by several descriptive studies that found an association between perceived norms and HPV vaccination (Gerend & Shepherd, 2012; Leader et al., 2009). However, it is challenging to change norms and beliefs. Another traditional approach to improving vaccination is school entry requirements; however, these laws are rare for the HPV vaccine in the U.S. (only 2 states, Virginia and Rhode Island, and 1 territory, DC); these laws are unlikely to garner political support until local norms favoring vaccination are strengthened.
4.3. Limitations
There are some limitations to this study. Data were only collected for the duration of the 12-month study window and may be censored. In addition, while the vaccine series should be completed over a 6-month time period, adolescents visit the doctor infrequently leading longer intervals between doses. Consequently, in our study, if a patient began the series late in the study window, we may have incomplete ascertainment of completion. However, this issue applied to few in our sample; over three-quarters completed the vaccine series under the guidelines recommending 2 doses for those under age 15. It was also possible, but unlikely, that a patient received the vaccine at a different clinic or moved out of the geographic area during the study.
We also did not know precisely the degree to which participants interacted with other participants who lived within 0.5 miles. However, participants who gave parental consent were asked about the vaccination behaviors of others in their neighborhood, and 43% reported knowing whether or not other parents were getting their daughters vaccinated at the time of their study visit. This question was posed to parents before receiving a recommendation to have their daughters vaccinated, and we suspect this proportion was likely to increase after parents made a vaccination decision during the visit. Nevertheless, we were unable to ask parents specifically about what factors influenced their HPV vaccination decisions. Future primary data collection related to parents’ attitudes and beliefs and the influence of others is an important area of investigation to clarify the context of HPV vaccination decisions.
Our study implicitly assumed that geographic neighbors attending the same clinic are the appropriate peer group when making HPV vaccination decisions. We contend this was a reasonable proxy, particularly since publicly-insured and un-insured patients in Dallas have few alternative options to receive the HPV vaccine. Further, for our low-income adolescent sample, school assignment is usually geographically based, again supporting geographic peers as a reasonable proxy. Future research should consider measuring parental awareness about others' HPV vaccine decisions at follow-up clinic visits to support our assertion that others’ vaccination decisions are internalized by a parent and influenced his/her decision. This research will advance our understanding of social mechanisms in health decision-making, particularly the health outcome contexts the traditional assumption of free riding behavior may be influenced by individuals considering the social consequences of their choices. Further, alternative specifications of the relevant peer or geographic network for adolescents should be tested.
Finally, our study was limited to a single urban county, Dallas Texas. External validity of results to other dense urban areas and to less dense suburban or rural areas is unknown. However, Dallas exhibits spatial clustering of low vaccination and low overall rates of HPV vaccination; both symptoms of sub-optimal vaccine uptake that are observed in other areas (Finney Rutten et al., 2017; Pruitt & Schootman, 2010; Wei et al., 2013). It is important to note that national surveys on adolescent HPV vaccination are unlikely to have a large enough sample size of proximally located individuals to test this hypothesis. We encourage other studies in other regions using our novel methods to better understand spatial patterns in vaccine uptake.
4.4. Conclusion
We found that individuals were more likely to complete the HPV vaccination series when others in their neighborhood had already completed the series, however this locally reinforcing behavior appeared to be limited. Our results along with other studies suggests that the limitation may occur because strong locally-held beliefs serve as a buffer against the “spread” of similar vaccination decisions. Our results provide no evidence to support the traditional economic theory of public good provision and provide suggestive evidence supporting the impure public goods model which provides a means for modeling complex HPV vaccination decisions that produce both social- and health-related externalities.
Funding
The 2-arm randomized controlled trial evaluating a multi-component intervention was funded by the Cancer Prevention and Research Institute of Texas (CPRIT PP 100047), PI was study author Tiro.
Declaration of interest
The authors have no financial disclosures to report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2019.100421.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Akerlof G.A. Social distance and social decisions. Econometrica: Journal of the Econometric Society. 1997:1005–1027. [Google Scholar]
- Allen J.D., Othus M.K., Shelton R.C., Li Y., Norman N., Tom L. Parental decision making about the HPV vaccine. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2187–2198. doi: 10.1158/1055-9965.EPI-10-0217. [DOI] [PubMed] [Google Scholar]
- Andreoni J. Why free ride?: Strategies and learning in public goods experiments. Journal of Public Economics. 1988;37:291–304. [Google Scholar]
- Bartlett J.A., Peterson J.A. The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States a review of the literature. The Journal of School Nursing. 2011;27:434–446. doi: 10.1177/1059840511415861. [DOI] [PubMed] [Google Scholar]
- Berezin M., Eads A. Risk is for the rich? Childhood vaccination resistance and a culture of health. Social Science & Medicine. 2016;165:233–245. doi: 10.1016/j.socscimed.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Beron K.J., Murdoch J.C., Vijverberg W.P. Why cooperate? Public goods, economic power, and the montreal protocol. The Review of Economics and Statistics. 2003;85:286–297. [Google Scholar]
- Bertrand M., Mullainathan S., Shafir E. Behavioral economics and marketing in aid of decision making among the poor. Journal of Public Policy and Marketing. 2006;25:8–23. [Google Scholar]
- Betsch C., Böhm R., Korn L. Inviting free-riders or appealing to prosocial behavior? Game-theoretical reflections on communicating herd immunity in vaccine advocacy. Health Psychology. 2013;32:978. doi: 10.1037/a0031590. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J., Hyde T., Tu P. Macmillan International Higher Education; 2013. Health economics. [Google Scholar]
- Boulier B.L., Datta T.S., Goldfarb R.S. Vaccination externalities. The B.E. Journal of Economic Analysis & Policy. 2007;7 [Google Scholar]
- Brewer N.T., Fazekas K.I. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Preventive Medicine. 2007;45:107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Browne K. The measles and free riders: California's mandatory vaccination law. Cambridge Quarterly of Healthcare Ethics. 2016;25:472–478. doi: 10.1017/S0963180116000116. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] 2016. CDC recommends only two HPV shots for younger adolescents. [Google Scholar]
- Centers for Disease Control and Prevention [CDC] 2016. Vaccines for children program (VFC) [Google Scholar]
- Cornes R., Sandler T. Easy riders, joint production, and public goods. The Economic Journal. 1984:580–598. [Google Scholar]
- Cornes R., Sandler T. The comparative static properties of the impure public good model. Journal of Public Economics. 1994;54:403–421. [Google Scholar]
- Cornes R., Sandler T. Cambridge University Press; 1996. The theory of externalities, public goods, and club goods. [Google Scholar]
- Ferris, R. (1/28/15). US measles outbreak is bad, and it's getting worse. In CNBC (Ed.).
- Finney Rutten L.J., Wilson P.M., Jacobson D.J., Agunwamba A.A., Radecki Breitkopf C., Jacobson R.M. A population-based study of sociodemographic and geographic variation in HPV vaccination. Cancer Epidemiology Biomarkers & Prevention. 2017;26(4):533. doi: 10.1158/1055-9965.EPI-16-0877. LP – 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbacher U., Gächter S., Fehr E. Are people conditionally cooperative? Evidence from a public goods experiment. Economics Letters. 2001;71:397–404. [Google Scholar]
- Garcini L., Galvan T., Barnack-Tavlaris J. The study of human papillomavirus (HPV) vaccine uptake from a parental perspective: A systematic review of observational studies in the United States. Vaccine. 2012;30:4588–4595. doi: 10.1016/j.vaccine.2012.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffard P.-Y., Philipson T. Disease eradication: Private versus public vaccination. The American Economic Review. 1997;87:222–230. [Google Scholar]
- Gerend M.A., Shepherd J.E. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Annals of Behavioral Medicine. 2012;44:171–180. doi: 10.1007/s12160-012-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C., Dempsey A.F. The rise (and fall?) of parental vaccine hesitancy. Human Vaccines & Immunotherapeutics. 2013;9(8):1755–1762. doi: 10.4161/hv.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix K.S., Sturm L.A., Zimet G.D., Meslin E.M. Ethics and childhood vaccination policy in the United States. American Journal of Public Health. 2016;106:273–278. doi: 10.2105/AJPH.2015.302952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman D.M., Benard V., Roland K.B., Watson M., Liddon N., Stokley S. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA pediatrics. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuka Y., Li M., Vietri J., Chapman G.B., Galvani A.P. Free-riding behavior in vaccination decisions: An experimental study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R.M., Walker J.M., Thomas S.H. Divergent evidence on free riding: An experimental examination of possible explanations. Public Choice. 1984;43:113–149. [Google Scholar]
- Karafillakis E., Simas C., Jarrett C., Verger P., Peretti-Watel P., Dib F. HPV vaccination in a context of public mistrust and uncertainty: A systematic literature review of determinants of HPV vaccine hesitancy in Europe. Human Vaccines & Immunotherapeutics. 2019;1–13 doi: 10.1080/21645515.2018.1564436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.M. Measles outbreak associated with a church Congregation . A Study of Immunization Attitudes of Congregation Members. 2008;123(April):126–134. doi: 10.1177/003335490812300205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac P., Megiddo I., Grenfell B.T., Laxminarayan R. Self-enforcing regional vaccination agreements. Journal of The Royal Society Interface. 2016;13:20150907. doi: 10.1098/rsif.2015.0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchen M.J., Moore M.R. Private provision of environmental public goods: Household participation in green-electricity programs. Journal of Environmental Economics and Management. 2007;53:1–16. [Google Scholar]
- Leader A.E., Weiner J.L., Kelly B.J., Hornik R.C., Cappella J.N. Effects of information framing on human papillomavirus vaccination. Journal of Women's Health. 2009;18:225–233. doi: 10.1089/jwh.2007.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. Housing upkeep and public good provision in residential neighborhoods housing upkeep and public good provision in residential neighborhoods. Housing Policy Debate. 2016 doi: 10.1080/10511482.2015.1137966. [DOI] [Google Scholar]
- LeSage J.P. 2010. Econometrics Toolbox. [Google Scholar]
- LeSage J.P., Kelley Pace R., Lam N., Campanella R., Liu X. New orleans business recovery in the aftermath of hurricane katrina. Journal of the Royal Statistical Society: Series A. 2011;174:1007–1027. [Google Scholar]
- LeSage J.P., Pace R.K. CRC Press; 2009. Introduction to spatial econometrics (statistics, textbooks and monographs) [Google Scholar]
- Lin G., Zhang T. Loglinear residual tests of Moran's I autocorrelation and their applications to Kentucky breast cancer data. Geographical Analysis. 2007;39:293–310. [Google Scholar]
- Marwell G., Ames R.E. Economists free ride, does anyone else?: Experiments on the provision of public goods, IV. Journal of Public Economics. 1981;15:295–310. [Google Scholar]
- McComb E., Ramsden V., Olatunbosun O., Williams-Roberts H. Knowledge, attitudes and barriers to human papillomavirus (HPV) vaccine uptake among an immigrant and refugee catch-up group in a western Canadian province. Journal of Immigrant and Minority Health. 2018:1–5. doi: 10.1007/s10903-018-0709-6. [DOI] [PubMed] [Google Scholar]
- Molina C., Earn D.J. Game theory of pre-emptive vaccination before bioterrorism or accidental release of smallpox. Journal of The Royal Society Interface. 2015;12:20141387. doi: 10.1098/rsif.2014.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullainathan S., Shafir E. 2014. Scarcity: The new science of having less and how it defines our lives. [Google Scholar]
- Murdoch J.C., Sandler T. Complementarity, free riding, and the military expenditures of NATO allies. Journal of Public Economics. 1984;25:83–101. [Google Scholar]
- Pace R.K., LeSage J. Chapman &Hall/CRC; Boca Raton, FL: 2009. Introduction to spatial econometrics. [Google Scholar]
- Pruitt S.L., Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. American Journal of Preventive Medicine. 2010;38:525–533. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri-Sheriff M., Hendrix K.S., Downs S.M., Sturm L.A., Zimet G.D., Finnell S.M.E. The role of herd immunity in parents' decision to vaccinate children: A systematic review. Pediatrics. 2012;130(3):522–530. doi: 10.1542/peds.2012-0140. [DOI] [PubMed] [Google Scholar]
- Sandler T., Murdoch J.C. Nash-cournot or lindahl behavior?: An empirical test for the NATO allies. Quarterly Journal of Economics. 1990;105:875–894. [Google Scholar]
- Sobo E.J. Social cultivation of vaccine refusal and delay among Waldorf (Steiner) school parents. Medical Anthropology Quarterly. 2015;29:381–399. doi: 10.1111/maq.12214. [DOI] [PubMed] [Google Scholar]
- Sobo E.J. What is herd immunity, and how does it relate to pediatric vaccination uptake? US parent perspectives. Social Science & Medicine. 2016;165:187–195. doi: 10.1016/j.socscimed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Tiro J.A., Pruitt S.L., Bruce C.M., Persaud D., Lau M., Vernon S.W. Multilevel correlates for human papillomavirus vaccination of adolescent girls attending safety net clinics. Vaccine. 2012;30:2368–2375. doi: 10.1016/j.vaccine.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Tiro J.A., Sanders J.M., Pruitt S.L., Stevens C.F., Skinner C.S., Bishop W.P. Promoting HPV vaccination in safety-net clinics: A randomized trial. Pediatrics. 2015;136:850–859. doi: 10.1542/peds.2015-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verelst F., Willem L., Kessels R., Beutels P. Individual decisions to vaccinate one's child or oneself: A discrete choice experiment rejecting free-riding motives. Social Science & Medicine. 2018;207:106–116. doi: 10.1016/j.socscimed.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Vicary S. Joint production and the private provision of public goods. Journal of Public Economics. 1997;63:429–445. [Google Scholar]
- Wei F., Moore P.C., Green A.L. Geographic variability in human papillomavirus vaccination among US young women. American Journal of Preventive Medicine. 2013;44:154–157. doi: 10.1016/j.amepre.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yiyi, Kockelman Kara M., Damien Paul. A spatial autoregressive multinomial probit model for anticipating land-use change in Austin, Texas. The Annals of Regional Science. 2014;52(1):251–278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.